Abstract
Context: Few studies have examined whether endogenous testosterone is associated with the development of coronary heart disease (CHD) in women.
Objective: This study tested the association of total testosterone (total T) and bioavailable T (BioT) levels with risk of incident coronary events among older community-dwelling women.
Design, Setting, and Participants: This was a prospective, population-based study of 639 postmenopausal women, aged 50–91 (mean, 73.8) yr who had serum testosterone measurements at baseline (1984–87) and who were followed for incident CHD events through 2004.
Main Outcome Measures: A total of 134 incident CHD events occurred during follow-up [45 nonfatal myocardial infarctions, 79 fatal myocardial infarctions, and 10 coronary revascularizations].
Results: The median follow-up was 12.3 yr. Age-adjusted CHD risk estimates were similar for the four highest total T quintiles relative to the lowest, suggesting a low threshold. In age-adjusted analyses, the lowest total T quintile (≤80 pg/ml) was associated with a 1.62-fold increased risk of incident CHD [95% confidence interval (CI), 1.10–2.39] compared to higher levels. BioT showed a U-shaped association with incident CHD. Age-adjusted risk for the lowest and highest BioT quintiles relative to the third were 1.79 (95% CI, 1.03–3.16) and 1.96 (95% CI, 1.13–3.41), respectively. Additional adjustment for lifestyle, adiposity, estradiol, and ovarian status, or for CHD risk factor covariates, had minimal influence on results.
Conclusions: An optimal range of testosterone may exist for cardiovascular health in women, with increased risk of CHD events at low levels of testosterone overall and at high levels of the bioavailable fraction of testosterone.
An optimal range of testosterone may exist for cardiovascular health in women, with increased risk of coronary heart disease events at both low and high levels.
Although many studies have investigated the role of estrogen in cardiovascular disease in women, less attention has been paid to testosterone. The testosterone to estrogen balance in women increases dramatically at menopause, a time when central adiposity and the incidence of cardiovascular disease also increase, suggesting that higher levels of testosterone may be atherogenic in women (1). The combination of hyperandrogenemia, an adverse coronary heart disease (CHD) risk profile, and endothelial dysfunction in young women with polycystic ovarian syndrome (PCOS) (2) supports the view that androgen excess may harm the female heart. However, the expected increase in CHD events and mortality in women with PCOS has not been consistently observed (3,4,5), and recent studies report low levels of testosterone in women with atherosclerotic disease (6,7,8,9,10), raising the possibility that testosterone may have beneficial effects on the heart or suggesting a U-shaped association with suboptimal levels at both extremes. The limited availability of sensitive and accurate assays for female testosterone levels has impeded resolution of whether testosterone increases or decreases the risk of CHD in older women. In addition, the association may depend on the fraction of testosterone examined.
Testosterone and estradiol circulate bound to albumin, to SHBG, and in the free (unbound) state. The free and non-SHBG-bound fractions are widely believed to be the most bioactive forms, although direct evidence supporting this thesis is sparse (11). An important difference in total and bioavailable (non-SHBG bound) testosterone (BioT) is that the relative proportion of the bioavailable fraction can be metabolically regulated. Central obesity and hyperinsulinemia have strong inhibitory influences on hepatic SHBG production (12), leading to higher circulating levels of BioT and providing a link between adiposity and androgen action in women.
We report here the prospective association of testosterone with 20-yr incident CHD events in community-dwelling postmenopausal women. Both total and bioavailable testosterone levels were considered, as well as the confounding influences of estradiol, SHBG, obesity and obesity-related comorbidities.
Subjects and Methods
Study population
The Rancho Bernardo Study is a population-based study of healthy aging in Caucasian residents of a southern California community. Between 1984 and 1987, 82% (n = 2480) of surviving community-dwelling older participants attended a research clinic visit. During this visit, information regarding medical history, date of final menstrual cycle, medication use, physical activity (exercise three or more times per week, yes/no), and alcohol consumption (one or more drinks per day vs. less or none) was obtained using standard questionnaires. Current medication use was validated by examination of pills and prescriptions brought to the clinic for that purpose. The study protocol was approved by the Institutional Review Board of the University of California, San Diego; all participants gave written informed consent.
Eligibility criteria for the women in the present analysis included: 1) age 50 or older when evaluated at the 1984–87 visit; 2) availability of stored sera; 3) postmenopausal status; 4) no estrogen or insulin use at the time of the clinic visit; and 5) no history of myocardial infarction (MI) or revascularization procedure. Of the 1094 women who attended the 1984–87 clinic visit, 332 were excluded for current estrogen use; 697 of the remaining women had sufficient stored sera for measurement of sex hormones. Of these, 13 were excluded for age less than 50 yr, eight for premenopausal status, two because of insulin use, 11 because they had sex hormone levels outside the normal physiological range (13), and 39 for prior MI or revascularization. The remaining 639 postmenopausal, non-estrogen-using women are the subject of this report. Compared with those without sex hormone measurements, participants included in this analysis were slightly older but did not differ in terms of weight, BMI, lifestyle characteristics, or prevalent heart disease.
Measurements
Height, weight, and waist and hip girth were measured in the clinic with participants wearing light clothing and no shoes in 1984–87. Body mass index (BMI) (kilograms/meter2) and waist to hip ratio were used as estimates of overall and central adiposity, respectively. Systolic blood pressure was measured twice in seated resting subjects using the Hypertension Detection and Follow–Up Program protocol (14); the mean of two readings was used in analyses.
Blood samples were obtained by venipuncture between 0730 h and 1100 h after a requested 12-h fast; serum and plasma were separated and frozen at −70 C. Sex hormone levels were measured on first-thawed serum samples between 1992 and 1994 in the University of California, San Diego, reproductive endocrinology research laboratory. Total testosterone and estradiol levels were measured by RIA after solvent extraction and celite column chromatography. SHBG levels were determined by the method of Rosner (15); bioavailable (non-SHBG-bound) testosterone and estradiol were measured by an adaptation of the Tremblay and Dube ammonium-sulfate precipitation technique (16).
Sensitivity and the intra- and interassay coefficients of variation were, respectively: 20 pg/ml, 4%, and 5% for T; 20 pg/ml × percentage free, 7%, and 11% for BioT; 3 pg/ml, 6%, and 7% for estradiol; 3 pg/ml × percentage free, 6.1%, and 7.9% for bioavailable estradiol; and 5 nmol/liter, 7.5%, and 11.4% for SHBG. T levels for all participants were above the assay sensitivity. Twenty-six percent (n = 176) of women had estradiol levels below the sensitivity of the assay and were assigned values equivalent to the assay sensitivity.
Fasting plasma total cholesterol, high-density lipoprotein(HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol and triglyceride levels were measured in a Center for Disease Control Certified Lipid Research Clinic Laboratory. Total cholesterol and triglyceride levels were measured by enzymatic techniques using an ABA-200 biochromatic analyzer (Abbott Laboratories, Irving, TX). HDL was measured after precipitation of the other lipoproteins with heparin and manganese chloride. LDL was estimated using the Friedewald formula (17). Plasma glucose levels were measured by the glucose oxidase method. Homeostasis model assessment for insulin resistance was used to estimate insulin resistance according to the formula: [insulin (milliunits per liter) × glucose (millimoles per liter)]/22.5.
Outcomes assessment
Medical history and prevalent and incident CHD events were obtained using standardized questionnaires at baseline, at clinic visits approximately every 4 yr thereafter, and from periodic mailings. Follow-up continued through 2004, a 20-yr period. Prevalent CHD was defined as doctor-diagnosed MI or coronary artery revascularization. Incident CHD was defined as the first occurrence of a coronary artery revascularization, nonfatal MI, or fatal MI. CHD event information (including date of the event) was available at a minimum of one follow-up time point for all participants and at two or more time points for 74% of the population. Validation of self-reported heart attack (by chest pain, enzyme elevation, and electrocardiogram) was achieved for 72% of a subset for whom hospital records could be obtained. In one third of the cohort, all death certificates with any mention of cardiovascular disease, hypertension, or diabetes were validated by interviews with next of kin, physicians, and hospital records. A mortality classification panel determined that these data supported the nosologist’s diagnosis in 85% of deaths classified as CHD.
Diabetes was defined by physician diagnosis, fasting plasma glucose of at least 7.0 mmol/liter (126 mg/dl), 2-h post-challenge glucose of at least 11.1 mmol/liter (200 mg/dl), or use of diabetes medications (18). The metabolic syndrome was defined according to the 2002 Adult Treatment Panel III criteria (19). Hypertension was defined as blood pressure of at least 130/85 mm Hg or use of antihypertensive medication. Comorbidities recorded included thyroid, liver, kidney, and heart disease; diabetes; cancer (non-skin); emphysema; arthritis; hip fracture; and hypertension.
Statistical analysis
Sex hormone, HDL cholesterol, and triglyceride levels were not normally distributed and were log10-transformed for analyses; reported values are geometric means or medians and interquartile ranges. The association between baseline total or bioavailable testosterone levels and incident CHD was investigated using Cox proportional hazards regressions; goodness of fit was confirmed by the May and Hosmer method (20). All models presented met the proportional hazards assumption. Based on an a priori hypothesis of either low (hormone deficiency) or high (hormone excess) threshold associations, time-to-event analyses were first performed using quintiles of total T and BioT levels for the entire population, which allows examination of threshold and nonlinear associations.
Multivariate models were performed to control for confounding or effect modification by other CHD risk factors. Three separate models were evaluated: the first adjusted for age, the second added adjustment for adiposity (BMI, waist to hip ratio), and the third added lifestyle characteristics (physical activity, alcohol consumption, and current smoking). Age-adjusted generalized linear models were performed to investigate covariates of low and high levels of testosterone. Additional Cox proportional hazards regression analyses tested the sensitivity of observed associations to significant covariates. There was no significant multicollinearity (variance inflation factor >2) between the independent variables.
All P values presented are two-tailed; P ≤ 0.05 was considered statistically significant. Data were analyzed using SPSS v15 (SPSS Inc., Chicago, IL).
Results
Baseline characteristics and CHD event rates
Baseline characteristics are shown in Table 1. The mean age of these women was 73.8 yr (range, 51–90 yr); the mean BMI was 24.3 kg/m2 (range, 15.4–39.8). Only 13% reported current smoking, 46% consumed at least one alcohol drink daily, and 77% reported moderate physical exercise three or more times per week. During a median follow-up of 12.3 yr, 134 women had a first CHD event; 45 were nonfatal MIs, 10 were coronary revascularizations, and 79 were CHD deaths.
Table 1.
Baseline characteristics for 639 postmenopausal women: the Rancho Bernardo Study
| Variable | |
|---|---|
| Demographic, anthropomorphic, mean (sd) | |
| Age (yr) | 73.8 (8.2) |
| BMI (kg/m2) | 24.3 (3.8) |
| Waist to hip ratio | 0.804 (0.063) |
| Lifestyle parameters (%) | |
| Alcohol (1+ drinks/day) | 36.5 |
| Current smoker | 13.5 |
| Physical activity (3+ times/week) | 76.5 |
| Prevalent conditions (%) | |
| Diabetes | 15.6 |
| Metabolic syndrome | 16.9 |
| Hypertension | 78.9 |
| Incident CHD events (n) | |
| Fatal MI | 79 |
| Non-fatal MI | 45 |
| Revascularization | 10 |
| Sex hormonesa | |
| Total T (pg/ml) | 142 (32, 490) |
| BioT (pg/ml) | 35 (8, 143) |
| Estradiol (pg/ml) | 5.0 (2.0, 17) |
| Bioavailable estradiol (pg/ml) | 3.0 (1.0, 9.0) |
| SHBG (nmol/liter) | 60.0 (12.6, 133.5) |
SI conversion factors: multiply testosterone by 0.00347 for nmol/liter; multiply estradiol by 3.671 for pmol/liter.
Log-transformed for analysis; values represent median (95% range).
Total T and incident CHD events
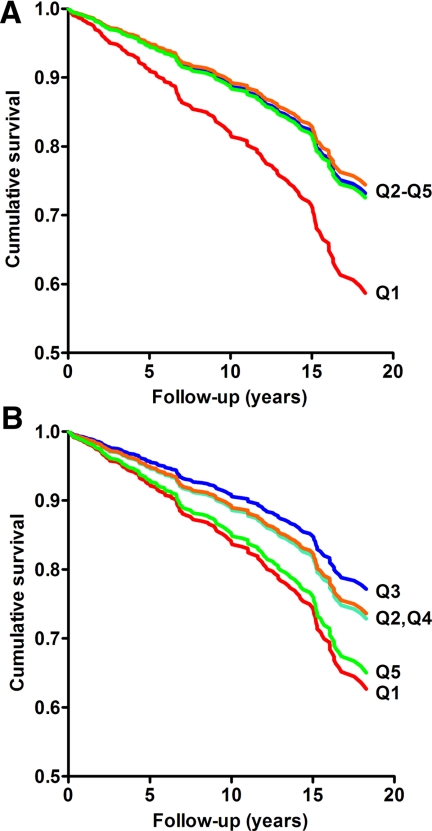
For total T, age-adjusted CHD risk estimates were similar for the four highest quintiles relative to the lowest, suggesting a low threshold (Table 2). We therefore derived risk estimates for the lowest quintile of total T (low total T) vs. all higher levels. In age-adjusted analyses (model 1), low total T was associated with a 62% (P = 0.017) increased risk of incident CHD. Adjusting for adiposity measures (model 2) and lifestyle characteristics (model 3; Fig. 1A) had a negligible effect on the results.
Table 2.
HRs for incident CHD events according to levels of total T
| No. of cases | HR (95% CI) | P value | |
|---|---|---|---|
| Total T quintile (pg/ml)a | |||
| Q1 (12–80) | 34 | 1.00 (ref) | |
| Q2 (81–121) | 23 | 0.60 (0.35–1.02) | 0.060 |
| Q3 (122–166) | 26 | 0.68 (0.41–1.13) | 0.135 |
| Q4 (167–227) | 25 | 0.58 (0.35–0.97) | 0.038 |
| Q5 (228–754) | 26 | 0.63 (0.37–1.05) | 0.075 |
| Quintile 1 vs. higher | |||
| Model 1a | 1.62 (1.10–2.39) | 0.017 | |
| Model 2b | 1.66 (1.12–2.46) | 0.013 | |
| Model 3c | 1.72 (1.15–2.57) | 0.008 |
Ref, Reference category.
Model 1, adjusted for age.
Model 2, adjusted for age, BMI, and waist-hip ratio.
Model 3, model 2 + alcohol use, current smoking, and exercise.
Figure 1.
Survival curves for incident CHD according to quintiles of total T (A) and BioT (B), adjusted for age, BMI, waist-to-hip ratio, current smoking, alcohol intake, and physical activity. The relative risk for the lowest quintile of total T vs. the higher quintiles was 1.72 (95% CI, 1.15–2.57; P = 0.008). The relative risks for the lowest and highest quintiles of BioT vs. quintile 3 were 1.93 (95% CI, 1.09–3.43; P = 0.025) and 1.84 (95% CI, 1.06–3.19; P = 0.032), respectively. Note that y-axes do not begin at zero.
BioT and incident CHD events
For BioT, age-adjusted quintile analyses suggested a U-shaped association with CHD risk (P for quadratic trend <0.001). Accordingly, the association of BioT with CHD risk was tested using the middle (third) quintile as the reference (Table 3). Both the lowest (low BioT) and the highest (high BioT) quintiles were associated with significantly increased age-adjusted risk of incident CHD, with a 79% (P = 0.046) increased risk for women with low BioT and a 96% (P = 0.022) increased risk for women with high BioT (model 1). This U-shaped association persisted after additional adjustment for adiposity (model 2) and lifestyle characteristics (model 3; Fig. 1B).
Table 3.
HRs of BioT for incident CHD events
| Hormone quintile | No. of cases | Model 1 HR (95% CI)a | Model 2 HR (95% CI)b | Model 3 HR (95% CI)c |
|---|---|---|---|---|
| BioT (pg/ml) | ||||
| Q1 (2-19) | 29 | 1.79 (1.01–3.16) | 1.92 (1.08–3.41) | 1.93 (1.09–3.43) |
| Q2 (20-29) | 25 | 1.31 (0.73–2.35) | 1.29 (0.72–2.33) | 1.29 (0.71–2.32) |
| Q3 (30-41) | 20 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q4 (42-62) | 25 | 1.39 (0.77–2.50) | 1.37 (0.76–2.47) | 1.34 (0.74–2.42) |
| Q5 (63-278) | 34 | 1.96 (1.13–3.16) | 1.88 (1.08–3.26) | 1.84 (1.06–3.19) |
Ref, Reference category.
Model 1, adjusted for age.
Model 2, adjusted for age, BMI, and waist-hip ratio.
Model 3, model 2 + alcohol use, current smoking, and exercise.
Independence of total T and BioT associations with CHD
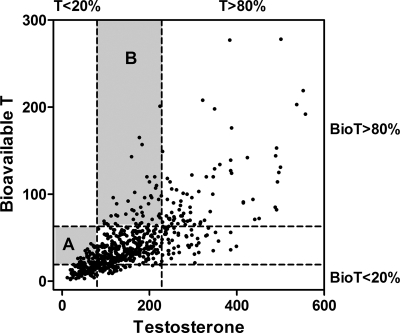
As shown in Fig. 2, the distribution of total T and BioT by quintiles was discordant; one third of women with low (≤20th percentile) total T did not have low BioT, and 36% of those with high (≥80th percentile) BioT did not have high total T (χ2, P < 0.001 for both). To examine the independence of BioT associations, total T levels were added as a continuous variable to the age, adiposity, and lifestyle-adjusted model of BioT quintiles. After adjusting for total T, the strength of the association for high BioT was increased from 1.84 to 2.59 [95% confidence interval (CI), 1.43–4.71]; however, low BioT was no longer significantly related to incident CHD events [hazard ratio (HR) = 1.09; 95% CI, 0.54–2.21]. In the converse analysis, adjustment for continuous BioT levels increased the strength of the low total T association from 1.72 to 2.03 (95% CI, 1.22–3.38), and BioT was not independently related to CHD events.
Figure 2.
Scatterplot demonstrating the discordance of total T and BioT levels. The dashed lines indicate cut points for the lowest (≤20%) and highest (≥80%) quintiles. Shaded area A encompasses data for the 33% of the women with low total T who do not have low BioT. Shaded area B encompasses data for the 36% of women with high BioT who do not have high total T.
In this population, BioT was negatively correlated with SHBG levels (Spearman R = −0.34; P < 0.001). Because SHBG has been shown to have independent associations with CHD risk (8,21,22,23), we tested whether SHBG accounted for BioT-CHD associations. Additional adjustment for SHBG in the age, adiposity, and lifestyle-adjusted model had minimal effect for low BioT (HR = 1.96; 95% CI, 1.10–3.48) and decreased the HR for the highest quintile of BioT from 1.96 to 1.66 (95% CI, 0.94–2.93) (P = 0.08). In this model, SHBG was not significantly related to incident CHD (HR = 0.88; 95% CI, 0.74–1.04 for a 1 sd increase in log SHBG; P = 0.14) independent of BioT. Sequential adjustment for total and bioavailable estradiol levels did not alter low total T or high BioT associations (data not shown). Finally, although age and BMI-adjusted SHBG levels were higher in the 14% of women reporting use of thyroid medications at baseline compared with nonusers (means, 61 vs. 52 nmol/liter; P = 0.02), adjustment for thyroid medication use did not influence BioT associations (data not shown).
In sum, these results suggest that low total T and high BioT are independently and specifically related to CHD risk; that is, the low BioT association seems to be a reflection of the covariance of BioT with total T levels, whereas the high BioT association is specific to the non-SHBG-bound testosterone fraction.
Covariates of low total T and high BioT
Next, we examined covariates of low total T and high BioT among demographic and lifestyle characteristics, health status markers, CVD risk factors, and comorbidities (Table 4). Women with low total T were younger and had lower BMI, lower total and HDL cholesterol, lower diastolic blood pressure, and higher triglycerides than women with higher T. They also were more likely to have had a hysterectomy or bilateral oophorectomy and less likely to be current cigarette smokers. In contrast, women with high BioT had higher BMI, diastolic and systolic blood pressure, total cholesterol, triglycerides, and fasting plasma glucose compared with women with lower BioT. They also had more comorbid conditions including a higher prevalence of hypertension, diabetes, and the metabolic syndrome and were less likely to have undergone hysterectomy or oophorectomy.
Table 4.
Characteristics of women with low total T or high BioT
| Variable | Total T >80 pg/ml | Low total T ≤80 pg/ml | P value | BioT <63 pg/ml | High BioT ≥63 pg/ml | P value |
|---|---|---|---|---|---|---|
| n | 510 | 129 | 514 | 125 | ||
| Demographic, anthropomorphic | ||||||
| Age (yr) | 74.2 | 72.0 | 0.01 | 73.7 | 73.8 | 0.84 |
| BMI (kg/m2) | 24.4 | 23.7 | 0.10 | 24.1 | 25.1 | 0.01 |
| Waist to hip ratio | 0.804 | 0.803 | 0.98 | 0.802 | 0.808 | 0.33 |
| Metabolic parameters | ||||||
| Systolic blood pressure (mm Hg) | 144.1 | 140.6 | 0.11 | 142.3 | 147.7 | 0.01 |
| Diastolic blood pressure (mm Hg) | 75.9 | 74.1 | 0.06 | 74.7 | 78.7 | <0.001 |
| Total cholesterol (mg/dl) | 231.1 | 224.7 | 0.10 | 227.8 | 238.6 | 0.01 |
| LDL cholesterol (mg/dl) | 141.9 | 138.0 | 0.28 | 140.0 | 145.4 | 0.15 |
| HDL cholesterol (mg/dl)a | 65.7 | 59.9 | 0.001 | 64.2 | 65.4 | 0.58 |
| Triglycerides (mg/dl)a | 98.3 | 109.8 | 0.03 | 99.2 | 107.2 | 0.08 |
| Fasting plasma glucose (mg/dl) | 100.3 | 99.0 | 0.58 | 99.3 | 103.4 | 0.08 |
| HOMA-IR (n = 382) | 2.58 | 2.62 | 0.87 | 2.61 | 2.51 | 0.63 |
| Health status parameters | ||||||
| Heart rate (BPM) | 64.9 | 65.4 | 0.72 | 65.0 | 64.8 | 0.79 |
| No. of comorbiditiesb | 1.93 | 1.86 | 0.58 | 1.83 | 2.22 | 0.01 |
| No. of doctor visits in past year | 5.11 | 6.37 | 0.28 | 5.23 | 5.14 | 0.73 |
| Hospitalized in past year (%) | 13.0 | 11.2 | 0.61 | 12.3 | 14.0 | 0.60 |
| Lifestyle parameters (%) | ||||||
| Alcohol (1+ drinks/day) | 37.3 | 33.3 | 0.41 | 37.0 | 34.4 | 0.59 |
| Current smoker | 14.9 | 7.8 | 0.03 | 13.0 | 15.2 | 0.53 |
| Physical activity (3+ times/week) | 75.7 | 79.8 | 0.32 | 76.8 | 75.2 | 0.70 |
| Prevalent conditions (%) | ||||||
| Hypertension | 79.4 | 76.7 | 0.51 | 76.7 | 88.0 | 0.01 |
| Diabetes | 16.7 | 11.6 | 0.16 | 13.8 | 23.2 | 0.01 |
| Metabolic syndrome | 16.7 | 17.8 | 0.75 | 14.6 | 26.4 | 0.01 |
| Ovarian status (%) | <0.001 | <0.001 | ||||
| Intact | 65.1 | 34.9 | 54.9 | 76.0 | ||
| Hysterectomy | 22.2 | 31.0 | 25.3 | 18.4 | ||
| Oophorectomy | 12.7 | 34.1 | 19.8 | 5.6 |
HOMA-IR, Homeostasis model assessment for insulin resistance; BPM, beats per minute.
Log10 transformed for analyses; values are geometric means.
Includes liver, kidney, and heart disease; diabetes; cancer (non-skin); emphysema; arthritis; hip fracture; and hypertension.
Potential confounding or effect modification by factors that distinguished women with low total T was examined in multivariate models adjusted for age, adiposity, and lifestyle. For total T, additional adjustment for total and HDL cholesterol, diastolic blood pressure, and triglycerides did not modify the low T association (HR = 1.68; 95% CI, 1.11–2.53). Risk estimates for low T were also similar after adjusting for ovarian status (HR = 1.61; 95% CI, 1.06–2.44) and after excluding the 109 women with bilateral oophorectomy (HR = 1.71; 95% CI, 1.06–2.75).
For BioT, additional adjustment for diastolic blood pressure, total cholesterol, triglycerides, and fasting plasma glucose had minimal influence on the high BioT association (HR = 1.89; 95% CI, 1.08–3.31). Risk estimates were also similar after excluding women with diabetes (n = 100; 25 were incident cases) (HR = 2.02; 95% CI, 1.07–3.81), but were attenuated by excluding those with the metabolic syndrome (n = 108; 34 were incident cases) (HR = 1.79; 95% CI, 0.97–3.32), or by adjusting for the total number of comorbid conditions (HR = 1.66; 95% CI, 0.94–2.95). Adjusting for ovarian status (HR = 2.04; 95% CI, 1.16–3.59) or excluding women with bilateral oophorectomy (HR = 1.93; 95% CI, 1.03–3.62) did not influence the high BioT association.
Finally, we tested the effect of comorbidity and occult disease by excluding women whose CHD event occurred in the first 5 yr of follow-up (n = 44). Results persisted for high BioT (HR = 2.04; 95% CI, 1.04–4.02), but were no longer significant for low total T (HR = 1.44; 95% CI, 0.85–2.43).
Discussion
To our knowledge, this is the first population-based study to show that low levels of testosterone overall and high levels of the bioavailable fraction of testosterone are independently related to higher risk of incident CHD events over a 20-yr follow-up in community-dwelling postmenopausal women. Women in the lowest 20% of total T for this population (≤80 pg/ml) had 62% elevated risk of a first-ever CHD event, whereas women in the highest 20% of BioT (≥63 pg/ml) had 96% elevated risk, compared with their counterparts without extreme testosterone levels. These results were not explained by age, adiposity, lifestyle, or ovarian status.
In this and other studies, higher levels of endogenous BioT in postmenopausal women are associated with adiposity and adiposity-related conditions including higher BMI, larger waist girth, insulin resistance (24,25), adverse lipid profiles (26,27), type 2 diabetes (25,28), and the metabolic syndrome (29,30). [It should be noted that although several of these prior studies report calculated free testosterone (based on mass action equations), and not BioT, levels of calculated free testosterone T and calculated BioT are mathematically equivalent when a constant albumin concentration is assumed and are essentially equivalent even when albumin is measured.] In almost all cases, total T was not related to these same CHD risk factors. For example, six of seven cross-sectional studies reviewed by Ding et al. (28) reported significant associations of BioT, free testosterone, or the free androgen index (T/SHBG) with diabetes in postmenopausal women; only one found a significant association for total T. Thus, higher circulating concentrations of the bioavailable (non-SHBG-bound) fraction of testosterone seem to be related to metabolic dysfunction and obesity-related conditions associated with CHD risk in older women, regardless of overall testosterone levels. Higher BioT predicted increased risk of CHD events in Rancho Bernardo women independent of SHBG levels and other CHD risk factors characteristic of women with elevated BioT. Although adjusting for the constellation of CHD risk factors represented by the metabolic syndrome, or for the number of comorbid conditions, eliminated the statistical significance of the high BioT association, risk estimates remained elevated. It is not clear whether high BioT is a marker or a mediator of CHD risk; our results suggest that both could be true, although residual confounding cannot be ruled out.
Rancho Bernardo women with low levels of total T also had increased risk of CHD events, irrespective of BioT concentrations. These women tended to be younger and thinner and to have lower blood pressure and total cholesterol than women with normal testosterone, all favorable CHD risk factors. However, they had higher triglycerides and lower HDL cholesterol (characteristic of atherogenic dyslipidemia), and eliminating events that occurred in the first 5 yr of follow-up attenuated the low total T–CHD association. Thus, low testosterone could be a marker of occult systemic illness or an indication of subclinical heart disease. Several clinic and population-based studies have examined the cross-sectional association of endogenous testosterone with unrecognized CHD in older women. Five investigations (7,8,9,31,32), including a case-control study of 364 postmenopausal women from ARIC (Atherosclerosis Risk in Communities Study) (8) and a study of almost 2000 postmenopausal women from MESA (Multi-Ethnic Study of Atherosclerosis) (31), found more carotid atherosclerosis (by intima-media thickness) in women with lower testosterone levels. In contrast, higher testosterone was associated with more extensive coronary artery calcium in the same MESA women (31), and with greater aortic atherosclerosis (by calcified deposits) in 528 postmenopausal women from the Rotterdam Study (33). In general, these results support the concept that testosterone deficiency may be characteristic of subclinical CHD in women, as has been observed repeatedly in men (reviewed in Ref. 34). Whether the underlying biology differs for arterial calcification in women requires further study.
To our knowledge, only one other population-based study has reported the prospective association of endogenous sex hormones with incident cardiovascular disease in postmenopausal women. A nested case-control study among women in the Women’s Health Study showed a trend for increased risk of cardiovascular events in the non-estrogen-using women (n = 115 1:1 matched pairs) across quartiles of the free androgen index (T/SHBG); the association was not independent of BMI and other cardiovascular risk factors (35). Neither low nor high total T was independently related to future cardiovascular events in the Women’s Health Study; however, levels were measured by a direct RIA considered to be suboptimal at the low ranges of testosterone found in postmenopausal women (36).
Androgens may influence CHD through direct effects on vascular processes. Androgen receptor expression in the vasculature seems to be predominantly determined by endogenous androgen levels, rather than genetic factors (37), and higher endogenous levels of testosterone are favorably associated with endothelial function in postmenopausal women (38). Other vascular mechanisms are also possible. Experimental studies show that androgens suppress proinflammatory cytokine activity, inhibit apoptosis, and enhance vascular smooth muscle cell proliferation, important factors in inhibiting atheroma formation and maintaining plaque integrity (reviewed by Malkin et al. in Ref. 39). Malkin and colleagues have proposed that androgen deficiency in men may impact coronary artery disease through these mechanisms; the same may be true for women with low total T.
The present study has limitations. Results were based on a largely Caucasian middle- to upper-middle-class community and may not apply to other ethnic and socioeconomic groups. Like most other epidemiological studies, hormone levels were based on a single assay. However, single measurements of androgens in postmenopausal women have been shown to reliably characterize average levels over a 2- to 3-yr period (40), and misclassification of women with low or high testosterone would be expected to underestimate, not cause, associations. In addition, blood for hormone measurements was obtained in the morning from fasting women (minimizing any diurnal variation), the laboratory methods used were the gold standard at the time the assays were performed, and testosterone levels were within the assay sensitivity for all participants. Assessment of CHD events was based on self-report, thus endpoint misclassification is also possible. However, 72% of nonfatal events and 85% of fatal events were confirmed by medical record review in subsets with available information, and any misclassification of CHD events would be likely to diminish associations, not cause them. Although it is possible that testosterone exerts its influence by conversion to estradiol, the addition of total or bioavailable estradiol to these analyses did not materially change results.
In summary, this study suggests that an optimal range of testosterone may exist for cardiovascular health in women, with increased risk of CHD events at low levels of testosterone overall and at high levels of the bioavailable fraction of testosterone. In part, the elevated risk associated with low total T may be related to preexisting subclinical heart disease, whereas high BioT may be a marker of underlying metabolic disease. However, direct effects of testosterone on CHD risk factors and vascular health cannot be ruled out and may even be likely. Given the current gap in knowledge, a cautious approach to the use of testosterone-modulating therapies in older women seems prudent.
Footnotes
G.A.L. was supported by American Heart Association Award 0930073N. The Rancho Bernardo Study was funded by research Grants AG028507 and AG018339 from the National Institute on Aging and Grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 24, 2009
Abbreviations: BioT, Bioavailable T; BMI, body mass index; CHD, coronary heart disease; CI, confidence interval; HDL, high-density lipoprotein; HR, hazard ratio; LDL, low-density lipoprotein; MI, myocardial infarction; PCOS, polycystic ovarian syndrome; T, total testosterone.
References
- Liu Y, Ding J, Bush TL, Longenecker JC, Nieto FJ, Golden SH, Szklo M 2001 Relative androgen excess and increased cardiovascular risk after menopause: a hypothesized relation. Am J Epidemiol 154:489–494 [DOI] [PubMed] [Google Scholar]
- Cussons AJ, Stuckey BG, Watts GF 2006 Cardiovascular disease in the polycystic ovary syndrome: new insights and perspectives. Atherosclerosis 185:227–239 [DOI] [PubMed] [Google Scholar]
- Wild S, Pierpoint T, McKeigue P, Jacobs H 2000 Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a retrospective cohort study. Clin Endocrinol (Oxf) 52:595–600 [DOI] [PubMed] [Google Scholar]
- Pierpoint T, McKeigue PM, Isaacs AJ, Wild SH, Jacobs HS 1998 Mortality of women with polycystic ovary syndrome at long-term follow-up. J Clin Epidemiol 51:581–586 [DOI] [PubMed] [Google Scholar]
- Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ 2008 Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: results from the National Institutes of Health–National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab 93:1276–1284 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Khatibi A, Agardh CD, Shakir YA, Nerbrand C, Nyberg P, Lidfeldt J, Samsioe G 2007 Could androgens protect middle-aged women from cardiovascular events? A population-based study of Swedish women: The Women’s Health in the Lund Area (WHILA) Study. Climacteric 10:386–392 [DOI] [PubMed] [Google Scholar]
- Bernini GP, Sgro' M, Moretti A, Argenio GF, Barlascini CO, Cristofani R, Salvetti A 1999 Endogenous androgens and carotid intimal-medial thickness in women. J Clin Endocrinol Metab 84:2008–2012 [DOI] [PubMed] [Google Scholar]
- Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M 2002 Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol 155:437–445 [DOI] [PubMed] [Google Scholar]
- Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F, Pujia A 2007 Role of endogenous androgens on carotid atherosclerosis in non-obese postmenopausal women. Nutr Metab Cardiovasc Dis 17:705–711 [DOI] [PubMed] [Google Scholar]
- Kaczmarek A, Reczuch K, Majda J, Banasiak W, Ponikowski P 2003 The association of lower testosterone level with coronary artery disease in postmenopausal women. Int J Cardiol 87:53–57 [DOI] [PubMed] [Google Scholar]
- Khosla S 2006 Editorial: sex hormone binding globulin: inhibitor or facilitator (or both) of sex steroid action? J Clin Endocrinol Metab 91:4764–4766 [DOI] [PubMed] [Google Scholar]
- Crave JC, Lejeune H, Brébant C, Baret C, Pugeat M 1995 Differential effects of insulin and insulin-like growth factor I on the production of plasma steroid-binding globulins by human hepatoblastoma-derived (Hep G2) cells. J Clin Endocrinol Metab 80:1283–1289 [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, May S 2007 Sex-specific determinants of serum adiponectin in older adults: the role of endogenous sex hormones. Int J Obes (Lond) 31:457–465 [DOI] [PubMed] [Google Scholar]
- 1976 The hypertension detection and follow-up program: hypertension detection and follow-up program cooperative group. Prev Med 5:207–215 [DOI] [PubMed] [Google Scholar]
- Rosner W 1972 A simplified method for the quantitative determination of testosterone-estradiol-binding globulin activity in human plasma. J Clin Endocrinol Metab 34:983–988 [DOI] [PubMed] [Google Scholar]
- Tremblay RR, Dube JY 1974 Plasma concentrations of free and non-TeBG bound testosterone in women on oral contraceptives. Contraception 10:599–605 [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS 1972 Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- 1999 Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO Consultation. Part 1: Diagnosis and classification of diabetes mellitus. Geneva: World Health Organization [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2002 Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421 [PubMed] [Google Scholar]
- May S, Hosmer DW 1998 A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal 4:109–120 [DOI] [PubMed] [Google Scholar]
- Reinecke H, Bogdanski J, Woltering A, Breithardt G, Assmann G, Kerber S, von Eckardstein A 2002 Relation of serum levels of sex hormone binding globulin to coronary heart disease in postmenopausal women. Am J Cardiol 90:364–368 [DOI] [PubMed] [Google Scholar]
- Weinberg ME, Manson JE, Buring JE, Cook NR, Seely EW, Ridker PM, Rexrode KM 2006 Low sex hormone-binding globulin is associated with the metabolic syndrome in postmenopausal women. Metabolism 55:1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RJ, Davison SL, Papalia MA, McKenzie DP, Davis SR 2007 Endogenous androgen levels and cardiovascular risk profile in women across the adult life span. Menopause 14:630–638 [DOI] [PubMed] [Google Scholar]
- Kalish GM, Barrett-Connor E, Laughlin GA, Gulanski BI 2003 Association of endogenous sex hormones and insulin resistance among postmenopausal women: results from the Postmenopausal Estrogen/Progestin Intervention Trial. J Clin Endocrinol Metab 88:1646–1652 [DOI] [PubMed] [Google Scholar]
- Oh JY, Barrett-Connor E, Wedick NM, Wingard DL 2002 Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care 25:55–60 [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, Pasternak RC, Lloyd-Jones D, Sowers MF, Torréns JI 2005 Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation 111:1242–1249 [DOI] [PubMed] [Google Scholar]
- Mudali S, Dobs AS, Ding J, Cauley JA, Szklo M, Golden SH 2005 Endogenous postmenopausal hormones and serum lipids: the atherosclerosis risk in communities study. J Clin Endocrinol Metab 90:1202–1209 [DOI] [PubMed] [Google Scholar]
- Ding EL, Song Y, Malik VS, Liu S 2006 Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295:1288–1299 [DOI] [PubMed] [Google Scholar]
- Golden SH, Ding J, Szklo M, Schmidt MI, Duncan BB, Dobs A 2004 Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: the atherosclerosis risk in communities study. Am J Epidemiol 160:540–548 [DOI] [PubMed] [Google Scholar]
- Maggio M, Lauretani F, Ceda GP, Bandinelli S, Basaria S, Paolisso G, Ble A, Egan JM, Metter EJ, Abbatecola AM, Zuliani G, Ruggiero C, Valenti G, Guralnik JM, Ferrucci L 2007 Association of hormonal dysregulation with metabolic syndrome in older women: data from the InCHIANTI study. Am J Physiol Endocrinol Metab 292:E353–E358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang P, Vaidya D, Dobs A, Golden SH, Szklo M, Heckbert SR, Kopp P, Gapstur SM 2009 Sex hormone levels and subclinical atherosclerosis in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 204:255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debing E, Peeters E, Duquet W, Poppe K, Velkeniers B, Van den Brande P 2007 Endogenous sex hormone levels in postmenopausal women undergoing carotid artery endarterectomy. Eur J Endocrinol 156:687–693 [DOI] [PubMed] [Google Scholar]
- Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA 2002 Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 87:3632–3639 [DOI] [PubMed] [Google Scholar]
- Nettleship JE, Jones RD, Channer KS, Jones TH 2009 Testosterone and coronary artery disease. Front Horm Res 37:91–107 [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, Buring JE 2003 Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation 108:1688–1693 [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, Cho MM, Endres DB, Morrison JL, Patel S, Paulson RJ 2003 Limitations of direct estradiol and testosterone immunoassay kits. Steroids 68:1173–1178 [DOI] [PubMed] [Google Scholar]
- Ng MK, Liu PY, Williams AJ, Nakhla S, Ly LP, Handelsman DJ, Celermajer DS 2002 Prospective study of effect of androgens on serum inflammatory markers in men. Arterioscler Thromb Vasc Biol 22:1136–1141 [DOI] [PubMed] [Google Scholar]
- Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F, Pujia A 2007 Endogenous testosterone and endothelial function in postmenopausal women. Coron Artery Dis 18:9–13 [DOI] [PubMed] [Google Scholar]
- Malkin CJ, Pugh PJ, Jones RD, Jones TH, Channer KS 2003 Testosterone as a protective factor against atherosclerosis—immunomodulation and influence upon plaque development and stability. J Endocrinol 178:373–380 [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE 1995 Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer Epidemiol Biomarkers Prev 4:649–654 [PubMed] [Google Scholar]