Abstract
Stress has significant adverse effects on health and is a risk factor for many illnesses. Neurobiological studies have implicated the amygdala as a brain structure crucial in stress responses. Whereas hyperactive amygdala function is often observed during stress conditions, cross-sectional reports of differences in gray matter structure have been less consistent. We conducted a longitudinal MRI study to investigate the relationship between changes in perceived stress with changes in amygdala gray matter density following a stress-reduction intervention. Stressed but otherwise healthy individuals (N = 26) participated in an 8-week mindfulness-based stress reduction intervention. Perceived stress was rated on the perceived stress scale (PSS) and anatomical MR images were acquired pre- and post-intervention. PSS change was used as the predictive regressor for changes in gray matter density within the bilateral amygdalae. Following the intervention, participants reported significantly reduced perceived stress. Reductions in perceived stress correlated positively with decreases in right basolateral amygdala gray matter density. Whereas prior studies found gray matter modifications resulting from acquisition of abstract information, motor and language skills, this study demonstrates that neuroplastic changes are associated with improvements in a psychological state variable.
Keywords: stress, amygdala, gray matter, MRI, mindfulness
INTRODUCTION
Acute stress initiates hormonal and behavioral responses that enable an organism to make adaptations to environmental demands (Chrousos, 2000). The amygdala has been implicated in both human and animal studies as playing a crucial role during stress responses, including the detection of stressful and threatening stimuli and the initiation of adaptive coping responses (LeDoux, 2000; Hasler et al., 2007). Amygdala-dependent cognition is facilitated during stressful conditions—a useful function for fear-related learning (Shors and Mathew, 1998; Sapolsky, 2003). However, prolonged exposure to stress increases the risk of being affected by a number of mental and physical illnesses (Johnson et al., 1992; Chrousos, 2000; Sapolsky, Romero, & Munck, 2000).
Aberrant amygdala function has been consistently demonstrated across several stress-related psychopathologies. For example, exaggerated amygdala activation has been found in trait anxiety (Stein et al., 2007), post-traumatic stress disorder (PTSD; Rauch et al., 2000; Shin et al., 2004, 2005), social phobia (Birbaumer et al., 1998; Evans et al., 2008; Phan et al., 2006), depression (Drevets et al., 1992; Abercrombie et al., 1998; Sheline et al., 2001; Siegle et al., 2002; Dougherty et al., 2004) and impulsive aggression (Coccaro et al., 2007).
Reports of differences in gray matter structure of the amygdala in pathologic stress conditions have been less consistent (Drevets et al., 2008). While some studies found enlarged amygdala volumes in subjects with affective disorders (Altshuler et al., 1998; Strakowski et al., 1999; Frodl et al., 2002; Lange and Irle, 2004; Weniger et al., 2006), others did not find altered volumes or reported volume reductions (Sheline et al., 1998; Mervaala et al., 2000; Frodl et al., 2003; Frodl et al., 2008). Amygdala findings for patients suffering from PTSD and other anxiety disorders have also been mixed (Gurvits et al., 1996; De Bellis et al., 2000; Gilbertson et al., 2002; Massana et al., 2003; Siegle et al., 2003; Wignall et al., 2004; Milham et al., 2005; Karl et al., 2006; Atmaca et al., 2008; Woon and Hedges, 2008; Hayano et al., 2009). One study with healthy individuals failed to find a correlation between chronic life stress and gray matter volume in the amygdala (Gianaros et al., 2007). These inconsistencies in the literature might result from a number of factors that can impact gray matter measures, such as gender (Wilke et al., 2007), genetics (Meyer-Lindenberg et al., 2006) and volumetry method (Doty et al., 2008).
In contrast to studies of humans, the stress literature with animals is more consistent. Several studies have shown that prolonged stress exposure leads to increases in measures of amygdala structure in rodents (Vyas et al., 2002, 2003; Mitra et al., 2005). Increased dendritic length and increased arborization were reported within the basolateral complex of the amygdala and in the extended amygdala as a result of exposure to chronic immobilization stress (Vyas et al., 2002, 2003). Differences between the results from the human and animal studies might be due to methodological differences. First, the human studies have often investigated amygdaloid volume using MRI, while animal studies have used invasive techniques to look at specific cellular changes within this structure. Second, while most human studies have been cross-sectional investigations of pathologic conditions, the animal studies have been longitudinal, with presumably healthy animals undergoing a controlled chronic stress manipulation. While individual differences are difficult to control and can confound findings in cross-sectional studies, in longitudinal studies these variables remain constant, allowing researchers to selectively vary the factor of interest. However, to our knowledge, no longitudinal neuroimaging studies have examined the influence of stress on amygdala morphology in healthy human beings.
Here, we report a longitudinal MRI study in humans that investigated the correlation between changes in perceived stress and changes in amygdaloid gray matter density following a stress-reduction intervention. Mindfulness-based stress reduction (MBSR; Kabat-Zinn, 1990) is a popular 8-week program developed to help individuals reduce their stress levels and increase psychological well-being. Mindfulness is defined as the non-judgmental awareness of present moment experiences (Kabat-Zinn, 1990). Participants practise meditation techniques designed to increase awareness of present moment experiences such as thoughts, emotions and physical sensations. They also learn to use this awareness in responding more skillfully to stress in their everyday lives. Numerous studies have demonstrated the efficacy of this program in reducing subjective reports of stress and increasing well-being (e.g. Chang et al., 2004; Carmody and Baer, 2008). However, the underlying neural mechanisms of these changes are largely unknown. Since the amygdala has been repeatedly shown to be involved in, and responsive to, an individual’s experience of stress, we hypothesized that changes in perceived stress would be associated with changes in amygdala gray matter density. Correlations within the whole brain were also explored on an exploratory basis.
METHODS AND MATERIALS
Twenty-seven participants (41% males; mean age 35.2 years; SD 6.7 years) who reported high levels of stress during the previous month were enrolled in the study. Individuals were eligible if their score on the perceived stress scale (PSS; Cohen and Williamson, 1988) was ≥1 SD above the population mean. The PSS is a validated self-report questionnaire widely used for assessing an individual’s self-perception of stress. The PSS has 14-, 10- and 4-item versions and has been shown to yield adequate reliability and validity (Cohen et al., 1983; Cohen and Williamson, 1988). In this study, the 4-item version was used to screen potential subjects while the 10-item version was used to assess change in perceived stress before and after the training. Participants gave their responses on a 5-point Likert scale, ranging from never (0) to very often (4). Inclusion criteria was based on the population means according to Cohen et al. (1983; Cohen and Williamson, 1988), namely 4.2 (SD 2.8) for females and 4.7 (SD 3.1) for males.
Further exclusion criteria were: current psychiatric illness or medical illness, ineligibility for MRI scanning (claustrophobia, metallic implants, pregnancy, etc.), or significant previous meditation or yoga experience. The protocol was approved by the Massachusetts General Hospital Institutional Review Board. Written informed consent was obtained from all study participants and they were compensated for completion of assessment procedures.
All participants completed the 8-week MBSR program, consisting of weekly group meetings and daily home mindfulness practises, including sitting meditation and yoga. The sample described here includes participants from two similar studies that both assess the effect of MBSR on brain structure. Sixteen participants received the standard MBSR class held at the Center for Mindfulness at the University of Massachusetts Medical School. Eleven subjects received a shorter version of the MBSR course held at Massachusetts General Hospital that consisted of only 12 contact hours (versus the standard 23 h) and 20 min daily homework practise (versus the standard 40 min). The intervention has been comprehensively described elsewhere (Kabat-Zinn, 1990). Classes took place between April 2005 and June 2008 and were led by several instructors. One enrolled participant was excluded from the data analyses due to non-adherence to home practise requirements (<4 h total of home practise). Data from 26 healthy, right-handed individuals (44% males; mean age 35.7 years, SD 6.3 years) were included in the analyses. Home practise logs demonstrated that participants reported an average of 19.77 h (SD 6.53 h) of prescribed out-of-class mindfulness practise over the 8-week study period. To test whether the amount of practise had an influence on the improvement in stress, a Pearson correlation between the number of hours of mindfulness home practise and the change in PSS scores was performed in SPSS (‘Statistical Package for Social Sciences, Release 12.0.2.’, 2004).
Participants were scanned at the Martinos Center for Biomedical Imaging in Charlestown, MA. Pre-intervention scans were acquired approximately 1 week before the intervention began and post-intervention scans were acquired within the 2 weeks following the intervention. High-resolution MRI data were acquired with a Siemens Magnetom Avanto 1.5 T scanner, using a T1-weighted, magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence, consisting of 128 sagittal slices (voxel size: 1.0 × 1.0 × 1.3 mm, TI = 1000 ms; TE = 3.39 ms; TR = 2730 ms; flip angle 7° and matrix 256 × 256 mm).
Anatomical MR images were compared for differences in gray matter density using voxel-based morphometry (VBM; Gaser, 2008), within the SPM5 neuroimaging statistical software (www.fil.ion.ucl.ac.uk/spm/software/spm5/) based in MATLAB 7.1, release 14 (Mathworks Inc., Natick, MA, USA). VBM permits an automated voxel-wise whole-brain statistical comparison of MRI scans. Images were first manually aligned to the anterior commissure after which gray matter, white matter and cerebral spinal fluid components were segmented within native space. We analyzed unmodulated images, which contain the probability within each voxel for being gray matter, i.e. the proportion of gray matter to other tissue types within a region (Good et al., 2001). For each individual, the gray matter segmentations of the post-intervention time-point were co-registered to the image of the pre-intervention time-point. The normalization parameters were calculated for the pre-intervention image only and then applied to the post-intervention image to make sure that regional differences between the images were not removed because of scan-specific normalization. Images were smoothed at 8-mm full width at half maximum with an Isotropic Gaussian Kernel.
Improvement in PSS (post-intervention score minus pre-intervention score; where negative values indicate decreases in PSS scores and positive values indicate increases) was used as the predictive regressor for changes in gray matter density (post-intervention image minus pre-intervention image; where negative values represent a decrease in gray matter density and positive values indicate increases) in a regression analysis. The significance threshold was defined as P < 0.05, corrected for multiple comparisons (false discovery rate) within the search region (height threshold = 0.01, uncorrected). The region of interest was defined as the bilateral amygdalae, according to Tzourio-Mazoyer et al. (2002). Exploratory correlation with gray matter density in the whole brain was performed at a significance threshold of P < 0.01 (uncorrected, 10 voxels).
RESULTS
PSS scores decreased pre- (mean 20.7; SD 5.6) to post-intervention (mean 15.2; SD 4.7; T = 3.7; df = 25; P < 0.001), indicating that the participants benefited from the course. The internal consistency of the PSS was high at both the pre- and post-intervention evaluation (Cronbach’s-α values 0.85 and 0.81, respectively), confirming an adequate reliability of the scale.
To assess whether the amount of individual meditation home practise predicted the improvement in stress, the number of minutes of meditation practise that participants reported on daily logs was correlated with the magnitude of their reduction in stress. With a Pearson correlation coefficient of r = 0.35, the amount of training was mildly correlated with the improvement in stress, though this correlation did not reach statistical significance (P = 0.079; df = 25).
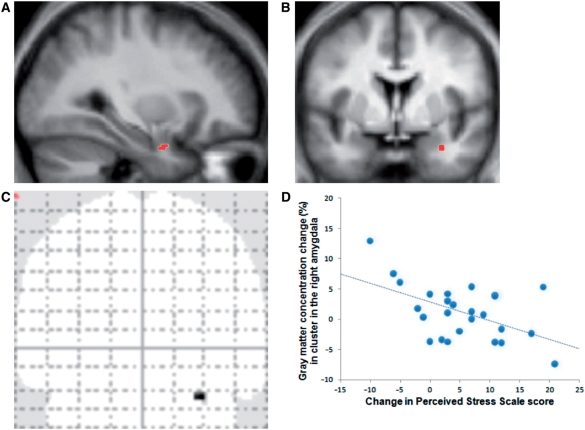
Pre- to post-intervention analyses of the MRI data in SPM confirmed a correlation between change in PSS scores and change in gray matter density within the right amygdala (cluster size: 10 voxels, MNI coordinates of peak voxel x = 32, y = 0, z = −26; voxel-level T = 3.18; P = 0.042, multiple comparisons correction within the amygdala search territory; Figure 1). Larger decreases in perceived stress were associated with larger decreases in amygdaloid gray matter density. The identified region appears to be located in basolateral/lateral regions of the amygdala, based on the atlas by Mai et al. (1997). The correlation of the change in perceived stress and amygdala gray matter density within the left amygdala was not significant.
Fig. 1.
(A–C) Location of positive correlation between gray matter density change in right amygdala and change in PSS score. Identified cluster overlaid on group-averaged sagittal (A) (x = 32) and coronal (B) (y = 0) structural image. (C) The coronal glass brain image illustrates that no other brain regions were correlated even at a liberal statistical threshold of uncorrected P < 0.01. (D) Average percent change (post-intervention minus pre-intervention) in gray matter density within the identified cluster extracted from each individual plotted against change in PSS scores. For illustrative purposes, voxel values within the identified cluster in the right amygdala were extracted and averaged using Marsbar (Brett et al., 2002), and values on the x-axis were reversed.
Controlling for age and gender did not change the significance of the results in the right amygdala (cluster size: 9 voxels, MNI coordinates of peak voxel x = 32, y = 0, z = –26; T = 3.13; P = 0.045). There were no significant correlations between change in gray matter density and age, nor any group differences between males and females, either in the amygdala or within the whole brain.
No other brain loci were significantly correlated with PSS change scores when exploratory whole brain analyses were performed in SPM, even at a liberal significance threshold of P < 0.01 (uncorrected, 10 voxels). There was also no correlation between PSS values and gray matter density at the pre-intervention time-point. Finally, there was no significant pre- to post-intervention decrease in amygdala gray matter density, i.e. no main effect of the MBSR intervention in the amygdala; however, pre- to post-changes were identified in other brain regions and are reported elsewhere (Hölzel et al., under review).
DISCUSSION
The present study investigates the potential relationship between changes in perceived stress and morphological changes in the amygdala. As predicted, there was a significant correlation between changes in PSS scores and changes in amygdaloid gray matter density. The more participants’ stress levels decreased, the greater the decrease of gray matter density in the right amygdala.
The amygdala is widely regarded as one of the most important limbic structures in prevailing models of stress states and anxiety disorders. It receives information from sensory modalities and projects to other subcortical structures, thereby mediating stress-related behavioral and physiological effects such as stress-hormone release, blood pressure elevation and facial expression of fear (LeDoux, 2000). The cluster identified here appears to be located in the lateral/basolateral region of the amygdala (Mai et al., 1997). The basolateral region has been proposed to serve as the site for the relay of sensory information from subcortical and cortical sensory areas to the central nucleus of the amygdala during anxiety responses (Campeau and Davis, 1995). Evidence of stress-related plasticity in these regions has previously been found in animal studies, including increased dendritic length and arborization within the basolateral complex of the amygdala (Vyas et al., 2002; Mitra et al., 2005). Strikingly, the basolateral amygdaloid sub-region identified in these rodent studies corresponds to the region identified here. Cytoarchitectural modifications such as those observed in rodent studies could potentially contribute to the increased gray matter density observed in a subset of the individuals in the present study. However, studies designed to establish the cellular mechanisms underlying the observed differences in amygdaloid gray matter in humans would require postmortem investigations.
Our results indicated an association between changes in stress levels and morphometric changes in the right, but not the left amygdala. It has been suggested that the right amygdala mediates an initial, fast and perhaps automatic stimulus detection, followed by a more evaluative and discriminative response by the left amygdala (Morris et al., 1998; Wright et al., 2001; Glascher and Adolphs, 2003; Costafreda et al., 2008). Based on this model, our data suggest that this stress reduction intervention may strongly impact the participants’ initial reaction to stimuli. This is consistent with a recent study demonstrating decreased autonomic arousal (skin conductance response) to affective stimuli following a stress reduction course similar to the one in this study (Ortner et al., 2007). However, further research will be required to directly test any relationship between gray matter changes and reactions to stimuli.
Previous longitudinal structural MRI studies in humans have shown that repeated activation of a neural region, either while learning new skills (Draganski et al., 2004; Ilg et al., 2008) or through transcranial magnetic stimulation (May et al., 2007), leads to an increase in the corresponding regional gray matter, whereas cessation of activation leads to a decrease. It seems plausible that this pattern could apply to the present findings—that changes in stress facilitate changes in amygdala activity, which in turn mediate changes in gray matter density. Interestingly, in rats, removal of experimental stressors after a period of chronic exposure did not lead to a reversal of the identified amygdaloid neuronal hypertrophy, or to the reversal of the associated enhanced anxiety-like behaviors within the observed time-frame of 21 days (Vyas et al., 2004). Our results suggest that ameliorating the subjective experience of stress through a behavioral intervention may actually decrease amygdala gray matter density in humans. This finding is particularly interesting as it suggests that an active re-learning of emotional responses to stress (such as taught in MBSR) can lead to beneficial changes in neural structure and well-being even when there is presumably no change in the person’s external environment. Future research will be required to address whether stress-induced alterations in the basolateral complex of the amygdala might influence a person’s susceptibility to anxiety and other affective disorders (Sajdyk et al., 1999; Shekhar et al., 2003).
Gianaros et al. (2008) recently reported that lower gray matter volume in the bilateral amygdala predicted greater stressor-related amygdala activation, as well as greater blood pressure reactivity. However, the complexity and heterogeneity of amygdala subnuclei, in addition to the low spatial resolution of neuroimaging methods, make interpreting this seemingly contradictory finding difficult. As methods and technology improve, future studies could consider how effects of stress may vary across the several heterogeneous subregions of the amygdala. It should also be noted that Gianaros et al. (2008) investigated gray matter volume, which is distinct from gray matter density examined in the present study. The biological differences underlying these two neuroimaging techniques remain unclear.
Although a correlation was found between changes in amygdaloid structure and perceived stress, the present study did not show a significant overall main effect of the training on amygdaloid gray matter density. Thus, the results do not support the conclusion that MBSR training per se leads to decreases in gray matter in this region. As reported elsewhere (Hölzel et al., under review), main effect analyses on a sub-cohort of the study participants did reveal significant changes in hippocampal, inferior temporal lobe, posterior cigulate, temporo-parietal and cerebellar gray matter density, though these regions were not correlated with changes in perceived stress.
The scatter plot (Figure 1D) illustrates that amygdaloid gray matter density increased for some participants, though it should be noted that a lot of those subjects also reported increases in perceived stress following the MBSR program. Some of the participants with improved perceived stress scores appear to have slight increases in gray matter density, but these small deviances may reflect noise. Alternatively, changes in amygdala gray matter may be temporally delayed relative to changes in perceived stress, perhaps requiring habitual activation in this region to subside prior to longer term structural changes. The results do support a bidirectional correlation; further work will be required to determine the precise relationship between the self-report measure and cellular changes. PSS values and gray matter density were not correlated at the pre-intervention time-point. This is in line with previous findings (Gianaros et al., 2007) and is not unexpected, as numerous factors can influence brain gray matter variables (Meyer-Lindenberg et al., 2006; Wilke et al., 2007). Importantly, we assessed the relationship between the change in one variable, namely perceived stress and changes in gray matter density within the amygdala. By employing a longitudinal design most within-subject variables were kept relatively constant, while the factor of interest, perceived stress, varied. Some behavioral variables, such as smoking, diet or exercise, and psychological factors (e.g. neuroticism) can also co-vary with changes in perceived stress, however, and might mediate or drive the relationship between changes in perceived stress and structural changes (cf., Gianaros et al., 2007). These variables were not assessed in the current study, and so it is unknown if the relationship between perceived stress and gray matter observed here is direct or indirect.
Several previous cross-sectional studies have investigated the impact of mindfulness meditation on brain morphology by comparing groups of experienced mindfulness meditators to nonmeditators (Lazar et al., 2005; Pagnoni and Cekic, 2007; Hölzel et al., 2008; Luders et al., 2009). These studies identified several regions of altered brain morphology, but none within the amygdalae. However, none of these studies assessed the participants’ perceived stress levels. Again, these data highlight the limitations of the cross-sectional study design. The unique hypothesis-driven, focused analysis employed in the present study revealed a novel link between changes in amygdaloid gray matter density and decreases in self-reported stress following stress-reduction training, marking a significant advance in our understanding of the association between both. Whereas previous studies have demonstrated that gray matter modifications can result from the acquisition of abstract information (Draganski et al., 2006), motor skills (Draganski et al., 2004) and language skills (Mechelli et al., 2004), this is the first study to demonstrate neuroplastic changes associated with changes in a measure of a psychological state.
Conflict of interest
None declared.
Acknowledgments
This research was funded by the National Institutes of Health-NCCAM (R21-AT003425-01A2) and the British Broadcasting Company (BBC). B.K.H. is supported by a Marie Curie International Outgoing Fellowship within the 7th European Community Framework Programme. S.W.L. was supported by National Institutes of Health funding K01AT00694. The funders had no role in study design, data collection and analysis, decision to publisher preparation of the manuscript. We thank C. Legro and the Center for Mindfulness for conducting the MBSR intervention, J. Bates, L. Shin and C. Linnman for critical reading of the manuscript; and S. Yerramsetti, N. Olendzki, C. Congleton, D. McCaffrey and A. McCallister for technical support.
REFERENCES
- Abercrombie HC, Schaefer SM, Larson CL, et al. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9(14):3301–7. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Archives of General Psychiatry. 1998;55(7):663–4. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, et al. Hippocampus and amygdalar volumes in patients with refractory obsessive-compulsive disorder. Progress in neuro-psychopharmacology and Biological Psychiatry. 2008;32(5):1283–6. doi: 10.1016/j.pnpbp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, et al. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9(6):1223–6. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. 8th International Conference on Functional Mapping of the Human Brain. 2. Vol. 16. Sendai, Japan: Neuroimage; 2002. Region of interest analysis using an SPM toolbox [abstract] [Google Scholar]
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. Journal of Neuroscience. 1995;15(3 Pt 2):2301–11. doi: 10.1523/JNEUROSCI.15-03-02301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. Journal of Behavioral Medicine. 2008;31(1):23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- Chang VY, Palesh O, Caldwell R, et al. The effects of a mindfulness-based stress reduction program on stress, mindfulness self-efficacy, and positive states of mind. Stress and Health: Journal of the International Society for the Investigation of Stress. 2004;20(3):141–7. [Google Scholar]
- Chrousos GP. The stress response and immune function: clinical implications. The 1999 Novera H. Spector Lecture. Annals of the New York Academy of Sciences. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 2007;62(2):168–78. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–96. [PubMed] [Google Scholar]
- Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The Social Psychology of Health. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, et al. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry. 2000;48(1):51–7. doi: 10.1016/s0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- Doty TJ, Payne ME, Steffens DC, Beyer JL, Krishnan KR, LaBar KS. Age-dependent reduction of amygdala volume in bipolar disorder. Psychiatry Research. 2008;163(1):84–94. doi: 10.1016/j.pscychresns.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Rauch SL, Deckersbach T, et al. Ventromedial prefrontal cortex and amygdala dysfunction during an anger induction positron emission tomography study in patients with major depressive disorder with anger attacks. Archives of General Psychiatry. 2004;61(8):795–804. doi: 10.1001/archpsyc.61.8.795. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Changes in grey matter induced by training. Nature. 2004;427:311–12. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. Journal of Neuroscience. 2006;26(23):6314–7. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure and Function. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. Journal of Neuroscience. 1992;12(9):3628–41. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety. 2008;25(6):496–505. doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- Frodl T, Jager M, Smajstrlova I, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. Journal of Psychiatry Neuroscience. 2008;33(5):423–30. [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl E, Zetzsche T, et al. Enlargement of the amygdala in patients with a first episode of major depression. Biological Psychiatry. 2002;51(9):708–14. doi: 10.1016/s0006-3223(01)01359-2. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, et al. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy control subjects. Biological Psychiatry. 2003;53(4):338–44. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Gaser C. VBM5 (for SPM5). [Computer software]. Available at: http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5/ (accessed on 30 September, 2008) [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35(2):795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. Journal of Neuroscience. 2008;28(4):990–9. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5(11):1242–7. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. Journal of Neuroscience. 2003;23(32):10274–82. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biological Psychiatry. 1996;40(11):1091–9. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Fromm S, Alvarez RP, Luckenbaugh DA, Drevets WC, Grillon C. Cerebral blood flow in immediate and sustained anxiety. Journal of Neuroscience. 2007;27(23):6313–9. doi: 10.1523/JNEUROSCI.5369-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano F, Nakamura M, Asami T, et al. Smaller amygdala is associated with anxiety in patients with panic disorder. Psych Clin Neurosci. 2009;63(3):266–76. doi: 10.1111/j.1440-1819.2009.01960.x. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Carmody J, Congleton C, McCallister A, Yerramsetti SM, Lazar S. (under review). Meditation practice leads to increases in regional brain gray matter concentration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Ott U, Gard T, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience. 2008;3(1):55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg R, Wohlschlager AM, Gaser C, et al. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance Imaging study. Journal of Neuroscience. 2008;28(16):4210–5. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neuroscience and Biobehavioral reviews. 1992;16(2):115–30. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full Catastrophe Living. New York: Delta Publishing; 1990. [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioral Reviews. 2006;30(7):1004–31. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychological Medicine. 2004;34(6):1059–64. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16(17):1893–7. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009;45(3):672–8. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. San Diego: Academic Press; 1997. [Google Scholar]
- Massana G, Serra-Grabulosa JM, Salgado-Pineda P, et al. Amygdalar atrophy in panic disorder patients detected by volumetric magnetic resonance imaging. Neuroimage. 2003;19(1):80–90. doi: 10.1016/s1053-8119(03)00036-3. [DOI] [PubMed] [Google Scholar]
- May A, Hajak G, Gaenssbauer S, et al. Structural brain alterations following 5 days of intervention: Dynamic aspects of neuroplasticity. Cerebral Cortex. 2007;17:205–10. doi: 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, et al. Structural plasticity in the bilingual brain. Proficiency in a second language and age at acquisition affect grey-matter density. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Kononen M, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychological Medicine. 2000;30(1):117–25. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(16):6269–74. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biological Psychiatry. 2005;57(9):961–6. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9371–6. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393(6684):467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Ortner CNM, Kilner SJ, Zelazo PD. Mindfulness meditation and reduced emotional interference on a cognitive task. Motivation and Emotion. 2007;31(4):271–83. [Google Scholar]
- Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiology of Aging. 2007;28(10):1623–7. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59(5):424–9. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47(9):769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behavioural Brain Research. 1999;100(1–2):207–15. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Stress and plasticity in the limbic system. Neurochemical Research. 2003;28(11):1735–42. doi: 10.1023/a:1026021307833. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Annals of the New York Academy of Sciences. 2003;985:308–25. doi: 10.1111/j.1749-6632.2003.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50(9):651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Price JL. Amygdala core nuclei volumes are decreased in recurrent major depression. Neuroreport. 1998;9(9):2023–8. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Archives of General Psychiatry. 2004;61(2):168–76. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62(3):273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Mathew PR. NMDA receptor antagonism in the lateral/basolateral but not central nucleus of the amygdala prevents the induction of facilitated learning in response to stress. Learning & Memory. 1998;5(3):220–30. [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals: an fMRI investigation. Annals of the New York Academy of Sciences. 2003;985:481–4. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Statistical Package for Social Sciences, Release 12.0.2. Chicago: SPSS Inc; 2004. [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. The American Journal of Psychiatry. 2007;164(2):318–27. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999;56(3):254–60. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Research. 2003;965(1–2):290–4. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22(15):6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–3. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Weniger G, Lange C, Irle E. Abnormal size of the amygdala predicts impaired emotional memory in major depressive disorder. Journal of Affective Disorders. 2006;94(1–3):219–29. doi: 10.1016/j.jad.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Wignall EL, Dickson JM, Vaughan P, et al. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biological Psychiatry. 2004;56(11):832–6. doi: 10.1016/j.biopsych.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Wilke M, Krageloh-Mann I, Holland SK. Global and local development of gray and white matter volume in normal children and adolescents. Experimental Brain Research. 2007;178(3):296–307. doi: 10.1007/s00221-006-0732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18(8):729–36. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12(2):379–83. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]