Abstract
Research has shown that thoughts of one's; own death (i.e. mortality salience; MS) increase aspects of intergroup bias. However, the extent to which MS influences neural activity underlying basic person perception processes has not been examined. In the current study, event-related brain potentials were used as measures of online attentional and evaluative processes as White participants categorized ingroup (White) and outgroup (Black) faces according to expression (happy vs angry) following either MS or a control induction. Results showed that MS affected the amplitude of the P2 and N2 components elicited by ingroup faces but had no effect on the processing of outgroup faces. Processing of angry ingroup relative to angry outgroup faces was pronounced in the MS condition, reflected both in N2 amplitude and in longer latency of the P3 component, suggesting heightened sensitivity to threats to positive ingroup. Overall, findings suggest that MS intensifies perception of social category features, primarily by enhancing processing of ingroup cues.
INTRODUCTION
Although death is inevitable for every organism, conscious awareness of this fact places humans in a unique existential predicament (e.g. Becker, 1973). Yearning to live, yet knowing one is destined to die, is posited as a fundamental underlying motivator of human behavior. Terror management theory (TMT; Solomon et al., 1991) suggests that people attempt to manage the anxiety engendered by the awareness of death by (i) investing in a cultural worldview that imbues the universe with order, meaning and permanence, securing either literal immortality (e.g. Heaven) or symbolic immortality (e.g. living on through one’s achievements); and (ii) garnering self-esteem—the belief that one is a person of value, a significant contributor to a meaningful universe.
Supporting these ideas, research indicates that reminders of death (mortality salience; MS) engenders self-esteem striving [see Pyszczynski et al. (2004) for a review] and leads people to engage in worldview defense—bolstering faith in their belief system by reacting positively to those who uphold their beliefs and negatively toward those who disparage them (Greenberg et al., 2007). Such reactions have been demonstrated with respect to a wide variety of social groups (e.g. sports team affiliation, ethnicity, nationality). Studies also show that reminders of death can affect stereotype-relevant judgments (e.g. Nodera et al., 2007; Renkema et al., 2008). For example, Schimel et al. (1999) found that MS led to enhanced stereotypic thinking and less favorable attitudes toward individuals who violated group-based stereotypes. Further, Greenberg et al. (2001) found that whereas a white person expressing racial pride was typically seen as racist, following MS white participants were more sympathetic to such expressions and viewed them as less racist.
However, little is known about whether, when and how reminders of death impact neurocognitive processes that unfold quickly during perception of both similar and dissimilar others. Investigating these issues could provide important insights into whether, for example, MS primarily influences early-stage attentional processes, somewhat later-stage evaluative processes, or both, which could provide a broader foundation for understanding effects of MS on behavioral expressions of intergroup bias. The current research investigated these issues using event-related brain potential (ERP) measures of attentional and evaluative categorization processes.
Person perception and ERPs
Research on the building blocks of person perception recently has focused on understanding the neurocognitive processes involved in attending to and evaluating social categories such as race and gender (e.g. Dickter and Bartholow, 2007; Ito and Urland, 2003 and 2005) and how social cues such as facial expression may effect these processes (Kubota and Ito, 2007). ERPs reflect electro-cortical activity associated with processing of stimulus and response events (Fabiani et al., 2007). Given their excellent temporal resolution and that overt behavioral responses are not required for their generation, ERPs offer a measure of rapidly occurring cognitive and motivational processes involved in person perception that people may be unable or unwilling to report (Bartholow and Dickter, 2007; Ito and Cacioppo, 2007). These features make ERPs an excellent tool for studying the covert processes involved in intergroup perception that might be affected by MS.
Several ERP components have proven particularly useful for understanding ingroup and outgroup categorization processes. The P2 (or P200) and N2 (or N200) are of specific interest because, in the context of person perception, their amplitude is thought to reflect early direction of selective attention (Luck et al., 2000) to outgroup and ingroup cues, respectively (Dickter and Bartholow, 2007; Ito and Urland, 2003 and 2005; Ito et al., 2004). Additionally, the amplitude of these components also has been associated with other aspects of social perception. For instance, the P2 has been associated with threat perception, as its amplitude often is enhanced for threat-related stimuli, such as fierce dogs (Carretie et al., 2001a), angry faces (Eimer et al., 2003; Kubota and Ito, 2007) and armed men (Correll et al., 2006). Thus, the P2 may serve as an indicator of whether MS affects the degree to which ingroup and outgroup members are perceived as threatening.
Similarly, in addition to ingroup bias, the N2 also is sensitive to manipulations of stimulus infrequency (e.g. Nieuwenhuis et al., 2003) and response conflict (e.g. Botvinick et al., 2001; Liotti et al., 2000). Some recent preliminary work suggests that N2 amplitude is particularly enhanced when ingroup cues are associated with conflict. Dickter and Bartholow (in press) had White participants categorize the race (white or black) of target faces flanked on either side by distracter faces (i.e. a flanker task; Eriksen and Eriksen, 1974). The N2 was largest on incompatible (i.e. high conflict) trials in which White (ingroup) targets were flanked by Black (outgroup) faces, particularly when such trials were relatively infrequent. In the current research, target faces contained additional social cues—varying facial expressions—that, when combined with racial cues, could lead to differences in conflict and/or subjective probability experiences. Recent work by Kubota and Ito (2007) showed that the N2 was larger to happy than to angry White (i.e. ingroup) faces in a task involving categorization of emotional expressions. However, to the extent that MS motivates seeking support from ingroup members who bolster one’s worldview (e.g. Castano et al., 2002), encountering an ingroup member who appears threatening (i.e. angry) could lead to heightened conflict. This suggests that the N2 elicited by angry White (ingroup) targets will be enhanced following MS.
The P3 (or P300) component also is useful here because of its sensitivity to evaluative categorization. The latency at which the P3 peaks provides a measure of the ease or speed with which a stimulus can be categorized and its evaluative implications understood (e.g. Kutas et al., 1977; Coles and Rugg, 1995), which occurs independently of response output processes (Magliero et al., 1984; McCarthy and Donchin, 1981). Research has shown that P3 latency is a sensitive measure of racial stereotype activation or accessibility, as the P3 peaks more quickly for stereotype-congruent than for stereotype-incongruent information (Bartholow et al., 2006). Given research suggesting that MS increases stereotype accessibility (e.g. Schimel et al., 1999), it was hypothesized here that MS would reduce P3 latencies for targets whose expressions are consistent with group stereotypes (e.g. angry Black; happy White) compared with other targets.
An issue of particular theoretical relevance is whether MS equally influences perceptions of the ingroup and outgroup, or differentially influences one or the other. The extant literature provides reasons to predict that effects of MS on neurocognitive reflections of early attention processes might be especially likely to emerge with respect to processing of ingroup members. Although MS-induced worldview defense effects are often understandably seen as showing negative reactions to outgroup members, it is important to recognize that in the vast majority of these studies such targets are actually expressing a view, or hold a characteristic, that is threatening to the participants’ belief system. With regard to race, in reviewing the TMT literature, Solomon et al. (2000) suggested that the mere presence of an outgroup cue (e.g. a picture of a Black man for a White participant) does not necessarily constitute a threat to an individual’s worldview, and thus MS might not affect processing of such cues. Previous studies are consistent with this possibility. In Greenberg et al. (2001), MS was found to intensify ingroup (i.e. White) identification but did not significantly reduce simple liking of an outgroup (i.e. Black) target. Also, evidence suggests that MS effects on outgroup evaluations largely depend on whether the target behaves in a stereotype-consistent or -inconsistent manner (Schimel et al., 1999).
Ingroup members, however, may offer symbolic comfort in the face of mortality reminders. For instance, social identity theory (Tajfel and Turner, 1986) suggests that in seeking to derive meaning and self-esteem from their group memberships, people show favoritism toward members of their ingroup without necessarily derogating outgroups (e.g. Allport, 1954; Brewer, 1979; Mummendey and Wenzel, 1999). Thus, given extensive research showing that MS enhances self-esteem striving (Pyszczynski et al., 2004), the effect of MS on person perception may manifest as enhanced processing of ingroup targets without necessarily influencing processing of outgroup targets. Such a pattern would be consistent with the idea that MS provokes self-esteem striving and a search for affiliative meaning, a sense of security that may be best derived from ingroup identification (Castano, 2004; Castano et al., 2002). Further, reminders of death have been found to enhance perceived attitudinal similarity with others (Pyszczynski et al., 1996), which may heighten attention to signs of ingroup connections. Taken together, past research and theory suggest that MS is more likely to affect initial processing of ingroup cues than outgroup cues.
The current research
The present study provides the first neurocognitive investigation of how MS affects rapid attentional and evaluative processes believed to be associated with biases in person perception. Participants subjected to MS or a control manipulation and categorized pictures of ingroup (White) and outgroup (Black) faces displaying neutral, happy and angry expressions while ERPs were recorded. Varying the facial expressions of the targets served two purposes. First, this factor allowed tests of whether MS effects on person perception differ for ingroup and outgroup cues as a function of whether those cues appear threatening (angry) vs safe (happy). Second, focusing participants’ explicit task on categorizing facial expressions (rather than race) allowed investigation of the effects of MS on the implicit (rather than explicit) categorization of racial ingroup and outgroup features (Ito and Cacioppo, 2000 and 2007).
This approach affords the opportunity to test a number of basic hypotheses. First, as described previously, the P2 is sensitive to both outgroup cues (e.g. Dickter and Bartholow, 2007; Ito and Urland, 2003) and to threat-related cues (e.g. Carretie et al., 2001a; Kubota and Ito, 2007). If MS enhances intergroup categorization differences, the difference in P2 amplitude elicited by outgroup vs ingroup targets should be exacerbated in the MS condition relative to the control condition. Second, as reviewed previously, ingroup targets generally elicit a larger N2 than outgroup targets (e.g. Dickter and Bartholow, 2007; Ito and Urland, 2003; Kubota and Ito, 2007). If MS leads to enhanced sensitivity to ingroup targets, the N2 elicited by ingroup relative to outgroup faces should be larger in the MS condition than the control condition. Moreover, given that death awareness increases preferences for comprehension goals and coherent and consistent meaning (e.g. Landau et al., 2004; Renkema et al., 2008), MS should enhance conflict during the processing of angry (i.e. threatening) ingroup faces and/or happy outgroup faces, resulting in an interactive effect of condition, target race and facial expression on the N2. Finally, to the extent that MS affects the ease or speed with which social targets can be categorized (e.g. Schimel et al., 1999), we expect P3 latency to be longer among MS participants for targets whose facial expressions counteract stereotype-based assumptions (i.e. threatening/angry ingroup targets and happy/safe outgroup targets) relative to those that support stereotypic beliefs.
METHODS
Participants
Thirty undergraduates (13 females) recruited from introductory psychology classes participated for partial course credit. All participants identified their ethnicity as White.1 Participants were all predominantly right-handed (Oldfield, 1971), had normal or corrected-to-normal vision, and reported no history of head injury or neurological disorders.
Condition manipulation
Participants were randomly assigned to either the MS or control condition (n = 15 each). As in previous research (Rosenblatt et al., 1989), participants in the MS condition wrote responses to two open ended questions: ‘Please briefly describe the emotions that the thought of your own death arouses in you’, and ‘Jot down, as specifically as you can, what you think will happen to you as you physically die and once you are physically dead’. Control condition participants responded to parallel questions focused on dental pain. Subsequently, all participants engaged in a brief delay task (reading an innocuous passage) to allow thoughts of death to fade from focal attention (Pyszczynski et al., 1999).
Facial expressions task
Presentation of ingroup and outgroup cues occurred within the context of a visual oddball task in which pictures of men’s faces were shown, one at a time, in sequences of five. Half the photos portrayed Black males and half portrayed White males (varying randomly). Within each sequence, four of the five faces displayed a neutral expression (i.e. context), and one ‘target’ face displayed either a happy, angry or morphed (part happy, part angry) expression. So that participants could not easily anticipate its occurrence, the target always appeared in positions 3, 4, or 5 (randomized across trial sequences). Participants’ task was to identify happy and angry facial expressions by pressing one of two keys on the keyboard (counterbalanced across participants), and to do nothing with faces displaying neutral or ambiguous expressions. Each picture was presented for 50 ms with an interstimulus interval of 1000 ms. Altogether, 10 different individuals’ faces were shown, each displaying each of the four expressions, for a total of 40 pictures. There were 30 repetitions of each trial type and 8 types of trials (i.e. Black Angry, Black Happy, Black Morph, Black Neutral, White Angry, White Happy, White Morph and White Neutral), resulting in 240 total trials.2
Electrophysiological recording
The electroencephalogram (EEG) was recorded continuously from 28 tin electrodes fixed in a stretch-lycra cap and placed at standard scalp locations (American Encephalographic Society, 1994), referenced online to the right mastoid; an average mastoid reference was calculated offline. All signals were filtered on-line at 0.05–30 Hz at a sampling rate of 1000 Hz. Impedance was kept below 8 kΩ in all channels. Ocular artifacts (i.e. blinks) were corrected from the EEG signal off-line. After artifact elimination (trials containing voltage deflections of ±75 µV were discarded), EEG data were averaged off-line according to participant, electrode and stimulus conditions.
Procedure
Upon arrival, participants read and signed informed consent forms and were told that the study would involve identifying facial expressions while brain activity was recorded. Once electrodes were in place, participants completed either the MS or control induction, depending on random assignment, followed by the delay task. Participants then completed the facial expressions task while EEG was recorded, after which participants were debriefed, thanked and dismissed.
RESULTS
Analytic approach
Analyses of P2 and N2 data were based on mean amplitude values.3 These values were determined by visually inspecting each participant’s average waveforms for each condition and tailoring the epochs for each component accordingly (average epoch range was 130–220, and 210–310 ms for the P2 and N2, respectively). Average amplitudes within these epochs were then computed. P3 latency was derived from individual subject averages using a procedure that determined the peak amplitude value within participant-specific P3 epochs and identifying the latency at which the peaks occurred. The participant-specific P3 epochs were created by examining grand average waveforms to get an idea as to when, on average, the P3 peaked. With this in mind, we then went through each individual subject's; data to ensure that their P3 was consistent with what was found in the grand average. Thus, if a participant’s P3 did not conform to the group average, adjustments were made for that subject’s epoch. Only trials in which facial expressions were correctly identified were included in each average, resulting in an average of 25 trials per condition. (Although we had no hypotheses concerning effects of MS on behavioral responses in this paradigm, analyses of behavioral data are included as Supplementary material.)
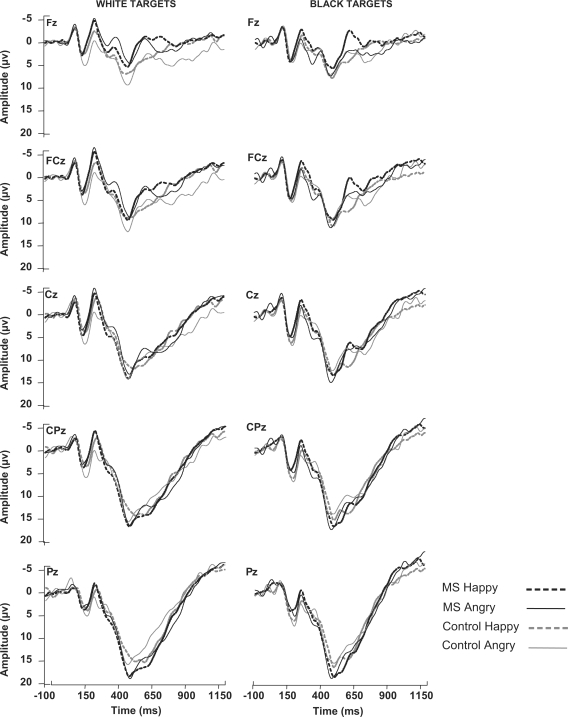
Figure 1 presents the waveforms elicited at midline electrodes by Black and White targets as a function of facial expression and condition. Initial analyses indicated that the components of interest were largest at and near midline scalp locations. Thus, analyses focused on data from electrodes representing those locations (F3, Fz, F4, FC3, FCz, FC4, C3, Cz, C4, CP3, CPz, CP4, P3, Pz & P4). Mean amplitudes of the P2 and N2 components were analyzed using separate 2 (Condition) × 2 (Race) × 2 (Facial Expression) × 5 (Coronal location) × 3 (Sagittal location) mixed factorial ANOVAs with repeated measures on all but the first factor.
Fig. 1.
ERP waveforms measured from midline electrode locations as a function of condition and facial expression for Black targets (left column) and White targets (right column). MS—participants in the mortality salience condition; control—participants in the control condition.
P2 amplitude
The ANOVA showed significant main effects of Coronal and Sagittal locations, F(4, 112) = 10.44, P < 0.002, and F(2, 56) = 13.46, P < 0.001, respectively, indicating that P2 amplitude increased linearly from the frontal electrode sites to the parietal sites and was largest along the midline (especially Pz), consistent with previous work using similar paradigms (e.g. Dickter and Bartholow, 2007; Ito and Urland, 2003 and 2005; Kubota and Ito, 2007). The analysis also showed a significant main effect of Expression, F(1, 28) = 5.38, P < 0.05, indicating that the P2 was larger for angry faces (M = 6.44 µV) than for happy faces (M = 5.44 µV), consistent with previous research (Kubota and Ito, 2007). The main effect of Race also was significant, F(1, 28) = 5.80, P < 0.05. The P2 was larger for Black faces (M = 6.52 µV) than White faces (M = 5.33 µV), replicating previous findings (e.g. Dickter and Bartholow, 2007; Ito and Urland, 2003 and 2005; Kubota and Ito, 2007). The interaction between Race and Expression was not significant, consistent with previous research (Kubota and Ito, 2007), nor was the interaction between Race and Condition (F < 1).
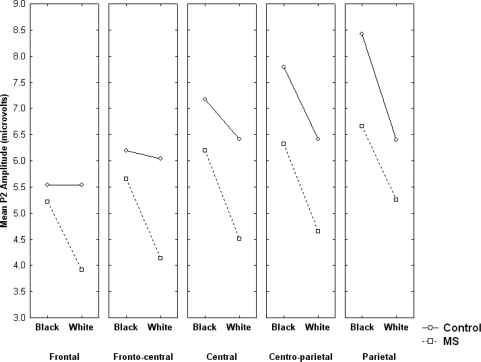
However, the Race × Condition × Coronal location interaction was significant, F(4, 112) = 3.43, P = 0.01 (Figure 2). The pattern of means in Figure 2 suggests that, as predicted, the effect of Target race (i.e. the difference between the P2 elicited by Black targets and White targets) was more pronounced for MS participants than for control participants. Moreover, the effect appeared to be more wide-spread across scalp locations in the MS condition. Follow-up contrast analyses confirmed that the race effect was significant for MS participants at central, centro-parietal and parietal locations (Fs ≥ 4.03, Ps ≤ 0.05), but was significant for control participants only at parietal locations, F(1, 14) = 9.43, P < 0.01. Inspection of Figure 2 shows that this pattern is due to White targets eliciting smaller P2 amplitude in the MS condition vs the control condition (ts ≥ 2.05, Ps ≤ 0.05), not to Black targets eliciting larger P2 amplitude in the MS condition (ts < 1), consistent with the notion (articulated previously) that MS should influence perceptions of the ingroup. Given prior research linking P2 amplitude with threat, this pattern may signify that ingroup targets are perceived as less threatening following MS, as opposed to outgroup targets being viewed with increased threat. No other effects of interest were significant.
Fig. 2.
Mean P2 amplitude as a function of condition, target race and coronal scalp location.
N2 amplitude
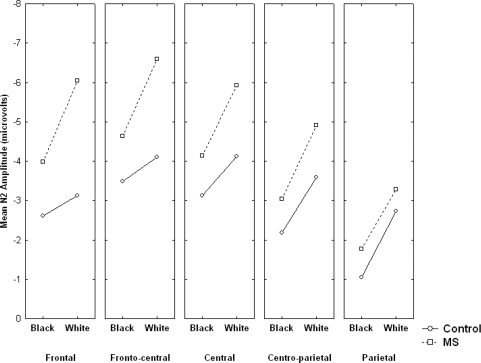
A significant Coronal location main effect, F(4, 112) = 13.14, P < 0.001, indicated that the N2 was largest at fronto-central electrodes, particularly at FCz (M = −4.71 µV), as is typical. The ANOVA also showed a significant main effect of Race, F(1, 28) = 9.67, P < 0.01. White targets elicited a larger (more negative) N2 (M = −4.44 µV) than Black targets (M = −3.00 µV), consistent with previous research (e.g. Dickter and Bartholow, 2007; Ito and Urland, 2003 and 2005; Kubota and Ito, 2007). The Race × Condition effect was not significant (F < 1). However, the Race × Condition × Coronal location interaction was significant, F(4, 112) = 2.61, P < 0.05. Figure 3 shows that, as predicted, the enhancement of the N2 for White relative to Black targets was more pronounced in the MS condition compared with the control condition. Follow-up contrast analyses confirmed that whereas the MS group showed a large race effect at all scalp locations, (Fs > 8.70, Ps < 0.01), the race effect in the control group was significant only at centro-parietal, F(1, 14) = 4.35, P = 0.055, and parietal locations, F(1, 14) = 8.56, P < 0.01.
Fig. 3.
Mean N2 amplitude as a function of condition, target race and coronal scalp location. Note that larger (more negative) N2 amplitude is plotted ‘up’ on y-axis.
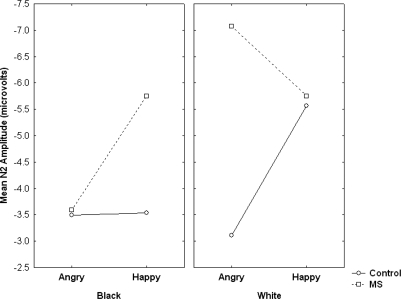
Finally, the ANOVA showed a significant Expression × Race × Condition × Coronal location × Sagittal location interaction F(8, 224) = 2.16, P < 0.05. Although this interaction is very complex, visual inspection of the means suggested that the interaction primarily was driven by larger differences between groups and target conditions at midline locations, particularly frontal and fronto-central midline electrodes, than at other locations. Given that the amplitude of the N2 was largest at FCz, we conducted a follow-up ANOVA focused on data from FCz only. This analysis showed a significant Expression × Race × Condition interaction, F(1, 28) = 5.01, P < 0.05 (Figure 4). To better understand this interaction, we conducted follow-up Race × Expression ANOVAs separately for the MS and Control groups. For the MS group, this analysis showed a marginal Race × Expression interaction, F(1, 14) = 4.33, P = 0.056. Inspection of the means showed that angry White targets elicited a larger N2 (M = −7.07 µV) than angry Black targets (M = −3.59 µV), F(1, 14) = 6.46, P < 0.01, but happy Black and happy White targets elicited identical N2s (Ms = −5.75 µV). Additionally, happy Black targets elicited a larger N2 than angry Black targets, F(1, 14) = 4.91, P < 0.05. Although angry White targets elicited directionally larger N2s than happy White targets, this difference was not significant (F < 1).
Fig. 4.
Mean N2 amplitude as a function of condition, target race and expression. White angry faces elicited larger N2 in the MS condition than in the control condition. Larger (more negative) N2 amplitude is plotted ‘up’ on y-axis.
For the control group, however, the Race × Expression interaction was not significant, F(1, 14) = 1.41, P = 0.26. Nevertheless, a simple effect test of control group means showed that happy White targets elicited a somewhat larger N2 (M = −5.37 µV) than angry White targets (M = −3.11 µV), F(1, 14) = 3.75, P = 0.07. An additional follow-up contrast based on data from both groups showed that angry White targets elicited a larger N2 in the MS condition than in the control condition, F(1, 28) = 6.02, P < 0.05. No other effects were significant. When considered in the context of previous research suggesting that the N2 reflects biased attention to ingroup cues (e.g. Dickter and Bartholow, 2007), as well as work showing the sensitivity of the N2 to conflict (e.g. Botvinick et al., 2001), these findings suggest that MS enhances attention to ingroup features, particularly when those features are combined with information (e.g. angry expressions) that conflicts with offering psychological comfort (e.g. positive ingroup esteem).
P3 latency
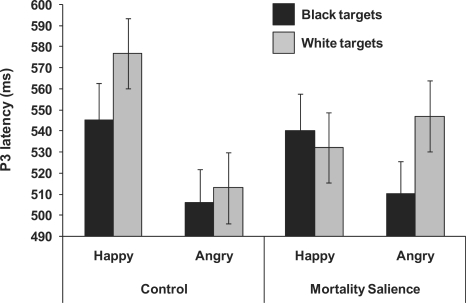
P3 latency values were analyzed using a 2 (Condition) × 2 (Race) × 2 (Expression) mixed factorial ANOVA focused on data from Pz, where the component was largest and peaked most quickly (M = 534 ms). The analysis showed a main effect of Race, F(1, 28) = 5.34, P < 0.05, indicating that the P3 peaked more quickly to Black faces (M = 524 ms) than to White faces (M = 544 ms). The ANOVA also showed a significant Expression × Condition interaction F(1, 28) = 5.89, P < 0.05, which was qualified by a Race × Expression × Condition interaction F(1, 28) = 4.91, P < 0.05 (Figure 5). To unpack this three-way interaction, we computed separate Race × Expression ANOVAs for the control and MS groups. For the MS group, the analysis showed a significant Race × Expression interaction, F(1, 14) = 4.78, P < 0.05. Follow-up contrast analyses showed that P3 latency was slower for angry White (M = 547 ms) than for angry Black targets (M = 510 ms), t(14) = 2.07, P < 0.05, and was marginally quicker for angry Black than for happy Black targets (M = 541), t(14) = 1.70, P < 0.10. No other effects were significant. For the control group, the Race × Expression interaction was not significant (F < 1). However, it is noteworthy that both groups showed a very similar pattern of P3 latency for Black targets (i.e. faster for angry than happy faces), but that latency patterns differ for White targets as a function of MS. Specifically, the P3 peaked somewhat more quickly for happy White targets in the MS condition than in the control condition, t(28) = 1.89, P < 0.07, and the P3 elicited by angry White targets was directionally slower in the MS than in the control condition, though this difference was not significant, t(28) = 1.42, P = 0.16. No other effects of interest were significant. The pattern of P3 latencies is consistent with the idea that MS leads to more difficulty in categorizing and evaluating negative ingroup targets, but has no effect on categorization on outgroup targets.
Fig. 5.
Mean P3 latency as a function of condition, target race and expression. In the MS condition, the P3 peaked significantly more quickly for angry Black targets than angry White targets, whereas in the control condition the P3 peaked more quickly for happy Black targets than for happy White targets. Additionally, happy White targets elicited a quicker P3 in the MS condition than in the control condition.
DISCUSSION
The main purpose of this experiment was to provide initial insights into the stage of perceptual processing at which reminders of death affect person perception, using relatively non-reactive, neurocognitive measures. As in previous research, the amplitude of the P2 and N2 ERP components revealed biases in early categorization of outgroup and ingroup targets, respectively. Specifically, outgroup (in this case, Black) targets elicited enhanced P2 amplitudes and ingroup (in this case, White) targets elicited larger N2 amplitudes (e.g. Dickter and Bartholow, 2007; Ito et al., 2004; Ito and Urland, 2003 and 2005; Kubota and Ito, 2007; Willadsen-Jensen and Ito, 2006). More interestingly for our purposes, these early electrophysiological manifestations of group categorization were moderated by MS. After reminders of death, participants experienced larger amplitudes across electrode locations to Black targets than to White targets, whereas control group participants showed this target race effect only at posterior locations. Interestingly, the race effect in the MS group was driven by smaller P2 to White targets, not enhanced P2 to Black targets. This pattern suggests that effects of MS on early group categorization processes manifest primarily via differences in categorization of the ingroup. One plausible interpretation of this finding, given the association between P2 amplitude and threat perception (e.g. Carretie et al., 2001a, b; Eimer et al., 2003), is that ingroup targets are perceived as less threatening (cf. Correll et al., 2006) following MS.
The pattern of N2 amplitudes further suggests MS primarily affects ingroup categorization. MS participants showed consistently larger N2s to White (ingroup) than Black (outgroup) targets across electrodes, an effect restricted to more posterior locations in the control condition. Overall, the pattern seen in both the P2 and N2 components could reflect an initial effort to derive psychological security by bolstering one’s worldview with a perceptual focus on similar others, and is consistent with the general tendency, outlined in social identity theory (Hogg and Abrams, 1990), for esteem-driven ingroup preference and outgroup indifference rather than outgroup derogation per se (Allport, 1954; Brewer, 1999; Dovidio and Gaertner, 2000).
On the one hand, the effects of MS on ERP markers of person perception could be described as simply amplifying typical outgroup and ingroup categorization biases, in that patterns of electrocortical target race effects appear to be more widespread across the scalp in the MS vs the control condition (Figures 2 and 3). On the other hand, the interaction of racial group and facial expression in the N2 reveals more than just a quantitative difference between the MS and control conditions, suggesting that reminders of death can qualitatively alter early person perception processes. Specifically, whereas previous research indicated that happy ingroup faces elicited larger N2 than neutral or angry ingroup faces (Kubota and Ito, 2007), a pattern seen among the control participants in this study (Figure 4), participants in the MS condition showed somewhat larger N2s to angry than to happy ingroup targets. As reviewed previously, the extant literature on the N2 provides a basis for expecting such a pattern. The N2 component is highly sensitive to relative stimulus infrequency (see Folstein and van Petten, 2008) and to stimuli that elicit conflict (Botvinick et al., 2001; van Veen and Carter, 2002; Yeung et al., 2004). To the extent that MS promotes ingroup favoritism or a desire to view ingroup members positively (e.g. Harmon-Jones et al., 1996), it makes sense that, following MS, threatening ingroup members might be perceived as less likely than threatening outgroup members. Even though the actual probability of happy/safe White and angry/threatening White targets was equivalent here, it could be that MS enhances the stereotypical perception that the probability of a threatening ingroup member is low. The larger N2 response to happy Black than to angry Black targets among the MS group also fits with this interpretation. Additionally, the requirement to overtly categorize ingroup members as angry/threatening, coupled with an underlying desire to view ingroup members positively, could elicit conflicting response tendencies.
However, it is important to note that the N2 and P2 findings from this study differ in some respects from past research on the neural markers of ingroup and outgroup categorization. For example, whereas effects of ingroup categorization on the N2 have tended to be largest at fronto-central scalp locations in previous work (Dickter and Bartholow, 2007; Ito and Urland, 2003), among control participants here the difference in N2 amplitude elicited by White and Black targets was significant only at more posterior locations. Similarly, the outgroup categorization effect in the P2 was localized primarily to posterior electrodes here, whereas this effect in previous work has been evident in more locations. Such differences in the scalp topography of the effects could be due to differences in the present paradigm. For example, participants in the control condition here were not completing the face perception task ‘cold’, but rather underwent a manipulation (i.e. think and write about the experience of dental pain) commonly used to control for negative valence associated with the MS manipulation. To our knowledge no previous studies of race categorization effects in the ERP have used manipulations like this. Thus, it is difficult to directly compare the scalp distribution of our effects with previous work. Ideally, future studies might include a ‘true’ control condition in addition to an aversive control to determine the extent to which other aversive contemplation produces unique effects on scalp distribution.
MS also influenced person perception at a somewhat later stage associated with evaluative categorization, reflected here in P3 latency. As reviewed previously, the latency at which the P3 peaks is considered a response-independent (e.g. Magliero et al., 1984; McCarthy and Donchin, 1981) index of the ease with which a stimulus is categorized and evaluated (e.g. Kutas et al., 1977). Previous work has shown that P3 latency is sensitive to violations of racial stereotypes, such that stereotype-violating targets are categorized more slowly (i.e. slower P3 latency) than stereotype-consistent targets (e.g. Bartholow et al., 2006). The present P3 latency data indicate that MS affected evaluative categorization of ingroup targets but not outgroup targets. Specifically, whereas participants in both groups showed faster P3 latencies to angry Black (i.e. stereotype-consistent) than happy Black targets, only those in the MS group showed slower P3 latencies to angry vs happy White targets (though this difference was not significant). Additionally, compared with the control group, MS participants showed faster P3s to happy White targets and slower P3s to angry White targets. This effect generally mirrors the pattern of effects seen in the earlier stage of processes reflected in the N2 component, and more generally is consistent with the broader theme evident across components of MS effects being limited to processing of ingroup targets. This pattern is also consistent with previous work indicating that death awareness leads to more difficulty processing information that appears to violate group-based norms or expectations (Schimel et al., 1999), though in the present case limited to expectations about the ingroup.
Taken together, the current findings suggest that reminders of death heighten sensitivity to cues important for person perception. Compared with participants in the control group, MS decreased the extent to which ingroup members were perceived as threatening (P2 amplitude), increased attention to ingroup features and conflict associated with threatening ingroup members (N2 amplitude), and facilitated evaluative categorization of ingroup members whose expressions conformed to stereotypic expectations. In short, activating mortality cognition enhances intergroup categorization differences at very early stages of processing, which previous work has linked to intergroup biases (e.g. Dickter and Bartholow, 2007; Ito et al., 2004). The current work provides the first evidence concerning how motivational factors generally, and deeply rooted existential insecurity specifically, can affect rapidly-occurring, neurophysiological representations of attention to and categorization of ingroup and outgroup faces. Perhaps just as importantly, this study suggests a link between neural responses to social targets and broader areas of inquiry to which terror management theory recently has been applied, such as reactions to terrorist acts committed by ingroup and outgroup members and intergroup political conflicts (e.g. Pyszczynksi et al., 2003). Thus, the present findings offer a fertile foundation for future efforts aimed at investigating the psychological mechanisms affected by death awareness at the social, cognitive and neural levels of analysis, and the relevance of these effects for intergroup relations.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared
Footnotes
1One participant was of mixed ethnicity (Asian and White). Given that his behavioral and neurocognitive responses were highly similar to the other participants in the sample, we retained his data and believe it appropriate to consider ‘ingroup’ and ‘outgroup’ distinctions used here to apply to him as well.
2Targets displaying morphed facial expressions were included to test hypotheses not relevant to the current report. Thus, responses to the morphed faces will not be considered here.
3Analyses were also conducted using peak amplitudes. Both analyses produced similar findings, thus only mean amplitude analyses are reported.
REFERENCES
- Allport G. The Nature of Prejudice. Reading, MA: Addison-Wesley; 1954. [Google Scholar]
- American Encephalographic Society (1994) American Encephalographic Society, guideline thirteen: guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1994;11:111–13. [PubMed] [Google Scholar]
- Bartholow BD, Dickter CL. Social cognitive neuroscience of person perception: a selective review focused on the event-related brain potential. In: Harmon-Jones E, Winkielman P, editors. Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior. New York: Guilford Press; 2007. pp. 376–400. [Google Scholar]
- Bartholow BD, Dickter CL, Sestir MA. Stereotype activation and control of race bias: cognitive control of inhibition and its impairment by alcohol. Journal of Personality and Social Psychology. 2006;90:272–87. doi: 10.1037/0022-3514.90.2.272. [DOI] [PubMed] [Google Scholar]
- Becker E. The Denial of Death. New York: Free Press; 1973. [Google Scholar]
- Botvinick M, Braver T, Barch D, Carter C, Cohen J. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brewer M. In-group bias in the minimal intergroup situation: a cognitive-motivational analysis. Psychological Bulletin. 1979;86:307–24. [Google Scholar]
- Brewer M. The psychology of prejudice: ingroup love or outgroup hate? Journal of Social Issues. 1999;55:429–44. [Google Scholar]
- Carretie L, Martin-Loeches M, Hinojosa JA, Mercado F. Emotion and attention interaction studied through event-related potentials. Journal of Cognitive Neuroscience. 2001a;13:1109–28. doi: 10.1162/089892901753294400. [DOI] [PubMed] [Google Scholar]
- Carretie L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention and the “negativity bias,” studied through event-related potentials. International Journal of Psychophysiology. 2001b;41:75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Castano E. In case of death, cling to the ingroup. European Journal of Social Psychology. 2004;34:375–84. [Google Scholar]
- Castano E, Yzerbyt, Paladino M, Sacchi S. I belong, therefore, I exist: ingroup identification, ingroup entitativity, and ingroup bias. Personality and Social Psychology Bulletin. 2002;28:135–43. [Google Scholar]
- Coles MGH, Rugg MD. Event-related brain potentials: an introduction. In: Rugg MD, Coles MGH, editors. Electrophysiology of Mind: Event-Related Brain Potentials and Cognition. New York: Oxford University Press; 1995. pp. 1–26. [Google Scholar]
- Correll J, Urland GR, Ito TA. Event-related potentials and the decision to shoot: the role of threat perception and cognitive control. Journal of Experimental and Social Psychology. 2006;42:120–8. [Google Scholar]
- Dickter CL, Bartholow BD. Event-related brain potential evidence of ingroup and outgroup attention biases. Social, Cognitive, and Affective Neuroscience. 2007:2, 189–98. doi: 10.1093/scan/nsm012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickter CL, Bartholow BD. Ingroup categorization and response conflict: Interactive effects of target race, flanker compatibility and infrequency on N2 amplitude. Psychophysiology. doi: 10.1111/j.1469-8986.2010.00963.x. in press. [DOI] [PubMed] [Google Scholar]
- Dovidio JF, Gaertner SL. Aversive racism and selection decisions: 1989 and 1999. Psychological Science. 2000;11:315–31. doi: 10.1111/1467-9280.00262. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A, McGlone FP. The role of spatial in the processing of facial expression: an ERP study of rapid brain responses to six basic emotions. Cognitive, Affective, and Behavioral Neuroscience. 2003;3:97–110. doi: 10.3758/cabn.3.2.97. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–9. [Google Scholar]
- Fabiani M, Gratton G, Federmeier FD. Event-related brain potentials: methods, theory, and applications. In: Cacioppo JT, Tassinary L, Bernston GG, editors. Handbook of psychophysiology. third. Cambridge, UK: Cambridge University Press; 2007. pp. 85–119. [Google Scholar]
- Folstein JR, van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP. Psychophysiology. 2008;45:152–70. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J, Schimel J, Martens A, Solomon S, Pysczynski T. Sympathy for the devil: evidence that reminding Whites of their mortality promotes more favorable reactions to White racists. Motivation and Emotion. 2001;25:113–33. [Google Scholar]
- Greenberg J, Solomon S, Arndt J. A uniquely human motivation: terror management. In: Shah J, Gardner W, editors. Handbook of Motivation Science. New York: Guilford; 2007. pp. 113–34. [Google Scholar]
- Harmon-Jones E, Greenberg J, Solomon S, Simon L. The effects of mortality salience on intergroup bias between minimal groups. European Journal of Social Psychology. 1996;25:781–5. [Google Scholar]
- Hogg MA, Abrams D. Social motivation, self-esteem, and social identity. In: Abrams D, Hogg MA, editors. Social identity theory: Constructive and critical advances. New York: Harvester Wheatsheaf; 1990. pp. 28–47. [Google Scholar]
- Ito TA, Cacioppo JT. Electrophysiological evidence of implicit and explicit categorization processes. Journal of Experimental Social Psychology. 2000;36:660–76. [Google Scholar]
- Ito TA, Cacioppo JT. Attitudes as mental and neural states of readiness: using physiological measures to study implicit attitudes. In: Wittenbrink B, Schwarz N, editors. Implicit measures of attitudes. New York: Guilford Press; 2007. pp. 125–58. [Google Scholar]
- Ito TA, Larsen J, Smith K, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. Journal of Personality and Social Psychology. 1998;76:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Ito TA, Thompson E, Cacioppo J. Tracking the timecourse of social perception: the effects of racial cues on event-related brain potentials. Personality and Social Psychology Bulletin. 2004;30:1267–79. doi: 10.1177/0146167204264335. [DOI] [PubMed] [Google Scholar]
- Ito TA, Urland G. Race and gender on the brain: electrocortical measures of attention to the race and gender of multiply categorizable individuals. Journal of Personality and Social Psychology. 2003;85:616–26. doi: 10.1037/0022-3514.85.4.616. [DOI] [PubMed] [Google Scholar]
- Ito TA, Urland G. The influence of processing objectives on the perception of faces: an ERP study of race and gender perception. Cognitive, Affective, and Behavioral Neuroscience. 2005;5:21–36. doi: 10.3758/cabn.5.1.21. [DOI] [PubMed] [Google Scholar]
- Kubota JT, Ito T. Multiple cues in social perception: the time course of processing race and facial expression. Journal of Experimental Social Psychology. 2007;43:738–52. doi: 10.1016/j.jesp.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197:792–5. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Landau MJ, Johns M, Greenberg J, Pyszczynski T, Solomon S, Martens A. A function of form: terror management and structuring of the social world. Journal of Personality and Social Psychology. 2004;87:190–210. doi: 10.1037/0022-3514.87.2.190. [DOI] [PubMed] [Google Scholar]
- Liotti M, Woldorff MG, Perez R, III, Mayberg HS. An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia. 2000;38:701–11. doi: 10.1016/s0028-3932(99)00106-2. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends in Cognitive Sciences. 2000;4:432–40. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Magliero A, Bashore TR, Coles MGH, Donchin E. On the dependence of P300 latency on stimulus evaluation processes. Psychophysiology. 1984;21:171–86. doi: 10.1111/j.1469-8986.1984.tb00201.x. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric of thought: a comparison of P300 latency and reaction time. Science. 1981;21:171–86. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- Mummendey Wenzel. Social discrimination and tolerance in intergroup relations: reactions to intergroup difference. Personality and Social Psychology Review. 1999;3:158–74. doi: 10.1207/s15327957pspr0302_4. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Van Den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Nodera A, Karasawa K, Numazaki M, Takabayashi K. An examination of the promoter of gender role stereotype-activation based on terror management theory. Japanese Journal of Social Psychology. 2007;23:195–201. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pyszczynski T, Greenberg J, Solomon S. A dual-process model of defense against conscious and unconscious death-related thoughts: an extension of terror management theory. Psychological Review. 1999;106:835–45. doi: 10.1037/0033-295x.106.4.835. [DOI] [PubMed] [Google Scholar]
- Pyszczynksi T, Greenberg J, Solomon S, Arndt J, Schimel J. Why do people need self-esteem?: a theoretical and empirical review. Psychological Bulletin. 2004;130:435–68. doi: 10.1037/0033-2909.130.3.435. [DOI] [PubMed] [Google Scholar]
- Pyszczynski T, Soloman S, Greenberg J. In the wake of 9/11: The Psychology of Terror. Washington, DC: American Psychological Association; 2003. [Google Scholar]
- Pyszczynski T, Wicklund RA, Floresky S, et al. Whistling in the dark: exaggerated estimates of social consensus in response to incidental reminders of mortality. Psychological Science. 1996;7:332–6. [Google Scholar]
- Renkema LJ, Stapel DA, Maringer M, Van Yperen NW. Terror management and stereotyping: why do people stereotype when mortality is salient? Personality and Social Psychology Bulletin. 2008;34:553–64. doi: 10.1177/0146167207312465. [DOI] [PubMed] [Google Scholar]
- Rosenblatt A, Greenberg J, Solomon S, Pyszczynski T, Lyon D. Evidence for terror management theory I: the effects of mortality salience on reactions to those who violate or uphold cultural values. Journal of Personality and Social Psychology. 1989;57:681–90. doi: 10.1037//0022-3514.57.4.681. [DOI] [PubMed] [Google Scholar]
- Schimel J, Simon L, Greenberg J, et al. Stereotypes and terror management: evidence that mortality salience enhances stereotypic thinking and preferences. Journal of Personality and Social Psychology. 1999;77:905–26. doi: 10.1037//0022-3514.77.5.905. [DOI] [PubMed] [Google Scholar]
- Solomon S, Greenberg J, Pyszczynski T. A terror management theory of social behavior: the psychological functions of self-esteem and cultural worldviews. In: Zanna MP, editor. Advances in Experimental Social Psychology. Vol. 24. New York: Academic Press; 1991. pp. 93–159. [Google Scholar]
- Solomon S, Greenberg J, Pyszczynski T. Pride and prejudice: fear of death and social behavior. Current Directions in Psychological Science. 2000;9:200–4. [Google Scholar]
- Tajfel H, Turner JC. The social identity of intergroup behavior. In: Worchel S, Austin W, editors. Psychology of Intergroup Relations. Chicago: Nelson-Hall; 1986. pp. 7–24. [Google Scholar]
- van Veen, Carter CS. The timing of action-monitoring processes in the anterior cingulated cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Willadsen-Jensen E, Ito TA. Ambiguity and the timecourse of racial perception. Social Cognition. 2006;24:580–606. [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural bases of error detection: conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–59. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]