Abstract
A major controversy in the social cognitive neurosciences evolved around the question whether activity in the posterior superior temporal sulcus and adjacent temporoparietal junction (pSTS/TPJ-region) evoked by various tasks represents a common process or distinct processes. To investigate this question, we employed functional magnetic resonance imaging (fMRI) while participants performed Biological Motion (BM), Theory-of-Mind (ToM) and Moral Judgment (MJ) tasks. Importantly, for each task we used the same newly developed animated stimuli. Indicative of a common process, we identified small clusters of overlapping activity for BM and ToM in right pSTS and for ToM and MJ in bilateral pSTS and left TPJ. Indicative of distinct processes, on the contrary, we detected extensive dissociable activity for BM in right pSTS, for ToM in bilateral pSTS and left TPJ, and for MJ in bilateral pSTS and TPJ. Thus, our data provide strong evidence for a combined two-staged process account: (i) the parsing of a stream of visual-spatial information, represented by activity in right pSTS, where neighboring and overlapping clusters of increased responses were found for all three tasks; (ii) increasingly more complex processing of the communicative significance of other people’s behavior, represented by hierarchically increasing activity in left pSTS and bilateral TPJ elicited by ToM and MJ.
Keywords: biological motion, theory-of-mind, moral judgment, fMRI, posterior superior temporal sulcus and temporoparietal junction, intention-reading-process
INTRODUCTION
The function of the posterior superior temporal sulcus (pSTS) and the adjacent temporoparietal junction (TPJ)—together referred to as the pSTS/TPJ-region—is a topic of hot debate in the social cognitive neurosciences. Numerous neuroimaging studies employing social cognitive tasks such as Biological Motion (BM), Theory-of-Mind (ToM) and Moral Judgment (MJ) consistently found activation of the posterior end of the STS ascending into the TPJ. Perceiving BM, the movement of bodies or body parts, evokes increases in activity in the pSTS (Puce et al., 1998; Grossman et al., 2000; Hoffman and Haxby, 2000; Grezes et al., 2001; Beauchamp et al., 2003; Saygin et al., 2004; Peelen et al., 2006). Making inferences concerning the intentions, desires, emotions or thoughts of another person, a function that is referred to as ‘theory-of-mind’ or mentalizing, also leads to robust increases in activity in the posterior end of the STS extending into the TPJ (Fletcher et al., 1995; Baron-Cohen et al., 1999;Gallagher et al., 2000; Saxe and Kanwisher, 2003; Rilling et al., 2004; Hynes et al., 2006; Marjoram et al., 2006; Saxe and Powell, 2006; Vollm et al., 2006; Wolf et al., 2010). Furthermore, making moral judgments, deciding whether a person violates a social norm or not, evokes increase in BOLD signal in pSTS (Greene et al., 2001; Berthoz et al., 2002; Moll et al., 2002; Heekeren et al., 2003; Greene et al., 2004; Heekeren et al., 2005; Moll et al., 2005; Borg et al., 2006; Harenski and Hamann, 2006; Prehn et al., 2008).
Different accounts conceive of the function of the pSTS/TPJ-region as representing one common process. Allison et al. (2000) posit that the STS region is responsible for the perception and representation of socially relevant stimuli. Frith and Frith (1999) proposed that the STS serves in the detection and analysis of goals and outcomes of behavior. Gallagher et al. (2000) suggested that the activity in the TPJ-region elicited by different social cognitive tasks could be explained in terms of a shared intention reading process.
Importantly, the accounts postulating the engagement of a common process in the pSTS/TPJ-region by different tasks such as BM, ToM and MJ rest on the assumption of a common neural substrate, i.e. the activation foci for BM, ToM and MJ should overlap extensively in the pSTS/TPJ- region. Alternatively, as suggested by Saxe (2006), the respective neural substrates could be distinct from one another, i.e. the activation foci in the pSTS/TPJ-region should dissociate clearly. In this case, these tasks may engage distinct processes in the pSTS/TPJ-region and thereby challenge the common process accounts.
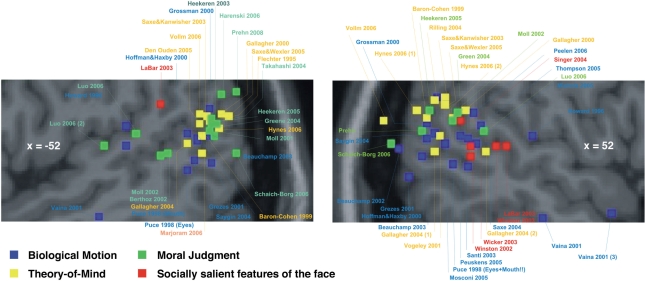
Figure 1 summarizes the results from previous neuroimaging studies on BM, ToM and MJ (Figure 1). There is a considerable overlap of activation clusters, though the location of activation maxima for BM is slightly anterior and inferior to those of ToM and MJ. For any of the tasks there is a high degree of variability of the location of activation maxima, which renders a clear-cut interpretation with respect to common or distinct representations difficult. Potential sources of the observed variability are different samples of participants, stimulus materials, scanning techniques, or analysis methods used in the different studies.
Fig. 1.
Meta-analysis of studies on BM, ToM and MJ. The activation maxima as reported by previous studies on BM (blue), ToM (yellow) and MJ (green) are superimposed on a left sagittal (left panel) and right sagittal slice (right panel) of the posterior superior temporal sulcus and the temporo–parietal junction. Note, that the location of activation maxima for BM is slightly anterior and inferior to those of either ToM or MJ.
The question of whether BM, ToM and MJ indeed share a common representation in the pSTS/TPJ-region or whether they rather rely on distinct representations can only be answered by using all of these tasks in a single experiment. Indeed, a recent study employing both a false belief and a social animation task provided first evidence for a dissociation of representations (Gobbini et al., 2007). Since the stimulus material in that study was different for the two tasks, however, the observed differences in neural activity may have been induced by variations of the respective material instead of differences between the tasks. To resolve this ambiguity, inherent to multi-task comparisons in a single experiment, we developed a new set of animated stimuli, each of which allows for the processing of BM, ToM and MJ.
To elucidate the question how these different tasks are represented in the pSTS/TPJ-region, we used the novel animated stimuli to investigate the relation of fMRI activation patterns of BM, ToM and MJ in the pSTS/TPJ-region with respect to their overlap or dissociation. Since the involvement of an intention reading process is plausible in all three tasks, we conceived of it as the most probable of social cognitive common process accounts. To ascertain whether such intention reading, i.e. ToM process, may serve as a process common to the three tasks, we tested whether task-induced changes in the BOLD signal would predict individual differences in ToM competence in those locations, where overlapping activity was identified.
MATERIALS AND METHODS
Participants
Twenty-five healthy participants [mean age (M) = 26, standard deviation (s.d.) = 4.43, three female] participated in this study. All participants were native German speakers, right-handed as assessed using the Montreal Handedness Questionnaire (Crovitz and Zener, 1962), and without any history of previous neurological or psychiatric diseases. The study was approved by the local ethics committee of the Charité University Medicine Berlin. Subjects were paid for their participation and gave written informed consent prior to the investigation according to the Declaration of Helsinki (1991).
Material
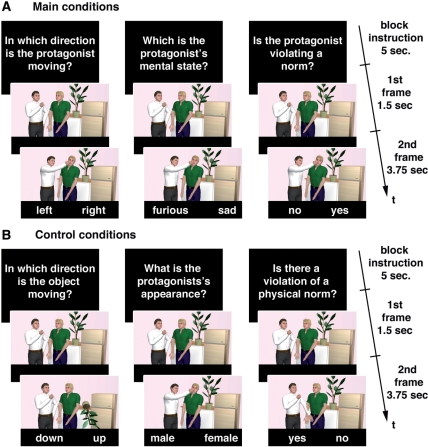
To directly compare the neural correlates of BM, ToM and MJ, we developed a set of animated stimuli, each depicting two people in a social interaction (Figure 2). Each stimulus consisted of two picture frames presented sequentially thereby creating the impression of movement of the characters. A white shirt marked the protagonist of the interaction. His or her behavior was either in accordance with or violating a social norm. To identify the neural correlate for each of the three functions of interest, we implemented the main conditions biological motion (bm), theory-of-mind (tom), moral judgment (mj) and respective matched control conditions, which we based on those used by previous studies. For the bm condition we used an object motion (om) condition (cf. Beauchamp et al., 2003), for the tom condition we used an appearance judgment (aj) condition (cf. Baron-Cohen et al., 1999), and for the mj condition we used a physical norm judgment (pnj) condition (cf. Prehn et al., 2008) as matched control condition, respectively. In the main conditions, the participants were instructed to make a judgment concerning the direction of the protagonist’s movement (bm), concerning his or her mental state (tom), or concerning the norm-congruency of his or her behavior (mj). In the control conditions participants were asked to make a judgment about the direction of motion of an object (om), or the gender, age, ethnicity of the protagonist (aj), or whether the scene depicted a violation of a rule of physics, e.g. a fusion of two discrete objects (pnj).
Fig. 2.
Stimulus material. Each stimulus consisted of two picture frames, which were presented rapidly one after the other. The top panel shows the main conditions (a) bm, tom and mj from left to right. The lower panel shows the control conditions (b) om, aj and pnj from left to right. The timeline of presentation is reported on the right.
A response pair was shown at the bottom of each second picture frame. Participants were asked to select the option that best accorded with their judgment and to respond by pressing either the left or right button of an MRI compatible response device with the middle or index finger of the right hand as quickly and correctly as possible. Note that the same stimulus material was used for all three main conditions.
Experimental procedure
Prior to the experiment, participants completed a practice session with similar stimulus material from a different material set. Stimuli were presented visually in a mixed blocked/event-related design using customized experimental control software (Presentation, Neurobehavioral Systems Inc., Albany, CA) running on a Microsoft Windows 98 operating system. The experiment was evenly divided into two runs. For each of the six conditions, there were four blocks. Each block comprised eight trials. Consequently, for each condition 32 trials (16 violations and 16 non-violations of social norms) were presented resulting in a total of 192 trials. The order of the experimental blocks and the order of trials were pseudo-randomized and counterbalanced across participants. Each block lasted for 105 s and was preceded by an introductory question of 5 s duration. Each trial lasted for 6.25 s and consisted of a green preparatory fixation cross and the two picture frames presented sequentially. The green preparatory fixation cross was presented for 1 s, the first picture frame for 1.5 s and the second frame for 3.75 s (Figure 2). Trials were presented in a pseudo randomized order with jittered interstimulus intervals (ISI, minimum 1.25 s, maximum 21.25 s, mean 7.45 s) optimized using OptSeq2 (www.surfer.nmr.mgh.harvard.edu). Between two trials participants were instructed to fixate a gray cross presented foveally. We monitored brain activity using fMRI and recorded performance rates and response times (RTs).
Measuring ToM competence
To assess individual differences in ToM competence we used the Movie for the Assessment of Social Cognition [MASC, (Dziobek et al., 2006)]. The MASC is a computerized test for the assessment of mindreading abilities (i.e. ToM competency). It involves watching a 15 min film about four characters getting together for a dinner party and it requires subjects to make inferences about the featured characters’ mental states. The film is stopped at 46 points during the plot and questions referring to the characters’ feelings, thoughts and intentions are asked (e.g. “What is Betty feeling?”, “What is Cliff thinking?”). Participants’ correct responses are scored as one point and added to an overall score. We used the multiple-choice version of the MASC that offers four options for each query. The MASC is a reliable and highly sensitive instrument for the assessment of mindreading abilities (Dziobek et al., 2006).
fMRI data acquisition and analyses
We used a 1.5-T MRI scanner (Siemens Magnetom Sonata, Erlangen, Germany) with a standard head coil to acquire whole brain MRI data. Head movement was minimized using a vacuum pad. Axially oriented functional images (T2*-weighted volumes) were acquired using standard parameters (TE: 40 ms; TR: 2500 ms; flip angle: 90°; FOV: 256 mm; matrix: 64 × 64; voxel size: 4 × 4 × 4 mm; 26 slices). After acquisition of functional images, two sagittally oriented T1-weighted volumes (TE: 3.56 ms; TR: 12.24 ms; flip angle: 23°; matrix: 256 × 256; voxel size: 1 × 1 × 1 mm3) were acquired for registration of the functional images.
MRI data were analyzed using a mixed effects approach within the framework of the general linear model as implemented in FMRI Expert Analysis Tool (FEAT), part of FSL [FMRIB’s Software Library; www.fmrib.ox.ac.uk/fsl; (Smith et al., 2004)] and AFNI [http://www.afni.nimh.nih.gov; (Cox, 1996)].
Prior to statistical analyses the following processing steps were applied: slice time correction and motion correction using MCFLIRT (Jenkinson et al., 2002), nonbrain removal using BET (Smith, 2002), spatial smoothing using a Gaussian kernel of 8 mm FWHM, and high pass temporal filtering (Gaussian-weighted LSF straight line fitting, with sigma = 100.0 s). Registration to high resolution and standard images was done using FLIRT (Jenkinson and Smith, 2001).
Time series were modeled for each individual subject using event-related regressors for all six conditions (bm, tom, mj, om, aj, pnj) as well as error trials and convolved with a hemodynamic response function. The error trials were modeled as regressors of no interest, whereas correct trials (1st and 2nd picture frame combined) were modeled as regressors of interest. The duration of the regressors for the correct trials was adjusted according to the response time of the particular trial to account for significant differences in RTs observed between the tasks. As mentioned earlier, we aimed to identify neural activity involved in the three functions of interest by computing contrast images between all main conditions and the respective control conditions (bm > om; tom > aj; mj > pnj). Contrast images were computed for each subject and, after spatial normalization, transformed into standard space (Jenkinson et al., 2002). All group analyses were performed using the transformed contrast images in a mixed effects model treating participants as random. In the higher-level analyses we report clusters of maximally activated voxels that (i) survived statistical thresholding at Z = 3.09 and (ii) had a cluster size of at least 221 mm3, resulting in a corrected mapwise P < 0.05 as determined using Monte Carlo simulations as implemented in AFNI’s AlphaSim.
To attain clusters of overlapping and of distinct activity associated with BM, ToM and MJ in the pSTS/TPJ-Region, we performed a conjunction analysis according to the criteria put forth by Nichols et al. (2005). First, we thresholded the contrasts between each main condition and its respective control condition (e.g., bm > om) at Z = 3.09 (P ≤ 0.001). Then we created an intersection of these thresholded statistical maps through logical AND operations to yield a single overlay image.
Since the common process accounts suggest that the three main tasks used in this study may rely on a common intention reading, i.e. ToM, process, we tested whether task-induced changes in the BOLD signal in ROIs of overlapping activity would predict individual differences in ToM competence as assessed using the MASC. To this end, we correlated the BOLD-response (parameter estimates) of the overlap identifying tasks (i.e. of the relevant contrast between main and control condition) extracted from the clusters of overlapping activity with the individual scores of the MASC. All analyses were two-tailed and a rejection criterion of P < 0.05 was chosen.
RESULTS
Behavioral data and ToM competence
Mean performance rates and mean RTs (only for correctly answered trials) were computed for each of the six experimental conditions and averaged across participants (Table 1). To compare performance rates and RTs for the six conditions, repeated measures ANOVAs (n = 25) and post-hoc t-tests were calculated. Bonferroni-correction was applied for post-hoc t-tests. The overall performance rate was high (93% correct). There were significant differences for performance rates between the conditions [F(5,24) = 3.799; P = 0.003]. The performance rate was lowest during identification of mental states (tom condition) and during identification of the direction of a moving object (om condition, see Table 1), performance rates in the tom condition and the pnj condition differed significantly (P < 0.05). There were significant differences for RTs between the conditions [F(5,24) = 89.626; P < 0.001]. RTs were longest for the tom, the bm and the om condition (Table 1).
Table 1.
Performance rates and response times
| BM | ToM | MJ | OM | AJ | PNJ | |
|---|---|---|---|---|---|---|
| Performance (mean ± s.d.) | 0.93 ± 0.06 | 0.91 ± 0.06 | 0.93 ± 0.06 | 0.91 ± 0.08 | 0.95 ± 0.03 | 0.96 ± 0.04 |
| RT in ms (mean ± s.d.) | 1791 ± 293 | 2158 ± 296 | 1594 ± 304 | 1779 ± 286 | 1545 ± 354 | 1552 ± 269 |
The ToM competence assessed with the MASC was well pronounced in our sample (mean score = 35 out of 45 possible points; s.d. = 4.97) and the distribution of scores was right skewed.
fMRI data
Main effects
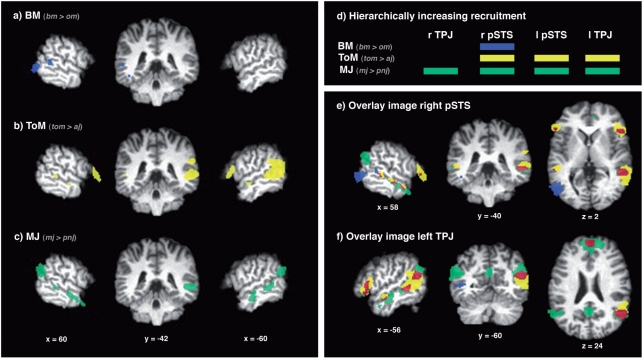
In a first step, we analyzed the fMRI data in a mixed effects group analysis (n = 25). The contrast of bm > om revealed a greater BOLD response in the right pSTS (BA 21) and in the occipito–temporal cortex bilaterally (BA 18/19, Table 2, Figure 3a). The contrast of tom > aj revealed greater BOLD responses in the pSTS bilaterally, though strongly left-lateralized (BA 22) and in the left TPJ (BA 39/40). Furthermore, increases in BOLD response were found in the inferior frontal gyrus bilaterally (BA 47), as well as in the superior frontal gyrus (BA 6/9) and in the temporal pole bilaterally (BA 21/38, Table 2, Figure 3b). The contrast of mj > pnj showed greater BOLD-response in the pSTS bilaterally (BA 21) and in the TPJ bilaterally (BA 39/40). Additionally, we detected increases in the BOLD response in the middle and inferior frontal gyrus on the left (BA 6/47), as well as in the superior frontal gyrus (BA 6/8/9), in the posterior cingulate cortex (BA 31), and in the temporal pole bilaterally (BA 21/38, Table 2, Figure 3c).
Table 2.
Anatomical locations and coordinates of activations
| Anatomical region | L/R | BA | Number of voxels in cluster | Z score of local maximum | MNI coordinates |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| BM > OM | |||||||
| Middle temporal gyrus, pSTS | R | 21 | 43 | 3.66 | 60 | −46 | 8 |
| Lateral occipital cortex | R | 19 | 594 | 4.66 | 56 | −70 | 0.0 |
| Occipital pole | L | 18 | 59 | 3.50 | −26 | −94 | −12 |
| TOM > AJ | |||||||
| Inferior frontal gyrus, pars orbitalis | L | 47 | 1218 | 5.01 | −46 | 28 | −10 |
| R | 47 | 354 | 4.95 | 54 | 32 | 0 | |
| Superior frontal gyrus | L | 9 | 301 | 4.53 | −4 | 56 | 28 |
| L | 6 | 279 | 4.43 | −6 | 12 | 60 | |
| Middle temporal gyrus, TP | L | 21/38 | 303 | 3.85 | −52 | 4 | −26 |
| R | 21/38 | 105 | 4.14 | 54 | 0 | −24 | |
| Middle temporal gyrus, pSTS-peak and | L | 22/39/40 | 1945 | 5.22 | −58 | −44 | 4 |
| TPJ-peaka | 4.57 | −56 | −58 | 22 | |||
| Superior temporal gyrus, pSTS | R | 22 | 328 | 4.06 | 52 | −36 | 2 |
| MJ > PNJ | |||||||
| Medial frontal cortex | L | 11/12 | 96 | 3.64 | −2 | 50 | −14 |
| Middle frontal gyrus | L | 6 | 361 | 4.26 | −44 | 10 | 46 |
| Inferior frontal gyrus, pars orbitalis | L | 47 | 769 | 4.01 | −46 | 36 | −12 |
| Superior frontal gyrus | L | 6/8/9 | 4118 | 5.26 | −8 | 48 | 36 |
| Precuneus, posterior cingulate cortex | R | 31 | 395 | 4.19 | 2 | −56 | 30 |
| Middle temporal gyrus | L | 21 | 268 | 3.90 | −56 | −14 | −16 |
| Middle temporal gyrus, TP | R | 21/38 | 636 | 4.46 | 54 | 6 | −28 |
| Middle temporal gyrus, pSTS | L | 21 | 367 | 4.34 | −58 | −38 | −4 |
| R | 21 | 24b | 3.44 | 62 | −38 | −2 | |
| Angular gyrus, TPJ | L | 39/40 | 879 | 4.90 | −54 | −64 | 28 |
| R | 39/40 | 479 | 4.27 | 58 | −64 | 32 | |
BA = Brodmann area, MNI coordinates = coordinates referring to the standard brain of the Montreal Neurological Institute, pSTS = posterior superior temporal sulcus, TPJ = temporoparietal junction, TP = temporal pole. Clusters of maximally activated voxels that (i) survived statistical thresholding at Z = 3.09 (P < 0.001) and (ii) had a cluster size of at least 221 mm3 (=28 voxels), resulting in a corrected mapwise P < 0.05.
aActivity in left pSTS was contiguous and extended up into the left TPJ.
bLocation listed despite a voxel count below the threshold, for prior hypotheses existed for its activation.
Fig. 3.
Imaging results. The left panel shows the activity elicited by the three contrasts between each main condition and respective control condition, the upper right panel represents these results schematically. The lower right panel shows the intersection of the three contrast maps. Left panel: Focusing on the pSTS-region, a right sagittal, a coronal, and a left sagittal slice are shown at the coordinates (x = 60, y = −42, z = 6, MNI coordinates) and (−60/−42/6), respectively, for each of the contrasts. The activations of each contrast were thresholded at Z = 3.09. Blue (a): BM (bm > om), yellow (b): ToM (tom > aj), green (c): MJ (mj > pnj). Right upper panel: A schematic representation (d) highlights the hierarchically increasing recruitment of pSTS/TPJ sub-locations by the contrasts for BM (bm > om), ToM (tom > aj) and MJ (mj > pnj). Right lower panel: The upper three intersection images (e) show the sagittal, coronal and axial slices of a location in the right pSTS, the lower three (f) show the slices of a location in the left TPJ. The contrasts of BM, ToM and MJ were integrated into a single dataset. Distinct activity: blue: BM (bm > om), yellow: ToM (tom > aj), green: MJ (mj > pnj). Overlapping activity: orange: conjunction of BM and ToM (bm > om and tom > aj), red: conjunction of ToM and MJ (tom > aj AND mj > pnj).
Activation patterns in the pSTS/TPJ-region
We focused our further analysis on the activation patterns within the pSTS/TPJ-region, where we identified in both hemispheres two separable sub-locations, one around the ascending limb of the superior temporal sulcus (pSTS) and another, more posterior and superior, at the end of the superior temporal sulcus, where the temporal and parietal lobe meet (TPJ). A comparison of the three activation patterns within the pSTS/TPJ-region revealed a hierarchically increasing recruitment of the respective sub-locations (Figure 3d). The increased activity associated with BM processing (bm > om) was confined to the right pSTS. ToM processing (tom > aj) was associated with additional activity in the left pSTS and TPJ. The activity in the left pSTS and TPJ was contiguous. However, there were two local maxima, one in the pSTS and one in the TPJ. The processing of MJ (mj > pnj) was associated with further evoked activity in the right TPJ. Also see Table S1, Figure S1 in the supplementary material section provided online for further details on the hierarchical activation pattern.
Clusters of overlapping and distinct activity of the three contrasts of interest
The intersection of contrast images for BM (bm > om), ToM (tom > aj) and MJ (mj > pnj) identified regions of both overlapping and distinct activity. In the pSTS/TPJ-region, clusters of overlapping activity were found for the contrasts of BM and ToM (bm > om AND tom > aj) in the right pSTS and for those of ToM and MJ (tom > aj AND mj > pnj) in the pSTS bilaterally and in the left TPJ (Figure 3e,f). A cluster of overlapping activity of all three contrasts (bm > om AND tom > aj AND mj > pnj) could not be identified. Clusters of distinct activity were found for the BM contrast in the right pSTS, for ToM in the pSTS bilaterally and in the left TPJ, and for MJ bilaterally in both the pSTS and the TPJ.
Correlation of BOLD signal changes in clusters of overlapping activity with ToM competence
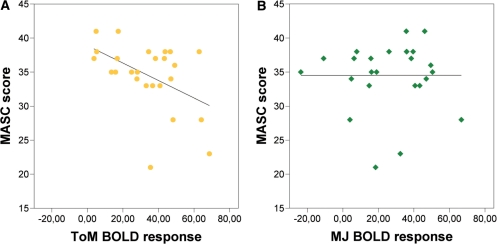
In the right pSTS-clusters of overlapping activity and in the left TPJ cluster we did not find significant correlations of the amplitude of the BOLD response of any contrast with the MASC, our ToM competence measure (Table 3). In the left pSTS-cluster of the contrasts of ToM and MJ (tom > aj AND mj > pnj), there was a significant negative correlation between the amplitude of the BOLD response of the ToM contrast and the MASC scores, but no significant correlation with BOLD changes in the MJ contrast (Table 3, Figure 4).
Table 3.
Correlations of BOLD response in clusters of overlapping activity with MASC
| Contrasts | Clusters of overlapping activity |
|||
|---|---|---|---|---|
| Right pSTS BM/ToM | Right pSTS ToM/MJ | Left pSTS ToM/MJ | Left TPJ ToM/MJ | |
| BM (bm > om) | −0.377/0.063 | – | – | – |
| ToM (tom > aj) | −0.340/0.096 | −0.075/0.723 | −0.477/0.016 | −0.328/0.110 |
| MJ (mj > pnj) | – | 0.085/0.686 | 0.001/0.998 | 0.002/0.991 |
The table shows the correlation coefficients and respective sigma values for the correlation of the amplitude of the BOLD response for the two contrasts, which identified a region of overlapping activation, with the MASC. The significant correlation is highlighted by bold font.
Fig. 4.
Correlations of the BOLD response with MASC scores in the left pSTS-ROI of ToM and MJ (tom > aj and mj > pnj). Scatter plots with regression lines reveal (a) a significant negative correlation between the amplitude of the BOLD response of the ToM contrast (tom > aj) and the MASC, but (b) no correlation with the BOLD response of the MJ contrast (mj > pnj).
DISCUSSION
To address the question of how BM, ToM and MJ are represented in the pSTS/TPJ-region, we ascertained their respective activation patterns with respect to their overlap or dissociation. A considerable overlap would be suggestive of a common process, while a clear dissociation would be indicative of distinct processes. Further, to test whether overlapping activity may be explained by a common intention-reading process, we correlated the BOLD response in clusters of overlapping activity with individual scores of the MASC. By contrasting the three main conditions with specifically matched control conditions (bm > om; tom > aj; mj > pnj), we replicated whole-brain activation patterns for each of the tasks as reported by previous studies. Within the pSTS/TPJ-region, small clusters of overlapping activity were identified for BM and ToM in the right pSTS and for ToM and MJ in the bilateral pSTS and left TPJ. A cluster of overlapping activity of all three tasks could not be detected. The right pSTS, however, showed increased activity during all three tasks. Clusters of dissociable activity were identified for BM in the right pSTS, for ToM in the bilateral pSTS and left TPJ, and for MJ in the bilateral pSTS and TPJ. Interestingly, the sub-locations of the pSTS/TPJ-region were recruited in a hierarchically increasing manner by the three tasks, i.e. for ToM more than for BM and for MJ more than for ToM. We found a significant correlation between the BOLD response in the left pSTS and the MASC. The partially overlapping and partially dissociating activation patterns of BM, ToM and MJ suggest that the activation of the pSTS/TPJ-region represents both a common process and distinct processes.
Cortical networks activated during the BM, ToM and MJ task
We replicated whole-brain activation patterns for each of the tasks as reported by previous studies indicating that our materials were effective. In accordance with previous studies, BM processing was associated with increased activity in the right pSTS and the occipito–temporal cortex bilaterally (Puce et al., 1998; Grossman et al., 2000; Hoffman and Haxby, 2000; Beauchamp et al., 2002, 2003). Replicating previous studies, ToM was associated with greater activation in the pSTS bilaterally, the left TPJ and bilaterally in the medial prefrontal cortex, and the temporal poles (Frith and Frith, 1999; Gallagher and Frith, 2003). In addition to these regions, the inferior frontal gyrus was activated bilaterally. Judging the mental state of a protagonist during our ToM task included the identification of emotional states, which has previously been shown to involve the IFG (Adolphs, 2002). MJ was associated with increased activity in the regions which are considered part of the functional network involved in moral judgments: the pSTS and TPJ were activated bilaterally and also the IFG, the superior frontal gyrus, the temporal poles and the posterior cingulate cortex (Greene and Haidt, 2002; Moll et al., 2005; Prehn and Heekeren, forthcoming).
Overlapping activity—representation of a common process
Since the conjunction analysis did not show a cluster of overlapping activity of BM, ToM and MJ, evidence for a common process shared by diverse social cognitive tasks is scant. If one were to identify at least the most likely target region for a common process involved in BM, ToM and MJ, this would be the right pSTS; for it was the only sub-location where we observed an increased response to all three tasks. Nevertheless, because clusters of overlapping activity were observed for BM and ToM in the right pSTS and for ToM and MJ in the bilateral pSTS and left TPJ, there is support for processes common to BM and ToM and to ToM and MJ, respectively.
To ascertain whether an intention-reading process may serve as such a common process in any of the clusters of overlapping activity, we correlated the BOLD-responses of our contrasts with the MASC scores, our measure of ToM competence. However, significant correlations for both of the two contrasts, whose activation was overlapping, could not be found in any of the clusters of overlapping activity. This finding is incompatible with the notion that an intention reading process could serve as a common process shared by diverse social cognitive tasks (Gallagher et al., 2000). The only significant correlation was observed between the BOLD-response in the ToM contrast and the MASC in the left pSTS cluster, highlighting the importance of this location for an intention reading process, which is in line with lesion studies establishing a causal link between the left pSTS/TPJ-region and ToM processes (Apperly et al., 2004; Samson et al., 2004). Yet, the BOLD-response in the MJ contrast was not significantly correlated in that location. Therefore, it is unlikely that an intention reading process serves as a process common to these two tasks.
Instead, the overlapping activity detected for BM and ToM and for ToM and MJ in the right pSTS may represent an initial analysis of social cues, focused on discerning movement characteristics, as suggested by Allison et al. (2000), rather than on inferring higher level representations such as intentions or mental states. Since both ToM and MJ are viewed as higher level social cognitive tasks, their overlapping activity, alternatively, may be construed in terms of a higher level process for the analysis of the social significance of the observed behavior, a function that has previously been suggested for the STS (Singer et al., 2004). The finding that the STS responds to the social salience of stimuli supports this view. For example, greater STS activation was also observed during the explicit judgment of trustworthiness or of attractiveness as opposed to age judgments of facial stimuli (Winston et al., 2002; Winston et al., 2007).
One limitation of the present study is, that the use of specific, individual control conditions for each of the main conditions in our study may have introduced a bias towards the detection of differences between tasks. For example, the material included a slight variation in the eye gaze direction of the protagonist (i.e. the protagonist was showing varying degrees of averted gaze relative to the observing study participant) in the control conditions om and pnj as compared to the main conditions. Contrasts of qualitatively different eye gaze direction conditions can lead to changes in BOLD signal in the STS (Puce et al., 1998; Hoffman and Haxby, 2000; Pelphrey et al., 2004), therefore, we cannot rule out that the subtle differences in eye gaze direction between the main and control condition of the contrasts bm > om and mj > pnj may have contributed to the observed signal changes in STS. A greater extent of overlapping activity may be revealed with further improved material, that uses a single, valid control condition for all three main conditions.
Domain-general account of a common process
In a recent review, integrating evidence from the domain of language on the one hand and from the domain of social cognition on the other, Redcay offers an intriguing new proposal for a common process carried out by the pSTS/TPJ-region (2008). She posits a two-staged process for (i) the parsing of a stream of information, whether auditory or visual, into meaningful discrete elements; and (ii) for the interpretation of the communicative significance from such input. During language perception a stream of sound of changing frequencies is decomposed into discrete elements, such as phonemes, syllables, words and phrases. Similarly, during social perception a stream of sequenced movements, for example of a facial expression, is dissected into individual facial cues such as the raise of an eyebrow. The activity in the right pSTS, which showed an increased response in all three tasks, may represent the first stage of this process, namely, the parsing of the stream of visual-spatial information, characteristic to our animated stimuli.
Furthermore, Redcay describes a hierarchy of both degree and location of activation. The greatest degree of activation is observed for dynamic, complex and socially meaningful stimuli. The location of activation (along the STS extending up into the TPJ) is also determined by the complexity of the task. This aspect will be addressed below.
Previously, Haxby et al. proposed a complex model of a distributed human neural system for face perception, in which they attributed the representation of changeable aspects of faces to the STS (Haxby et al., 2000). In our view, the conception of the two-staged process account extends Haxby et al.’s model, further specifying the processes carried out by the STS.
Dissociable activity—representation of distinct processes
The marked extent of dissociable activity observed for BM, ToM and MJ in the pSTS/TPJ-region indicates that diverse social cognitive tasks might draw upon distinct processes. This refutes the conception that one common process alone could sufficiently explain the activity in the pSTS/TPJ-region evoked by social cognitive tasks. Instead, the pSTS/TPJ-region appears to be functionally subdivided in a complex manner, at least into a more anterior and inferior pSTS-location and another more posterior and superior TPJ-location. These data do not support an attribution of function to the pSTS/TPJ-region as a whole, whether pertaining to social perception (Allison et al., 2000) or intention reading processes (Gallagher et al., 2000).
The observed dissociation between anterior/inferior and posterior/superior parts of the pSTS/TPJ-region is in line with the results by Gobbini et al. (2007), who found dissociable activity in the pSTS in response to social animations and in the TPJ in response to false belief stories. The complexity of pSTS/TPJ-activation patterns in the present study clearly extends beyond this dichotomy. Actually, the above-described differential activation of sub-locations follows a pattern of hierarchically increasing recruitment. While the processing of BM elicited increased activity in the right pSTS only, ToM led to additional activity in the left pSTS and left TPJ and MJ further evoked activity in the right TPJ. Importantly, the increasing recruitment of sub-locations can neither be related to differences in material, since it was identical for the three tasks, nor to increasing task difficulty, since performance rates did not differ among the three tasks and response times did not show an according pattern. Instead, this indicates that additional task-related cognitive resources are recruited when processing ToM or MJ as opposed to BM, or MJ as compared to ToM, respectively.
The most notable additional activity evoked by either ToM or MJ in comparison to BM was located in the TPJ. This dissociation of activation patterns between BM on the one hand and both ToM and MJ on the other is congruent with a recent proposal for a functional divide of the pSTS/TPJ-region by Saxe (2006), who posited neighboring but distinct areas for the processing of goal-directed actions in the pSTS and for the representation of the specific contents of mental states, such as beliefs, in the TPJ. Our BM task, which focused on the direction of a protagonist’s actions, differed from either the ToM or the MJ task in that it did not demand any higher level processing such as inferring the protagonist’s mental states.
The most important further activity elicited by MJ in comparison to ToM was found in the right TPJ. Within the framework of this experiment this region may appear to be specific for a MJ process. Recent studies, which demonstrated a considerable overlap in the right TPJ for activity elicited by lower-level processes such as attention reorienting and higher-level processes such as ToM, however, strongly argue for a domain-general account of right TPJ function (Decety and Lamm, 2007; Mitchell, 2007). At present it is not clear what the exact nature of this domain-spanning process could be. Nevertheless, selectivity of this region for a high level social cognitive process such as MJ is improbable. The fact that our ToM task did not evoke increased activity in the right TPJ may seem surprising, since some authors have stressed the relevance of the right TPJ for the attribution of mental states such as desires (Saxe and Wexler, 2005) or thoughts (Saxe and Powell, 2006). Possibly, this discrepancy may be explained by differences in the argumentative structure and complexity of mental states. The mental states, which had to be attributed in our study, were simple adjective descriptions of a particular state of mind (e.g. “The protagonist is sad”). In contrast, the mental states, which were used in the above named studies that identified activity in the right TPJ (Saxe and Wexler, 2005; Saxe and Powell, 2006), were characterized by having a specific representational content (e.g. “Person A thinks that it is raining”). To our knowledge, however, the neural activation patterns for representational and non-representational mental states have not been explicitly compared yet. In any case, our ToM task did elicit increased activity in the left TPJ. The importance of this latter region for ToM processes, in fact, is supported by lesion studies, which established a causal link between the left pSTS/TPJ and the ability to reason about others’ mental states (Apperly et al., 2004; Samson et al., 2004).
The observed distinct clusters of activity are evidence that BM, ToM and MJ draw upon distinct processes. Note, however, that just because the cognitive functions involved in BM, ToM and MJ, respectively, do not engage the same brain region, it does not mean that the different regions they engage must carry out different cognitive functions. The distinct clusters of activity evoked by the three tasks, could still subserve the same cognitive process.
Domain-general account of hierarchically increasing recruitment
There is a notable correspondence between the hierarchical recruitment of pSTS/TPJ sub-locations observed in our experiment and a recent study of language comprehension, in which the location of activation along the STS and extending up into the TPJ was determined by the linguistic complexity of the task (Xu et al., 2005). The reading of words led to activation in the left STS, which increased in intensity and posterior extent during reading of sentences. Reading a narrative evoked further activation in the left STS and TPJ and also in the right TPJ. The activation patterns for reading sentences and reading a narrative correspond well with those observed for judgments on mental states and norm-conformity of behavior, respectively, in our study. This correspondence offers an interesting alternative interpretation of the hierarchically increasing recruitment of the left STS and bilateral TPJ by ToM and MJ. Instead of construing the additional activity in terms of the respective eliciting task, it may be understood as representing increasingly more complex processing of the social situation depicted in the stimuli. Though not formally assessed in this study, one would expect that formulating a judgment concerning the mental state of another person is conceptually more demanding and complex than focusing on the direction of movement. Likewise, judging the permissibility of another person’s behavior appears to be a more complex task than identifying the mental content.
CONCLUSION
In sum, the differentiated representation of BM, ToM and MJ in the pSTS/TPJ-region refutes the conception that one common process alone could sufficiently explain the function of this region. Instead, the partially overlapping and partially dissociating activation patterns suggest that the activity in the pSTS/TPJ-region represents both common and distinct processes. There is no evidence that an intention reading process could serve as the common process for the different social cognitive tasks. We propose an interpretation of the differentiated activation patterns elicited by BM, ToM and MJ in terms of Redcay’s domain-spanning conceptualization of a two-staged process (2008). We suggest that the activity in the right pSTS, where an increased response was found for all three tasks, represents the first stage of this process, namely, the parsing of the stream of visual-spatial information, characteristic of our animated stimuli, which were identical for all three tasks. The hierarchically increasing activity in the left pSTS and bilateral TPJ elicited by ToM and MJ may represent the second stage of the process, that is, the increasingly more complex processing of the communicative significance, which is modulated by the context set by each task.
Acknowledgments
This study was financially supported by grants from the Bundesministerium für Bildung und Forschung (Berlin NeuroImaging Center, BNIC) and the Deutsche Forschungsgemeinschaft [Emmy-Noether-Program (HE 3347/2-1)]. The authors thank Dorit Kliemann, Rebecca Saxe and Julia Schooler for helpful comments.
REFERENCES
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12(2):169–77. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in Cognitive Science. 2000;4(7):267–78. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Apperly IA, Samson D, Chiavarino C, Humphreys GW. Frontal and temporo-parietal lobe contributions to theory of mind: neuropsychological evidence from a false-belief task with reduced language and executive demands. Journal of Cognitive Neuroscience. 2004;16(10):1773–84. doi: 10.1162/0898929042947928. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11(6):1891–8. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. Parallel visual motion processing streams for manipulable objects and human movements. Neuron. 2002;34(1):149–59. doi: 10.1016/s0896-6273(02)00642-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. FMRI responses to video and point-light displays of moving humans and manipulable objects. Journal of Cognitive Neuroscience. 2003;15(7):991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Armony JL, Blair RJ, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125(Pt 8):1696–708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- Borg JS, Hynes C, Van Horn J, Grafton S, Sinnott-Armstrong W. Consequences, action, and intention as factors in moral judgments: an FMRI investigation. Journal of Cognitive Neuroscience. 2006;18(5):803–17. doi: 10.1162/jocn.2006.18.5.803. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crovitz HF, Zener K. A group-test for assessing hand- and eye-dominance. American Journal of Psychology. 1962;75(2):271–6. [PubMed] [Google Scholar]
- Decety J, Lamm C. The role of the right temporo–parietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13(6):580–93. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Dziobek I, Fleck S, Kalbe E, et al. Introducing MASC: a movie for the assessment of social cognition. Journal of Autism and Developmental Disorders. 2006;36(5):623–36. doi: 10.1007/s10803-006-0107-0. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, et al. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57(2):109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds – a biological basis. Science. 1999;286(5445):1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Science. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. Journal of Cognitive Neuroscience. 2007;19(11):1803–14. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Greene J, Haidt J. How (and where) does moral judgment work? Trends in Cognitive Science. 2002;6(12):517–23. doi: 10.1016/s1364-6613(02)02011-9. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44(2):389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293(5537):2105–8. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Grezes J, Fonlupt P, Bertenthal B, Delon-Martin C, Segebarth C, Decety J. Does perception of biological motion rely on specific brain regions? Neuroimage. 2001;13(5):775–85. doi: 10.1006/nimg.2000.0740. [DOI] [PubMed] [Google Scholar]
- Grossman E, Donnelly M, Price R, et al. Brain areas involved in perception of biological motion. Journal of Cognitive Neuroscience. 2000;12(5):711–20. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30(1):313–24. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Science. 2000;4(6):223–33. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Prehn K, Schwintowski HP, Villringer A. Influence of bodily harm on neural correlates of semantic and moral decision-making. Neuroimage. 2005;24(3):887–97. doi: 10.1016/j.neuroimage.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Schwintowski HP, Villringer A. An fMRI study of simple ethical decision-making. Neuroreport. 2003;14(9):1215–9. doi: 10.1097/00001756-200307010-00005. [DOI] [PubMed] [Google Scholar]
- Henson R. What can functional neuroimaging tell the experimental psychologist? Q J Exp Psychol A. 2005;58(2):193–233. doi: 10.1080/02724980443000502. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2000;3(1):80–4. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Hynes CA, Baird AA, Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. 2006;44(3):374–83. doi: 10.1016/j.neuropsychologia.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Marjoram D, Job DE, Whalley HC, et al. A visual joke fMRI investigation into theory of mind and enhanced risk of schizophrenia. Neuroimage. 2006;31(4):1850–8. doi: 10.1016/j.neuroimage.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Mitchell JP. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cereb Cortex. 2008;18(2):262–71. doi: 10.1093/cercor/bhm051. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, et al. The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. Journal of Neuroscience. 2002;22(7):2730–6. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: the neural basis of human moral cognition. Nature Reviews Neuroscience. 2005;6(10):799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Peelen MV, Wiggett AJ, Downing PE. Patterns of fMRI activity dissociate overlapping functional brain areas that respond to biological motion. Neuron. 2006;49(6):815–22. doi: 10.1016/j.neuron.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: processing of mutual and averted social gaze in the superior temporal sulcus. Psychological Science. 2004;15(9):598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Prehn K, Heekeren HR. Moral judgment and the brain: A functional approach to the question of emotion and cognition in moral judgment integrating psychology, neuroscience and evolutionary biology. In: Braeckman J, Verplaetse J, De Schrijver J, editors. The moral brain. Netherlands: Springer; 2009. pp. 129–54. [Google Scholar]
- Prehn K, Wartenburger I, Meriau K, et al. Individual differences in moral judgment competence influence neural correlates of socio-normative judgments. Social Cognitive and Affective Neuroscience. 2008;3(1):33–46. doi: 10.1093/scan/nsm037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. Journal of Neuroscience. 1998;18(6):2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E. The superior temporal sulcus performs a common function for social and speech perception: implications for the emergence of autism. Neurosci Biobehav Rev. 2008;32(1):123–42. doi: 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Sanfey AG, Aronson JA, Nystrom LE, Cohen JD. The neural correlates of theory of mind within interpersonal interactions. Neuroimage. 2004;22(4):1694–703. doi: 10.1016/j.neuroimage.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporo–parietal junction is necessary for representing someone else's; belief. Nature Neuroscience. 2004;7(5):499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16(2):235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo–parietal junction in “theory of mind”. Neuroimage. 2003;19(4):1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It's; the thought that counts: specific brain regions for one component of theory of mind. Psychological Science. 2006;17(8):692–9. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A. Making sense of another mind: the role of the right temporo–parietal junction. Neuropsychologia. 2005;43(10):1391–9. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Wilson SM, Hagler DJ, Jr, Bates E, Sereno MI. Point-light biological motion perception activates human premotor cortex. Journal of Neuroscience. 2004;24(27):6181–8. doi: 10.1523/JNEUROSCI.0504-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Kiebel SJ, Winston JS, Dolan RJ, Frith CD. Brain responses to the acquired moral status of faces. Neuron. 2004;41(4):653–62. doi: 10.1016/s0896-6273(04)00014-5. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Vollm BA, Taylor AN, Richardson P, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29(1):90–8. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Winston JS, O'D;oherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45(1):195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O'D;oherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5(3):277–83. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Wolf I, Dziobek I, Heekeren HR. Neural correlates of social cognition in naturalistic settings: A model-free analysis approach. Neuroimage. 2010;49(1):894–904. doi: 10.1016/j.neuroimage.2009.08.060. [DOI] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. Neuroimage. 2005;25(3):1002–15. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]