Abstract
Neuroimaging studies have revealed a consistent overlap between brain regions involved in self-processing and those implicated in autobiographical memory. However, no study has directly tested how the degree of self-involvement with an event being remembered alters the neural circuitry engaged during memory retrieval. The present study compared hockey players’ memories for game elements in which they were highly involved (e.g. scoring a goal) versus less involved (e.g. watching a goal from the bench). Specifically, we examined how the effective connectivity of a network of brain regions known to be involved in autobiographical memory retrieval varied based upon the players’ level of self-involvement with the remembered event. During remembering of high self-involvement events, connections between the left hippocampus and medial prefrontal cortex were ‘in synchrony’ with connections between the medial prefrontal cortex and the right amygdala–hippocampal complex. By contrast, the hippocampal–prefrontal connection was ‘out-of-sync’ with the prefrontal–amygdala connection during retrieval of low self-involvement memories. This result is discussed in terms of two memory systems (one that is hippocampal-based and one that is amygdala–hippocampal-based) that may be involved to varying degrees depending upon the characteristics of a remembered event.
Keywords: self-involvement, self, autobiographical memory, memory, effective connectivity
Although memory is typically thought of in terms of its cognitive properties and functions, a wealth of research suggests that memory also plays an important role in the social domain. For example, reflecting on personal experiences contributes to our sense of identity, guides our present and future behavior, and facilitates our social bonding when we discuss personal memories with others (Bluck, 2003; Addis and Tippett, 2004, 2008). Autobiographical memories (AM) of events in which we had a high degree of personal involvement are likely to contribute significantly to these self-related processes.
In line with the suggestion that AM retrieval may be closely linked to our sense of self, recent neuroimaging work reveals that some brain regions activated as part of the AM retrieval network also support self-referential processing. For example, neuroimaging studies have consistently revealed a set of cortical-midline structures, including the medial prefrontal cortex (MPFC) and posterior cingulate cortex (PCC; Gusnard et al., 2001; Johnson et al., 2002; Kelley et al., 2002; Macrae, et al., 2004), to be activated during self-referential tasks such self-reflection, self-referential encoding and trait judgments. Similar regions of MPFC and PCC are also activated during retrieval of AMs, relative to other types of memory (for a meta-analysis, see Svoboda et al., 2006; for reviews, see Cabeza and St. Jacques, 2007; Maguire, 2001). Moreover, a recent study examining the effective connectivity of the AM retrieval network found strong connectivity between a left posterior midline region (centered on the PCC/retrosplenial cortex, BA 23/30) and left MPFC (BA 10), via the left hippocampus (Addis et al., 2007). In temporal lobe epilepsy patients exhibiting left hippocampal damage, the direct connection between left PCC and MPFC strengthened significantly, suggesting this is a critical pathway supporting AM retrieval (for a similar result in a hippocampal amnesic patient, see Maguire et al., 2001).
Given that MPFC and PCC support both self-referential processing and AM retrieval, it is possible that the degree of self-involvement a person had in a remembered event may influence the connectivity between these and other regions within the AM network during retrieval. Indeed, there are many studies that suggest a link between self-referential processing and memory retrieval (e.g. Fossati et al., 2004; Magno and Allen, 2007). However, the majority of these studies focus on personal semantic information (e.g. I am a Democrat). By contrast, AM studies focus on the self within the context of personal episodes (i.e. personal episodic memory; e.g. remembering getting married on September 15; remembering how I found out that my first paper was accepted last month), leading to the question of whether AM retrieval will be modulated by the self-referential nature of a memory in a similar fashion to retrieval of semantic self-relevant information.
Even though research indicates that both the retrieval of personal episodic AMs and self-referential tasks focusing on personal semantics engage MPFC, a prior study suggests some distinction between retrieval of personal episodic and semantic information. In particular, a comparison of the brain regions recruited specifically in retrieving AMs, relative to personal semantic information, reveals that left MPFC is specifically recruited during retrieval of personal episodic memories (Maguire, 2001). Even among personal episodic memories, though, a person can have been highly involved in an event (e.g. my wedding), or more remotely involved (e.g. a friend’s wedding). Recent research has highlighted that this may be an important distinction to make when studying AM, as Sharot et al. (2007) found that, when remembering the experiences of September 11th, a region of the amygdala was modulated by how close to ground zero the participant was that day. This can be seen as an index of self-involvement—those close to ground zero were even more involved in the event (e.g. they may have taken cover from falling debris) than those spatially removed from it (e.g. watching the events unfold on TV). However, this prior study also had a potential confound, given that those closer to ground zero also found the event more directly threatening (i.e. they felt they were in harm’s way) and more arousing.
The present study examined how effective connectivity between regions within the AM retrieval network varies depending upon the level of self-involvement with the event being recalled, even when the arousal level of the experiences do not differ as a function of self-involvement. Although regions typically associated with self-referential processing (e.g. MPFC, PCC) may be necessary to support retrieval of AMs regardless of self-involvement, it is possible that those regions might interact differently with regions supporting memory processes (e.g. the hippocampus) depending upon an individuals’ self-involvement with the memory being retrieved. Moreover, other brain regions such as the amygdala, that do not comprise the network engaged when retrieving fairly neutral or low-arousing AMs, may be recruited into the AM network under conditions of high self-involvement. We reasoned that retrieval of events in which a person was highly involved should lead to stronger connectivity between regions mediating self-referential processing, the amygdala and hippocampal memory mechanisms than retrieval of events in which a person was less directly involved.
In order to investigate this issue, we asked members of the 2006–2007 Boston College Women’s Ice Hockey team to recall events from the hockey season in response to cue words while they underwent an functional magnetic resonance imaging scan. College athletes were selected as an ideal population in which to study this phenomenon, because during any game a number of sub-events occur (e.g. goals, penalties, etc.) that an individual player may be more or less involved in (e.g. being the person scoring the goal, versus watching from the bench as a goal is scored). This allows for a within-subjects analysis of self-involvement in AM, as opposed to the between-subjects design employed in other studies (e.g. Sharot et al., 2007). In our connectivity analyses, we used a well-validated network of brain regions typically found to be engaged during AM retrieval (e.g. Svoboda et al., 2006; Cabeza and St. Jacques, 2007), and we additionally included regions responding to emotion and possibly self-involvement (e.g. amygdala; Sharot et al., 2007).
METHODS
Participants
Participants were 13 female members of the 2006–2007 Boston College Women’s Ice Hockey team, ranging in age from 18 to 21 years (M = 19.15, SD = 1.07). All were right-handed, native English speakers with normal vision and no history of neurological or psychiatric problems. Participants gave written informed consent following the guidelines of the Boston College and Massachusetts General Hospital Institutional Review Boards, and they were paid $25/h for their time.
Procedure
AM trials
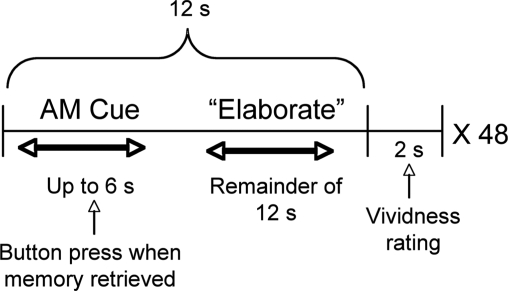
Prior to scanning, we identified 48 hockey-related words (e.g. ‘goal’, ‘intermission’, ‘warm-up’, ‘penalty’), which served as generic cues to trigger participants’ retrieval of AMs. Participants were instructed to ‘recall an event from the 2006–2007 hockey season, specific in time and place, related to the cue’. Behavioral results demonstrated that these cues were effective at triggering AMs; participants reported successfully retrieving a specific AM for, on average, 95% of cues (range = 85–100%; trials for which a memory was not retrieved were excluded from the analyses). During each AM trial, participants viewed a word cue printed in white font on a black screen. Participants were instructed to make a button press when they had identified the AM they would think about for the cue. If no button press was made, the cue disappeared after 6 s. Following the button press (or once 6 s had passed), the word ‘Elaborate’ was displayed on the screen, and participants were instructed to retrieve as much detail about the AM as possible. The elaboration phase lasted a minimum of 6 s, and its duration depended upon the reaction time of the button press (i.e. if a participant indicated she had identified an AM after viewing the cue for 3 s, then the elaboration phase would last a total of 9 s). Twelve seconds after the initial presentation of the cue, the word ‘Vividness?’ appeared on the screen for 2 s, and participants were instructed to press the button that corresponded with the vividness of their memory for that trial (1–4 scale). Thus, AM trials lasted a total of 14 s from cue onset to vividness rating (see Figure 1).
Fig. 1.
Experimental design used to elicit retrieval of autobiographical memories.
Control trials
As a control task, participants were asked to view and read a sentence that was printed on the screen and to imagine the depicted scenario. Sentences were positive, negative or neutral in emotional valence (for a description of the stimuli, see Kensinger et al., 2002), though for the current analyses we restricted our focus to the neutral sentence trials. The structure of these trials paralleled the AM trials: Participants were presented with each sentence; after 4 s the word ‘Imagine’ appeared on the screen and participants were asked to continue to imagine the scene. After 8 s of the initial presentation of the sentence, the word ‘Vividness?’ appeared on the screen, and participants made a button press indicating the vividness of their imagery. Thus, control sentence trials lasted a total of 10 s from cue onset to vividness rating.
Post-scan survey
Following scanning, participants wrote a brief description of each memory they retrieved during scanning, and they used a Likert-type scale to rate each AM on seven factors thought to characterize the recollective experience of AM: valence, arousal, rehearsal, reliving, perspective (personal/field perspective or outside observer perspective), and imagery (was the AM more like a verbal narrative or a film; see Rubin et al., 2003). Critically, we also asked participants to report, on a 7-point scale, how involved they were with the event when it first occurred. This rating was used to classify reported events into high self-involvement (rating of 6–7) and low self-involvement (rating of 1–3).
Scanning parameters
Anatomical images were acquired on a 1.5 Tesla Avanto whole-body MR System with a standard birdcage head coil. Experimental stimuli were projected from a Macintosh iBook G4 to a Sharp200 color LCD projector through a collimating lens that projected onto a screen mounted in the magnet bore. Participants viewed the screen through mirrors located on the head coil.
Anatomic images were acquired with a multi-planar rapidly acquired gradient echo (MP-RAGE) sequence (TR = 2730 ms, TE = 3.31 ms, flip angle = 40°, field of view = 256 × 256 mm, acquisition matrix 256 × 256, number of slices = 128, slice thickness = 1.33 mm, no gap, 1 × 1 × 1.33 mm resolution). Co-planar and high-resolution T1 weighted localizer images were acquired. In addition, a T1 weighted inversion recovery echo planar image was acquired for auto alignment.
Four functional scans were acquired (T2*-weighted echo planar imaging sequence, repetition time of 2000 ms, echo time of 40 ms and a flip angle of 90°). Twenty-six interleaved axial-oblique (parallel to the line between the anterior commisure and the posterior commisure) slices were collected in a 3.125 mm × 3.125 mm × 3.72 mm matrix with a 3.12 mm thickness and a 0.63 mm skip between slices. Each functional scan lasted 8 min 49 s.
Functional imaging data
Preprocessing and univariate analyses were performed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK). Functional images were slice-time corrected, realigned and unwarped for motion correction, co-registered to a structural image, spatially normalized and smoothed using a Gaussian kernel of 7.6 mm full-width half maximum (i.e. a kernel that was two times the size of the functional voxel). Linear slope was removed to correct for drift. Each stimulus event was modeled by SPM2’s canonical hemodynamic response function (applied at task onset).
Our first contrast analysis compared all AM retrieval trials (collapsing across emotional valence and level of involvement) to all neutral sentence control trials. This contrast enabled us to identify the AM retrieval network and to select the majority of nodes for the connectivity analysis (see below). Despite an a priori hypothesis predicting engagement of the hippocampus during AM retrieval (in line with the AM literature demonstrating a critical role of the hippocampus in AM retrieval, e.g. Maguire et al., 2001; Svoboda et al., 2006), hippocampal activity was not robust in this contrast. Since this study was designed and the data collected, reports have shown that the hippocampus is engaged when imagining scenarios (e.g. Schacter et al., 2007). Thus, it is not surprising that the hippocampal activity associated with AM retrieval was not evident when contrasted against the sentence task requiring participants to imagine the scenario depicted by the sentence. Thus, we defined the hippocampal ROI from a contrast analysis comparing all AM retrieval trials (collapsing across emotion and involvement) to the implicit baseline. Moreover, we had a specific a priori prediction regarding the involvement of the amygdala in personally important events (Sharot et al., 2007), and given that this region is not typically a part of the core AM network identified by contrasting AM retrieval with a control task (Svoboda et al., 2006), we defined the amygdala ROI from a contrast comparing emotional (positive and negative) to neutral AM trials.
A significance threshold of P < 0.001 (uncorrected for multiple comparisons), and an extent threshold of five contiguously active voxels (2 mm × 2 mm × 2 mm) was applied to the contrast of AMs versus neutral sentences (Addis et al., 2007). As we had a priori hypotheses regarding the hippocampus and the amygdala, we used a threshold of P < 0.005 (Addis et al., 2007). For all contrasts, MNI coordinates were converted to Talairach space and regions of activations were localized in reference to a standard stereotaxic atlas (Talairach and Tournoux, 1988).
Region of Interest (ROI) analyses were conducted using the MarsBar toolbox in SPM (Brett et al., 2002). Event-related time-courses were extracted from active clusters by creating ROIs that included all significant voxels within a 5 mm radius of the maximum voxel. A hemodynamic response function was calculated within each ROI for each individual participant and condition, as a function of peristimulus time (0–16 s). Statistics were calculated on the average percent signal change within peristimulus time 6–8 s. T-tests examined whether there was an effect of self-involvement within each ROI. These percent signal change values were also used for the connectivity analyses, as described below.
Effective connectivity analysis
The primary aim of the current study was to investigate how the effective connectivity of the AM network changes according to the level of self-involvement in the episode being remembered. This was accomplished by using structural equation modeling (SEM; McIntosh and Gonzalez-Lima, 1994). This multivariate technique assesses the fit of a neuroanatomical model of connections with the interregional covariances observed in the BOLD signal. Unlike correlations, SEM allows for a consideration of connections across multiple nodes of a network, and it provides information about the directionality and strength of influences between different regions.
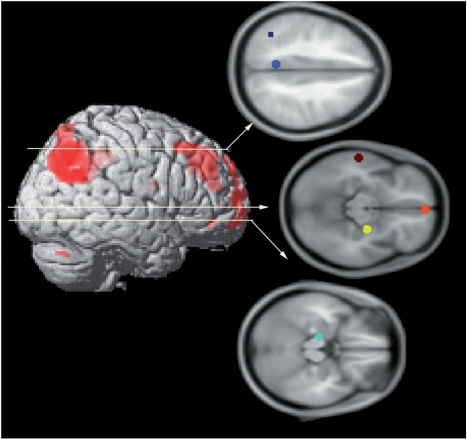
The first step of this SEM analysis was to specify the anatomical model. Selection of regions for this model was based upon the results of the contrasts described above. Of the regions identified by the contrast of the AM and sentence control tasks (see Table 1), a limited set of key nodes known to comprise the AM retrieval network were selected for our anatomical model (see Figure 2 ; Maguire et al., 2001; Addis et al., 2007). Additionally, the hippocampal ROI (x, y, z = −14, −18, −18; as identified by the contrast of AM versus the implicit baseline) was included, as this is a critical node of the AM retrieval network (Addis et al., 2007). Moreover, the amygdala ROI (x, y, z = 26, −12, −11; as identified by the contrast of emotional vs. neutral AMs) was included given the findings of Sharot et al. (2007) suggesting this region may respond to self-involvement. Our anatomical model thus included MPFC, lateral middle temporal lobe, right amygdala-hippocampal complex, left hippocampus, left temporoparietal junction (TPJ), and a region of medial parietal cortex that encroached on both PCC and the precuneus. The average percent signal change for each of these regions was extracted from a 5 mm sphere around the peak voxel in each region (see Figure 2 for co-ordinates of peak voxels). Then, on the basis of primate neuroanatomy and previous connectivity studies of the AM network (Maguire et al., 2001; Addis et al., 2007), the anatomical connections (including multi-synaptic connections) between these regions, and the direction of those connections, were specified (see Figure 3 ). Next, a functional model was constructed for each of two conditions (high self-involvement and low self-involvement) by calculating the inter-regional correlations of percent signal change values across subjects.
Table 1.
Brain regions activated during AM retrieval (AM trials > control trials)
| Brain region | Co-ordinates |
Z-score | ||
|---|---|---|---|---|
| x | y | z | ||
| L. Medial frontal gyrus (BA 8) | 2 | 33 | 41 | 4.89 |
| R. Medial frontal gyrus (BA 10/11)a,b | 2 | 56 | −13 | 4.41 |
| L. Superior frontal gyrus (BA 10) | −20 | 64 | −7 | 4.81 |
| R. Superior frontal gyrus (BA 10) | 26 | 60 | 3 | 3.30 |
| L. Superior frontal gyrus (BA 9) | −18 | 58 | 32 | 3.89 |
| L. Middle frontal gyrus (BA 8) | −28 | 27 | 37 | 4.52 |
| R. Middle frontal gyrus (BA 8/9) | 26 | 29 | 45 | 3.51 |
| L. Caudate | −12 | −1 | 18 | 4.05 |
| R. Caudate | 10 | 1 | 18 | 3.38 |
| L. Middle temporal gyrus (BA 20/21)a | −60 | −22 | −11 | 6.77 |
| L. Inferior temporal gyrus (BA 20) | −57 | −7 | −20 | 3.54 |
| L. Posterior cingulate/precuneus (BA 31/7)a | −6 | −50 | 37 | 6.88 |
| L. Temporoparietal junction (BA 40)a | −40 | −56 | 43 | 7.94 |
| R. Angular gyrus (BA 39) | 50 | −70 | 33 | 4.60 |
Note. The Talairach co-ordinates of the maximally activated focus within each different structure are reported, as indicated by the highest Z-score. BA = Brodmann area.
aRegions included in the anatomical model for the effective connectivity analysis.
bActivation in this region extended bilaterally.
Fig. 2.
Six regions were included in the anatomical model; these regions were selected based upon prior studies of autobiographical memory retrieval (e.g. Maguire et al., 2001; Sharot et al., 2007). Regions included medial parietal cortex (encroaching upon posterior cingulated cortex and precuneus; center of ROI = −6, −50, 37; in blue), left temporoparietal junction (center of ROI = −40, −56, 43; in navy), medial PFC (center of ROI = 2, 55, −13; in orange), lateral middle temporal lobe (center of ROI = −60, −22, −10; in brown), right amygdala-hippocampal complex (center of ROI = 26, −12, −11; in yellow), and left hippocampus (center of ROI = −14, −18, −18; in turquoise).
Fig. 3.
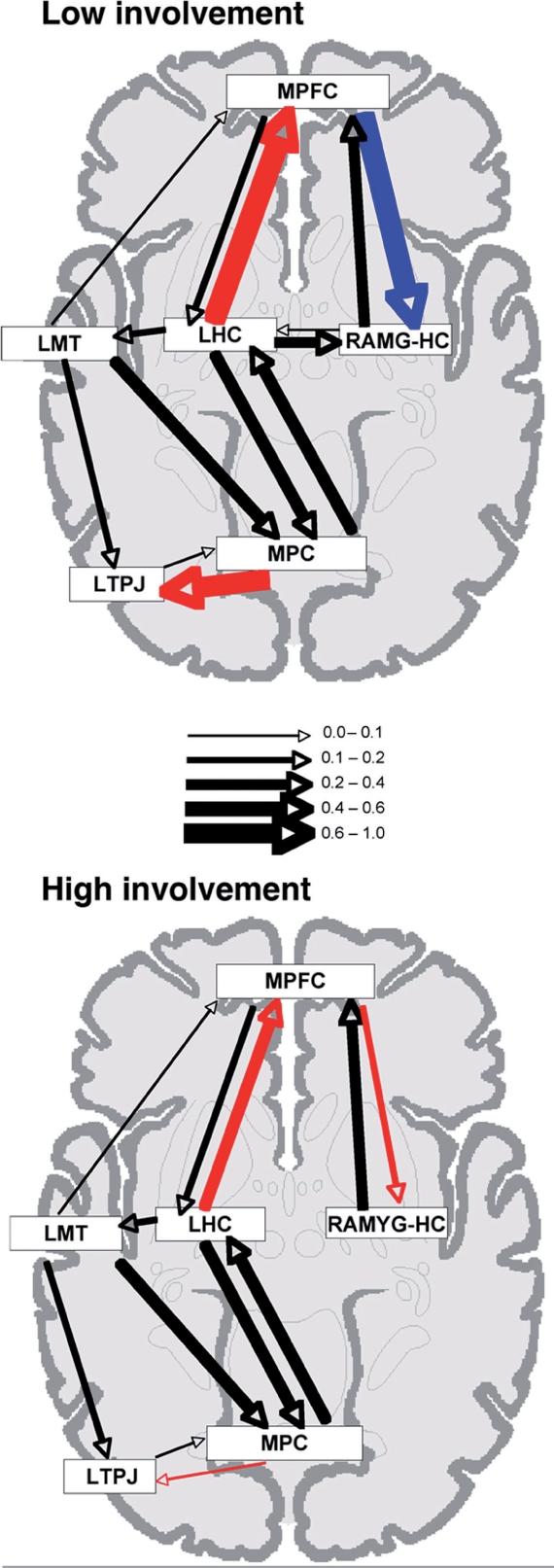
Diagrammatic representation of the effective connections within the neural network mediating AM retrieval for (top) low self-involvement events and (bottom) high self-involvement events. Connections which differed significantly across conditions are depicted in color (red = positive influence, increasing activity in the target node, blue = negative influence, decreasing activity in the target node). Connections which did not differ significantly between groups are depicted in black. Arrow thickness represents the strength of the connections (i.e. the value of the path coefficient), as described in the key.
All SEM calculations were performed using Lisrel 8.30 (Joreskog and Sorbom, 1993). First, estimates of path coefficients were calculated based upon correlations of percent signal change values within each condition and across subjects from the regions in the anatomical model. The resulting path coefficients indicate the strength and direction of the effect of that link in the model. Significant differences across the conditions were then assessed using the stacked-model approach (McIntosh and Gonzalez-Lima, 1994). In an omnibus test, a null model was first constructed in which the path coefficients from both conditions were set to be equal across conditions. This was compared with a second, alternative model in which all path coefficients were allowed to differ across conditions. The differences between the models were assessed by subtracting their goodness-of-fit χ2 values to obtain a χ2diff A significantly lower χ2 value for the alternative model (i.e. a greater χ2diff between the models) indicated there were significant differences between conditions (P < 0.05). To determine which individual connections contributed significantly to the difference between the null and alternative model, each connection was allowed to vary in a stepwise manner. Those connections that did increase the significance of the difference across conditions (as evidenced by a decrease of the P-value associated with the χ2diff) were then set to vary for the remainder of the analysis. Any connection which did not contribute to the significance of the difference across conditions was set to be equal across condition as the analysis progressed to the next connection. Given that this is a stepwise analysis, it is possible that the order the connections are entered in to the analysis could affect the results (i.e. whether a particular connection was found to be significant). Thus, four orders of connections were used: connections involving anterior to posterior cortical regions then subcortical regions; posterior to anterior cortical then subcortical; subcortical, then anterior to posterior cortical; subcortical, then posterior to anterior cortical (Addis and McAndrews, 2006; Addis et al., 2007). The stepwise analysis which resulted in the largest difference between the null and alternate chi-square values was ‘posterior to anterior cortical then subcortical’; this analysis was used to determine significant connections.
RESULTS
Behavioral data
AM trials were sorted into high and low self-involvement conditions on the basis of the self-involvement ratings provided during the post-scan interview. Specifically, those trials with involvement ratings of 1–3 comprised the low condition while those trials rated 6–7 comprised the high condition. Participants contributed, on average, 13.85 trials to the low self-involvement condition (SD = 7.71) and 25.23 trials to the high self-involvement condition (SD = 8.83). Paired t-test analyses of the other post-scan AM ratings (see Table 2) revealed no significant differences between high and low self-involvement events on ratings of emotional valence, arousal, amount of rehearsal, extent of reliving, or type of imagery recalled (all P > 0.30). AMs of high self-involvement events were rated as more vivid than low self-involvement events (P < 0.04), and individuals were more confident in the accuracy of their AMs of high self-involvement events than low self-involvement events (P < 0.001). High self-involvement events also were more likely to be recalled from a strictly personal perspective, whereas low self-involvement events were seen less exclusively from a personal perspective (P < 0.02).
Table 2.
Characteristics of the recollective experience of autobiographical memories
| Memory characteristic | High involvement AM |
Low involvement AM |
||
|---|---|---|---|---|
| M | SD | M | SD | |
| Valence | 4.55 | 0.83 | 4.67 | 0.56 |
| Arousal | 5.03 | 0.54 | 4.82 | 0.53 |
| Vividness* | 3.39 | 0.29 | 3.14 | 0.43 |
| Rehearsal | 2.86 | 0.76 | 2.80 | 0.79 |
| Confidence* | 5.56 | 1.1 | 4.04 | 1.1 |
| Reliving | 5.55 | 0.80 | 4.43 | 0.83 |
| Perspective* | 1.82 | 0.89 | 2.49 | 1.1 |
| Imagery | 5.46 | 0.95 | 5.46 | 0.86 |
Note. Asterisk denotes a statistically significant difference between high and low involvement AMs (P < 0.05). For valence, higher numbers are more positive; for perspective, higher numbers indicate more outside-observer perspective (relative to first-person perspective); for imagery, higher numbers indicate more visual imagery (relative to narrative imagery).
Main effect of self-involvement
To examine if there was a main effect of self-involvement in modulating neural activity in the six regions of interest to be used in connectivity analyses, paired t-tests were conducted comparing activity in the six ROIs for high versus low self-involvement AMs. Results of these analyses revealed no significant differences between high and low self-involvement AMs in any of the six ROIs (all P > 0.35).
Effective connectivity of the AM retrieval network
SEM was used to assess differences in the effective connectivity of the AM retrieval network during the retrieval of high and low self-involvement AMs. The omnibus SEM analysis revealed a significant effect of condition on the effective connectivity of the AM retrieval network (P < 0.001). A stepwise assessment of connections was conducted to determine which connections differed significantly across conditions (P < 0.01). This assessment revealed three connections that differed significantly between low and high self-involvement memories: the influence of medial parietal cortex on left TPJ, the influence of the left hippocampus on MPFC, and the influence of MPFC on the right amygdala-hippocampal complex (see Figure 3, bottom panel). Of particular interest are the differences in connectivity between medial temporal lobe structures (left hippocampus, right amygdala-hippocampal complex) and MPFC. During retrieval of high self-involvement memories, there is a positive influence of the left hippocampus on MPFC, and a positive influence of MPFC on the amygdala-hippocampal complex (see Figure 3, bottom panel). By contrast, during retrieval of low self-involvement memories, there is a stronger, positive influence of the left hippocampus on MPFC, and a strong, negative influence of MPFC on the amygdala-hippocampal complex (see Figure 3, top panel).
DISCUSSION
Recent research has indicated significant overlap between brain regions that support self-referential processing and those that support AM retrieval. It has been unclear, however, how the relationship between self-referential and mnemonic retrieval processes varies depending upon the extent of self-involvement in the event being remembered. The present study revealed differences in effective connectivity of the MPFC and medial temporal lobe regions of the AM network depending upon self-involvement. Specifically, two circuits, one connecting the left hippocampus to the MPFC, the other connecting MPFC to right amygdala, appeared to be ‘out of sync’ during recall of low self-involvement events (i.e. left hippocampus positively influences MPFC, while MPFC negatively influences right amygdala), whereas they appeared to be ‘in sync’ during recall of high self-involvement events (i.e. both connections are positive). This result emphasizes how the interactions among the nodes of the AM network can be modulated, within a single person, based upon the types of experiences that person is retrieving. Because extensive neuroimaging and neuropsychological research has linked the hippocampus to the retrieval of memories rich with contextual, ‘external’ detail (Maguire, 2001; Addis et al., 2004; Svoboda et al., 2006) and the amygdala to the processing of affectively-rich, ‘internal’ information (reviewed by Phan et al., 2004; Buchanan, 2007), this finding perhaps suggests that when recalling an event of high self-involvement, we rely on both a memory system that retrieves primarily external details (supported by left hippocampus and connected regions such as MPFC) and a memory system that retrieves internal details (supported by right amygdala-hippocampal complex and connected regions such as MPFC). By contrast, when recalling an event of lower self-involvement, we may rely more exclusively on an external-detail oriented memory system and less on an internal-detail oriented system. Indeed, the strong positive influence of the left hippocampus on the MPFC is consistent with the pattern of connectivity previously found during AM retrieval in young adults (Addis et al., 2007) suggesting this may be the default pattern unless an event is of unusually high self-involvement.
The present results are consistent with extensive research suggesting that there are distinct ‘hot’ and ‘cold’ processing systems, supported by amygdala and hippocampal processes, respectively (Metcalfe and Jacobs, 1996, 1998). The current study suggests that degree of self-involvement with a prior event can modulate whether these processing systems work together to support memory retrieval (as is true for high-involvement memories) or remain distinct from one another (as for low-involvement memories). Critically, the present results suggest that the effects of self-involvement can occur even when the events are rated as equally high in arousal. These results suggest that the amygdala-based system may be tied not only to the arousal level of the event but also to the types of details that are remembered about the event (e.g. whether more internal or external details are retrieved; see also Sharot et al., 2007).
There has been extensive debate about the best way to characterize the role of the amygdala during episodic retrieval (e.g. Is it tied to accurate memory? To memory for some details but not others? To the subjective feeling of re-experiencing an event?). The present results are consistent with claims that the amygdala can be tied to the subjective vividness of a memory and to a person’s confidence in a memory (Phelps and Sharot, 2008). The high-involvement events, which recruited the amygdala into the AM network, were remembered more vividly, and with more confidence, than the low-involvement events. Though these functions of the amygdala have traditionally been tied to its role in arousal-based processing, the present results emphasize the importance of examining broader, social processes when considering the amygdala’s role in memory retrieval.
Beyond the amygdala-MPFC connection, the connectivity between the medial parietal cortex and the left TPJ also varied based on self-involvement: There was a weak, positive connection between the two regions during recall of high self-involvement events, but the connection became significantly stronger during the retrieval of low self-involvement events. Previous research has shown that medial parietal regions support episodic imagery (Fletcher et al., 1995; Wagner et al., 2005) and the processing of contextual information (Bar and Aminoff, 2003). As such, the increase of the influence of medial parietal regions on TPJ fits with the idea that the retrieval of low self-involvement AMs may rely more on episodic and contextual processing than retrieval of higher self-involvement AMs. Additionally, the TPJ is implicated in theory-of-mind processing (Saxe et al., 2006), and so this finding may reflect a recruitment of theory-of-mind processes to remember events in which one is not strongly personally involved. It makes sense that regions mediating theory-of-mind processes could also assist with the retrieval of information regarding others’ actions and vocalizations, especially when the self is not the main ‘agent’ in the remembered episode. Indeed, our behavioral data reveal that low self-involvement memories were remembered from a less exclusively first-person perspective than high self-involvement memories. Thus, the stronger medial parietal cortex-TPJ connection when remembering low self-involvement events may reflect participants’ retrieval of more third-person-oriented details.
Thinking about these results more broadly, we believe that they are interesting in a few respects. To our knowledge, this is the first study to demonstrate differences in effective connectivity of AM based on the characteristics of the autobiographical memories being retrieved; the other AM effective connectivity studies published to date have either focused on between-group differences in connectivity (between patients with brain damage and normal controls; see Maguire et al., 2001; Addis et al., 2007) or on within-subject differences based upon the type of memory being retrieved (episodic, semantic; Maguire et al., 2000). Furthermore, the finding of task-related connectivity differences between regions that do not show a main effect of high and low self-involvement demonstrates that interpretation of main effects alone may not always provide the most complete picture of how a neural network responds to memory characteristics. Indeed, others have also demonstrated that a region can exhibit differing patterns of effective connections under different task conditions even if the region fails to show a main effect of condition (e.g. Stephan et al., 2003; Rowe et al., 2005). The current findings again highlight the complementary information provided by examining how interactions between regions differ according to task, in this case, within-subjects differences among autobiographical memories.
Second, the present results corroborate those of Sharot et al. (2007), reinforcing the importance of self-involvement as a characteristic that influences the neural systems, particularly the medial temporal regions, involved in AM retrieval. Though the within-subject design of the present study was quite different from the between-subject design of Sharot et al. (2007), both studies converge on the conclusion that the amygdala plays a more dominant role in the retrieval of events with a high level of personal involvement and may be less involved for the retrieval of events with low personal involvement.
An important caveat with the present data is that we cannot disentangle if the observed connectivity differences reflect the self-involvement of the event per se, or some other mnemonic feature of highly self-relevant events. As mentioned previously, events high in self-involvement were remembered more vividly and confidently than events low in self-involvement. It is, therefore, possible that the connectivity differences observed here would arise whenever an event is remembered more vividly or confidently, and that modulation of self-involvement is not necessary for these changes in the AM network to occur. Because the MPFC is not a region whose activity typically varies based upon the detail of AM retrievals, we think this alternate explanation is less viable; however, it will be important for future research to examine whether similar changes in the connectivity of the AM network can occur when self-relevance is held constant but the subjective features of the AMs (e.g. their vividness or confidence) vary.
In sum, results from the present study demonstrate that, when remembering autobiographical experiences, the level of self-involvement in the event being remembered critically impacts the connectivity amongst brain regions used to retrieve those memories and appears to result in other regions, such as the amygdala, being recruited into the AM retrieval network and positively connected with regions such as MPFC. The extent to which frontal-MTL circuits are working in synchrony depends upon the amount of self-involvement in the memory being retrieved. Future research should examine if other memory characteristics also influence the effective connectivity of the AM retrieval network and should attempt to further specify what specific features of the broad concept of ‘self-involvement’ distinguish the neural processes supporting their recall.
Acknowledgments
This research was supported by National Institute of Mental Health grant MH080833 to E.A.K.
REFERENCES
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Addis DR, Moscovitch M, McAndrews MP. Consequences of hippocampal damage across the autobiographical memory network. Brain. 2007;130:2327–2342. doi: 10.1093/brain/awm166. [DOI] [PubMed] [Google Scholar]
- Addis DR, Tippet LJ. Memory of myself: autobiographical memory and identity in Alzheimer’s disease. Memory. 2004;12:56–74. doi: 10.1080/09658210244000423. [DOI] [PubMed] [Google Scholar]
- Addis DR, Tippet LJ. The contributions of autobiographical memory to the content and continuity of self: a social-cognitive neuroscience approach. In: Sani F, editor. Self-continuity: Individual and Collective Perspectives. New York: Psychology Press; 2008. pp. 71–84. [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bluck S. Autobiographical memory: exploring its functions in everyday life. Memory. 2003;11:113–123. doi: 10.1080/741938206. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valbregue R, Poline JB. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16:1140–1141. [Google Scholar]
- Buchanan TW. Retrieval of emotional memories. Psychological Bulletin. 2007;133:761–779. doi: 10.1037/0033-2909.133.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, St. Jacques P. Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Fletcher P, Frith C, Baker SC, Shallice T, Frackowiak RS, Dolan R. The mind’s eye—precuneus activation in memory-related imagery. Neuroimage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, et al. Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22:1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences, USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Joreskog KG, Sorbom D. LISREL 8: Structural Equation Modeling with the SIMPLIS Command Language. Hillsdale, NJ: Earlbaum Associates; 1993. [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. The effect of normal aging and Alzheimer’s disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Magno E, Allan K. Self-reference during explicit memory retrieval: an event-related potential analysis. Psychological Science. 2007;18:672–677. doi: 10.1111/j.1467-9280.2007.01957.x. [DOI] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ, Buchel C. Patterns of hippocampal-cortical interaction dissociate temporal lobe memory subsystems. Hippocampus. 2000;10:475–482. doi: 10.1002/1098-1063(2000)10:4<475::AID-HIPO14>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damade on fMRI regional activations and interaction during memory retrieval. Brain. 2001;2001:1156–1170. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Human Brain Mapping. 1994;2:2–22. [Google Scholar]
- Metcalfe J, Jacobs WJ. A “hot-system/cool-system” view of memory under stress. PTSD Research Quarterly. 1996;7:1–3. [Google Scholar]
- Metcalfe J, Jacobs WJ. Emotional memory: The effects of stress on “cool” and “hot” memory systems. In: Medin DL, editor. The Psychology of Learning and Motivation: Advances in Research and Theory. San Diego: Academic Press; 1998. pp. 187–222. Vol. 38. [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectrums. 2004;9:258–266. doi: 10.1017/s1092852900009196. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Sharot T. How (and why) emotion enhances the subjective sense of recollection. Current Directions in Psychological Science. 2008;17:147–152. doi: 10.1111/j.1467-8721.2008.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JB, Stephan KE, Friston KJ, Frackowiak RSJ, Passingham RE. The prefrontal cortex shows context-specific changes in effective connectivity to motor or visual cortex during the selection of action or colour. Cerebral Cortex. 2005;15:85–95. doi: 10.1093/cercor/bhh111. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Schrauf RW, Greenberg DL. Belief and recollection of autobipographical memories. Memory & Cognition. 2003;31:887–901. doi: 10.3758/bf03196443. [DOI] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social, Cognitive, and Affective Neuroscience. 2006;1:229–234. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. The prospective brain: remembering the past to imagine the future. Nature Reviews Neuroscience. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Sharot T, Martorella EA, Delgado MR, Phelps EA. How personal experience modulates the neural circuitry of memories of September 11. Proceedings of the National Academy of Sciences USA. 2007;104:389–394. doi: 10.1073/pnas.0609230103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Marshall JC, Friston KJ, Rowe JB, Ritzl A, Zilles K, et al. Lateralised cognitive processes and lateralised task control in the human brain. Science. 2003;301:384–386. doi: 10.1126/science.1086025. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory. Trends in Cognitive Sciences. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]