Abstract
Context: Fibroblast growth factor 23 (FGF23) regulates phosphorus homeostasis and vitamin D metabolism. Circulating FGF23 levels are elevated in inherited and acquired hypophosphatemic disorders that can cause rickets or osteomalacia. Particularly increased concentrations of FGF23 are observed in patients with chronic kidney disease (CKD), in which increased FGF23 is associated with more rapid disease progression, improved bone mineralization, the development of left ventricular hypertrophy, and increased mortality.
Objective: Our objective was to determine whether the markedly elevated levels of immunoreactive FGF23 in CKD represent accumulation of intact, biologically active hormone, C-terminal cleavage fragments, or both.
Design: Biologically active FGF23 in plasma from CKD patients treated by peritoneal dialysis was quantified using a cell-based Egr-1 reporter assay; bioactive FGF23 levels were compared with those measured with immunometric FGF23 assays detecting either intact hormone alone or intact hormone and C-terminal fragments.
Setting and Patients: Adult and pediatric patients with end-stage renal disease treated with peritoneal dialysis participated in the study at a tertiary referral center.
Results: Serially diluted patient samples revealed levels of bioactive FGF23 that ran in parallel to CHO cell-derived recombinant human FGF23. FGF23 bioactivity was inhibited by an anti-FGF23 antibody. Levels of bioactive and immunoreactive FGF23 were tightly correlated, and Western blot analysis of FGF23 immunoprecipitated with anti-FGF23 antibodies from plasma of dialysis patients revealed only a single prominent protein band, which was indistinguishable from recombinant intact FGF23, without clear evidence for FGF23 fragments.
Conclusions: Our results provide strong evidence for the conclusion that virtually all circulating FGF23 in dialysis patients is intact and biologically active.
Circulating FGF23 in end stage renal disease patients treated by peritoneal dialysis is intact and biologically active as revealed by immunoassay, bioassay, and Western blot analysis.
Fibroblast growth factor 23 (FGF23) is an endocrine hormone that regulates phosphorus homeostasis by altering the expression of NPT2a and NPT2c, the sodium-dependent phosphate transporters in the renal proximal tubules, and thereby modifies the urinary excretion of phosphorus (1,2,3). FGF23 is also the most important negative regulator of renal 1α-hydroxylase expression, which prevents the circulating levels of 1,25(OH)2 vitamin D from becoming inappropriately high (3). After the molecular cloning of the gene encoding FGF23 (4,5,6), different assays were developed allowing its immunological detection in serum or plasma (7,8). Elevated or inappropriately high circulating FGF23 levels in association with increased urinary phosphate excretion and inappropriately low 1,25(OH)2 vitamin D levels were found in patients with tumor-induced osteomalacia (TIO) (7,8) and in patients with several rare human genetic disorders, including X-linked hypophosphatemia (XLH) (7,8,9), autosomal recessive hypophosphatemia (10,11), and McCune-Albright syndrome (12,13).
The most common condition associated with markedly elevated circulating levels of FGF23 is chronic kidney disease (CKD) (14,15). FGF23 levels are elevated already in early-stage CKD and increase progressively because renal function declines such that patients on dialysis manifest levels that are frequently 100- to 10,000-fold above the normal range for healthy controls (9,14,15). Although FGF23 levels in CKD are much higher than those observed in TIO or in genetic syndromes with FGF23-induced hypophosphatemia, it is uncertain whether immunoreactive FGF23 levels accurately reflect bioactivity, particularly when using assays employing antibodies that are directed against epitopes within the C-terminus of FGF23. Addressing this latter question is thought to be particularly relevant because several observational studies demonstrated relationships between high FGF23 levels and improved bone mineralization (16), more rapid CKD progression (17), left ventricular hypertrophy in both predialysis and dialysis patients (18,19), and an increased risk of mortality among incident and prevalent hemodialysis patients (18,20,21). It remains unclear from these studies, however, whether elevated FGF23 in CKD patients is simply an indicator of disordered phosphorus homeostasis, which itself is associated with many of the adverse outcomes observed in CKD patients (22,23,24,25,26), or whether FGF23 contributes directly to these clinical outcomes through as yet undefined mechanisms.
Several in vitro studies using transfected cell lines have documented that mutations at the RXXR site of FGF23, identified in patients affected by autosomal dominant hypophosphatemic rickets (4), render the mutant protein much less sensitive to cleavage by subtilisin-like proprotein convertases (27,28,29), i.e. the enzymes that typically lead to the generation of N- and C-terminal FGF23 fragments with as yet uncertain biological relevance (30,31). Because of the well-documented accumulation of C-terminal PTH fragments as CKD progresses (32), it has been widely assumed that C-terminal FGF23 fragments also accumulate as renal function declines and contribute to a large extent to the markedly elevated FGF23 levels observed in end-stage renal disease (ESRD). Indeed, Weber et al. (9) showed the presence of a C-terminal FGF23 fragment in a patient with ESRD. However, different studies in ESRD patients have shown very strong correlations between the FGF23 levels measured by an assay detecting either intact FGF23 alone (iFGF23 assay) and an assay detecting intact as well as C-terminal FGF23 (cFGF23 assay) (16,21). These findings suggested that accumulation of C-terminal FGF23 fragments may not contribute significantly to the markedly elevated concentrations of circulating immunoreactive FGF23.
To clarify whether elevated FGF23 in CKD retains its bioactivity and whether significant amounts of FGF23 fragments can be found in the circulation, we analyzed plasma FGF23 levels in patients treated with peritoneal dialysis by three different approaches: 1) measuring immunoreactivity by iFGF23 and cFGF23 assay, 2) assessing quantitatively the biological activity of FGF23 with a cell-based reporter assay, and 3) characterizing the molecular nature of circulating FGF23 by Western blot analyses.
Materials and Methods
Cell-based reporter assay
HEK cells were transfected with the cDNA encoding full-length human Klotho, and pGL4.20 (Promega, Madison, WI), in which the luciferase gene is downstream of approximately 1 kb of promoter for early growth response-1 (Egr-1) (33). A stable clone, selected by 1 mg/ml G418 (GIBCO, Carlsbad CA) and 2 μg/ml puromycin, was studied further. To quantify FGF23 activity, reporter cells were plated in 96-well plates at 104 cells per well in a final volume of 100 μl/well of DMEM (GIBCO) supplemented with 10% fetal bovine serum, 1 mg/ml G418, and 2 μg/ml puromycin. After cells had attached, recombinant basic FGF (R&D Systems, Minneapolis, MN), recombinant FGF23, or plasma (final concentration, 2%) was added in the absence or presence of a recently described mouse monoclonal antibody raised against human FGF23 (34), and cell culture was continued for another 24 h at 37 C. Cells were then lysed by adding Steady-Glo luciferase assay reagent (Promega) for 20 min, and the whole lysates were transferred to an assay plate (Corning, Lowell, MA) to measure luciferase activity using a Victor3 multilabel plate reader (PerkinElmer, Waltham, MA) for 5 sec.
Immunoassays for the detection of FGF23
Twenty-one adult and 13 pediatric chronic peritoneal dialysis patients were recruited. Plasma samples and clinical data were collected at a single random visit to the peritoneal dialysis clinic. Samples were immediately centrifuged, aliquoted, and stored at −80 C until batched assays were performed. Concentrations of FGF23 in plasma samples were determined by two assays: 1) an assay using two monoclonal antibodies that recognize an N-terminal and a C-terminal portion of FGF23, respectively, i.e. epitopes are located within the regions comprising amino acid residues 25-179 and 180-251, respectively (8) (Kainos, Tokyo, Japan; referred to as iFGF23 assay), and 2) an assay using two affinity-purified antibodies recognizing epitopes located within the C-terminal region of FGF23; amino acid residues 206-222 and 225-244, respectively (7) (Immutopics, San Clemente, CA; referred to as cFGF23 assay). Measurements were performed by a single investigator without knowledge of any of the subjects’ clinical data. The study was approved by the Institutional Review Board at Massachusetts General Hospital and University of California, Los Angeles, and all subjects provided written informed consent. In the case of the children under 18 yr of age, parents provided written informed consent after the children assented to participate in the study.
Immunoprecipitation analysis
The mouse monoclonal antibody, FC1 (34), recognizing the C-terminal domain of FGF23, was immobilized to the N-hydroxysuccinimide activated Sepharose according to the manufacture’s guidance (GE Healthcare, Piscataway, NJ). The conditioned medium containing recombinant human FGF23 was obtained from UMR106 osteosarcoma cells that had been transiently transfected with expression vector encoding human FGF23 using FuGENE 6 (Roche, Indianapolis, IN). Eighty microliters of conditioned medium or patients’ plasma was incubated with the FC1-immobilized resin for 2 h at 4 C. The resin was washed in PBS, and bound proteins were subjected to Western blot analysis using an affinity-purified, biotinylated polyclonal antibody raised in goats against a C-terminal FGF23 epitope (amino acid residues 225-244) (Immutopics). The signal was visualized with streptavidin-conjugated horseradish peroxidase (Pierce, Rockford, IL) and Western Lightning Plus-ECL system (PerkinElmer).
Results
Plasma levels of FGF23 in peritoneal dialysis patients
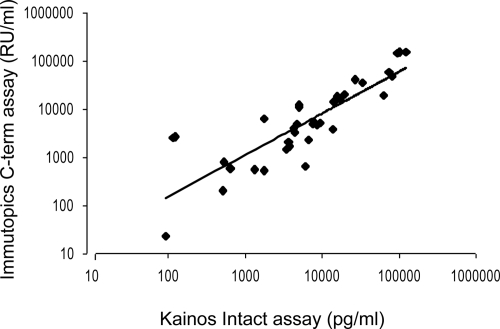
Clinical characteristics and laboratory data of 34 patients treated with peritoneal dialysis are summarized in Table 1. The mean concentrations of immunoreactive FGF23 determined by iFGF23 and cFGF23 assays were 20,033 ± 5,146 (mean ± sem; median, 6081) pg/ml and 20,126 ± 6,183 (median, 4573) Reference Units (RU/ml), respectively. There was a significant linear correlation between iFGF23 and cFGF23 (Fig. 1; r = 0.92; P < 0.01).
Table 1.
Description of adult and pediatric subjects
| Adults (n = 21) | Children (n = 13) | |
|---|---|---|
| Demographics | ||
| Age (yr) | 52 (17) | 16 (3) |
| White (%) | 86 | 46 |
| Female (%) | 52 | 75 |
| Anuric (no. of patients) | 2 | 7 |
| Dialysis vintage (months) | 15 (8–22) | 16 (12–28) |
| Comorbidities (no. of patients) | ||
| Diabetes | 5 | 0 |
| Hypertension | 18 | 3 |
| Smoking | 3 | 0 |
| Cardiovascular disease (all)a | 4 | 0 |
| Arrhythmia | 4 | 0 |
| Medications (%) | ||
| Phosphorus binders | 81 | 100 |
| Active vitamin D | 90 | 100 |
| Ergocalciferol | 19 | 0 |
| Measurements | ||
| Body mass index (kg/m2) | 27 (6) | 20 (8) |
| Systolic blood pressure (mm Hg) | 131 (21) | 121 (18) |
| Residual creatinine clearance (ml/min · 1.73 m2)b | 2.3 (0.4–5.3) | 2.9 (1.7–4.0) |
| Calcium (mg/dl) | 9.2 (0.5) | 9.7 (0.6) |
| Phosphorus (mg/dl) | 4.4 (1.4) | 7.1 (1.9) |
Values are means (standard deviation) or medians (interquartile range).
Abstracted from chart review and included history of coronary artery disease, congestive heart failure, cardiac arrest, or myocardial infarction.
Calculated for those patients with residual urine output.
Figure 1.
Comparison of FGF23 levels in peritoneal dialysis patients measured by immunometric iFGF23 (Kainos) or cFGF23 assay (Immutopics). The paired results were plotted logarithmically, and an exponential regression curve was calculated.
Development of a cell-based reporter assay for evaluating FGF23 activity in plasma from peritoneal dialysis patients
We used a cell-based reporter assay to quantitatively assess the bioactivity of FGF23 in plasma samples of peritoneal dialysis patients. Because FGF23 can up-regulate the renal expression of Egr-1 only in the presence of the membrane-anchored coreceptor Klotho (33,35), we engineered HEK cells that stably express both Klotho and a luciferase reporter gene downstream of the Egr-1 promoter. This enabled us to quantitatively detect the biological activity of FGF23 (Fig. 2A).
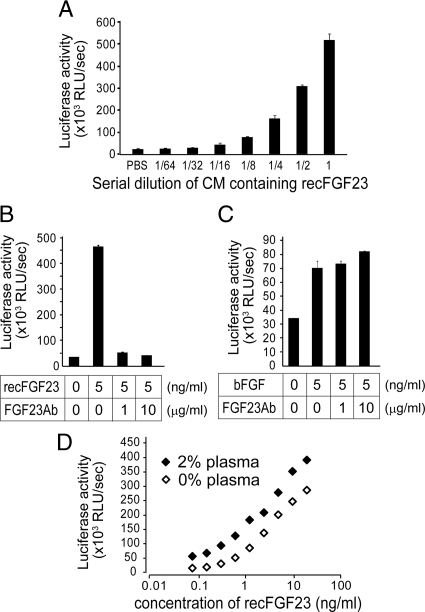
Figure 2.
Validation of the cell-based reporter assay to measure biologically active FGF23. A, Luciferase reporter activities of recombinant FGF23 (recFGF23) in the diluted conditioned medium (CM) of HEK cells; B and C, inhibitory effects of the FGF23Ab (0, 1, or 10 μg/ml, final concentration) on the luciferase activities induced by recombinant FGF23 (B) or basic FGF (C), both 5 ng/ml; D, luciferase activities induced by recombinant FGF23 with or without addition of plasma from a normal subject. All data are presented as mean relative luminescence per second ± sd (n = 3).
Several cytokines and growth factors up-regulate Egr-1, and patients with CKD can have high concentrations of such factors (36,37). Therefore, to establish that the observed luciferase effects were FGF23 specific, we used an anti-FGF23 neutralizing antibody (FGF23Ab) that specifically blocks the biological activity of FGF23 in vitro and in vivo (34). The presence of FGF23Ab completely blocked the luciferase activities evoked by addition of recombinant FGF23 (Fig. 2B). In contrast, the FGF23Ab had no effect on the luciferase activities induced by basic FGF (bFGF, FGF2) (Fig. 2C).
We then assessed which concentration of plasma provided optimal activation of the luciferase promoter using plasma from a healthy control subject. At plasma concentrations higher than 5% of the final assay volume, nonspecific activation of the reporter and morphologically visible cell changes were observed (data not shown). At plasma concentrations less than 5%, there was minimal change in basal luciferase reporter activity and no change in cell morphology; therefore, we chose 2% plasma as the maximal concentration for our assays. When increasing doses of recombinant FGF23 were added, slightly higher levels of luciferase activity were observed in the presence of 2% plasma, yet the slopes of the dose-response curves were similar for recombinant FGF23 with and without plasma (Fig. 2D). These findings indicated that 2% plasma did not affect the cellular response or the biological activity of FGF23 in this assay.
Biological activity of FGF23 in plasma samples from peritoneal dialysis patients
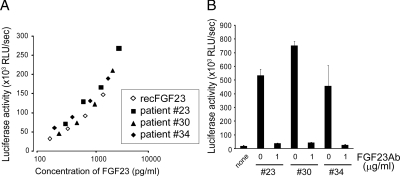
We next compared the biological activity of circulating FGF23 in plasma samples from several patients to the activity of recombinant FGF23 derived from stably transfected CHO cells (Fig. 3A). Three patient-derived plasma samples were serially diluted with normal plasma, and their biological FGF23 activities were compared with that of increasing concentrations of CHO cell-derived recombinant FGF23 (final plasma concentration was 2% for all samples). FGF23 bioactivity plotted against the concentrations of recombinant FGF23 as determined by the iFGF23 assay revealed almost identical titration curves for the three patients’ plasma samples that were tested. To confirm that the reporter activities were indeed FGF23-specific, we incubated these plasma samples with FGF23Ab before assessing their FGF23 bioactivity (Fig. 3B). This treatment with FGF23Ab resulted in an obvious reduction of the reporter activities indicating that the bioactivity measured in plasma samples is specific for FGF23. These data indicate that FGF23 in patient samples is biologically as active as recombinant FGF23 produced by CHO cells.
Figure 3.
Bioactive FGF23 in plasma from peritoneal dialysis patients. A, Titration curves of plasma samples from ESRD patients. CHO cell-derived recombinant FGF23 or patient-derived plasma samples were serially diluted, and their FGF23 bioactivities were assessed in the reporter assay; the levels of bioactive FGF23 (luciferase activity) were plotted against the levels of immunoreactive FGF23 determined by the iFGF23 assay. The data are presented as mean ± sd (n = 3). B, Inhibitory effects of the FGF23Ab (1 μg/ml, final concentration) on the luciferase activities induced by circulating FGF23 in patients. The data are presented as mean ± sd (n = 3).
Correlation between immunoreactive and bioactive FGF23 levels
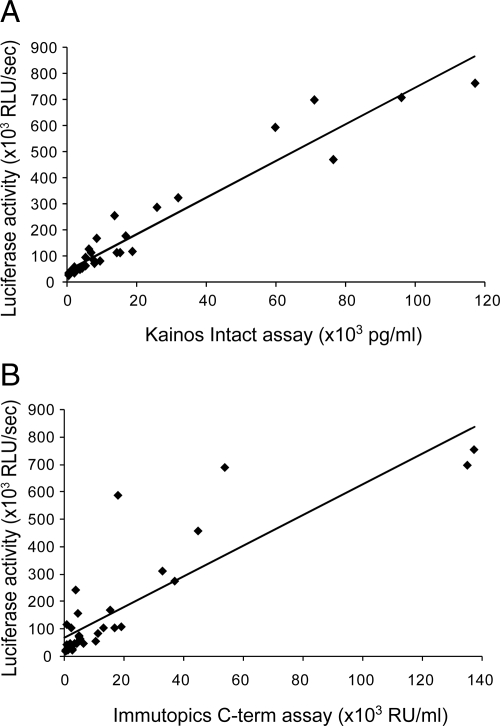
Using the cell-based reporter assay, we assessed the FGF23 bioactivity in all plasma samples from peritoneal dialysis patients. Similar to the wide range of immunoreactive FGF23 concentrations, FGF23-dependent reporter activities varied also significantly; average was 164,166 ± 210,739 (mean ± sd; median; 71,765) relative luciferase units (RLU)/sec. However, FGF23 bioactivities (bioFGF23) were highly correlated to immunoreactive FGF23 concentrations determined by iFGF23 or cFGF23 assays (Fig. 4) (bioFGF23 vs. iFGF23: r = 0.965, P < 0.01; bioFGF23 vs. cFGF23: r = 0.858, P < 0.01). These results indicate that FGF23 bioactivity is determined almost exclusively by the amount of immunoreactive FGF23 measured using either the iFGF23 or the cFGF23 assay.
Figure 4.
Correlation between levels of bioactive (luciferase activities) and immunoreactive FGF23 in plasma of peritoneal dialysis patients. Levels of bioactive FGF23 were well correlated with the levels of immunoreactive FGF23 determined by either the Kainos (iFGF23) (A) or the Immutopics (cFGF23) (B) assay. Simple linear regression curves were calculated. C-term, C-terminal.
Circulating forms of FGF23 in peritoneal dialysis patients
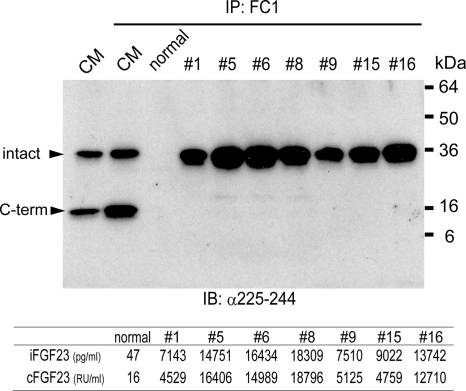
The strong correlation between bioactive and immunoreactive FGF23, along with the strong correlation between the FGF23 measurements obtained with the iFGF23 and the cFGF23 assay, made the presence of large amounts of circulating C-terminal FGF23 fragments unlikely. To confirm this hypothesis, we performed immunoprecipitations of plasma samples from patients using the previously described antibody FC1 (34). Subsequent Western blot analysis revealed only the 32-kDa band representing the intact FGF23 molecule, yet little or no evidence for the 10-kDa band representing the C-terminal degradation product (Fig. 5). Furthermore, the intensities of the 32-kDa band representing the intact FGF23 molecule correlated well with immunoreactive and bioactive FGF23 levels (Fig. 5, panel below the Western blot). To demonstrate that the FC1 antibody is able to immunoprecipitate intact FGF23 as well as C-terminal fragments, conditioned medium was obtained from cells transiently transfected with a cDNA encoding wild-type human FGF23 (no mutation). Consistent with previous reports (34), 10- and 32-kDa protein bands were detected representing the C-terminal fragment and intact FGF23, respectively.
Figure 5.
Western blot analysis of FGF23 immunoprecipitated from plasma of CKD patients. Conditioned medium (CM) containing recombinant FGF23 and plasma samples from healthy individual (normal) or ESRD patients were immunoprecipitated (IP) with the FC1 antibody before the precipitated proteins were subjected to Western blot analysis (IB) using the polyclonal anti-FGF23 antibody recognizing an epitope within the C-terminal portion (amino acid residues 225–244) of human FGF23. Two major bands (arrowheads) in CM represent intact FGF23 (intact) and its C-terminal fragment (C-term). The individual levels of immunoreactive FGF23 determined by iFGF23 and cFGF23 assays are shown in the panel below the blot.
Discussion
In this study, we used a cell-based reporter system (33) to demonstrate that circulating FGF23 in peritoneal dialysis patients retains its bioactivity and that the levels of bioactive FGF23 are tightly correlated with the plasma concentrations of immunoreactive FGF23, as measured by either iFGF23 or cFGF23 assays. Consistent with these findings, Western blot analysis of immunoprecipitated FGF23 revealed that the circulating form of FGF23 in dialysis patients is indistinguishable in size from intact recombinant FGF23, without clear evidence for the presence of significant amounts of C-terminal fragments. We cannot exclude the presence of N-terminal FGF23 fragments that escape detection by either the iFGF23 or the cFGF23 assay and that are not detected by the Western blot analysis performed herein. However, because of the strong correlation between bioactive and immunoreactive FGF23 determined by the cFGF23 assay, and because of the good correlation between the immunoreactive FGF23 and the intensity of the 32-kDa protein band detected by Western blot, intact FGF23 appears to be the dominant circulating hormonal form in peritoneal dialysis patients.
These data differ from similar analyses of immunoreactive PTH in patients with CKD in whom the accumulation of PTH fragments initially limited the utility of PTH as a prognostic biomarker of bone disease and potentially other adverse outcomes (38,39). In fact, the accumulation of PTH fragments that are generated within the parathyroid gland and, after secretion, in few organs, particularly the liver (40,41), limited in dialysis patients the clinical utility of RIAs. Only after the introduction of immunometric assays did PTH measurements become reasonably good predictors of hyperparathyroid and adynamic bone disease in ESRD (38,39).
Intact FGF23 is most likely the major circulating hormonal form in patients with TIO and XLH, where measurements using the iFGF23 and cFGF23 assays correlated well, at least when FGF23 levels were significantly elevated (42,43). Furthermore, studies in Hyp mice, the murine equivalent of human XLH (44), and the analysis of aqueous extracts of a tumor isolated from a patient with TIO revealed only evidence for intact FGF23 by Western blot analysis (34,45). To date, rare genetic forms of tumoral calcinosis, in which FGF23 undergoes insufficient O-glycosylation, thus represent the only known diseases with increased circulating levels of FGF23 fragments but little or no intact FGF23 (46,47,48,49).
Our data now indicate that most of the circulating FGF23 in CKD is intact and biologically active, even at the dramatically elevated concentrations observed in patients treated with dialysis. Similar to other reports (16,21), iFGF23 and cFGF23 levels were highly correlated in the current study, and both measurements correlated well with FGF23 bioactivity, suggesting that the level of immunoreactive FGF23 in ESRD patients, determined by either assay, is a reasonably accurate predictor of circulating bioactive FGF23. Immunoassays detecting either iFGF23 or cFGF23 thus appear to be equally suitable to further evaluate the pathophysiological role of this hormone in patients with CKD. Measurements with the iFGF23 assay, however, showed overall better correlation to the level of bioactive FGF23 than the cFGF23 assay. This may be related to the previously reported better performance of the iFGF23 assay for establishing the diagnosis in TIO or XLH patients, a finding that could be related to the higher sensitivity and/or lower intraassay and interassay coefficients of variation of this assay (42,43).
The present results have important implications for understanding the recently described associations between FGF23 levels and the rapidity of CKD progression as well as left ventricular hypertrophy and mortality in dialysis patients (14,17,18,20). Although the mechanisms underlying the findings from these observational studies are currently unknown, there are several possible explanations for the described relationships between FGF23 and adverse clinical outcomes in CKD. First, FGF23 may represent a more sensitive biomarker of abnormal phosphorus metabolism and the associated cardiovascular risk than the serum phosphate level itself, which has been implicated in each of these processes (22,23,24,25,26). Furthermore, it is conceivable that the extraordinarily high concentrations of bioactive FGF23 observed in numerous ESRD patients may activate FGF receptors in the presence of only small amounts of Klotho, or even in its absence. Consistent with this hypothesis, FGF23 was shown to bind in the absence of Klotho to different FGF receptors, albeit with low affinity (50). FGF23 may therefore have functions that are independent from its role in the regulation of renal phosphate handling and vitamin D synthesis (33,35). This raises the possibility that the elevated levels of biologically active FGF23 in CKD could have as yet unknown functions that contribute to the progression of kidney disease or could have detrimental effects in ESRD.
Taken together, our findings strongly support the conclusion that circulating FGF23 in ESRD patients is mostly intact and biologically active. Although it is conceivable that as yet undefined FGF23 fragments with agonist or antagonist properties exist, our findings suggest that measuring immunoreactive FGF23 with currently available assays is likely to be sufficient for predicting FGF23 bioactivity in CKD. FGF23 immunoassays will thus be of considerable importance for further exploring the pathophysiological roles of this novel hormone in patients with impaired renal function.
Footnotes
This work was supported by the National Institutes of Health (PO1 DK11794, DK67563, and RO1DK076116).
Disclosure Summary: T.I., M.E., K.W.-P., M.W., and I.B.S. have nothing to declare. T.S., I.U., and Y.Y. are employed by Kyowa Hakko Kirin Co., Ltd., Japan. H.J. is named as a coinventor on the patent describing the cFGF23 assay described in this report (U.S. Patent 7,094,551).
First Published Online December 4, 2009
Abbreviations: bioFGF23, Bioactive FGF23; cFGF23 assay, intact hormone and C-terminal fragments; CKD, chronic kidney disease; Egr-1, early growth response-1; ESRD, end-stage renal disease; FGF23, fibroblast growth factor 23; FGF23Ab, anti-FGF23 neutralizing antibody; iFGF23 assay, intact FGF23 alone; TIO, tumor-induced osteomalacia; XLH, X-linked hypophosphatemia.
References
- Segawa H, Kawakami E, Kaneko I, Kuwahata M, Ito M, Kusano K, Saito H, Fukushima N, Miyamoto K 2003 Effect of hydrolysis-resistant FGF23-R179Q on dietary phosphate regulation of the renal type-II Na/Pi transporter. Pflügers Arch 446:585–592 [DOI] [PubMed] [Google Scholar]
- Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, Tomoe Y, Aranami F, Matsumoto N, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K 2009 Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol 20:104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T 2004 FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435 [DOI] [PubMed] [Google Scholar]
- The ADHR Consortium 2000 Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348 [DOI] [PubMed] [Google Scholar]
- Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T 2001 Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98:6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Yoshioka M, Itoh N 2000 Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun 277:494–498 [DOI] [PubMed] [Google Scholar]
- Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H 2003 Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348:1656–1663 [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S 2002 Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87:4957–4960 [DOI] [PubMed] [Google Scholar]
- Weber TJ, Liu S, Indridason OS, Quarles LD 2003 Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res 18:1227–1234 [DOI] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE 2006 Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Jüppner H, Strom TM 2006 DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38:1248–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron-Robey P 2003 FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112:683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Imanishi Y, Kinoshita E, Nakagomi Y, Shimizu N, Miyauchi A, Satomura K, Koshiyama H, Inaba M, Nishizawa Y, Jüppner H, Ozono K 2005 The role of fibroblast growth factor 23 for hypophosphatemia and abnormal regulation of vitamin D metabolism in patients with McCune-Albright syndrome. J Bone Miner Metab 23:231–237 [DOI] [PubMed] [Google Scholar]
- Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Jüppner H, Wolf M 2005 Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16:2205–2215 [DOI] [PubMed] [Google Scholar]
- Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB 2003 Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64:2272–2279 [DOI] [PubMed] [Google Scholar]
- Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, Gales B, Jüppner H, Salusky IB 2009 Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab 94:511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, König P, Kraatz G, Mann JF, Müller GA, Köhler H, Riegler P 2007 Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18:2600–2608 [DOI] [PubMed] [Google Scholar]
- Hsu HJ, Wu MS 2009 Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. Am J Med Sci 337:116–122 [DOI] [PubMed] [Google Scholar]
- Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, Defilippi C, Wolf M 2009 Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119:2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C 2009 High levels of serum fibroblast growth factors (FGF)-23 are associated with increased mortality in long haemodialysis patients. Nephrol Dial Transplant 24:279–2796 [DOI] [PubMed] [Google Scholar]
- Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M 2008 Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359:584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM 2004 Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15:2208–2218 [DOI] [PubMed] [Google Scholar]
- Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK 2001 Association of elevated serum PO4, Ca × PO4 product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12:2131–2138 [DOI] [PubMed] [Google Scholar]
- Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB 2000 Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342:1478–1483 [DOI] [PubMed] [Google Scholar]
- Isakova T, Gutiérrez OM, Chang Y, Shah A, Tamez H, Smith K, Thadhani R, Wolf M 2009 Phosphorus binders and survival on hemodialysis. J Am Soc Nephrol 20:388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal AR, Sullivan C, Leon JB, Bialostosky K 2008 Public health approach to addressing hyperphosphatemia among dialysis patients. J Ren Nutr 18:256–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet-Pagès A, Lorenz-Depiereux B, Zischka H, White KE, Econs MJ, Strom TM 2004 FGF23 is processed by proprotein convertases but not by PHEX. Bone 35:455–462 [DOI] [PubMed] [Google Scholar]
- Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T 2002 Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology 143:3179–3182 [DOI] [PubMed] [Google Scholar]
- White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ 2001 Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60:2079–2086 [DOI] [PubMed] [Google Scholar]
- Berndt TJ, Craig TA, McCormick DJ, Lanske B, Sitara D, Razzaque MS, Pragnell M, Bowe AE, O'Brien SP, Schiavi SC, Kumar R 2007 Biological activity of FGF-23 fragments. Pflugers Arch 454:615–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Shi M, Goetz R, Mohammadi M, Kuro OM, Moe OW 2008 C-terminal fragment of fibroblast growth factor (FGF) 23 inhibits renal phosphate (Pi) excretion as an FGF23 antagonist by displacing FGF23 from its receptor. J Am Soc Nephrol 19:78A [Google Scholar]
- Jüppner H, El-Hajj Fuleihan G 2006 Measurement of parathyroid hormone. In: Seibel M, Robins S, Bilezikian J, eds. Dynamics of bone and cartilage metabolism. 2nd ed. Philadelphia: Elsevier; 507–512 [Google Scholar]
- Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T 2006 Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774 [Google Scholar]
- Yamazaki Y, Tamada T, Kasai N, Urakawa I, Aono Y, Hasegawa H, Fujita T, Kuroki R, Yamashita T, Fukumoto S, Shimada T 2008 Anti-FGF23 neutralizing antibodies show the physiological role and structural features of FGF23. J Bone Miner Res 23:1509–1518 [DOI] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M 2006 Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281:6120–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachigian LM, Collins T 1998 Early growth response factor 1: a pleiotropic mediator of inducible gene expression. J Mol Med 76:613–616 [DOI] [PubMed] [Google Scholar]
- Nguyen HQ, Hoffman-Liebermann B, Liebermann DA 1993 The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell 72:197–209 [DOI] [PubMed] [Google Scholar]
- Goodman WG, Jüppner H, Salusky IB, Sherrard DJ 2003 Parathyroid hormone (PTH), PTH-derived peptides, and new PTH assays in renal osteodystrophy. Kidney Int 63:1–11 [DOI] [PubMed] [Google Scholar]
- Wesseling K, Coburn J, Salusky I 2005 The renal osteodystrophies. In: DeGroot L, Jameson J, eds. Endocrinology. 5th ed. Philadelphia: WB Saunders; 1697–1718 [Google Scholar]
- D'Amour P, Brossard JH, Rousseau L, Nguyen-Yamamoto L, Nassif E, Lazure C, Gauthier D, Lavigne JR, Zahradnik RJ 2005 Structure of non-(1–84) PTH fragments secreted by parathyroid glands in primary and secondary hyperparathyroidism. Kidney Int 68:998–1007 [DOI] [PubMed] [Google Scholar]
- Jüppner H, Gardella TJ, Brown EM, Kronenberg HM, Potts Jr J 2005 Parathyroid hormone and parathyroid hormone-related peptide in the regulation of calcium homeostasis and bone development. In: DeGroot L, Jameson J, eds. Endocrinology. 5th ed. Philadelphia: WB Saunders; 1377–1417 [Google Scholar]
- Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D, Kassem M, Rackoff P, Zimering M, Dalkin A, Drobny E, Colussi G, Shaker JL, Hoogendoorn EH, Hui SL, Econs MJ 2006 Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab 91:2055–2061 [DOI] [PubMed] [Google Scholar]
- Ito N, Fukumoto S, Takeuchi Y, Yasuda T, Hasegawa Y, Takemoto F, Tajima T, Dobashi K, Yamazaki Y, Yamashita T, Fujita T 2005 Comparison of two assays for fibroblast growth factor (FGF)-23. J Bone Miner Metab 23:435–440 [DOI] [PubMed] [Google Scholar]
- Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer CG, Tenenhouse HS 1997 Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest 99:1200–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KE, Jonsson KB, Carn G, Hampson G, Spector TD, Mannstadt M, Lorenz-Depiereux B, Miyauchi A, Yang IM, Ljunggren O, Meitinger T, Strom TM, Jüppner H, Econs MJ 2001 The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide overexpressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab 86:497–500 [DOI] [PubMed] [Google Scholar]
- Benet-Pagès A, Orlik P, Strom TM, Lorenz-Depiereux B 2005 An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet 14:385–390 [DOI] [PubMed] [Google Scholar]
- Frishberg Y, Topaz O, Bergman R, Behar D, Fisher D, Gordon D, Richard G, Sprecher E 2005 Identification of a recurrent mutation in GALNT3 demonstrates that hyperostosis-hyperphosphatemia syndrome and familial tumoral calcinosis are allelic disorders. J Mol Med 83:33–38 [DOI] [PubMed] [Google Scholar]
- Larsson T, Davis SI, Garringer HJ, Mooney SD, Draman MS, Cullen MJ, White KE 2005 Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology 146:3883–3891 [DOI] [PubMed] [Google Scholar]
- Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E 2004 Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet 36:579–581 [DOI] [PubMed] [Google Scholar]
- Yu X, Ibrahimi OA, Goetz R, Zhang F, Davis SI, Garringer HJ, Linhardt RJ, Ornitz DM, Mohammadi M, White KE 2005 Analysis of the biochemical mechanisms for the endocrine actions of fibroblast growth factor-23. Endocrinology 146:4647–4656 [DOI] [PMC free article] [PubMed] [Google Scholar]