Abstract
Context: Thiazolidinedione (TZD) use has recently been associated with an increased risk of fractures.
Objective: The aim of this study was to determine the time-dependent relationship between TZD use and fracture risk.
Design: We conducted a retrospective cohort study in a large health system in southeast Michigan.
Patients: Patients who received care from the health system were included if they were at least 18 yr of age, had a diagnosis of diabetes, and had at least one prescription for an oral diabetes medication. These criteria identified 19,070 individuals (9,620 women and 9,450 men).
Intervention: This study compared patients treated with TZDs to patients without TZD treatment. Cox proportional hazard models were used to assess the relationship between exposure and outcomes.
Main Outcome Measures: The primary outcome was the time to fracture. Secondary analyses examined the risk of fractures in subgroups defined by sex and age.
Results: TZD use was associated with an increased risk of fracture in the cohort overall [adjusted hazard ratio (aHR), 1.35; 95% confidence interval (CI), 1.05–1.71] and in women (aHR, 1.57; 95% CI, 1.16–2.14), but not in men (aHR, 1.05; 95% CI, 0.70–1.58). Women more than 65 yr of age appeared to be at greatest risk for fracture (aHR, 1.72; 95% CI, 1.17–2.52). Among women, the increased fracture risk was not apparent until after 1 yr of TZD treatment.
Conclusions: TZD use was associated with an increased risk for fractures in women, particularly at ages above 65 yr. Clinicians should be aware of this association when considering TZD therapy so as to appropriately manage and counsel their patients.
Thiazolidinedione use is associated with an increased risk for fractures, particularly in older women.
Osteoporosis-related fractures represent a significant health and economic burden in the United States. In the year 2005, the incidence of fractures in the United States was predicted at 2 million, with an estimated cost of $17 billion, and these numbers are projected to increase by 50% by the year 2025 (1). In recent years, numerous medications have been implicated in causing bone loss and increasing fracture risk (2), such as antiepileptic drugs (3,4), antidepressants (5), proton pump inhibitors (6), and recently, thiazolidinediones (TZDs) (7,8,9,10,11,12,13).
TZDs are mainly used to treat insulin resistance in patients with type 2 diabetes mellitus (DM), and they have accounted for up to 21% of prescribed oral antidiabetic agents in the United States (14). The potential increased fracture risk associated with TZDs further compounds the complex relationship between type 2 DM and fractures. Although initially thought to be protective due to the associated high body mass index, type 2 DM is now known to be independently associated with rapid bone loss and an increased risk of fragility fractures (15,16,17,18). Furthermore, insulin use was found to be an independent predictor of fracture risk in diabetic individuals (19). Patients with DM may also be predisposed to develop fractures due to microvascular and macrovascular sequelae of DM (20). For example, diabetic neuropathy and retinopathy as well as cerebrovascular accidents lead to an increased propensity to fall (19,21,22); diabetic neuropathy may result in regional osteopenia (23); and chronic or end-stage kidney disease due to diabetic nephropathy is associated with osteodystrophy and bone loss (24).
Most of the published literature on TZDs and fractures in humans consists of adverse event reporting from large randomized controlled trials (8,10,12), small observational studies (7,13), a case-control study (9), and a meta-analysis (11). To date, there has been only one randomized controlled trial to assess the effect of rosiglitazone on bone turnover (25). This trial, however, looked at markers of bone turnover, not fractures. There was also a recent longitudinal, observation study of TZD use on fracture risk, but this was restricted to older adults and estimates presented in comparison to other oral hypoglycemic medications (26).
Given the prevalent use of TZDs in patients with diabetes and that this patient population is already at risk for fractures, it is important to assess the effect of these drugs on fracture risk. Therefore, we examined the longitudinal relationship between TZD use and fracture events in a large, population-based cohort of patients followed for type 2 DM. We also attempted to identify population subgroups most susceptible to this potential adverse drug event.
Patients and Methods
Study design, setting, and population
This study was approved by the Institutional Review Board at Henry Ford Health System. The study was also in compliance with the health system’s Health Insurance Portability and Accountability Act policy. This study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology recommendations (27). Patients were members of a large health maintenance organization (HMO) in southeast Michigan and received their care from a large multispecialty medical group. All patients had prescription coverage, with tiered copayments based on the covering entity’s formulary. Inclusion criteria for the cohort were as follows: age of at least 18 yr; at least one clinical encounter with a coded diagnosis of diabetes [international classification of diseases (ICD)-9 code 250.xx] between January 1, 2000, and May 31, 2007; and at least one prescription for an oral diabetes medication during this same time period. For patients meeting these criteria, the index date was the first date during the observation period that the patient had a clinical encounter coded as diabetes or the filling of an oral diabetes medication. We required patients to have at least 12 months of continuous enrollment in the HMO before the index date to establish baseline characteristics. Patients were observed until the date of the first fracture event, disenrollment from the HMO, or the last date of observation (i.e. May 31, 2007), whichever came first.
Demographic, clinical, and exposure variables
Demographic data (e.g. age, sex, and race-ethnicity), laboratory results, encounter diagnoses, and pharmacy claims were all maintained electronically by the health system. Race-ethnicity was usually patient self-identified, but on occasion could have been assigned by health care personnel. Household income was estimated using software that mapped the patient’s address to a census block (Pitney Bowes MapInfo, Troy, NY). Individuals’ household income was taken as the median household income for their census block using year 2000 data from the U.S. Census Bureau. We abstracted laboratory data for percentage glycated hemoglobin (HbA1c). Baseline clinical status was based on diagnoses and procedures extending 1 yr before the index date for the following conditions: coronary heart disease (CHD), congestive heart failure (CHF), cerebrovascular accident (CVA) and transient ischemic attack (TIA), peripheral vascular occlusive disease (PVOD), chronic kidney disease (CKD), and end-staged renal disease (ESRD). We also used diagnosis and procedural codes to calculate an adaptation of the Charlson comorbidity index as an estimate of baseline comorbidity (28).
Prescription fills for TZDs, insulin, oral steroids, and lipid-lowering agents were identified through pharmacy claims. Any patient with one or more prescription fills for a TZD was considered to be exposed from the time of their first fill.
Outcome assessment
Outcomes were assessed without knowledge of the individuals’ medication exposure. We identified fractures from electronically maintained medical claims data in a manner similar to that which has been reported elsewhere (29). These data included diagnosis and procedural codes for events that occurred both within and outside the health system. Each individual event had to be identified by the presence of at least two fracture-specific ICD-9 codes within a 3-month period, along with an appropriate procedural code, to be included in the analysis. Codes representing fractures with trauma (ICD-9 E codes) were excluded.
Statistical analysis
The primary study outcome was the time to first fracture at any location. Student’s t test and the χ2 test were used to compare characteristics between study individuals treated with a TZD and persons not so treated. To examine the relationship between TZDs and fracture risk, we calculated unadjusted event rates per patient-years of follow-up for the TZD-treated and untreated individuals in the entire cohort, among females only, and among males only. We performed a time-to-event analysis starting from the index date; however, individuals treated with TZDs were considered exposed from the time of their first prescription fill. This affected the calculation of rates with follow-up time preceding the TZD use counting toward the untreated group. Similarly, TZD exposure was entered into the regression models as a time-updated covariate, such that exposure in the TZD-treated group began after the first TZD fill. Patients were followed until one of the following events occurred: a fracture at any location, disenrollment from the HMO, or the last date of observation. The unadjusted hazard ratio (HR) was shown with a Kaplan-Meier curve of the proportion of patients without fractures over time comparing TZD-treated patients with those without TZD treatment. To account for other potential confounders, we used Cox proportional hazards models to calculate adjusted HRs (aHRs) for TZD exposure, adjusting for age, sex, race-ethnicity, marital status, median household income, HbA1c levels, insulin use, oral corticosteroid use, lipid-lowering agent use, baseline clinical diagnoses (i.e. CHD, CHF, CVA/TIA, PVOD, CKD, and ESRD), and the Charlson comorbidity index. Secondary analyses included the same covariates predicting the time to first fracture, but stratified by sex, age (≤65 yr and >65 yr), and both. As a post hoc analysis, we assessed the adjusted relationship between TZD exposure and the time to fractures at particular locations (i.e. the combination of humeral and distal upper extremity and distal lower extremity fractures and the combination of femur and vertebral fractures) in all women and in women more than 65 yr of age. All analyses were performed using SAS v9.1 (SAS Institute Inc., Cary, NC) (30). A P value <0.05 was considered statistically significant.
Role of the funding source
The funding source had no role in the design of the study, the collection of data, analysis, review of the manuscript for critical content, or the decision to publish.
Results
We identified 19,070 patients with diabetes who met our study criteria between January 1, 2000, and May 31, 2007. Of the 19,070 patients, 4,511 had at least one fill for a TZD during the study period. Baseline comparisons between individuals treated with a TZD and individuals who did not receive TZD treatment are shown in Table 1. Significant differences between the two groups were observed in age, marital status, race-ethnicity, insulin use, oral corticosteroid use, HbA1c, and baseline clinical diagnoses of CHD, CHF, and CVA. Without accounting for the period of follow-up, the number of fractures appeared to be higher in patients who did not use TZDs when compared with those who did. During the observation period, 477 patients had at least one fracture (95 patients in the TZD-treated group and 382 patients not treated with a TZD).
Table 1.
Characteristics of study patients with type 2 DM stratified by TZD use
| Characteristic | All patients | Patients who used a TZD | Patients who did not use a TZD | P valuea |
|---|---|---|---|---|
| n | 19,070 | 4,511 | 14,559 | |
| Age (yr) | 58.3 ± 13.0 | 57.4 ± 12.2 | 58.6 ± 13.2 | <0.001 |
| Females | 9,620 (50.4) | 2,222 (49.3) | 7,398 (50.8) | 0.068 |
| Race | <0.001 | |||
| African-American | 7,965 (41.8) | 1,946 (43.1) | 6,019 (41.3) | |
| White | 9,887 (51.8) | 2,290 (50.8) | 7,597 (52.2) | |
| Other/unknown | 1,218 (6.4) | 275 (6.1) | 943 (6.5) | |
| Married (398 with missing marital status) | 12,068 (64.6) | 2,956 (66.7) | 9,112 (64.0) | 0.001 |
| Household income (374 with missing income) | $48,258 ± 21,858 | $48,454 ± 21,731 | $48,197 ± 21,898 | 0.495 |
| Charlson comorbidity score | 1.6 ± 1.4 | 1.6 ± 1.4 | 1.6 ± 1.4 | 0.261 |
| TZD use (≥1 fill during observation period)b | ||||
| Rosiglitazone alone | 999 (5.2) | 999 (22.1) | ||
| Pioglitazone alone | 3,170 (16.6) | 3,170 (70.3) | ||
| Both rosiglitazone and pioglitazone | 342 (1.8) | 342 (7.6) | ||
| Oral corticosteroid medication use (≥1 fill during observation period)b | 5,501 (28.9) | 1,479 (32.8) | 4,022 (27.6) | <0.001 |
| Baseline insulin use (≥1 fill during baseline period)c | 2,042 (10.7) | 682 (15.1) | 1,360 (9.3) | <0.001 |
| Baseline lipid-lowering medication use (≥1 fill during baseline period)c | 5,775 (30.3) | 1,417 (31.4) | 4,358 (29.9) | 0.059 |
| Baseline clinical diagnosesc | ||||
| CHDd | 1,383 (7.3) | 363 (8.1) | 1,020 (7.0) | 0.019 |
| CHF | 1,698 (8.9) | 356 (7.9) | 1,342 (9.2) | 0.006 |
| CVAs/TIAs | 1,391 (7.3) | 295 (6.5) | 1,096 (7.5) | 0.026 |
| PVOD | 1,000 (5.2) | 236 (5.2) | 764 (5.2) | 0.967 |
| CKD | 620 (3.3) | 165 (3.7) | 455 (3.1) | 0.078 |
| ESRD | 100 (0.5) | 31 (0.7) | 69 (0.5) | 0.083 |
| Baseline HbA1c levele | 8.3 ± 2.3 | 8.7 ± 2.2 | 8.2 ± 2.2 | <0.001 |
| Fracture (≥1 during observation period)b | ||||
| Any location | 477 (2.5) | 95 (2.1) | 382 (2.6) | 0.052 |
| Vertebral | 55 (0.3) | 9 (0.2) | 46 (0.3) | |
| Femur | 99 (0.5) | 21 (0.5) | 78 (0.5) | |
| Humerus | 89 (0.5) | 23 (0.5) | 66 (0.5) | |
| Distal upper or lower extremity | 189 (1.0) | 32 (0.7) | 157 (1.1) | |
| Pelvis | 18 (0.1) | 7 (0.2) | 11 (0.1) | |
| Ribs | 26 (0.1) | 3 (0.1) | 23 (0.2) | |
| Other | 1 (0.01) | 0 (0.0) | 1 (0.01) |
Data are expressed as mean ± sd or number (percentage).
P value for the comparison of individuals who did and did not use a TZD during the study period.
The observation period refers to the time between the index date and the date of censoring. The index date is the first diagnosis of diabetes or the first fill of an oral diabetes medication between January 1, 2000 and May 31, 2007. The last day of any follow-up was May 31, 2007.
Occurring up to 1 yr before index date.
Includes a previous diagnosis of myocardial infarction, other acute coronary syndromes, and coronary revascularization procedures 1 yr before the index date.
Based on the HbA1c measurement in closest proximity to index date.
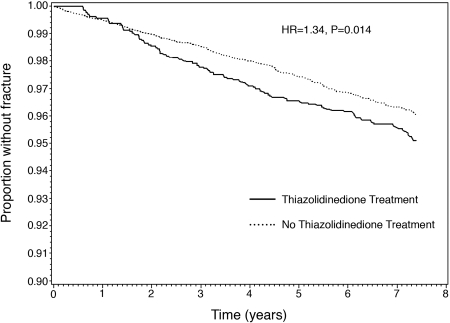
Because TZD exposure was assessed from the time of the first fill, the calculation of fracture rates had to account for between-group differences in patient-years of follow-up. The 4,511 TZD-treated individuals had a total of 13,498 patient-years of follow-up (i.e. average follow-up of 2.99 yr), and 95 patients with at least one fracture for an unadjusted first event rate of 7.0 per 1000 patient-years. The 14,559 individuals without TZD treatment had a total of 72,625 patient-years of follow-up (i.e. average follow-up, 4.99 yr) and 382 patients with at least one fracture for an unadjusted first event rate of 5.3 per 1000 patient-years. The Kaplan-Meier curve comparing the proportion of patients without fractures over time between the TZD-exposed and nonexposed individuals is shown in Fig. 1. The unadjusted risk of fracture was higher in those treated with TZDs when compared with those not so treated [HR, 1.34; 95% confidence interval (CI) 1.06–1.70]. At approximately 1.5 yr, the curves for the two treatment groups began to separate and remained separated for the remaining duration of observation.
Figure 1.
Kaplan Meier curve showing the relationship between TZD use and time to first fracture among patients with type 2 DM. Includes all individuals in the study cohort (n = 19,070). Individuals treated with a TZD are shown in the solid line, and individuals without TZD treatment are shown in the dashed line. HR denotes the unadjusted hazard ratio.
Of 19,070 study patients, 18,309 (9,286 women and 9,023 men) had complete data for inclusion in the multivariable Cox regression analysis. There were 463 events identified in this group, and the results are shown in Table 2. In the adjusted analysis, TZD use was again associated with an elevated fracture risk when compared with patients not treated with a TZD (aHR, 1.34; 95% CI, 1.05–1.71). With the exception of oral steroid use (aHR, 1.23; 95% CI, 0.99–1.51), we observed significant relationships with other factors commonly associated with fracture risk, such as female sex (aHR, 1.71; 95% CI, 1.41–2.09), age (aHR, 1.05; 95% CI, 1.04–1.05), ESRD (aHR, 4.49; 95% CI, 2.09–9.66), CVA (aHR, 1.75; 95% CI, 1.35–2.27), and insulin use (aHR, 1.74; 95% CI, 1.37–2.21). African-American race-ethnicity was protective for fractures when compared with other race-ethnic groups (aHR, 0.49; 95% CI, 0.39–0.61), which were predominately white. With the exception of the aforementioned factors and CHF, none of the other covariates was significantly associated with fracture risk.
Table 2.
Relationship of TZD use and other covariates to fracture risk among patients with type 2 DM (n = 18,309; 463 events)a
| aHRb (95% CI) | P value | |
|---|---|---|
| TZD use | 1.34 (1.05–1.71) | 0.019 |
| Oral steroid use | 1.23 (0.99–1.51) | 0.056 |
| African-American race | 0.49 (0.39–0.61) | <0.001 |
| Married | 0.95 (0.78–1.16) | 0.606 |
| Female | 1.71 (1.41–2.09) | <0.001 |
| Charlson score | 1.05 (0.98–1.11) | 0.138 |
| Age | 1.05 (1.04–1.05) | <0.001 |
| Median household income | 1.00 (0.95–1.04) | 0.848 |
| Baseline CHD | 0.98 (0.71–1.34) | 0.877 |
| Baseline CHF | 1.41 (1.06–1.86) | 0.016 |
| Baseline PVOD | 1.12 (0.81–1.56) | 0.491 |
| Baseline ESRD | 4.49 (2.09–9.66) | <0.001 |
| Baseline CVAs | 1.75 (1.35–2.27) | <0.001 |
| Baseline CKD | 0.91 (0.55–1.49) | 0.693 |
| Baseline insulin usec | 1.74 (1.37–2.21) | <0.001 |
| Baseline lipid-lowering medication usec | 0.91 (0.75–1.11) | 0.366 |
| Baseline HbA1cd | 1.04 (0.99–1.09) | 0.080 |
A total of 761 patients were excluded from the multivariable analysis due to missing data.
Multivariable models adjusted for all covariates shown in the table.
Defined as any prescription fill for these medications in the year prior to the index date.
HbA1c measurement closest to index date.
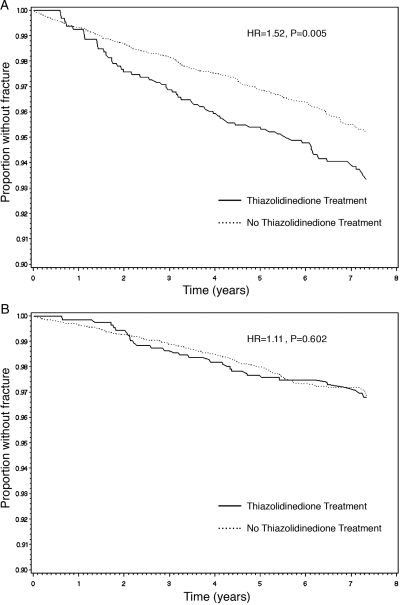
Because previous studies have suggested a heightened risk of TZD-associated fractures in women (8,12), we stratified our analyses by sex. For the 9,620 women included in our cohort, we had 6,600 patient-years of follow-up and 63 patients with at least one fracture for an unadjusted first event rate of 9.5 per 1000 patient-years for TZD users. In comparison, we had 36,264 patient-years of follow-up and 236 patients with at least one fracture for unadjusted first event rate of 6.5 per 1000 patient-years in women who did not use TZDs. The Kaplan-Meier curve comparing the proportion of women without fractures over time between the TZD-exposed and nonexposed individuals is shown in Fig. 2A. The unadjusted risk of fracture was higher in women treated with TZDs when compared with those not so treated (HR, 1.52; 95% CI, 1.13–2.30; P value, 0.005). Among women, the curves for the two treatment groups appear to separate after 1 yr of TZD exposure.
Figure 2.
Kaplan Meier curves showing the relationship between TZD use and time to first fracture among women (A) and men (B) with type 2 DM. Persons treated with a TZD are shown in the solid line and those without TZD treatment are shown in the dashed line. Includes all women (n = 9,620) and men (n = 9,450) in the cohort study. HR denotes the unadjusted hazard ratio.
For the 9,450 men included in our cohort, there were 6,897 patient-years of follow-up and 32 patients with at least one fracture for an unadjusted first event rate of 4.6 per 1000 patient-years for TZD users, and there were 36,361 patient-years of follow-up and 146 patients with at least one fracture for an unadjusted first event rate of 4.0 per 1000 patient-years for men who did not use TZDs. The Kaplan-Meier curve comparing the proportion of men without fractures over time between the TZD-exposed and nonexposed individuals is shown in Fig. 2B. The unadjusted risk of fracture was not significantly higher in men treated with TZDs when compared with those not so treated (HR, 1.11; 95% CI, 0.75–1.65; P value, 0.602). Accordingly, the curves for the two treatment groups do not appear to separate over time.
We calculated aHRs for TZD use on the time to first fracture stratified by sex. Results for women and men with complete data (i.e. n = 9,286 and n = 9,023, respectively) are shown in Table 3. Compared with no TZD use, TZD treatment was associated with an elevated risk among women (aHR, 1.57; 95% CI, 1.16–2.14), but not in men (aHR, 1.05; 95% CI, 0.70–1.58). In these stratified analyses, other covariates were again associated with increased fracture risk in the expected direction. In addition, CHF was associated with an increased fracture risk (aHR, 1.53; 95% CI, 1.09–2.17) among women, and higher baseline HbA1c was associated with an increased risk of fracture (aHR, 1.08; 95% CI, 1.01–1.16) in men.
Table 3.
Relationship of TZD use and other covariates to fracture risk among female (n = 9,286; 292 events) and male (n = 9,023; 171 events) patients with type 2 DM
| Females
|
Males
|
|||
|---|---|---|---|---|
| aHRa (95% CI) | P value | aHRa (95% CI) | P value | |
| TZD use | 1.57 (1.16–2.14) | 0.004 | 1.05 (0.70–1.58) | 0.817 |
| Oral steroid use | 1.18 (0.92–1.53) | 0.196 | 1.32 (0.92–1.91) | 0.136 |
| African-American race | 0.47 (0.35–0.62) | <0.001 | 0.55 (0.38–0.79) | 0.002 |
| Married | 0.96 (0.76–1.23) | 0.756 | 1.02 (0.71–1.47) | 0.894 |
| Charlson score | 1.05 (0.97–1.14) | 0.225 | 1.05 (0.96–1.16) | 0.306 |
| Age | 1.06 (1.04–1.07) | <0.001 | 1.03 (1.01–1.04) | <0.001 |
| Median household income | 1.00 (0.94–1.07) | 0.930 | 0.98 (0.91–1.06) | 0.666 |
| Baseline CHD | 0.92 (0.60–1.41) | 0.696 | 1.13 (0.71–1.82) | 0.609 |
| Baseline CHF | 1.53 (1.09–2.17) | 0.015 | 1.18 (0.73–1.89) | 0.497 |
| Baseline PVOD | 1.01 (0.65–1.57) | 0.973 | 1.31 (0.81–2.17) | 0.259 |
| Baseline ESRD | 3.68 (1.05–12.96) | 0.043 | 5.31 (1.95–14.49) | 0.001 |
| Baseline CVAs | 1.73 (1.26–2.38) | 0.001 | 1.71 (1.15–2.78) | 0.011 |
| Baseline CKD | 0.81 (0.42–1.59) | 0.548 | 1.03 (0.49–2.18) | 0.931 |
| Baseline insulin useb | 1.77 (1.31–2.37) | <0.001 | 1.71 (1.15–2.56) | 0.009 |
| Baseline lipid-lowering medication useb | 0.95 (0.74–1.21) | 0.675 | 0.84 (0.61–1.17) | 0.309 |
| Baseline HbA1cc | 1.01 (0.94–1.07) | 0.885 | 1.08 (1.01–1.16) | 0.020 |
Multivariable models adjusted for all covariates shown in the table.
Defined as any prescription fill for these medications in the year prior to the index date.
HbA1c measurement closest to index date.
We additionally stratified by age and by both age and sex; these results are shown in Table 4. The positive association between TZD use and fracture risk was seen in patients older than 65 yr (aHR, 1.54; 95% CI, 1.11–2.12), but not patients 65 yr or younger (aHR, 1.15; 95% CI, 0.79–1.66). In the age by sex subgroups, only women older than 65 yr demonstrated a significantly heightened risk (aHR, 1.72; 95% CI, 1.17–2.52). The association in women 65 yr old or younger did not reach statistical significance (aHR, 1.35; 95% CI, 0.82–2.23), and men regardless of age strata did not appear to be at marked increased risk.
Table 4.
Relationship between TZD use and fracture risk among patients with type 2 DM stratified by age and sex
| Strata | No. of patients included in the model | No. of events | aHRa (95% CI) | P value |
|---|---|---|---|---|
| Age ≤65 | 12,396 | 188 | 1.15 (0.79–1.66) | 0.465 |
| Age >65 | 5,913 | 275 | 1.54 (1.11–2.12) | 0.009 |
| Age ≤65, male | 6,197 | 85 | 0.95 (0.54–1.67) | 0.869 |
| Age ≤65, female | 6,199 | 103 | 1.35 (0.82–2.23) | 0.234 |
| Age >65, male | 2,826 | 86 | 1.18 (0.64–2.15) | 0.599 |
| Age >65, female | 3,087 | 189 | 1.72 (1.17–2.52) | 0.006 |
Adjusted for race-ethnicity, marital status, and median household income; HbA1c; insulin, oral steroid, and lipid-lowering agent use; baseline clinical status for CHD, CHF, CVAs/TIAs, PVOD, CKD, and ESRD; and the Charlson comorbidity index.
In a post hoc analysis looking at fracture subtypes in women, we found TZD use to be associated with fractures of the upper extremity and distal lower extremity combined (aHR, 1.55; 95% CI, 1.05–2.29), but the association with femur and vertebral fractures combined did not reach statistical significance (aHR, 1.34; 95% CI, 0.77–2.33). Similar findings were observed in women over 65 yr of age where TZD use was again associated with fractures of the upper extremity and distal lower extremity combined (aHR, 1.72; 95% CI, 1.03–2.89) but did not reach statistical significance for femur and vertebral fractures combined (aHR, 1.37; 95% CI, 0.70–2.68). In addition, we repeated all analyses accounting for the use of other oral hypoglycemic agents, and this had no substantive effect on any of the aforementioned relationships presented in the tables.
Discussion
As the evidence incriminating TZD use as a risk factor for fractures accumulates (7,8,9,10,11,12), clinicians may be inclined to avoid using these drugs in treating their diabetic patients, especially those who are at high risk for low bone mass and fractures. This study comprises one of the largest cohorts to examine the longitudinal relationship between TZD use and fractures to date, and unlike earlier studies was not limited to either particular age groups of adults with type 2 DM or comparisons with other hypoglycemic agents. Our results are consistent with the published literature in that TZD use was found to be associated with an increased risk of fractures, particularly in women above 65 yr of age (7,8,9,10,11,12). Based on the unadjusted event rates for the entire cohort and women alone, the approximate number needed to harm (i.e. the number needed to be treated with TZDs to have one excess fracture) would be 588 and 333, respectively. The number needed to harm may be even lower for women, however, given the higher adjusted rate in this group. Of interest, the current study also suggests that the increased risk of fracture among women does not accrue until after 1 yr of TZD treatment.
Although initial studies suggested that troglitazone, a TZD no longer available in the United States market (31), had a favorable effect on bone turnover (32), rosiglitazone has been shown to induce bone loss in rodent models (33,34). Subsequently, observation of diabetic patients from both the Health, Aging, and Body Composition observational study and the UK General Practice Research Database suggested that rosiglitazone and pioglitazone are associated with increased bone loss and a higher risk of fracture (7,9). In addition, an increased fracture risk has been reported from large randomized controlled trials involving both rosiglitazone and pioglitazone (8,10,12). Therefore, both TZDs available in the United States appear to possess an increased risk for fractures.
TZDs are agonists of the peroxisome proliferator-activated receptor-γ (PPARγ), a nuclear transcription factor expressed in adipocytes. Activation of the PPARγ by TZDs regulates adipocyte differentiation and promotes insulin sensitivity (35). However, PPARγ is also expressed in bone marrow stromal cells, osteoblasts, and osteoclasts (36). Activation of PPARγ in bone marrow stromal cells has been shown to promote the differentiation of mesenchymal stem cells into adipocytes and inhibit their differentiation into osteoblasts (36,37). In addition, PPARγ activation can inhibit osteoblast differentiation while inducing osteoclast differentiation, thus resulting in decreased bone formation and increased bone resorption (36,38). These functions of PPARγ are the likely mechanism of TZD-associated bone loss.
A post hoc analysis of A Diabetes Outcome Progression Trial (ADOPT) suggested that the increased risk of fractures is similar among pre- and postmenopausal women exposed to TZDs (39). In a recent case-control study, the association between TZD use and the higher fracture risk was also found to be independent of age (9). In our cohort, however, TZD use was associated with a higher risk of fractures in all women, but it only reached statistical significance among women more than 65 yr of age. Yet, older women are already at a higher risk of osteoporosis and osteoporosis-related fractures, and this might explain why they appeared to be the most affected by TZDs. In addition, one rodent study found that rosiglitazone induced bone loss in ovariectomized, but not intact rats (40). This finding suggests that estrogen deprivation may permit or potentiate the detrimental skeletal effects of TZDs (41). Similar to the ADOPT study (39), we also show that the increased risk of fractures appears after approximately 1 yr of TZD use among women but is not apparent among men over time. Although two recent studies suggest that men may also be at increased risk for fractures after TZD exposure (42,43), we did not observe this association for men despite having nearly equal numbers of men and women in our analyses.
We also observed that the increased fracture risk appeared to involve primarily the upper extremity and distal lower extremity. These findings are similar to those noted in ADOPT and RECORD (8,12,39); however, some observational data suggest a generalized increased risk of fractures (9). Animal models may again profer a potential mechanism because bone denervation in rats has been shown to result in regional osteopenia (44). Because patients with diabetes are at risk for peripheral neuropathy, TZD use may act synergistically to increase the likelihood of distal extremity fractures.
This study must be interpreted in light of its limitations. Similar to the methods of others (9,29), we relied on claims data and electronic medical records to identify our outcomes. As such, we did not review the radiological evidence of fractures in our study patients. Likewise, because we primarily relied on pharmacy claims data from one insurance carrier to identify TZD exposure, medications obtained from alternative sources (e.g. dual insurance coverage) may have been missed. Fortunately, previous analyses by our group showed that very few patients filled medications via other coverage (45).
Because adverse events may occur during or after treatment, we considered individuals to be continually exposed to TZDs from the time of their first fill. However, among those persons exposed to TZDs, the average time between initiation of therapy and completion of the last TZD fill was 1.9 yr (sd, 1.8 yr) (data not shown). In contrast, the average follow-up time for this group was 3.0 yr (sd, 2.1 yr). This may explain why the separation between Kaplan-Meier curves for TZD-treated and untreated individuals appeared to stabilize, rather than continue to diverge, over time. However, this potential misclassification implies that our estimates are conservative, such that the actual risk of fracture in TZD-treated individuals is higher than reported here.
As an observational study, treatment groups may have differed in important ways that were not accounted for in our regression models. For example, we did not have information on other important risk factors for fractures such as body mass index, smoking, diabetic retinopathy and nephropathy, and bone mineral density, and we were therefore unable to account for these variables in our models. Moreover, despite our large study size, the numbers of patients and events became much smaller after further stratification. This may have limited power for some of our subanalyses. Nonetheless, the consistency of our results with the finding of others suggests that estimates are correct. Moreover, we found that other covariates, such as age, race-ethnicity, insulin use, CVA, and ESRD, were significantly associated with fractures in the direction expected (19,22,46). These findings lend further credence to the reliability of our reported findings.
In summary, our study suggests that TZD use in patients with diabetes is associated with an increased risk of fractures in women, particularly over 65 yr of age and after an exposure duration of approximately 1 yr. Given that this population is already at high risk for fractures, more data are needed to guide physicians on the frequency and modality of screening when TZDs are used. For example, the increase in bone marrow fat caused by TZDs may result in underestimating dual-energy x-ray absorptiometry-measured bone mineral density (41). Nevertheless, it is essential for the clinicians to be aware of this potential adverse drug association so as to appropriately counsel their patients when prescribing TZDs.
Footnotes
This study was funded in part through the Fund for Henry Ford Hospital and grants from the National Heart Lung and Blood Institute (R01HL79055) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK64695), National Institutes of Health.
Disclosure Summary: The authors have no conflicts of interest to disclose.
First Published Online January 8, 2010
Abbreviations: aHR, Adjusted HR; CHD, coronary heart disease; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; CVA, cerebrovascular accident; DM, diabetes mellitus; ESRD, end-staged renal disease; HbA1c, glycated hemoglobin; HMO, health maintenance organization; HR, hazard ratio; PPARγ, peroxisome proliferator-activated receptor-γ; PVOD, peripheral vascular occlusive disease; TIA, transient ischemic attack; TZD, thiazolidinedione.
References
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A 2007 Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22:465–475 [DOI] [PubMed] [Google Scholar]
- Schubert C 2009 Bone seems susceptible to range of drugs. Nat Med 15:588 [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Walczak TS, Blackwell T, Ensrud ER, Bowman PJ, Stone KL 2004 Antiepileptic drug use increases rates of bone loss in older women: a prospective study. Neurology 62:2051–2057 [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Walczak TS, Blackwell TL, Ensrud ER, Barrett-Connor E, Orwoll ES 2008 Antiepileptic drug use and rates of hip bone loss in older men: a prospective study. Neurology 71:723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand MW, Samson MM, Pouwels S, van Staa TP, Thio B, Cooper C, Leufkens HG, Egberts AC, Verhaar HJ, de Vries F 2009 Use of anti-depressants and the risk of fracture of the hip or femur. Osteoporos Int 20:1705–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine L 2009 Proton pump inhibitors and bone fractures? Am J Gastroenterol 104 Suppl 2:S21–S26 [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka- Czernik B, Feingold KR, Strotmeyer ES, Resnick HE, Carbone L, Beamer BA, Park SW, Lane NE, Harris TB, Cummings SR 2006 Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab 91:3349–3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G 2006 Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443 [DOI] [PubMed] [Google Scholar]
- Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR 2008 Use of thiazolidinediones and fracture risk. Arch Intern Med 168:820–825 [DOI] [PubMed] [Google Scholar]
- Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR 2009 Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf 32:187–202 [DOI] [PubMed] [Google Scholar]
- Loke YK, Singh S, Furberg CD 2009 Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ 180:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ 2009 Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373:2125–2135 [DOI] [PubMed] [Google Scholar]
- Yaturu S, Bryant B, Jain SK 2007 Thiazolidinedione treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care 30:1574–1576 [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H 2004 Thiazolidinediones. N Engl J Med 351:1106–1118 [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Sellmeyer DE, Strotmeyer ES, Tylavsky FA, Feingold KR, Resnick HE, Shorr RI, Nevitt MC, Black DM, Cauley JA, Cummings SR, Harris TB 2005 Diabetes and bone loss at the hip in older black and white adults. J Bone Miner Res 20:596–603 [DOI] [PubMed] [Google Scholar]
- Leslie WD, Lix LM, Prior HJ, Derksen S, Metge C, O'Neil J 2007 Biphasic fracture risk in diabetes: a population-based study. Bone 40:1595–1601 [DOI] [PubMed] [Google Scholar]
- Schwartz AV 2006 Diabetes, TZDs, and bone: a review of the clinical evidence. PPAR Res 2006:24502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins J, Rodriguez BL, Johnson KC, Margolis KL 2006 Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. J Clin Endocrinol Metab 91:3404–3410 [DOI] [PubMed] [Google Scholar]
- Ivers RQ, Cumming RG, Mitchell P, Peduto AJ 2001 Diabetes and risk of fracture: The Blue Mountains Eye Study. Diabetes Care 24:1198–1203 [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Sellmeyer DE 2004 Women, type 2 diabetes, and fracture risk. Curr Diab Rep 4:364–369 [DOI] [PubMed] [Google Scholar]
- Casellini CM, Vinik AI 2007 Clinical manifestations and current treatment options for diabetic neuropathies. Endocr Pract 13:550–566 [DOI] [PubMed] [Google Scholar]
- Jørgensen L, Engstad T, Jacobsen BK 2002 Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke 33:542–547 [DOI] [PubMed] [Google Scholar]
- Grey A 2008 Skeletal consequences of thiazolidinedione therapy. Osteoporos Int 19:129–137 [DOI] [PubMed] [Google Scholar]
- Nickolas TL, Leonard MB, Shane E 2008 Chronic kidney disease and bone fracture: a growing concern. Kidney Int 74:721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR 2007 The peroxisome proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab 92:1305–1310 [DOI] [PubMed] [Google Scholar]
- Solomon DH, Cadarette SM, Choudhry NK, Canning C, Levin R, Stürmer T 2009 A cohort study of thiazolidinediones and fractures in older adults with diabetes. J Clin Endocrinol Metab 94:2792–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M 2007 Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med 147:W163–W194 [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA 1992 Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619 [DOI] [PubMed] [Google Scholar]
- Islam S, Liu Q, Chines A, Helzner E 2009 Trend in incidence of osteoporosis-related fractures among 40- to 69-year-old women: analysis of a large insurance claims database, 2000–2005. Menopause 16:77–83 [DOI] [PubMed] [Google Scholar]
- 2004 SAS/STAT users guide, version 9.1. Cary, NC: SAS Institute Inc. [Google Scholar]
- 2000 From the Food and Drug Administration. JAMA 283:2228 [Google Scholar]
- Watanabe S, Takeuchi Y, Fukumoto S, Fujita H, Nakano T, Fujita T 2003 Decrease in serum leptin by troglitazone is associated with preventing bone loss in type 2 diabetic patients. J Bone Miner Metab 21:166–171 [DOI] [PubMed] [Google Scholar]
- Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B 2004 Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145:401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorocéanu MA, Miao D, Bai XY, Su H, Goltzman D, Karaplis AC 2004 Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis. J Endocrinol 183:203–216 [DOI] [PubMed] [Google Scholar]
- Spiegelman BM 1998 PPAR-γ: adipogenic regulator and thiazolidinedione receptor. Diabetes 47:507–514 [DOI] [PubMed] [Google Scholar]
- Grey A 2009 Thiazolidinedione-induced skeletal fragility–mechanisms and implications. Diabetes Obes Metab 11:275–284 [DOI] [PubMed] [Google Scholar]
- Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H 2004 PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113:846–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Chong LW, Evans RM 2007 PPAR-γ regulates osteoclastogenesis in mice. Nat Med 13:1496–1503 [DOI] [PubMed] [Google Scholar]
- Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G 2008 Rosiglitazone-associated fractures in type 2 diabetes: an analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care 31:845–851 [DOI] [PubMed] [Google Scholar]
- Sottile V, Seuwen K, Kneissel M 2004 Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARγ agonist BRL49653 (rosiglitazone). Calcif Tissue Int 75:329–337 [DOI] [PubMed] [Google Scholar]
- Schwartz AV 2008 TZDs and bone: a review of the recent clinical evidence. PPAR Res 2008:297893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas IJ, Evans SJ, Pocock S, Smeeth L 2009 The risk of fractures associated with thiazolidinediones: a self-controlled case-series study. PLoS Med 6:e1000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormuth CR, Carney G, Carleton B, Bassett K, Wright JM 2009 Thiazolidinediones and fractures in men and women. Arch Intern Med 169:1395–1402 [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Boulet JM, Sellers R, Bunton TE, Walker K 2006 Neuropathy-induced osteopenia in rats is not due to a reduction in weight born on the affected limb. Bone 38:387–393 [DOI] [PubMed] [Google Scholar]
- Williams LK, Joseph CL, Peterson EL, Wells K, Wang M, Chowdhry VK, Walsh M, Campbell J, Rand CS, Apter AJ, Lanfear DE, Tunceli K, Pladevall M 2007 Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol 120:1153–1159 [DOI] [PubMed] [Google Scholar]
- Bishara SE, Cummins DM, Jorgensen GJ, Jakobsen JR 1995 A computer assisted photogrammetric analysis of soft tissue changes after orthodontic treatment. Part I: methodology and reliability. Am J Orthod Dentofacial Orthop 107:633–639 [DOI] [PubMed] [Google Scholar]