Abstract
Objectives
This study investigates the effects of controlled reoxygenation cardiopulmonary bypass on oxidative stress, inflammatory response, and organ function in children undergoing repair of cyanotic congenital heart defects.
Methods
Sixty-seven cyanotic patients (median age 15 months, interquartile range 6–49 months) undergoing corrective cardiac surgery were randomized to receive either controlled normoxic (50–0 mm Hg; n = 35) or hyperoxic (150–180 mm Hg; n = 32) cardiopulmonary bypass. Troponin I and 8-isoprostane, C3a, interleukins 6, 8, and 10, cortisol, protein S100, and alpha-glutamate transferase were measured preoperatively and 10 and 30 minutes after starting bypass, on removal of the aortic crossclamp, and 12 and 24 hours thereafter.
Results
Overall, troponin I and 8-isoprostane levels were lower in the controlled normoxic group (−29%, 95% CI −48% to −3%, P = .03, and −26%, 95% CI −44% to −2%, P = .03, respectively). Protein S100 release was also lower in the normoxic group 10 minutes after starting bypass (−26%, 95% CI −40% to −9%, P = .005) and 10 minutes after aortic crossclamp removal (−23%, 95% CI −38% to −3%, P = .02, respectively), but similar at other time points in the two groups (P ≥ .17). The alpha-glutamate transferase release was significantly lower in the normoxic group 10 minutes after aortic crossclamp removal (−28%, 95% CI −44% to −9%, P = .006, respectively) but was similar at other times (P ≥ .11). Release of C3a, interleukins 6, 8, and 10, and cortisol was similar in the two groups throughout (P ≥ .15).
Conclusion
Controlled reoxygenation on starting cardiopulmonary bypass is associated with reduced myocardial damage, oxidative stress, and cerebral and hepatic injury compared with hyperoxic bypaass and similar whole body inflammatory and stress response in cyanotic children undergoing open cardiac surgery.
Repair of heart defects causing cyanosis is performed with cardiopulmonary bypass (CPB) in early age with increasing frequency. Postoperative cardiac dysfunction is the major cause of morbidity and mortality despite successful surgical correction.1,2 There is substantial experimental evidence for the existence of a myocardial reoxygenation injury in the cyanotic, immature heart. Studies of hypoxemia/reoxygenation in immature piglets have provided evidence for oxygen-mediated myocardial injury as a result of hyperoxemia in the setting of previous cyanosis.3,4 We5 have shown that cyanotic children have more myocardial reperfusion injury and poorer clinical outcomes than do acyanotic ones after similar periods of ischemic cardioplegic arrest. More recently, we6 have shown that reintroduction of high oxygen levels to cyanotic patients supported by CPB leads to myocardial damage before ischemic cardioplegic arrest, suggesting a CPB-induced mechanism of reoxygenation injury.
Hypoxia and reoxygenation have also emerged in recent years as very important mechanisms of cerebral and hepatic injury.7,8 Matheis and associates,9 in a small, observational study, demonstrated that uncontrolled hyperoxic reoxygenation on CPB for surgical correction of congenital heart defects was associated with higher S100 levels in cyanotic infants as compared with acyanotic patients undergoing comparable operations, while hepatic reoxygenation injury has been demonstrated in experimental animal models.10
Overall, these experimental and clinical studies provide direct evidence that an unintended reoxygenation injury occurs in cyanotic patients with the initiation of CPB, resulting in organ damage. We hypothesized that controlling reoxygenation during CPB could prevent the deleterious effect of hyperoxic CPB on organ function. This randomized controlled trial, therefore, investigates the effects of controlled normoxic (50–80 mm Hg) and relatively hyperoxic (150–180 mm Hg) CPB on oxidative stress, inflammatory response, and organ damage in infants and children undergoing repair of cyanotic congenital heart defects.
PATIENTS AND METHODS
Sixty-seven cyanotic patients undergoing corrective cardiac surgery between January 2003 and November 2006 at the Bristol Royal Hospital for Children were randomized to receive either controlled normoxic (50–80 mm Hg) or hyperoxic (150–180 mm Hg) CPB. All patients were in a stable condition without preoperative respiratory or inotropic support. “Normoxia” referred to a pump prime, the Po2 of which is matched to the Po2 of the patient (ie, normoxic for the patient). “Hyperoxia” referred to a pump prime prepared to the current “best practice” protocol, which has a Po2 relatively hyperoxic for a cyanotic patient. The study was approved by the Research Ethics Committee of the Bristol Royal Hospital for Children and parental informed consent was gained for all patients. Treatment allocations, stratified by age (<6 months vs ≥6 months), were generated by computer in advance of starting the study, using block randomization with varying block sizes. Allocation details were concealed in sequentially numbered, opaque sealed envelopes. After consent was obtained the night before the operation, a patient was randomly assigned from the next numbered envelope. The surgical team, except for the perfusionists, was blind to the treatment allocation.
CPB, Anesthetic, and Surgical Technique
All operations were performed with CPB. Intraoperative anesthetic and operative techniques were standardized as previously reported.11 However, in the normoxic group, induction of anesthesia was started with a forced expiratory volume in 1 second (FIO2) of 21% and in the hyperoxic group with an FIO2 of 50%. Continuous pulse oximetry was used in every patient from the start of anesthesia.
Cold blood (4°C–6°C) St Thomas Hospital I–based cardioplegic solution (4:1 dilution blood/St Thomas Hospital I crystalloid cardioplegic solution) was used for myocardial preservation, with the following composition (mmol/L): 16 MgCl2, 2 CaCl2, 20 KCl, 147 NaCl, and 1.0 procaine HCl. Additional cardioplegic solution was administered after each 20 minutes of aortic crossclamping.
After the operation, all patients were admitted to the pediatric intensive care unit and were managed according to unit protocols5,11 by intensivists and pediatric cardiologists blinded to the treatment allocation.
The reoxygenation strategy on starting CPB was developed at our Department of Clinical Perfusion Science as follows:
Normoxic CPB group
The CPB circuit is set up and primed as per protocol,5,6 usually with a red blood cell/albumin prime or occasionally a clear prime, depending on the patient’s hemoglobin level. Just before the initiation of CPB, medical nitrogen is delivered to the gas exchange device (oxygenator) via a bacteriologic filter (0.2 μm) at a rate of between 100 and 200 mL/min while the prime is circulated at approximately 1000 mL/min. An in-line Po2 monitor is used to measure the Po2 of the prime (in air this will equilibrate at roughly 150 mm Hg). Using this technique, we are able to reduce the Po2 of the prime fluid to less than 100 mm Hg and actually match that of the patient’s own (cyanotic) arterial Po2 levels. Finally, before CPB has been established, the prime Po2 is confirmed using a point of care blood gas analyzer, and the in-line Po2 monitor is calibrated. CPB is initiated in this “iso-oxic” manner and the Po2 levels of the arterialized blood are adjusted accordingly during CPB, terminating CPB with an arterial Po2 of between 100 and 110 mm Hg.
Hyperoxic CPB group
Oxygen delivery is run at 100% to keep arterial oxygen saturation greater than 95% and Po2 levels between 150 and 180 mm Hg on starting CPB.
Outcome Variables
The primary end points were the release of troponin I (enzyme-linked immunosorbent assay; Access Immunoassay System; Beckman Instruments Inc, Fullerton, Calif) and 8-isoprostane (enzyme immunoassay [EIA]; Cayman Chemicals, Ann Arbor, Mich) as measurements of myocardial cell damage and oxidative stress, and the release of markers of the whole body inflammatory response (complement activation [C3a] and interleukin [IL] 6, IL-8, and IL-10) (enzyme-linked immunosorbent assay; Amersham Biosciences UK, Little Chalfont, United Kindom), and stress response (cortisol; Access Immunoassay System, Beckman Coulter). Cerebral injury was assed by the postoperative release of protein S100 (CanAg S100 EIA; CanAg Diagnostics AB, Goteborg, Sweden) and alpha-glutathione S-transferase (άGT) (Biotrin High Sensitivity Alpha GST EIA Assay; Biotrin International, Dublin, Ireland) was used to assess hepatic cell damage. Blood (5 mL) was drawn preoperatively, at 10 and 30 minutes after starting CPB, and 10 minutes, 12, and 24 hours after coming off CPB. This was immediately centrifuged at 4°C, at 4000 rpm for 15 minutes. The resulting plasma was then frozen in liquid nitrogen before storage at −80°C. The assays were performed by a laboratory technician blinded to the treatment allocation and clinical status of the patient. Clinical outcomes (duration of inotropic support after surgery, intubation time, intensive care unit stay, postoperative hospital stay) were also recorded. The sample size was calculated on the basis of previous similar studies carried out at our institution in which statistically significant reductions in troponin I release (effect size 0.48) and in 8-isoprostane (effect size 0.94) were found with 55 and 42 patients, respectively.11 With one preoperative and five postoperative measurements, a sample size of 32 per group has 90% power to detect effect sizes of .5 or more for both markers at the 5% level of statistical significance (2-tailed) assuming a correlation of .7 between preoperative and postoperative values and between postoperative measures.
Statistical Analysis
Continuous outcomes are summarized as an arithmetic mean and standard deviation if normally distributed or as a geometric mean or median and interquartile range if skewed. Categorical data are presented as actual counts and percentages. Operative and postoperative characteristics measured on a continuous scale were compared by regression analysis adjusting for age. Skewed measures were log-transformed to achieve normality and the results were back transformed to the original scale. Biochemical markers measured at multiple time points were analyzed with a mixed regression model. All the markers had skewed distributions and were analyzed on the logarithmic scale. These analyses were adjusted for age, baseline response, pathology, and the interactions between pathology and time and treatment and time. Interactions between treatment and pathology were investigated and, if statistically significant at the 5% level, the interaction term was retained in the model.
Differences with respect to pathology and age are not reported explicitly; estimates are pooled over pathologies and age. Effect sizes are reported as mean differences (if normally distributed) or as ratios of geometric means (if skewed) with corresponding 95% confidence intervals (CIs) and P values. If the treatment effect was consistent across time points, an overall estimate, pooled over all time points, is reported; otherwise the treatment effect is reported for each time point separately. Length of stay was compared by Cox regression with the effect size being reported as a hazard ratio for hospital discharge with its 95% CI. Categorical outcomes were not subject to statistical analysis owing to the small number of events (<10).
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Thirty-five patients were randomized to the normoxic and 32 to the hyperoxic CPB group. Overall, the median age was 15 months (interquartile range 6–49 months) with 60% male. Reasons for surgery included tetralogy of Fallot (42%), single ventricle (40%), and transposition of the great arteries (12%). Preoperative characteristics are shown in Table 1. Intraoperative and clinical outcomes are reported in Table 2.
TABLE 1. Baseline characteristics.
| Variable | Normoxic (n = 35) | Hyperoxic (n = 32) |
|---|---|---|
| Age (m, median [IQR]) | 15.0 (6.0–47.0) | 14.5 (5.0–51.0) |
| Male (%) | 22 (63) | 18 (56) |
| Weight (kg, median [IQR]) | 9.6 (7.5–13.9) | 9.5 (6.6–15.8) |
| Renal failure (%) | 2 (6) | 3 (9) |
| Pathology (%) | ||
| Single ventricle physiology | 14 (40) | 13 (41) |
| TOF | 14 (40) | 14 (44) |
| TAPVD and TGA | 7 (20) | 5 (16) |
Not all percentages sum to exactly 100% due to rounding. IQR, Interquartile range; TOF, tetralogy of Fallot; TAPVD, total anomalous pulmonary venous defect; TGA, transposition of the great arteries
TABLE 2. Operative and postoperative details.
| Variable | Normoxic (n = 35) | Hyperoxic (n = 32) | Effect size | (95% CI) | P value |
|---|---|---|---|---|---|
| Operative details | |||||
| Oxygen saturation (mm Hg)* | 79.9 (7.61) | 78.5 (7.22) | 1.34‡ | (−2.29 to 4.97) | .46 |
| Bypass time (min)† | 82.9 (41–147) | 89.7 (43–210) | 0.93§ | (0.77 to 1.11) | .40 |
| Crossclamp time (min)* | 52.0 (20.52) | 52.3 (24.66) | −0.39‡ | (−14.16 to 13.38) | .96 |
| Postoperative details | |||||
| Ventilation time (min)† | 21.5 (2–191) | 19.6 (3–72) | 1.12§ | (0.67 to 1.87) | .66 |
| Length of stay (d)(median [IQR]) | 10.5 (6.0–14.0) | 8.5 (6.5–12.5) | 0.90|| | (0.55 to 1.50) | .67 |
| Dopamine | |||||
| Coming off bypass (μg · kg−1 · min−1) | 6.3 (3.44) | 6.8 (4.84) | 0.53‡ | (−2.61 to 1.56) | .62 |
| Peak dose (μg · kg-1 · min-1)* | 11.8 (5.22) | 9.6 (5.36) | 2.19‡ | (−0.58 to 4.96) | .12 |
| Duration (h)† | 33.5 (6–256) | 28.6 (0–366) | 1.19§ | (0.73 to 1.96) | .48 |
| Epinephrine (No. [%]) | 2 (6) | 4 (14) | |||
CI, Confidence interval; IQR, interquartile ratio.
Values are expressed as mean (SD).
Values are expressed as geometric mean with range.
Difference in means (normoxic/hyperoxic).
Ratio of geometric means (normoxic/hyperoxic).
Hazard ratio for hospital discharge (normoxic/hyperoxic).
Troponin I and 8-Isoprostane Release
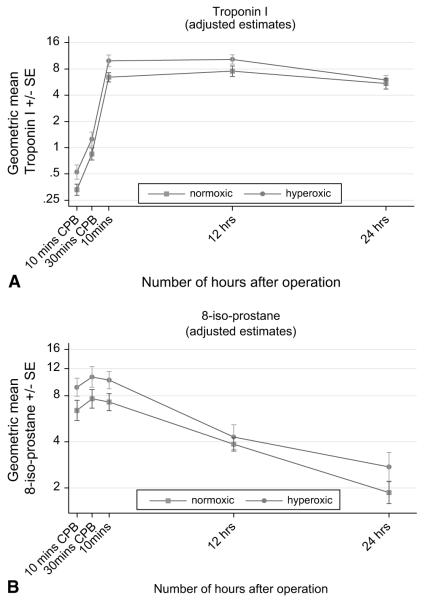
Troponin I was released in a time-dependent fashion as previously described11,12 (Figure 1, A). By 10 minutes after the release of the aortic crossclamp, troponin levels were significantly higher than baseline in both groups (Table 3). The levels continued to rise during the 12 hours after surgery and remained high (compared with baseline) at 24 hours. Overall, troponin I levels were significantly higher in the hyperoxic group (ratio [normoxic/hyperoxic] = 0.71, 95% CI 0.52–0.97, P = .03).
FIGURE 1.
A, Time-related plasma changes in the geometric mean troponin I in the hyperoxic (●) and normoxic (■) groups. B, Time-related plasma changes in the geometric mean 8-isoprostane in the hyperoxic (●) and normoxic (■) groups. CPB, Cardiopulmonary bypass; SE, standard error.
TABLE 3. Biochemical markers.
| Geometric mean* |
||||||
|---|---|---|---|---|---|---|
| Variable | Time | Normoxic | Hyperoxic | Ratio | (95% CI) | P value |
| Troponin I | (n = 32) | (n = 30) | ||||
| Preop | 0.03 | 0.02 | ||||
| 10 min on CPB | 0.3 | 0.5 | 0.63 | |||
| 30 min on CPB | 0.8 | 1.3 | 0.67 | |||
| 10 min | 6.4 | 9.9 | 0.65 | |||
| 4 h | 7.5 | 10.3 | 0.73 | |||
| 24 h | 5.4 | 6.0 | 0.91 | |||
| Test for interaction between treatment and time | .61 | |||||
| Treatment effect, pooled over all time points | 0.71 | (0.52–0.97) | .03 | |||
| 8-Isoprostane | (n = 34) | (n = 30) | ||||
| Preop | 2.8 | 2.3 | ||||
| 10 min on CPB | 6.4 | 9.1 | 0.71 | |||
| 30 min on CPB | 7.6 | 10.6 | 0.72 | |||
| 10 min | 7.3 | 10.1 | 0.72 | |||
| 4 h | 3.9 | 4.3 | 0.90 | |||
| 24 h | 1.9 | 2.7 | 0.68 | |||
| Test for common ratio | .73 | |||||
| Treatment effect, pooled over all time points | 0.74 | (0.56–0.98) | .03 | |||
| IL-6 | (n = 34) | (n = 32) | ||||
| Preop | 0.7 | 0.5 | ||||
| 10 min on CPB | 0.9 | 1.0 | 0.90 | |||
| 30 min on CPB | 1.1 | 1.1 | 1.02 | |||
| 10 min | 4.7 | 5.9 | 0.80 | |||
| 4 h | 37.6 | 43.7 | 0.86 | |||
| 24 h | 58.5 | 51.7 | 1.13 | |||
| Test for interaction between treatment and time | .72 | |||||
| Treatment effect, pooled over all time points | 0.94 | (0.66–1.33) | .71 | |||
| IL-8 | (n = 34) | (n = 32) | ||||
| Preop | 5.2 | 4.5 | ||||
| 10 min on CPB | 7.1 | 7.4 | 0.95 | |||
| 30 min on CPB | 8.1 | 9.0 | 0.90 | |||
| 10 min | 16.7 | 18.1 | 0.92 | |||
| 4 h | 36.7 | 32.2 | 1.14 | |||
| 24 h | 29.0 | 27.3 | 1.06 | |||
| Test for interaction between treatment and time | .72 | |||||
| Treatment effect, pooled over all time points | 0.99 | (0.83–1.18) | .93 | |||
| IL-10 | (n = 34) | (n = 32) | ||||
| Preop | 9.3 | 11.2 | ||||
| 10 min on CPB | 12.8 | 17.8 | 0.72 | |||
| 30 min on CPB | 15.0 | 22.4 | 0.67 | |||
| 10 min | 89.9 | 145.1 | 0.62 | |||
| 4 h | 89.6 | 105.7 | 0.85 | |||
| 24 h | 26.5 | 30.0 | 0.89 | |||
| Test for interaction between treatment and time | .67 | |||||
| Treatment effect, pooled over all time points | 0.74 | (0.49–1.12) | .15 | |||
| C3α | (n = 34) | (n = 32) | ||||
| Preop | 1416.7 | 1357.7 | ||||
| 10 min on CPB | 1247.4 | 1254.0 | 0.99 | |||
| 30 min on CPB | 1181.7 | 1467.0 | 0.81 | |||
| 10 min | 1663.4 | 1843.1 | 0.90 | |||
| 4 h | 1295.6 | 1462.2 | 0.89 | |||
| 24 h | 1029.1 | 977.6 | 1.05 | |||
| Test for interaction between treatment and time | .16 | |||||
| Treatment effect, pooled over all time points | 0.92 | (0.82–1.04) | .18 | |||
| Cortisol | (n = 29) | (n = 28) | ||||
| Preop | 485.6 | 410.2 | ||||
| 10 min on CPB | 255.2 | 262.1 | 0.97 | |||
| 30 min on CPB | 255.8 | 288.4 | 0.89 | |||
| 10 min | 216.1 | 261.6 | 0.83 | |||
| 4 h | 157.4 | 204.5 | 0.77 | |||
| 24 h | 368.4 | 297.3 | 1.24 | |||
| Test for interaction between treatment and time | .25 | |||||
| Treatment effect, pooled over all time points | 0.93 | (0.76–1.13) | .45 | |||
| S100 | (n = 33) | (n = 32) | ||||
| Preop | 226.4 | 229.5 | ||||
| 10 min on CPB | 355.7 | 481.0 | 0.74 | (0.60–0.91) | .005 | |
| 30 min on CPB | 668.6 | 695.3 | 0.96 | (0.78–1.18) | .71 | |
| 10 min | 1244.1 | 1610.0 | 0.77 | (0.62–0.97) | .02 | |
| 4 h | 327.0 | 368.0 | 0.89 | (0.75–1.05) | .17 | |
| 24 h | 228.3 | 217.8 | 1.05 | (0.80–1.37) | .73 | |
| Test for interaction between treatment and time | .004 | |||||
| αGT | (n = 33) | (n = 32) | ||||
| Preop | 3683.7 | 3810.1 | ||||
| 10 min on CPB | 3235.4 | 3830.7 | 0.84 | (0.69–1.04) | .11 | |
| 30 min on CPB | 4093.3 | 4166.0 | 0.98 | (0.82–1.18) | .85 | |
| 10 min | 6797.4 | 9496.7 | 0.72 | (0.56–0.91) | .006 | |
| 4 h | 7423.5 | 8966.9 | 0.83 | (0.60–1.15) | .26 | |
| 24 h | 3097.0 | 3943.4 | 0.79 | (0.58–1.07) | .12 | |
| Test for interaction between treatment and time | .02 | |||||
CI, Confidence interval; CPB, cardiopulmonary bypass; IL, interleukin; C3A, complement component 3a; aGT, alpha-glutathione S-transferase.
Geometric means for measurements during and after surgery are least squares estimates after adjusting for the preoperative values, age strata, pathology, the interaction between pathology and time and the interaction between treatment and pathology (where appropriate, see Statistical Methods section for further details).
The release of 8-isoprostane was also time dependent (Figure 1, B). In both groups, levels rose significantly from baseline 10 minutes after the start of CPB and remained high 10 minutes after the release of the aortic crossclamp, after which time levels declined. The pattern of response was the same in both groups. Throughout, 8-isoprostane levels were significantly higher in the hyperoxic group. On average, 8-isoprostane levels were 35% higher in the hyperoxic group (ratio ([normoxic/hyperoxic] = 0.74, 95% CI 0.56–0.98, P = .03). In addition, there was no evidence to suggest time-related differences between the two groups (P = .62).
Protein S100 and άGT
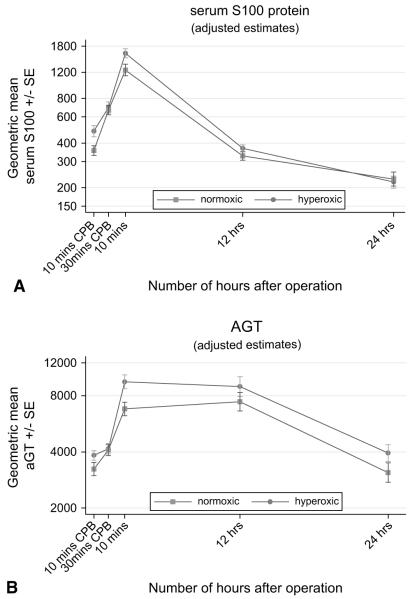
The protein S100 and άGT responses were also time dependent (Table 3, Figure 2, A and B), but in contrast to the other markers the difference between the hyperoxic and normoxic groups varied with time (P = .004 and P .02, respectively). For protein S100, there was a significant difference between the normoxic and hyperoxic groups after 10 minutes on CPB (ratio [normoxic/hyperoxic] = 0.74, 95% CI 0.60–0.91, P = .005) and 10 minutes after stopping CPB (ratio [normoxic/hyperoxic] = 0.77, 95% CI 0.62–0.97, P = .02), but at other time points the response was similar (P ≥ .17). The mean άGT was also significantly lower in the normoxic group 10 minutes after stopping CPB (ratio [normoxic/hyperoxic] = 0.72, 95% CI 0.56–0.91, P = .006), but at other time points the levels were similar in the two groups (P ≥ .12).
FIGURE 2.
A, Time-related plasma changes in the geometric mean protein S100 in the hyperoxic (●) and normoxic (■) groups. B, Time-related plasma changes in the geometric mean alpha-glutathione S-transferase (άGT) in the hyperoxic (●) and normoxic (■) groups. CPB, Cardiopulmonary bypass; SE, standard error.
Release of Inflammatory Markers (IL-6, IL-8, IL-10, C3a, and Cortisol)
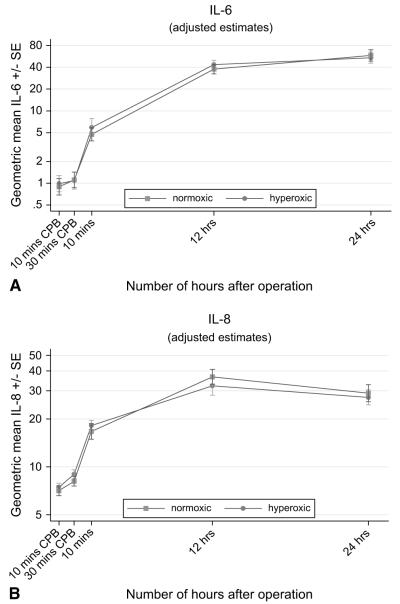
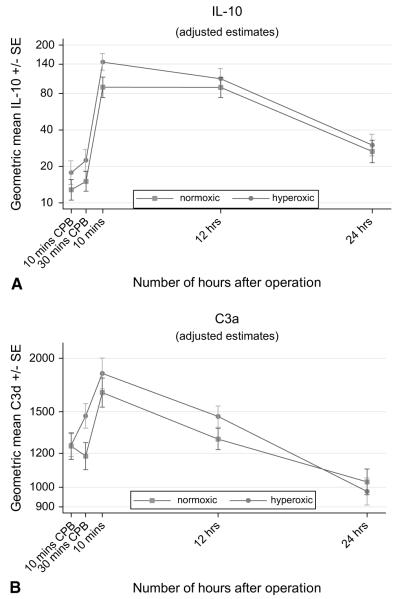
The release of the inflammatory markers IL-6, IL-8, IL-10, C3a, and cortisol was also time dependent (Table 3, Figures 3 to 5). In both groups, IL levels rose significantly after the release of the aortic crossclamp and remained significantly higher than at baseline. The C3a profile was thereafter similar to that for the ILs; the response was greatest 10 minutes after the release of the aortic crossclamp, then declined, and by 24 hours was lower than at baseline. In contrast, cortisol levels fell dramatically during surgery and remained lower than at baseline thereafter. For all five inflammatory markers, there was no evidence to suggest time-related differences between the two groups (P ≥ .16), and in all cases the response for children in the hyperoxic group was similar to that for the normoxic group (P ≥ .18, Table 3).
FIGURE 3.
A, Time-related plasma changes in the geometric mean interleukin 6 (IL-6) in the hyperoxic (●) and normoxic (■) groups. B, Time-related plasma changes in the geometric mean interleukin 8 (IL-8) in the hyperoxic (●) and normoxic (■) groups. CPB, Cardiopulmonary bypass; SE, standard error.
FIGURE 5.
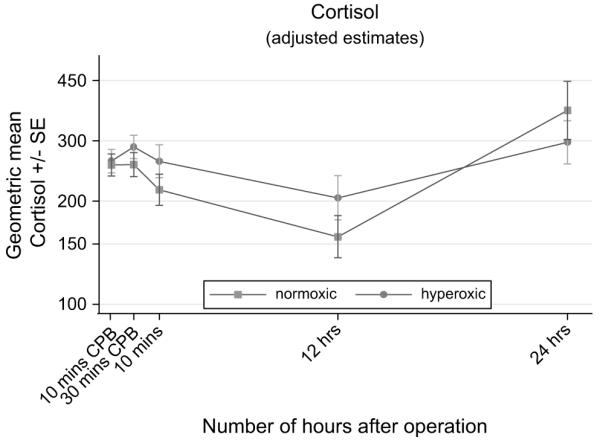
Time-related plasma changes in the geometric mean cortisol in the hyperoxic (●) and normoxic (■) groups. CPB, Cardiopulmonary bypass; SE, standard error.
DISCUSSION
This study demonstrates that “controlled reoxygenation” on starting CPB in cyanotic patients undergoing cardiac surgery significantly reduces reoxygenation injury. Normoxic CPB was indeed associated with reduced oxidative stress, myocardial, cerebral, and hepatic injury, and similar whole body inflammatory and stress response compared with hyperoxic CPB.
Myocardial Damage and Reoxygenation Injury
One of the strategies proposed to avoid reoxygenation injury is the use of controlled reoxygenation during CPB. Allen and associates13 demonstrated an improvement in the antioxidant reserve capacity with lower levels of oxygen in cyanotic infants undergoing cardiac surgery, and similar findings were obtained by Bulutcu and coworkers.14 Our results confirm these findings and further stress the importance of controlled reoxygenation on starting CPB in cyanotic patients. The perfusion strategy of keeping PO2 as close as possible to the patient’s preoperative values resulted in a reduced oxidative stress and myocardial cell damage. Reoxygenation injury is associated with the production of reactive oxygen species by the formation of oxygen-derived free radicals such as superoxide and peroxide. This leads to cell membrane degradation through lipid peroxidation.15,16 It is now evident that oxidative stress is a major part of the cellular mechanism of the resulting myocardial damage.17-20 8-Isoprostane has been shown to be a reliable marker for the volume of myocardium exposed to oxidant stress during acute myocardial infarction17,20 as well as a quantitative marker of oxidant stress during coronary reperfusion.19 The increased 8-isoprostane levels in the hyperoxic group were also associated with higher troponin I release, suggesting a direct effect of CPB oxygen levels on the degree of reperfusion injury.
Cerebral Injury
In this randomized trial, we found that controlling reoxygenation 10 minutes after the initiation of CPB was associated with significant increase of protein S100 release with values that returned to preoperative levels in both groups 24 hours after the operation. In support of our data is the clinical evidence of the effects of reoxygenation injury on cerebral function reported by Matheis and colleagues9 in a small, observational study. The authors demonstrated that uncontrolled hyperoxic reoxygenation on CPB for surgical correction of congenital heart defects was associated with higher S100 levels in cyanotic infants compared with acyanotic patients undergoing comparable operations.
In recent years, hypoxia and reoxygenation have emerged as very important mechanisms of cerebral injury. Indeed, Sher and Hu7 demonstrated in an in vitro cell model that gradual reoxygenation after prolonged hypoxia improves neuronal survival compared with rapid reoxygenation and delays the manifestations of metabolic dysfunction. Their findings are also consistent with the concept that a period of relative hyperoxia may contribute to hypoxia-induced neuronal injury. Similarly, Stauton and coworkers8 have shown that hypoxemia–reoxygenation causes endothelial dysfunction in intraparenchymal cerebral arterioles by impairing endothelium-dependent dilation of microvessels, which in turn may decrease oxygen delivery and increase neuronal injury. The use of protein S100 beta release as a marker of cerebral injury remains controversial inasmuch as it has been shown to be affected by potential contamination from sources such as bypass suckers and mediastinal fat.21 However, protein S100 is still considered one of the most specific and sensible markers of cerebral injury after cardiac surgery and is widely used in clinical trials.22 Furthermore, we23 have shown that there is a close correlation between S100 release and retinal embolization in patients undergoing coronary surgery.
Hepatic Injury
Hepatic reoxygenation injury has been demonstrated in experimental animal models of rats undergoing induced hypoxia for 60 minutes followed by 25 minutes of reflow.24 It has also been shown that during reoxygenation of perfused rat liver, there is an increased oxyradical production leading to liver injury.10 The άGTs are a group of cytosolic proteins that constitute up to 2% to 5% of the soluble protein in hepatocytes.25 The baseline level of άGTs in serum is extremely low, and as such it is easy to monitor any increases that may occur. άGT is a very sensitive and specific biomarker of hepatocyte injury.26 It is unaffected by muscle injury25 and other factors that can cause elevated transaminase levels. An elevated άGT level indicates hepatocyte injury even when other markers are normal. A normal serum άGT level almost excludes acute hepatocyte injury.25
Our study shows that the άGT levels are similar in the two groups during CPB but that there is a significant increase in άGT concentration in the hyperoxic compared with the controlled normoxic group immediately postoperatively. Thereafter, the levels of άGT are similar in each group. To the best of our knowledge, this represents the first clinical evidence of a reduced hepatic injury when a strategy of controlled reoxygenation CPB is adopted in cyanotic children undergoing cardiac surgery.
Inflammatory and Stress Response
Our study shows that the inflammatory and stress response triggered by CPB was similar in the hyperoxic and normoxic groups. The pro-inflammatory cytokine levels (IL-6 and IL-8) and cortisol increased significantly at the end of the ischemic time and remained significantly elevated after 24 hours, indicating the induction of a systemic inflammatory and stress response with either techniques of CPB. This was also associated with a significant increase in C3a at the end of the ischemic time, with subsequent decrease toward preoperative levels after 24 hours. In addition to the production of pro-inflammatory cytokines related to CPB, our results clearly demonstrate significant secretion of the anti-inflammatory cytokine IL-10. In contrast to the release of IL-6 and IL-8, the pattern of IL-10 release showed a significant peak as early as 10 minutes after the end of the ischemic time and tended to return toward preoperative levels after 24 hours.
These findings agree with the results of previously published studies indicating similar time-related inflammatory and stress response during pediatric cardiac surgical procedures.27-30
Clinical Implication of the Study and Conclusion
This study provides direct evidence that an unintended oxygen-mediated injury occurs in cyanotic patients with the initiation of CPB, resulting in myocardial, cerebral, and hepatic injury. This reoxygenation injury can be reduced by using a novel and simple CPB strategy of controlled reoxygenation. This strategy is simple and can be incorporated at no additional risk into the operative management, so long as the perfusionist is familiar with the technique and the equipment is appropriate. It does not interfere with the surgical procedure and, by limiting oxidative stress and reoxygenation injury in this very high-risk group of patients, might lead to a reduction in morbidity and mortality.
Our study cannot detect differences in clinical outcome between the two groups; the primary outcomes were related to differences in biochemical markers of organ dysfunction. Recruitment to a larger trial to evaluate clinical outcomes as primary end points is ongoing at our Institution.
FIGURE 4.
A, Time-related plasma changes in the geometric mean interleukin 10 (IL-10) in the hyperoxic (●) and normoxic (■) groups. B, Time-related plasma changes in the geometric mean C3a in the hyperoxic (●) and normoxic (■) groups. CPB, Cardiopulmonary bypass; SE, standard error.
Acknowledgments
The BUPA Foundation, National Heart Research Fund, Garfield Weston Trust, and the British Heart Foundation supported this work.
We thank Mark Ginty and Svitlana Korolchuk for performing the biochemical analyses, Professor A. Wolf, Mr. D Trivedi, the Perfusion Department, and the pediatric nursing staff for their support.
Abbreviations and Acronyms
- άGT
alpha-glutathione S-transferase
- C3a
complement activation
- CI
confidence interval
- CPB
cardiopulmonary bypass
- EIA
enzyme immunosorbent assay
- FIO2
forced expiratory volume in 1 second
Footnotes
All participants in this study have seen and approved the final version and have no conflicts of interest to disclose.
References
- 1.Castaneda AR, Jonas RA, Mayer JE, Hanley FL. Myocardial preservation in the immature heart. Cardiac surgery of the neonate and infant. Saunders; Philadelphia: 1994. p. 41. [Google Scholar]
- 2.Hammon JW. Myocardial protection in the immature heart. Ann Thorac Surg. 1995;60:839–42. doi: 10.1016/0003-4975(95)00573-4. [DOI] [PubMed] [Google Scholar]
- 3.Morita K, Ihnken K, Buckberg GD, Sherman MP, Young HH. Studies of hypoxemic/reoxygenation injury: without aortic clamping. IX. Importance of avoiding perioperative hyperoxaemia in the setting of previous cyanosis. J Thorac Cardiovasc Surg. 1995;110:1235–44. doi: 10.1016/s0022-5223(95)70010-2. [DOI] [PubMed] [Google Scholar]
- 4.Ihnken K, Morita K, Buckberg GD, Sherman MP, Young HH. Studies of hypoxemic/reoxygenation injury: without aortic clamping. III. Comparison of the magnitude of damage by hypoxemia/reoxygenation versus ischemia/reperfusion. J Thorac Cardiovasc Surg. 1995;110:1182–9. doi: 10.1016/s0022-5223(95)70004-8. [DOI] [PubMed] [Google Scholar]
- 5.Imura H, Caputo M, Parry A, Pawade A, Angelini GD, Suleiman MS. Age-dependent and hypoxia-related differences in myocardial protection during pediatric open heart surgery. Circulation. 2001;103:1551–6. doi: 10.1161/01.cir.103.11.1551. [DOI] [PubMed] [Google Scholar]
- 6.Modi P, Imura H, Caputo M, Pawade A, Parry A, Angelini GD, et al. Cardiopulmonary bypass–induced myocardial reoxygenation in pediatric patients with cynosis. J Thorac Cardiovasc Surg. 2002;124:1035–6. doi: 10.1067/mtc.2002.122536. [DOI] [PubMed] [Google Scholar]
- 7.Sher PK, Hu S. Neuroprotective effect of graded reoxygenation following chronic hypoxia in neuronal cell cultures. Neuroscience. 1992;47:979–84. doi: 10.1016/0306-4522(92)90045-4. [DOI] [PubMed] [Google Scholar]
- 8.Stauton M, Drexler C, Dulitz MG, Ekbom DC, Schmeling WT, Farber NE. Effects of hypoxia-reoxygenation on microvascular endothelial function in the rat hippocampal slice. Anaesthesiology. 1999;91:1462–9. doi: 10.1097/00000542-199911000-00040. [DOI] [PubMed] [Google Scholar]
- 9.Matheis G, Abdel-Rahman U, Braun S, Wimmer-Greinecker G, Esmaili A, Seitz U, et al. Uncontrolled reoxygenation by initiating cardiopulmonary bypass is associated with higher protein S100 in cyanotic versus acyanotic patients. Thorac Cardiovasc Surg. 2000;48:263–8. doi: 10.1055/s-2000-7879. [DOI] [PubMed] [Google Scholar]
- 10.Brass CA, Nunes F, Nagpal R. Increased oxyradical production during reoxygenation of perfused rat liver. Signal versus injury. Transplantation. 1994;58:1329–35. [PubMed] [Google Scholar]
- 11.Modi P, Suleiman MS, Reeves B, Pawade A, Parry AJ, Angelini GS, et al. Myocardial metabolic changes during pediatric cardiac surgery: a randomized controlled study of three cardioplegic techniques. J Thorac Cardiovasc Surg. 2004;128:67–75. doi: 10.1016/j.jtcvs.2003.11.071. [DOI] [PubMed] [Google Scholar]
- 12.Imura H, Modi P, Pawade A, Parry AJ, Suleiman MS, Angelini GD, et al. Cardiac troponin I in neonates undergoing the arterial switch operation. Ann Thorac Surg. 2002;74:1998–2002. doi: 10.1016/s0003-4975(02)04030-4. [DOI] [PubMed] [Google Scholar]
- 13.Allen BS, Rahman S, Ilbawi MN, Kronon M, Bolling KS, Halldorsson AO, et al. Detrimental effects of cardiopulmonary bypass in cyanotic infants: preventing the reoxygenation injury. Ann Thorac Surg. 1997;64:1381–8. doi: 10.1016/S0003-4975(97)00905-3. [DOI] [PubMed] [Google Scholar]
- 14.Bulutcu FS, Bayndir O, Polat B, Yalcin Y, öZbek U, Cakali E. Does normoxemic cardiopulmonary bypass prevent myocardial reoxygenation injury in cyanotic children? J Cardiothorac Vasc Anesth. 2002;16:330–3. doi: 10.1053/jcan.2002.124142. [DOI] [PubMed] [Google Scholar]
- 15.Ihnken K, Morita K, Buckberg GD. Studies of hypoxemic/reoxygenation injury: without aortic clamping. II. Evidence for reoxygenation damage. J Thorac Cardiovasc Surg. 1995;110:1171–81. doi: 10.1016/s0022-5223(95)70003-x. [DOI] [PubMed] [Google Scholar]
- 16.Dhaliwal H, Kirshenbaum LA, Randhawa AK, Singal PK. Correlation between antioxidant changes during hypoxia and recovery on reoxygenation. Am J Physol. 1991;261:H362–8. doi: 10.1152/ajpheart.1991.261.3.H632. [DOI] [PubMed] [Google Scholar]
- 17.Mehlhorn U, Krahwinkel A, Geissler HJ, LaRosee K, Fischer, Klass O, et al. Nitrotyrosine and 8-isoprostane formation indicate free radical–mediated injury in hearts of patients subjected to cardioplegia. J Thorac Cardiovasc Surg. 2003;125:178–83. doi: 10.1067/mtc.2003.97. [DOI] [PubMed] [Google Scholar]
- 18.Blasig IE, Grune T, Schönheit K. 4-Hydroxynonenal, a novel indicator of lipid peroxidation for reperfusion injury of the myocardium. Am J Physiol. 1995;69:H14–22. doi: 10.1152/ajpheart.1995.269.1.H14. [DOI] [PubMed] [Google Scholar]
- 19.Delanty N, Reilly MP, Pratico D, Lawson JA, McCarthy JF, Wood AE, et al. 8-Epi PGF2{alpha} generation during coronary reperfusion: a potential quantitative marker of oxidant stress in vivo. Circulation. 1997;95:2492–9. doi: 10.1161/01.cir.95.11.2492. [DOI] [PubMed] [Google Scholar]
- 20.Reilly MP, Delanty N, Roy L, Rokach J, Callaghan PO, Crean P, et al. Increased formation of the isoprostanes IPF2alpha-I and 8-epi-prostaglandin F2alpha in acute coronary angioplasty: evidence for oxidant stress during coronary reperfusion in humans. Circulation. 1997;96:3314–20. doi: 10.1161/01.cir.96.10.3314. [DOI] [PubMed] [Google Scholar]
- 21.Babin-Ebell J, Roth P, Reese J, Bechtel M, Mortasawi A. Serum S100 B levels in patients after cardiac surgery: possible sources of contamination. Thorac Cardiovasc Surg. 2007;55:168–72. doi: 10.1055/s-2006-924713. [DOI] [PubMed] [Google Scholar]
- 22.Ali MS, Harmer M, Vaughan R. Serum 100 protein as a marker of cerebral damage during cardiac surgery. Br J Anaesth. 2000;85:287–98. doi: 10.1093/bja/85.2.287. [DOI] [PubMed] [Google Scholar]
- 23.Ascione R, Ghosh A, Reeves BC, Arnold J, Potts M, Shah A, et al. Retinal and cerebral microembolization during coronary artery bypass surgery: a randomized, controlled trial. Circulation. 2005;112:3833–8. doi: 10.1161/CIRCULATIONAHA.105.557462. [DOI] [PubMed] [Google Scholar]
- 24.Videla LA. Respective roles of free radicals and energy supply in hypoxic rat liver injury after reoxygenation. Free Radic Res Commun. 1991;14:209–15. doi: 10.3109/10715769109088950. [DOI] [PubMed] [Google Scholar]
- 25.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Beckett GJ, Hayes JD. Glutathione S-transferase measurements and liver disease in man. J Clin Biochem Nutr. 1987;2:1–24. [Google Scholar]
- 27.Edmunds LH. Inflammatory and immunological response to cardiopulmonary bypass. In: Jonas RA, Elliot MJ, editors. Cardiopulmonary bypass in neonates, infants and young children. Butterworth-Heinemann; Oxford: 1994. pp. 225–41. [Google Scholar]
- 28.Hovels-Gurich HH, Vazquez-Jimenez JF, Silvestri A, Schumacher K, Minkenberg R, Duchateau J, et al. Production of proinflammatory cytokines and myocardial dysfunction after arterial switch operation in neonates with transposition of the great arteries. J Thorac Cardiovasc Surg. 2002;124:811–20. doi: 10.1067/mtc.2002.122308. [DOI] [PubMed] [Google Scholar]
- 29.Caputo M, Bays S, Rogers C, Pawade A, Parry A, Suleiman M-S, et al. The effects of normothermic and hypothermic cardiopulmonary bypass on myocardial injury, oxidative stress, and inflammatory response in pediatric open-heart surgery: a prospective randomized study. Ann Thorac Surg. 2005;80:982–8. doi: 10.1016/j.athoracsur.2005.03.062. [DOI] [PubMed] [Google Scholar]
- 30.Humphreys N, Bays SM, Parry AJ, Pawade A, Heyderman RS, Wolf AR. Spinal anesthesia with an indwelling catheter reduces the stress response in pediatric open heart surgery. Anesthesiology. 2005;103:1113–20. doi: 10.1097/00000542-200512000-00003. [DOI] [PubMed] [Google Scholar]