Abstract
Context: Radioiodine ablation is commonly used to treat thyroid cancer, but a major challenge is often the loss of radioiodine avidity of the cancer caused by aberrant silencing of iodide-handling genes.
Objectives: This study was conducted to test the therapeutic potential of targeting the aberrantly activated MAPK and PI3K/Akt/mTOR pathways and histone deacetylase to restore radioiodine avidity in thyroid cancer cells.
Experimental Design: We tested the effects of specific inhibitors targeting these pathways/molecules that had established clinical applicability, including the MAPK kinase inhibitor RDEA119, mTOR inhibitor temsirolimus, Akt inhibitor perifosine, and histone deacetylase inhibitor SAHA, individually or in combinations, on the expression of iodide-handling genes and radioiodide uptake in a large panel of thyroid cancer cell lines.
Results: The expression of a large number of iodide-handling genes could be restored, particularly the sodium/iodide symporter, TSH receptor, and thyroperoxidase, by treating cells with these inhibitors. The effect was particularly robust and synergistic when combinations of inhibitors containing SAHA were used. Robust expression of sodium/iodide symporter in the cell membrane, which plays the most important role in iodide uptake in thyroid cells, was confirmed by immunofluorescent microscopy. Radioiodide uptake by cells was correspondingly induced under these conditions. Thyroid gene expression and radioiodide uptake could both be further enhanced by TSH.
Conclusions: Targeting major signaling pathways could restore thyroid gene expression and radioiodide uptake in thyroid cancer cells. Further studies are warranted to test this therapeutic potential in restoring radioiodine avidity of thyroid cancer cells for effective ablation treatment.
Clinically applicable inhibitors that suppress MAP kinase, PI3K/Akt pathways, and histone deacetylase may restore expression of thyroid genes and radioiodine uptake in thyroid cancer cells, providing important clinical implications.
Thyroid cancer is a common endocrine malignancy that has seen a nearly linear increase in incidence in recent decades (1,2,3). After thyroidectomy, radioiodine ablation therapy is commonly pursued as the mainstay of medical treatment for this cancer (4,5). This radioiodine treatment takes advantage of the unique function of thyroid follicular cells to take up and concentrate iodide, a process that involves several key thyroid iodide-handling protein molecules, including sodium/iodide symporter (NIS), TSH receptor (TSHR), thyroperoxidase (TPO), thyroglobulin (Tg), and various thyroid transcription factors (6,7). NIS is localized in the basal membrane of follicular thyroid cells and transports iodide from blood stream into the thyroid cell. TPO is involved in organification and incorporation of iodide into Tg for iodide accumulation and thyroid hormone synthesis in the thyroid gland. Several thyroid transcription factors, including thyroid transcription factor 1 (TTF1 or TITF1) (8) and 2 (TTF2 or FOXE1) (9) and PAX8 (10) are involved in the regulation of thyroid genes. TSH, acting on TSHR, in thyroid cell membrane plays a central role in up-regulating these thyroid iodide-handling genes and iodide uptake in thyroid cells.
Thyroid cancer sometimes loses the ability to take up iodide, creating a major obstacle for radioiodine treatment to which there is currently no solution. The 10-yr survival rate of patients with nonradioiodine avid metastatic thyroid cancer is only 10% (11). In fact, thyroid cancer-caused death virtually always occurs in cases that have lost radioiodine avidity. The underlying molecular basis is aberrant silencing of iodide-handling genes. Numerous studies demonstrated impaired or lost expression of the genes for NIS, TSHR, TPO, Tg, and certain thyroid transcription factors (12,13,14,15,16,17). Interestingly, silencing of these genes is often associated with BRAF mutation in thyroid cancer, which promotes overactivation of the MAPK pathway (7,11,18,19,20). A recent study demonstrated that LY294002, an inhibitor of the phosphatidylinositol-3-kinase (PI3K)/Akt pathway, increased NIS expression in rat thyroid cells (21), suggesting that the PI3K/Akt pathway may also play a role in the regulation of thyroid iodide-handling genes. Inhibition of histone deacetylase (HDAC) was previously shown to induce expression of NIS (22,23,24), but many of the cell lines used in these studies are now known to be of nonthyroid origin (25). It therefore remains to be established whether targeting these major signaling pathways/molecules could restore expression of thyroid genes in thyroid cancer cells and hence may be therapeutically applicable in radioiodine treatment of thyroid cancer patients. In the present study, we used a large panel of authenticated thyroid cancer cell lines to test the therapeutic potential of targeting major signaling pathways to restore thyroid gene expression and radioiodide uptake using several clinically applicable inhibitors.
Materials and Methods
Human thyroid cancer cell lines
The thyroid cancer cell lines C643, Hth7, Hth74, and SW1736 were originally from Dr. N. E. Heldin (University of Uppsala, Uppsala, Sweden); KAT18 from Dr. Kenneth B. Ain (University of Kentucky Medical Center, Lexington, KY); OCUT-1 from Dr. Naoyoshi Onoda (Osaka City University Graduate School of Medicine, Osaka, Japan); BCPAP from Dr. Massimo Santoro (University of Federico II, Naples, Italy); K1 from Dr. David Wynford-Thomas (University of Wales College of Medicine, Cardiff, UK); WRO from Dr. G. J. F. Juillard (University of California-Los Angeles School of Medicine, Los Angeles, CA); and FTC133 from Dr. George Brabant (University of Manchester, Manchester, UK). The TPC1 cell line was provided by Dr. Alan P. Dackiw (The Johns Hopkins University, Baltimore, MD). These cancer cells have been recently authenticated to be unique thyroid cancer cell lines (25), except for the OCUT1 cell that has been used in other recent thyroid cancer studies (26,27). Their tumor origins and genetic alterations are presented in Supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). All these cells were grown at 37 C in RPMI 1640 medium with 10% fetal bovine serum, except for FTC133, which was cultured with DMEM/F-12 medium. In some experiments, cells were treated with the MAPK kinase (MEK) inhibitor RDEA119, the mammalian target of rapamycin (mTOR) inhibitor temsirolimus (CCI779), the Akt inhibitor perifosine, and the HDAC inhibitor SAHA with the indicated concentrations for 30 h, individually or in different combinations. Dimethylsulfoxide or PBS was used as the vehicle control. Cells were additionally treated with 20 mU/ml bovine TSH (Sigma, St. Louis, MO) in some experiments as indicated. RDEA119 was obtained from Ardea Biosciences, Inc. (San Diego, CA). Temsirolimus, perifosine, and SAHA (Vorinostat) were obtained from Cayman Chemical (Ann Arbor, MI).
Western blotting assay
Cells were lysed in RIPA buffer. Cellular proteins were collected by centrifugation at 4 C for 20 min, subjected to 10% SDS-PAGE, and transferred onto polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Piscataway, NJ), and membranes were probed with the indicated primary antibodies. Anti-phospho-ERK (sc-7383), anti-ERK1 (sc-94), anti-phospho-Akt (sc-7985-R), anti-phospho-p70S6 kinase (p70S6K) (sc-230), anti-Ac-histone (sc-8655-R), and anti-Actin (sc-1616-R) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antigen-antibody complexes were visualized using horseradish peroxidase-conjugated antimouse (sc-2005; Santa Cruz Biotechnology) or antirabbit (sc-2004; Santa Cruz Biotechnology), IgG antibodies, and the ECL Western Blotting Analysis System (Amersham Pharmacia Biotech).
RNA extraction and real-time quantitative RT-PCR analysis
Total RNA was isolated from cells using TRIzol reagent according to the instructions of the manufacturer (Invitrogen, San Diego, CA). Total RNA (2 μg) was converted to cDNA on an iCycler Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA) using Oligo-dT and SuperScript II according to the instructions of the manufacturer (SuperScript First-Strand Synthesis kit; Invitrogen). Real-time quantitative RT-PCR analysis was performed to evaluate the expression of thyroid genes on an ABI Prism 7900HT Sequence Detector (Applied Biosystems, Foster City, CA), using SYBR GreenER qPCR SuperMix according to the instructions of the manufacturer (Applied Biosystems). Primer pairs of thyroid-specific genes, NIS, TSHR, TPO, Tg, PAX8, FOXE1, and TTF1 were designed using Primer Express (Applied Biosystems). PCR primer pairs were designed to span a large intron, and the sequences are presented in Supplemental Table 2. The expression value of each gene was normalized to β-actin cDNA to calculate the relative amount of RNA present in each sample according to the 2−ΔΔCt method (28). The specificity of real-time quantitative PCR for all these genes was confirmed by running the PCR products on a 1.5% agarose gel to show single specific bands at the expected sizes and by sequencing (data not shown). Each sample was run in triplicate.
Immunofluorescent localization of NIS
Cells treated with specific inhibitors, individually or in different combinations, to induce the expression of NIS were incubated with VJ2 α-hNIS mAb (a gift from Dr. Sabine Costagliola at the Free University of Brussels) (29), and diluted at 1:20 in FACS buffer (3% fetal bovine serum, 0.02% NaN3 in PBS) at 4 C for 40 min. Cells were then washed twice with FACS buffer and incubated with fluorescein isothiocyanate-conjugated α-mouse IgG (Sigma) diluted at 1:100 in FACS buffer at 4 C for 40 min. Cells were washed again in FACS buffer, and then resuspended in 500 μl of FACS buffer with 7-amino-actinomycin D (7-AAD). Fluorescent microscopic examination was conducted to monitor NIS expression (Nikon Corporation, Tokyo, Japan).
Radioiodide uptake assay
Thyroid cancer cells (5 ×105 cells per well) were seeded in 12-well plates and treated with the indicated inhibitors for 30 h. After treatment with inhibitors, one well was counted for cell number for each group, and the remaining wells were incubated with 1 ml serum-free RPMI 1640 medium containing 1 μCi Na125I in 5 μm nonradioactive NaI for 1 h at 37 C with 5% CO2. To determine NIS-specific uptake, a group of wells were preincubated with NaClO4 at 300 μm for 30 min before the start of incubation with Na125I. The medium was subsequently aspirated, and cells were quickly washed twice with ice-cold Hank’s balanced salt solution. Cells were trypsinized with 200 μl of 0.25% trypsin and, once lifted, were lysed with 500 μl of NaOH (0.33 m) containing 1% sodium dodecyl sulfate. Cells were collected, and radioactivity was counted by a gamma-counter. Experiments were performed in triplicate.
Statistical analysis
All the experiments were similarly performed at least two or three times. Most of the measurements were conducted in triplicate and some in duplicate. Data were compared using the t test. Significance was defined as P < 0.05. Unless indicated, data shown in the figures are representatives.
Results
Induction of the expression of thyroid iodide-handling genes in thyroid cancer cells by suppressing the MAPK pathway, PI3K/Akt/mTOR pathway, and HDAC
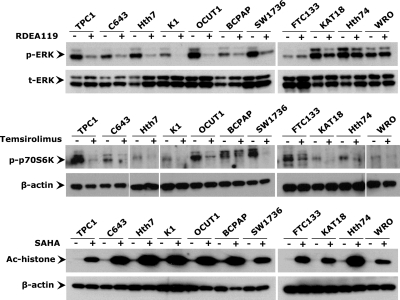
We tested the effects of the MEK inhibitor RDEA119, the mTOR inhibitor temsirolimus, and the HDAC inhibitor SAHA on 11 thyroid cancer cell lines, including TPC1, C643, Hth7, K1, OCUT1, BCPAP, SW1736, FTC133, KAT18, Hth74, and WRO cells. As shown in Fig. 1, treatment of cells with RDEA119 at 0.5 μm for 30 h strongly inhibited phosphorylation of ERK (p-ERK) in most thyroid cancer cells, particularly TPC1, C643, Hth7, K1, OCUT1, BCPAP, and SW1736 cells that harbored genetic alterations in the MAPK pathway, including RET/PTC, Ras mutation and BRAF mutation. In thyroid cancer cell lines FTC133, KAT18, Hth74, and WRO cells that did not harbor these genetic alterations, there was modest or no effect on p-ERK. At 10 nm for 30 h, temsirolimus remarkably inhibited phosphorylation of p70S6K, a substrate of mTOR that is involved in cell cycle control (30), in all of the 11 thyroid cancer cells (Fig. 1). Treatment of cells with the HDAC inhibitor SAHA at 5 μm for 30 h resulted in a marked increase in histone acetylation in all cell lines (Fig. 1). We next explored the ability of these inhibitors to induce the expression of thyroid genes in cells treated with these inhibitors, individually or in different combinations under the conditions used for the studies in Fig. 1. As shown in Figs. 2A and 3, expression of NIS, TSHR, TPO, and Tg was restored to various extents by RDEA119, temsirolimus, or SAHA in most of the 11 thyroid cancer cells. FOXE1 and TTF1 genes were also reexpressed to various extents in different cells. However, expression of PAX8 seemed somehow to be negatively affected by these inhibitors (Supplemental Fig. 1A). Among the three inhibitors, SAHA showed the most pronounced effect on the expression of thyroid genes. Interestingly, although RDEA119 and temsirolimus each alone had a small effect, a remarkable synergistic effect was seen between RDEA119 or temsirolimus and SAHA on most genes in most cells (Fig. 2A, Fig. 3, and Supplemental Fig. 1A). Combination of temsirolimus with RDEA119 even better synergized the effect of SAHA. Combined use of inhibitors of MAPK and PI3K/Akt pathways without SAHA did not show significant synergistic effects in thyroid cancer cells (data not shown).
Figure 1.
Effects of various inhibitors on the MAPK pathway, PI3K/Akt/mTOR pathway, and HDAC in thyroid cancer cells. Inhibition of ERK and p70S6K phosphorylation and histone deacetylation was achieved using the MEK-specific inhibitor RDEA119, mTOR-specific inhibitor temsirolimus (CCI779), and HDAC-specific inhibitor SAHA, respectively. Thyroid cancer cells were treated with 0.5 μm RDEA119, 10 nm temsirolimus, or 5 μm SAHA for 30 h. Dimethylsulfoxide or PBS was used as the vehicle control. After treatments, cells were lysed for Western blotting analyses. The effect of inhibition is reflected by the level of phosphorylated ERK and p70S6K and acetylated histone detected with specific anti-phosphorylated ERK (p-ERK), anti-phosphorylated p70S6K (p-p70S6K), and anti-acetylated histone H3 (Ac-histone) antibodies. Immunoblotting with antibodies against t-ERK or β-actin was used for quality control.
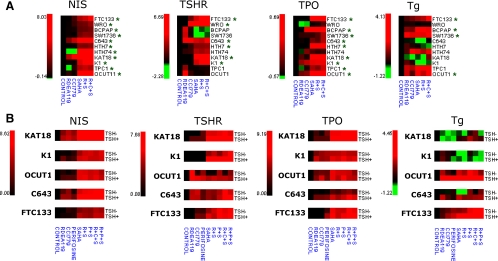
Figure 2.
Induction of the expression of NIS, TSHR, TPO, and Tg genes in thyroid cancer cells using various inhibitors, individually or in different combinations. A, Eleven authenticated thyroid cancer cells, as indicated, were treated with inhibitors (RDEA119, CCI779, and SAHA) as described in Fig. 1, individually or in different combinations, to induce the expression of NIS, TSHR, TPO, and Tg genes. Data were obtained using real-time RT-PCR and are presented as the mean of values from three assays for the ratios of specific gene expression with natural log conversion. The red color represents the highest expression, green is the lowest expression, and black is the intermediate. B, Effect of TSH stimulation on NIS, TSHR, TPO, and Tg expression induced by specific inhibitors (RDEA119, CCI779, perifosine, and SAHA), individually or in different combinations. Details are as described in Materials and Methods, and data were obtained and are presented as in panel A. Green asterisks show that the basal level of gene expression is low. R, RDEA119; C, CCI779 (temsirolimus); P, perifosine; S, SAHA.
Figure 3.
TSH enhancement of expression of NIS, TSHR, and TPO genes induced by various inhibitors, individually or in different combinations, in thyroid cancer cells. Various thyroid cancer cells were treated with specific inhibitors (RDEA119, CCI779, perifosine, and SAHA) as described in Fig. 1 and in Materials and Methods, individually or in different combinations, supplemented with or without 20 mU/ml TSH. Data are presented as the mean ± sd of values from three assays. R, RDEA119; C, CCI779 (temsirolimus); P, perifosine; S, SAHA. *, P < 0.05; **, P < 0.01 for comparison with control.
TSH stimulation enhanced thyroid gene expression induced by SAHA synergized by perifosine and other inhibitors in thyroid cancer cells
Because Akt is upstream of mTOR in the PI3K/Akt pathway and is coupled also to other signaling molecules, targeting Akt may also be a reasonable strategy in restoring the expression of thyroid genes. We therefore also examined the effect of the Akt inhibitor perifosine on selected thyroid cancer cells, including KAT18, K1, OCUT1, C643, and FTC133 cells. These cells were treated with 5 μm perifosine for 30 h. As shown in Supplemental Fig. 2, this inhibitor remarkably inhibited Akt phosphorylation in most cells. Correspondingly, like temsirolimus, although perifosine alone had only a minimal effect, it could enhance the effect of SAHA on the expression of the NIS, TSHR, TPO, and Tg genes in many of these cells (Figs. 2B and 3). The PAX8, FOXE1, and TTF1 genes were also reexpressed to various extents by treatment with perifosine in combination with SAHA in different cells (Supplemental Fig. 1B). Like temsirolimus, perifosine, when combined with RDEA119, even better synergized the effect of SAHA (Fig. 2B, Fig. 3, and Supplemental Figs. 1B and 3). Because TSHR plays an important role in up-regulating the expression of iodide-handling genes in thyroid cells (6,7), we investigated whether TSH treatment could affect the expression of thyroid genes in these cells. As shown in Fig. 2B, expression of the NIS, TSHR, TPO, and Tg genes induced by RDEA119, temsirolimus, perifosine, SAHA, and their various combinations was enhanced by TSH to various extents in most cells. The expression of the PAX8, FOXE1, and TTF1 genes induced by various inhibitors was also enhanced by TSH in many cases (Supplemental Figs. 1B and 3). A striking finding was the dramatic enhancing effect of TSH on thyroid gene expression induced by SAHA or SAHA combined with the inhibitors of the MAPK and PI3K/Akt pathways as shown in Figs. 2B and 3 for NIS, TSHR, TPO, and Tg genes and in Supplemental Figs. 1B and 3 for other thyroid genes. There was also an interesting dramatic synergistic effect of TSH on the expression of its own receptor TSHR induced by SAHA or by treatment combinations containing SAHA in most cells (Figs. 2B and 3).
NIS protein expression in the cell membrane and radioiodide uptake in thyroid cancer cells induced by simultaneously suppressing the MAPK and PI3K/Akt pathways and HDAC
Because NIS plays a central role in thyroid cellular iodide uptake, we next investigated further the expression of NIS, particularly its expression on cell membranes, using immunofluorescent microscopy in selected thyroid cancer cells, including C643, K1, and KAT18 cells. As a representative test, we chose to use the combination of RDEA119, perifosine, and SAHA because this induced a robust expression of NIS in many thyroid cancer cells per real-time quantitative RT-PCR analysis (Figs. 2 and 3). We used double immunofluorescence to examine NIS protein expression. The red color represented nuclear staining by 7-AAD, and the green color represented NIS expression. Because 7-AAD does not readily pass through intact cell membranes, cells with the green color alone were thus intact viable cells that did not have 7-AAD nuclear staining. NIS staining in these cells represented NIS protein expression on cell membranes. As shown in Fig. 4, robust expression of the NIS protein in cell membranes was induced by simultaneous treatment of cells with the three inhibitors.
Figure 4.
Immunofluorescent microscopic analysis of NIS expression induced by inhibitors in thyroid cancer cells. After a 30-h combined treatment with RDEA119, perifosine, and SAHA, cells were analyzed by immunofluorescent microscopy using anti-NIS antibody and fluorescein isothiocyanate-coupled secondary antibody. Double immunofluorescence was displayed with the red color representing 7-AAD nuclear staining and the green color representing NIS expression. Cells with the green color alone are intact living cells that do not have 7-AAD nuclear staining. NIS staining in these cells represents NIS protein expression on the cell membrane. Cells with double colors suggest that the cells were not intact and therefore both cell membrane NIS staining and 7-AAD nuclear staining occurred. Control cells did not show significant NIS staining, and the dead cells mostly showed nuclear staining with 7-ADD. R, RDEA119; P, perifosine; S, SAHA.
Given the induction of expression of thyroid iodide-handling genes in thyroid cancer cells by suppressing multiple signaling pathways/molecules, we finally tested the functional relevance of such gene expression by examining the ability of cells to take up radioiodide in three selected thyroid cancer cells C643, K1, and KAT18. As shown in Fig. 5, combined treatment of cells with RDEA119, perifosine, and SAHA robustly induced radioiodide uptake in all three cells. Additional treatment of cells with TSH significantly enhanced radioiodide uptake induced by treatment with the inhibitors (Fig. 5). These functional results were consistent with the robust expression of NIS and TSHR under similar conditions in these cells (Figs. 2–4). The induced radioiodide uptake could be inhibited by the NIS blocker NaClO4, suggesting that it was mediated specifically by the newly produced NIS protein. A small basal level of radioactivity was seen in nontreated control cells (Fig. 5). This radioactivity was largely insensitive to NaClO4, suggesting that it was not NIS-specific and probably represented nonspecific binding of radioiodide with cells.
Figure 5.
Radioiodide uptake in thyroid cancer cells induced by inhibitors targeting MEK, Akt, and HDAC in thyroid cancer cells. Thyroid cancer cells C643, K1, and KAT18 were treated with a combination of RDEA119, perifosine, and SAHA as described in Materials and Methods, supplemented with or without 20 mU/ml TSH. Cells were subsequently incubated with 1 μCi 125I/1 ml/well on 12-well plates for 1 h. Cells were then washed and harvested for radioactivity measurement using a gamma-counter as described in Materials and Methods. Data are expressed as mean ± sd of values from three assays. R, RDEA119; P, perifosine; S, SAHA; Ctr, control. •••, P < 0.001; ••, P < 0.01; •, P < 0.05, compared with NaClO4-treated cells. *, P < 0.001, compared with untreated cells.
Discussion
Radioiodine ablation therapy after thyroidectomy is the mainstay of medical treatment for thyroid cancer in many patients (4,5). This treatment takes the advantage of the unique iodide-handling machinery of thyroid cells, involving NIS, TSHR, TPO, Tg, and several thyroid transcription factors, to take up and accumulate radioiodide. T4 withdrawal to increase endogenous TSH or use of recombinant human TSH to enhance the ablation efficacy through the action of TSHR is commonly administered (4,5,31). This treatment, however, is ineffective in poorly differentiated and undifferentiated thyroid cancer and often even in differentiated thyroid cancer (32,33). The underlying molecular mechanism is the impairment or even complete loss of the expression of iodide-handling genes and aberrant localization of NIS in these thyroid cancers (12,13,14,15,16,17,34). This results in the loss of the ability of thyroid cancer cells to take up and concentrate radioiodide, thus failure of radioiodine treatment. This is a main cause of thyroid cancer-associated morbidity and mortality. There is currently no effective treatment for these thyroid cancer patients when the tumor becomes surgically inoperable.
The specific molecular mechanism underlying silencing of thyroid iodide-handling genes in thyroid cancer is unclear. Many recent studies demonstrated an association of BRAF mutation and, hence, activation of the MAPK pathway with silencing of thyroid genes in thyroid cancer (35,36). We previously showed that induced expression of the BRAF mutant and consequent activation of the MAPK pathway signaling in rat thyroid cells could silence the expression of thyroid iodide-handling genes and removal of BRAF mutant could restore the expression of these genes (37). We and others also used the luciferase reporting system to show that inhibition of the promoter activities of the NIS and TSHR genes could be directly linked to the MAPK pathway signaling (34,37). Thus, overactivation of the MAPK pathway, which is common in thyroid cancer, likely plays an important role in the aberrant silencing of thyroid genes in thyroid cancer. A recent study showed that the PI3K inhibitor LY294002 could induce expression of NIS in rat thyroid cells (21). Although the LY294002 compound is not clinically applicable, these results suggest that the PI3K/Akt pathway may also play a role in the silencing of thyroid genes in thyroid cancer. This may be the case particularly given the common occurrence of activating genetic alterations in the PI3K/Akt pathway in thyroid cancer (38,39,40,41). Interestingly, several previous studies demonstrated that HDAC inhibitors could induce expression of NIS in some human cancer cell lines (22,23,24). Many of the cell lines used in these studies are now known to be nonthyroid in origin (25), and the inhibitors used may not be clinically applicable. Nevertheless, these studies suggest that aberrant histone deacetylation may also play an important role in thyroid gene silencing in thyroid cancer.
We tested the hypothesis in the present study that targeting the MAPK and PI3K/Akt pathways and HDAC could be an effective strategy in restoring the expression of thyroid iodide-handling genes and, hence, uptake of radioiodine in thyroid cancer. Using a large panel of authenticated thyroid cancer cell lines, we indeed demonstrated that inhibitors of these signaling pathways/molecules could induce the expression of thyroid iodide-handling genes. We specifically chose the MEK inhibitor RDEA119, Akt inhibitor perifosine, mTOR inhibitor temsirolimus, and HDAC inhibitor SAHA because these agents have proven acceptable safety profiles and in vivo biological activities in clinical trials on other human cancers (42,43,44). They are therefore ready for clinical use in thyroid cancer if proven to be effective. Among these inhibitors when individually used, SAHA was the most effective in inducing thyroid gene expression. Interestingly, although RDEA119, perifosine, and temsirolimus each alone had only a small effect on thyroid gene expression in thyroid cancer cells, they all significantly synergized the effect of SAHA. Although a recent clinical trial showed no inhibitory effect of SAHA on the growth and progression of radioiodine-refractory thyroid cancer (44), our present study suggests that SAHA may be effective when used in conjunction with radioiodine ablation therapy for thyroid cancer.
It is particularly important to note that among the various thyroid genes tested, expression of NIS was generally most responsive to the treatment with the inhibitors in all the cells. Protein expression and cell membrane localization of NIS was confirmed using immunofluorescent microscopic analysis. This is clinically important because NIS, acting to transport iodide from the blood stream into the thyroid cell, plays the most important role in radioiodide uptake and accumulation in thyroid cancer cells. The functionality of this NIS gene expression was clearly shown by our demonstration of robust and NaClO4-sensitive radioiodide uptake in thyroid cancer cells in response to inhibitor treatments. It is worth noting that expression of TSHR was also robustly induced by the inhibitors in most cells, and treatment of cells with TSH further enhanced both the expression of thyroid genes and radioiodide uptake induced by the inhibitors targeting the multiple signaling pathways/molecules. This effect of TSH was most dramatic when SAHA was used to treat cells, either alone or in combination with other inhibitors, particularly the latter. These results suggest that use of these inhibitors, particularly in combination with SAHA, would most effectively confer the sensitivity of thyroid cancer cells to radioiodine ablation when the patients undergo T4 withdrawal to increase endogenous TSH or receive human recombinant TSH. This therapeutic strategy may be expected to be effective even in poorly differentiated and undifferentiated thyroid cancers because most of the cell lines used in the present study were derived from these types of thyroid cancer. In fact, even the thyroid cancer cell lines originally derived from differentiated thyroid cancers that were used in this study likely had lost differentiation because many such human thyroid tumor cell lines have evolved in vitro into phenotypes with gene expression profiles that were close to in vivo undifferentiated tumors (45). This further supports the idea that restoration of thyroid gene expression and radioiodine avidity are possible in undifferentiated thyroid cancers using the treatment strategies tested in the present study.
In summary, for the first time using a large panel of authentic thyroid cancer cells, we demonstrated robust expression of thyroid iodide-handling genes, particularly NIS and TSHR, driven synergistically by suppressing the MAPK and PI3K/Akt pathways and HDAC. The functional importance of this thyroid gene expression was demonstrated by restoration of the ability of cells to take up radioiodide that could be dramatically enhanced by TSH. These results provide important clinical implications for novel therapeutic strategies to restore radioiodine avidity of thyroid cancers. Because the inhibitors tested in the present study are clinically applicable, further studies, including appropriately designed clinical trials in thyroid cancer, with the goal to use them to restore the responsiveness to radioiodine ablation may be warranted.
Supplementary Material
Acknowledgments
We thank Drs. N. E. Heldin, K. B. Ain, N. Onoda, M. Santoro, D. Wynford-Thomas, G. Brabant, A. P. Dackiw, G. J. Juillard, R. E. Schweppe, and B. R. Haugen for kindly providing us or facilitating the accessibility to the cell lines used in this study. We also thank Dr. S. Costagliola for kindly providing us VJ2 α-hNIS mAb.
Footnotes
This work was supported by National Institutes of Health Grant RO-1 CA113507 (to M.X.).
Disclosure Summary: The authors have no potential conflict of interest to declare.
First Published Online December 11, 2009
Abbreviations: 7-AAD, 7-Amino-actinomycin D; HDAC, histone deacetylase; MEK, MAPK kinase; mTOR, mammalian target of rapamycin; NIS, sodium/iodide symporter; PI3K, phosphatidylinositol-3-kinase; Tg, thyroglobulin; TPO, thyroperoxidase; TSHR, TSH receptor; TTF, thyroid transcription factor.
References
- Leenhardt L, Grosclaude P, Chérié-Challine L 2004 Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid 14:1056–1060 [DOI] [PubMed] [Google Scholar]
- Davies L, Welch HG 2006 Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295:2164–2167 [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ 2009 Cancer statistics, 2009. CA Cancer J Clin 59:225–249 [DOI] [PubMed] [Google Scholar]
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM 2009 American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W 2006 European Thyroid Cancer Taskforce. European Consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 154:787–803 [DOI] [PubMed] [Google Scholar]
- Nilsson M 2001 Iodide handling by the thyroid epithelial cell. Exp Clin Endocrinol Diabetes 109:13–17 [DOI] [PubMed] [Google Scholar]
- Riesco-Eizaguirre G, Santisteban P 2006 A perspective view of sodium iodide symporter research and its clinical implications. Eur J Endocrinol 155:495–512 [DOI] [PubMed] [Google Scholar]
- Guazzi S, Price M, De Felice M, Damante G, Mattei MG, Di Lauro R 1990 Thyroid nuclear factor 1 (TTF-1) contains a homeodomain and displays a novel DNA binding specificity. EMBO J 9:3631–3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchia PE, Mattei MG, Lapi P, Fenzi G, Di Lauro R 1999 Cloning, chromosomal localization and identification of polymorphisms in the human thyroid transcription factor 2 gene (TITF2). Biochimie 81:433–440 [DOI] [PubMed] [Google Scholar]
- Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P 1990 Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development 110:643–651 [DOI] [PubMed] [Google Scholar]
- Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M 2006 Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab 91:2892–2899 [DOI] [PubMed] [Google Scholar]
- Sheils OM, Sweeney EC 1999 TSH receptor status of thyroid neoplasms-TaqMan RT-PCR analysis of archival material. J Pathol 188:87–92 [DOI] [PubMed] [Google Scholar]
- Venkataraman GM, Yatin M, Marcinek R, Ain KB 1999 Restoration of iodide uptake in dedifferentiated thyroid carcinoma: relationship to human Na+/I− symporter gene methylation status. J Clin Endocrinol Metab 84:2449–2457 [DOI] [PubMed] [Google Scholar]
- Lazar V, Bidart JM, Caillou B, Mahé C, Lacroix L, Filetti S, Schlumberger M 1999 Expression of the Na+/I− symporter gene in human thyroid tumors: a comparison study with other thyroid-specific genes. J Clin Endocrinol Metab 84:3228–3234 [DOI] [PubMed] [Google Scholar]
- Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H, Bernet V, Burman KD, Kohn LD, Saji M 2001 Overexpression and overactivation of Akt in thyroid carcinoma. Cancer Res 61:6105–6111 [PubMed] [Google Scholar]
- Arturi F, Russo D, Bidart JM, Scarpelli D, Schlumberger M, Filetti S 2001 Expression pattern of the pendrin and sodium/iodide symporter genes in human thyroid carcinoma cell lines and human thyroid tumors. Eur J Endocrinol 145:129–135 [DOI] [PubMed] [Google Scholar]
- Mirebeau-Prunier D, Guyétant S, Rodien P, Franc B, Baris O, Rohmer V, Reynier P, Tourmen Y, Malthièry Y, Savagner F 2004 Decreased expression of thyrotropin receptor gene suggests a high-risk subgroup for oncocytic adenoma. Eur J Endocrinol 150:269–276 [DOI] [PubMed] [Google Scholar]
- Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, Barbi F, Avenia N, Scipioni A, Verrienti A, Tosi E, Cavaliere A, Gulino A, Filetti S, Russo D 2007 BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab 92:2840–2843 [DOI] [PubMed] [Google Scholar]
- Di Cristofaro J, Silvy M, Lanteaume A, Marcy M, Carayon P, De Micco C 2006 Expression of tpo mRNA in thyroid tumors: quantitative PCR analysis and correlation with alterations of ret, Braf, ras and pax8 genes. Endocr Relat Cancer 13:485–495 [DOI] [PubMed] [Google Scholar]
- Mian C, Barollo S, Pennelli G, Pavan N, Rugge M, Pelizzo MR, Mazzarotto R, Casara D, Nacamulli D, Mantero F, Opocher G, Busnardo B, Girelli ME 2008 Molecular characteristics in papillary thyroid cancers (PTCs) with no 131I uptake. Clin Endocrinol (Oxf) 68:108–116 [DOI] [PubMed] [Google Scholar]
- Kogai T, Sajid-Crockett S, Newmarch LS, Liu YY, Brent GA 2008 Phosphoinositide-3-kinase inhibition induces sodium/iodide symporter expression in rat thyroid cells and human papillary thyroid cancer cells. J Endocrinol 199:243–252 [DOI] [PubMed] [Google Scholar]
- Kitazono M, Robey R, Zhan Z, Sarlis NJ, Skarulis MC, Aikou T, Bates S, Fojo T 2001 Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR901228), increase expression of the Na(+)/I(−) symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab 86:3430–3435 [DOI] [PubMed] [Google Scholar]
- Furuya F, Shimura H, Suzuki H, Taki K, Ohta K, Haraguchi K, Onaya T, Endo T, Kobayashi T 2004 Histone deacetylase inhibitors restore radioiodide uptake and retention in poorly differentiated and anaplastic thyroid cancer cells by expression of the sodium/iodide symporter thyroperoxidase and thyroglobulin. Endocrinology 145:2865–2875 [DOI] [PubMed] [Google Scholar]
- Puppin C, D'Aurizio F, D'Elia AV, Cesaratto L, Tell G, Russo D, Filetti S, Ferretti E, Tosi E, Mattei T, Pianta A, Pellizzari L, Damante G 2005 Effects of histone acetylation on sodium iodide symporter promoter and expression of thyroid-specific transcription factors. Endocrinology 146:3967–3974 [DOI] [PubMed] [Google Scholar]
- Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, Haugen BR 2008 Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab 93:4331–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogisawa K, Onoda N, Ishikawa T, Takenaka C, Inaba M, Ogawa Y, Chung KH 2002 Establishment and characterization of OCUT-1, an undifferentiated thyroid cancer cell line expressing high level of telomerase. J Surg Oncol 80:197–203 [DOI] [PubMed] [Google Scholar]
- Liu D, Hou P, Liu Z, Wu G, Xing M 2009 Genetic alterations in the phosphoinositide 3-kinase/Akt signaling pathway confer sensitivity of thyroid cancer cells to therapeutic targeting of Akt and mammalian target of rapamycin. Cancer Res 69:7311–7319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Pohlenz J, Duprez L, Weiss RE, Vassart G, Refetoff S, Costagliola S 2000 Failure of membrane targeting causes the functional defect of two mutant sodium iodide symporters. J Clin Endocrinol Metab 85:2366–2369 [DOI] [PubMed] [Google Scholar]
- Berven LA, Crouch MF 2000 Cellular function of p70S6K: a role in regulating cell motility. Immunol Cell Biol 78:447–451 [DOI] [PubMed] [Google Scholar]
- Duntas LH, Cooper DS 2008 Review on the occasion of a decade of recombinant human TSH: prospects and novel uses. Thyroid 18:509–516 [DOI] [PubMed] [Google Scholar]
- Sherman SI, Angelos P, Ball DW, Byrd D, Clark OH, Daniels GH, Dilawari RA, Ehya H, Farrar WB, Gagel RF, Kandeel F, Kloos RT, Kopp P, Lamonica DM, Loree TR, Lydiatt WM, McCaffrey J, Olson Jr JA, Ridge JA, Shah JP, Sisson JC, Tuttle RM, Urist MM 2007 National Comprehensive Cancer Network Thyroid Carcinoma Panel. Thyroid carcinoma. J Natl Compr Canc Netw 5:568–621 [DOI] [PubMed] [Google Scholar]
- Pacini F, Castagna MG, Brilli L, Jost L; ESMO Guidelines Working Group 2008 Differentiated thyroid cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 19 (Suppl 2):ii99–101 [DOI] [PubMed] [Google Scholar]
- Riesco-Eizaguirre G, Gutiérrez-Martínez P, García-Cabezas MA, Nistal M, Santisteban P 2006 The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr Relat Cancer 13:257–269 [DOI] [PubMed] [Google Scholar]
- Xing M 2007 BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev 28:742–762 [DOI] [PubMed] [Google Scholar]
- Xing M 2008 Recent advances in molecular biology of thyroid cancer and their clinical implications. Otolaryngol Clin North Am 41:1135–1146, ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hu S, Hou P, Jiang D, Condouris S, Xing M 2007 Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res 13:1341–1349 [DOI] [PubMed] [Google Scholar]
- García-Rostán G, Costa AM, Pereira-Castro I, Salvatore G, Hernandez R, Hermsem MJ, Herrero A, Fusco A, Cameselle-Teijeiro J, Santoro M 2005 Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res 65:10199–10207 [DOI] [PubMed] [Google Scholar]
- Hou P, Liu D, Shan Y, Hu S, Studeman K, Condouris S, Wang Y, Trink A, El-Naggar AK, Tallini G, Vasko V, Xing M 2007 Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res 13:1161–1170 [DOI] [PubMed] [Google Scholar]
- Santarpia L, El-Naggar AK, Cote GJ, Myers JN, Sherman SI 2008 Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab 93:278–284 [DOI] [PubMed] [Google Scholar]
- Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, Vasko V, El-Naggar AK, Xing M 2008 Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab 93:3106–3116 [DOI] [PubMed] [Google Scholar]
- Gills JJ, Dennis PA 2009 Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep 11:102–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings IR, Verweij J, Wiemer EA, Sleijfer S 2009 The applicability of mTOR inhibition in solid tumors. Curr Cancer Drug Targets 9:439–450 [DOI] [PubMed] [Google Scholar]
- Woyach JA, Kloos RT, Ringel MD, Arbogast D, Collamore M, Zwiebel JA, Grever M, Villalona-Calero M, Shah MH 2009 Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab 94:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Staveren WC, Solís DW, Delys L, Duprez L, Andry G, Franc B, Thomas G, Libert F, Dumont JE, Detours V, Maenhaut C 2007 Human thyroid tumor cell lines derived from different tumor types present a common dedifferentiated phenotype. Cancer Res 67:8113–8120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.