Abstract
Context: Inflammation is increasingly recognized as an important contributing factor in diabetes mellitus. Lipoxygenases (LOs) produce active lipids that promote inflammatory damage by catalyzing the oxidation of linoleic and arachidonic acid, and LO is expressed in rodent and human islets. Little is known about the differential effect of the various hydroxyeicosatetraenoic acids (HETEs) that result from LO activity in human islets.
Objective: We compared the effects of 12-LO products on human islet viability and function.
Design: Human islets were treated with stable compounds derived from LOs: 12(S)-HETE, 15HETE, 12HPETE, and 12RHETE and then examined for insulin secretion and islet viability. The p38-MAPK (p38) and JNK stress-activated pathways were investigated as mechanisms of 12-LO-mediated islet inhibition in rodent and human islets.
Results: Insulin secretion was consistently reduced by 12(S)-HETE and 12HPETE. 12(S)-HETE at 1 nm reduced viability activity by 32% measured by MTT assay and increased cell death by 50% at 100 nm in human islets. These effects were partially reversed with lisofylline, a small-molecule antiinflammatory compound that protects mitochondrial function. 12(S)-HETE increased phosphorylated p38-MAPK (pp38) protein activity in human islets. Injecting 12-LO siRNA into C57BL/6 mice reduced 12-LO and pp38-MAPK protein levels in mouse islets. The addition of proinflammatory cytokines increased pp38 levels in normal mouse islets but not in siRNA-treated islets.
Conclusions: These data suggest that 12(S)-HETE reduces insulin secretion and increases cell death in human islets. The 12-LO pathway is present in human islets, and expression is up-regulated by inflammatory cytokines. Reduction of 12-LO activity could thus provide a new therapeutic approach to protect human β-cells from inflammatory injury.
12-Lipoxygenase is an important inflammatory pathway leading to damage of human islets.
Lipoxygenases (LOs) are a family of iron-containing enzymes that catalyze the dioxygenation of polyunsaturated fatty acids in lipids. They are classified as 5-, 8-, 12-, and 15-LO according to the carbon atom of arachidonic acid at which the oxygen is inserted (1,2). The 12-LO enzyme, but not 5-LO or 15-LO, is specifically expressed in pancreatic β-cells (3). 12-LO activation can cause the formation of oxidized lipids such as 12(S)-hydroxyeicosatetraenoic acid [12(S)-HETE] (1). Human and rabbit 15-LO as well as the leukocyte-type 12-LO have high homology and are classified as 12-/15-LO (especially in rat and mouse) because they can form both 12(S)-HETE and 15(S)-HETE from arachidonic acid (1,2). Recently, there has been interest in the 12-/15-LO pathway because of the data implicating it in the pathogenesis of diabetes (4,5).
Cytokine-induced destruction of pancreatic β-cells seen in type 1 diabetes and islet graft rejection involves multiple intracellular signaling pathways that directly or indirectly lead to inflammatory damage or programmed cell death (6). Inflammation also is an important pathological process leading to β-cell dysfunction and death in type 2 diabetes (7). Recently, we have demonstrated that inflammatory cytokines rapidly activate 12-LO, and we showed that cytokines induce 12-LO translocation. The effects of 12-HETE on insulin secretion, cytotoxicity, and kinase activation were similar to the effects seen with cytokines in rodent islets (8). Clearly, there are major differences between rodent and human islets (9). Therefore, it remains important to check whether there is a differential effect of the various products that result from LO activity in human islets. In the current study, we directly tested the role of 12(S)-HETE, 15HETE, 12RHETE, and 12-hydroperoxyeicosatetraenoic acid (12HPETE) in insulin secretion, β-cell metabolism, and cell viability in human islets. We evaluated potential mechanisms of 12-LO product activation and possible in vivo significance.
Methods and Materials
Islet preparation
Human islets were acquired and sent to us from the Islet Cell Resource Consortium and the Juvenile Diabetes Research Foundation Basic Science Human Islet Distribution Program. Islets were incubated overnight in Miami medium at 37 C and 5% CO2 before experiments. Animal studies were approved by the Institutional Animal Care and Use Committee and conducted according to Institutional Animal Care and Use Committee-approved protocol. Mouse pancreatic islets were isolated from C57BL/6 mice by collagenase digestion using a previously described protocol (10) that was modified to include Histopaque centrifugation (11). After clamping the common bile duct at the duodenum, the pancreas was perfused through the common bile duct with 5 ml of 1.4 mg/ml collagenase P (Roche Diagnostics, Indianapolis, IN) in fresh Hanks’ balanced salt solution (HBSS) (Invitrogen, Carlsbad, CA) with 1% BSA and 4.2 mm sodium bicarbonate. The perfused pancreas was removed and incubated at 37 C for 8–11 min in 1 ml HBSS solution. After incubation, samples were shaken vigorously by hand. Samples were then washed with HBSS, and the pellet was resuspended. Samples were strained through mesh with 35 linear openings per inch. Samples were centrifuged and resuspended in room temperature Histopaque 1077 (Sigma-Aldrich, St. Louis, MO). Equal volumes of HBSS were gently added onto Histopaque layer, resulting in a discontinuous gradient. Samples were centrifuged for 20 min at 1200 rpm in Eppendorf Centrifuge 5810R (Eppendorf North America, Hauppauge, NY) at room temperature. Supernatant was washed, and islets were transferred to a petri dish containing RPMI 1640 supplemented with 11 mm glucose (Invitrogen). All islets were incubated overnight to allow sufficient recovery time from collagenase digestion before any experiments were performed.
Drug treatments
The cytokine combination chosen for this study is used widely as a means of inducing inflammatory responses in islets (12,13,14,15). Human forms of cytokines (B&D Scientific, Franklin Lakes, NJ) were used at the following concentrations: 10 ng/ml for TNF-α, 100 ng/ml for interferon-γ (IFN-γ), and 5 ng/ml for IL-1β in PBS. Lisofylline (LSF) was a generous gift of DiaKine Therapeutics (Charlottesville, VA). HETEs [12(S)-HETE, 15HETE, 12HPETE, and 12RHETE] and 12-LO inhibitor [cinnamyl-3, 4-dihydroxy-α-cyanocinnamate (CDC)] were purchased from Biomol (Plymouth Meeting, PA).
Glucose-stimulated insulin secretion
Islets were incubated in a modified Kreb-Ringer buffer with 11 mm glucose after 4-h HETE exposure. In other studies, human islets were cultured in CMRL medium-1066 (Invitrogen) with 5% fetal bovine serum overnight and then transitioned to serum free Kreb-Ringer buffer at 37 C, and islets were incubated with and without HETEs in 3 and 18 mm glucose for 4 h. The supernatant was collected after each treatment, and insulin concentration in the supernatant was measured by an enzyme immunoassay method (Mercodia, Uppsala, Sweden) with a mouse or human insulin standard. The intraassay variation was less than 4%, and the interassay variation was less than 10%.
Cell death measurements
Islets were treated with 20 μg/ml of propidium iodide (PI) and incubated for 10 min. Islets were imaged once under brightfield illumination to determine the islet borders and imaged again to measure PI fluorescence using 535-nm excitation and 617-nm emission as previously reported (11). Islets were circled, and the mean PI fluorescence intensity was determined for each islet individually. Three separate trials from three donors were conducted.
Small interfering RNA (siRNA) studies
Stabilized siRNAs for ip injections were synthesized by Dharmacon (Chicago, IL). Groups of 10-wk-old C57BL/6J male mice (Jackson Laboratory, Bar Harbor, ME) received daily ip injections of 1.6 mg/kg siRNA prepared in 0.9% saline or vehicle alone (0.9% saline) for 3 d as described in Ref. 16. For in vitro studies, islets from each group of injected mice were harvested on the fourth day and pooled before analysis. Injections with each siRNA were performed at least three different times. siRNA sequences were as follows: si-Control, 5′-AAAGUCGACCUUCAGUAAGGA-3′; and si-Alox15, 5′-GGAUAAGGAAAUUGAGAUU-3′.
Western immunoblotting
Equal amounts of whole islet cell protein extracts were separated on polyacrylamide-sodium dodecyl sulfate gels and transferred to polyvinylidene difluoride transfer membranes (GE Healthcare, Buckinghamshire, UK). The blots were probed with primary antibodies, and bands were detected using horseradish peroxide-conjugated secondary antibodies (GE Healthcare UK Limited, Chalfont, UK) and the enhanced chemiluminescence detection system (GE Healthcare).
Antibodies
Rabbit PAb anti-p38-MAPK (pTpY180/182) and anti-c-Jun N-terminal kinase (JNK)-1 and -2 (pTpY183/185) were purchased from Alpha BioSource/Invitrogen and were used at a 1:500 dilution. Mouse monoclonal anti-p38-MAPK (A-12) and rabbit polyclonal anti-actin (I-19) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). p38-MAPK was used at a 1:500 dilution, and actin was used at a 1:2500 dilution. Rabbit anti-12-LO (see Ref. 8) was used at a 1:1000 dilution.
Cell viability evaluations
Viability was assessed by detecting 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) metabolism as previously described (17).
Statistics
Statistical analysis was performed using Graph Pad Prism version 4.03 software (GraphPad Software, Inc., San Diego, CA). All data are presented as mean ± se, unless otherwise stated. One-way ANOVA followed by a Tukey posttest was used for comparing all groups unless otherwise stated. A P value <0.05 was used to indicate statistical significance.
RNA extraction and real-time PCR
RNA was prepared using the RNeasy Protect Mini Kit (QIAGEN, Gaithersburg, MD) for human islets. cDNA was made from 5 μg of total RNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen) in 20 μl reaction volume using random hexamers (Invitrogen). For quantitative measurement of PCR products, TaqMan was used (Applied Biosystems Inc., Foster City, CA). Three microliters of the cDNA reaction (5-fold diluted) were used as template for PCR in a reaction volume of 25 μl for PCR. Thermal cycling was performed using the iCycler (Bio-Rad, Hercules, CA). For the amplification of 12-LO, the cycling conditions were 95 C for 30 sec, 60 C for 1 min. All reactions were performed in triplicate, and the data were normalized to a housekeeping gene, actin, and evaluated using the 2−ΔΔCT method; Alox12 TaqMan probe was used. Expression levels are presented as fold induction of transcript related to control.
Results
Selective effects of LO products to inhibit insulin secretion
LOs catalyze the oxidation of linoleic acid and arachidonic acid, generating products of varying stability. These include HPETEs, which are subsequently reduced to more stable HETEs by glutathione peroxidase. 12(S)-HETE, 15(S)-HETE, 12HPETE, and 12RHETE were tested at 1 and 100 nm doses to examine their effects on insulin secretion. Human islets were incubated with the various HETEs for 4 h and then assessed for insulin secretion during a 1-h period incubated in 11 mm glucose. We also confirmed effects of 12(S)-HETE on basal (3 mm) and stimulated (18 mm) glucose concentration.
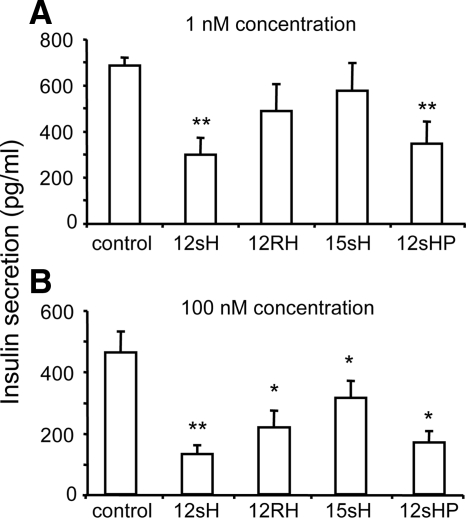
As shown in Fig. 1A, insulin secretion was significantly decreased by only 12(S)-HETE (down 51%; P < 0.01) and 12HPETE (down 43%; P < 0.01) at 1 nm concentrations. In contrast, 15(S)-HETE and 12RHETE (the inactive form) had no significant effect on insulin secretion. As shown in Fig. 1B at 100 nm, an inhibitory effect was observed with all compounds tested compared with control, with the greatest effect still observed with 12(S)-HETE and 12HPETE. There was no effect of 12(S)-HETE on basal insulin secretion (data not shown).
Figure 1.
HETE effects on human islet insulin secretion. Insulin secretion after treatment with several different LO products (HETEs) for 4 h in 11 mm glucose at either 1 nm (A) or 100 nm (B) concentrations. The 12-LO product 12(S)HPETE (12sHP) and its more stable derivative 12(S)-HETE (12sH) produced the most substantial reduction in insulin secretion. *, P < 0.05; **, P < 0.01.
12(S)-HETE reduces metabolic activity and insulin secretion: partial reversal with LSF
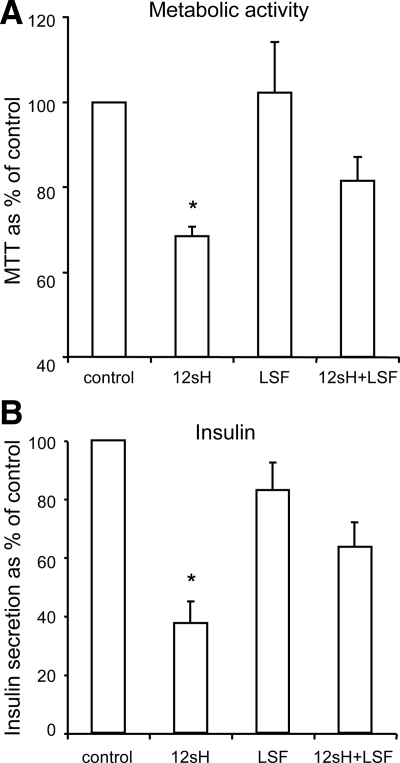
Because the most potent and consistent inhibition of islet function was observed with the 12-LO products, we performed additional experiments on human islets using 12(S)-HETE, the stable form of 12HPETE. As shown in Fig. 2A, 12(S)-HETE reduced human islet metabolic activity as measured by MTT assay. This effect was partially blocked by preincubation with the small molecule antiinflammatory compound LSF. LSF has previously been shown to protect islet mitochondrial function during the addition of inflammatory cytokines (10). Under the same test conditions, the 12(S)-HETE-induced reduction in insulin secretion was also reversed by LSF treatment (Fig. 2B).
Figure 2.
Effects of 12(S)-HETE (12sH) on metabolic activity and insulin secretion are partially reversed by the small molecular antiinflammatory compound LSF. Human islets from four different donors were pretreated with 50 μm LSF overnight or untreated as indicated. Islets were then treated with 1 nm 12(S)-HETE for 4 h and incubated in 11 mm glucose for 1 h to measure islet metabolic activity by MTT assay (A) or insulin secretion (B). Insulin secretion and metabolic activity were significantly inhibited by 12sH, but these effects were partially reversed by LSF pretreatment. *, P < 0.05.
12(S)-HETE induces cell death in human islets
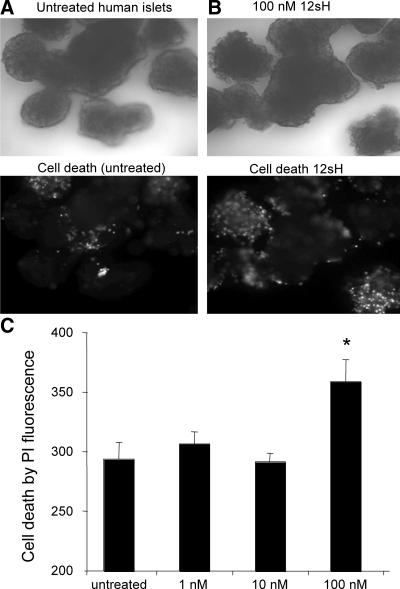
We next evaluated islet cell death by PI staining in islets after overnight incubation with 1, 10, or 100 nm 12(S)-HETE. As shown in Fig. 3, whereas human islets showed relatively little PI fluorescence in untreated conditions (Fig. 3A), a significant increase in PI fluorescence was observed among islets incubated overnight with 100 nm 12(S)-HETE (Fig. 3B). As summarized, a dose-response study is shown in Fig. 3C. A significant increase in cell death was produced by 100 nm 12(S)-HETE, as measured by PI staining intensity, whereas no significant increase in cell death was observed with 1 or 10 nm 12(S)-HETE. This finding is consistent with dose-response measures of cell death previously observed with mouse islets (8).
Figure 3.
12-LO products induce cell death in human islets. A, Human islets (top) cultured overnight in untreated conditions show relatively little cell death measured by PI fluorescence intensity (bottom). B, Overnight treatment with 12(S)-HETE causes a significant increase in cell death at 100 nm. C, Dose-dependent cell death response to 12(S)-HETE. *, P < 0.01. 12sH, 12(S)-HETE.
12(S)-HETE activates phosphorylated p38-MAPK (pp38) in human islets
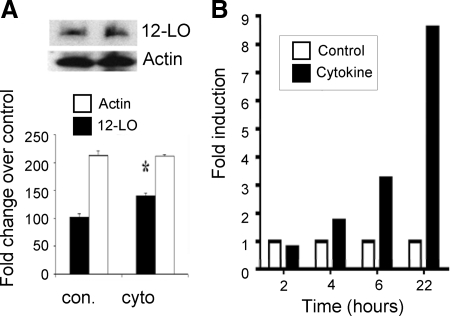
To investigate possible mechanisms of 12-LO-mediated effects, we investigated stress-mediated pathways linked to β-cell damage. We first demonstrated that treatment of human islets with inflammatory cytokines significantly increased 12-LO protein levels compared with the control untreated condition (Fig. 4A). Analysis by real-time PCR also showed that 12-LO mRNA expression was induced and that it peaked at 22 h after cytokine treatment (Fig. 4B). Interestingly, the cytokines did not induce significant expression of 5-LO or 15-lipoxygenase-1 expression (data not shown).
Figure 4.
Inflammatory cytokines induced 12-LO protein and gene expression in human islets. Human islets were treated with cytokines (10 ng/ml for TNF-α, 100 ng/ml for IFN-γ, and 5 ng/ml for IL-1β in PBS) and vehicle for 30 min and collected for Western blot (A) and for different time points and collected for real time RT-PCR. A, 12-LO protein levels after cytokine treatment compared with control, and its quantification was normalized to the actin control. The results are presented as the means ± sd. *, P < 0.05 compared with the corresponding control. B, 12-LO mRNA expression was determined by using Alox12 TaqMan probe by quantitative PCR at indicated time points. The data were normalized to total actin, and fold differences were calculated using the 2−ΔΔCT method. 12sH, 12(S)-HETE.
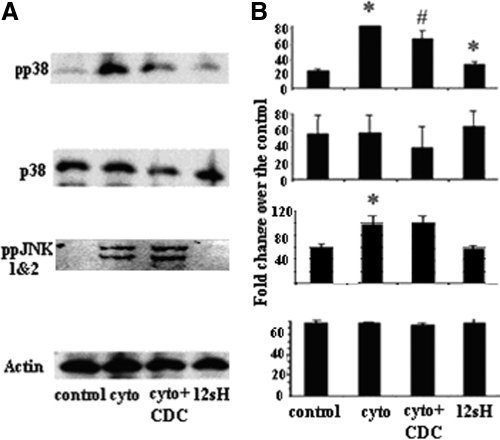
We next examined whether treating islets with 12(S)-HETE would affect the activity of two well-known stress-mediated pathways linked to β-cell damage, p38-MAPK and JNK in human islets. In Fig. 5A, treatments of cytokines and 12(S)-HETE increased phosphorylated p38 (pp38) without any change in total p38-MAPK (p38). In islets pretreated with the 12-LO inhibitor CDC, cytokine-induced pp38-MAPK activity was significantly reduced. Phosphorylated JNK (ppJNK1 and -2) activity in human islets was increased with cytokine addition but was not affected by 12(S)-HETE, suggesting selectivity of 12-LO products for p38-MAPK activation. The results are quantified in Fig. 5B and show that the 12-LO product 12(S)-HETE could be a mediator of cytokine-induced toxicity in human islets through activation of p38-MAPK signaling.
Figure 5.
Effects of 12(S)-HETE and 12-LO blockade on pp38-MAPK protein activity. Human islets were treated with cytokines (10 ng/ml for TNF-α, 100 ng/ml for IFN-γ, and 5 ng/ml for IL-1β in PBS), cytokines + CDC (1 μm), 12(S)-HETE (100 nm), or vehicle for 30 min and collected for Western blot. A, Representative Western blots showing pp38-MAPK, total p38-MAPK protein, ppJNK 1 and -2, and actin for each condition. B, Protein levels quantified for each protein in panel A and normalized to the actin control. The results are presented as the means ± sd. *, P < 0.05 compared with the corresponding control; #, P < 0.05 compared with the cytokine treatment group. 12sH, 12(S)-HETE.
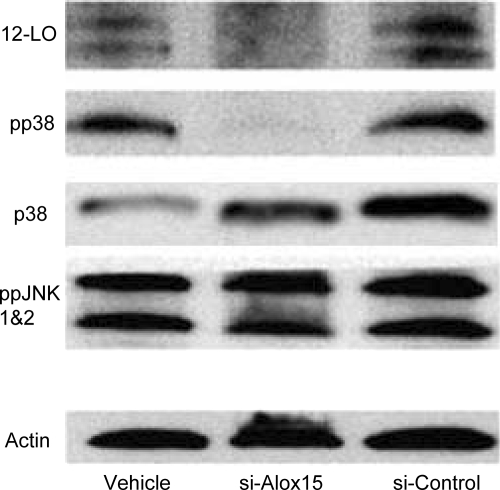
To further evaluate a role of 12-LO in vivo in mediating stress-induced pp38-MAPK in islets, we injected C57BL/6J mice ip with either stabilized siRNA against the mouse form of 12-LO (si-Alox15) or control siRNA (si-Control) daily for 3 d to produce short-term knockdown in 12-LO protein. As shown in Fig. 6, the repeated si-Alox15 injections caused a significant reduction of 12-LO protein in pancreatic islets, whereas si-Control produced similar results to saline-injected mice (vehicle). After 12-LO knockdown, pp38-MAPK activity was significantly reduced. This reduction in p38-MAPK was associated with a similar decrease in 12-LO expression. siRNA knockdown of 12-LO was selective for p38-MAPK because there was no effect on ppJNK1 and -2.
Figure 6.
siRNA knockdown of 12-LO reduces pp38-MAPK protein levels in mouse islets. Islets were isolated from mice that were ip injected on 3 consecutive days with a stabilized siRNA construct against leukocyte 12-LO. The siRNA against 12-LO (si-Alox15) considerably reduced 12-LO levels compared with untreated controls (vehicle) or mice injected with missense siRNA (si-Control). Similarly, pp38-MAPK was greatly reduced in the siRNA knockdown group, whereas the total p38-MAPK (p38) expression does not change. Phosphorylated JNK expression (ppJNK1 and -2) was similar in all groups.
Discussion
Emerging data suggest a major role of local inflammation in mediating β-cell damage in both type 1 and type 2 diabetes. However, the precise pathways inducing the damage in human islets have not been fully clarified. The role of LO in regulating β-cell function and development of diabetes continues to be elucidated (2,3,5,8,18). The LO pathway leads to the formation of leukotrienes and also catalyzes the conversion of arachidonic acid to HETE by glutathione peroxidase (19). 12-LO is present in the β-cells of the pancreas (3,5), and in that setting it converts arachidonic acid primarily into 12(S)-HETE. The current study also shows that inflammatory cytokines induce 12-LO RNA and protein expression. In contrast, human islets do not express 15-LO in the basal state or after cytokine addition (data not shown). In the current study, we show for the first time that 12(S)-HETE inhibits insulin secretion, reduces metabolic activity, and induces cell death in human islets. These effects on islets may be mediated by the p38-MAPK signaling pathway, which is activated by 12-LO and repressed in the absence of 12-LO. However, additional studies will be needed to fully evaluate the mechanism of 12(S)-HETE toxicity and the role of the p38-MAPK pathway in human islets.
Several studies have suggested that diabetes is associated with increased production of 12(S)-HETE (3,8,20). However, this is the first study clearly documenting the detrimental effects of 12(S)-HETE in human islets. Based on our findings, it can be concluded that 12(S)-HETE inhibits insulin secretion in human islets at low concentrations that can be produced in vivo. We also showed that 12(S)-HETE reduces metabolic activity in human islets, and those effects were partially blocked by preincubation with LSF, an antiinflammatory compound that improves islet viability and function (21). Moreover, we showed that 12(S)-HETE increased cell death in human islet cells. Thus, our results provide evidence that 12(S)-HETE leads to β-cell damage in human islets. The precise cell source of 12(S)-HETE in human islets will require additional study. However, isolated rodent β-cell lines express 12-LO and produce 12-HETE. In human islets, sources of 12-LO could include β-cells as well as possibly resident macrophages or dendritic cells.
Evidence suggests that MAPKs participate in cytokine-induced β-cell toxicity (22). Both p38-MAPK kinases and JNK are considered to be important signaling mechanisms in cytokine-induced damage and cell death in islets and β-cells (23,24,25,26). Here we used siRNA technology to examine the in vivo role of 12-LO in the p38-MAPK signaling pathway. When 12-LO was specifically knocked down with an siRNA target to that enzyme in C57BL/6J mice, there was a decrease in endogenous pp38-MAPK, whereas the phosphorylated JNK level was not changed. Of interest, islets from 12-LO-null mice also showed reduced pp38-MAPK protein activity compared with islets from control C57BL/6 mice but no differences in JNK activity (Ma, K., and J. L. Nadler, unpublished observation). Our results also showed that 12(S)-HETE and cytokines activated p38-MAPK kinases in human islets. In addition, p38-MAPK kinase activation in human islets by inflammatory cytokines was blocked by the 12-LO inhibitor, CDC, implicating 12(S)-HETE as one mediator of cytokine-induced p38-MAPK kinase activation.
The role of the LO pathway in human β-cell function has not been fully elucidated. Previous studies using high concentrations of nonspecific LO inhibitors suggested that 12-LO products play a role in insulin secretion in rodent islets (27). The important role of 12-LO in type 1 diabetes development in mice was demonstrated in global 12-LO knockout mice on the C57BL/6J background and congenic 12-LO null mice on the nonobese diabetic background (4,5). In these studies, 12-LO deletion led to a marked reduction of the rate of diabetes development. In β-cell lines such as β-TC3 cells, 12(S)-HETE added for a prolonged period also produced reduced insulin output (8). 12(S)-HETE-induced β-cell death in β-TC3 cells was possibly the result of the deregulation of the JNK and p38-MAPK kinase pathways in mouse cell lines (8). The present study provides new data that low concentrations of 12(S)-HETE, a specific product of 12-LO, reduce islet function in a manner consistent with p38-MAPK activation. Our data support the hypothesis that 12-LO products act directly on the β-cells in islets. However, because 12-HETE can dramatically increase inflammatory cytokine production from macrophages (28), it is possible that 12-HETE action also increases local inflammation in islets by the release of cytokines. Further studies will be needed to test this hypothesis.
12(S)-HETE biological actions on MAPK in diabetic models have been investigated in several cell types (29,30,31,32). Recent studies have indicated that 12(S)-HETE significantly activated Src, focal adhesion kinase, Akt, p38- MAPK, cAMP-responsive element-binding protein, and histone H3-Lys-9/14 acetylation in vascular cells (33). Additional studies in human islets will be needed to study the potential role of these additional signal transduction pathways in 12-LO product effects on β-cell function or viability.
In summary, our studies show for the first time that 12-LO products inhibit insulin secretion, reduce metabolic activity, and induce cell death in human islets. The data also suggest that 12-LO activation may be an important local pathway mediating β-cell dysfunction or reduced β-cell mass in diabetes. Therefore, pharmacological or molecular approaches to reduce 12-LO action could provide a novel therapeutic approach to protect human β-cells from inflammatory injury.
Acknowledgments
The authors thank the Islet Cell Resource Consortium and the Juvenile Diabetes Research Foundation Basic Science Human Islet Distribution Program for providing human islets.
Footnotes
This work was supported by National Institutes of Health Grants R-01 DK 55240 (to J.L.N.) and K01 DK081621 (to C.S.N.).
Disclosure Summary: Dr. Nadler is a consultant and founder of Diakine Therapeutics who holds the license for Lisofylline.
First Published Online January 20, 2010
Abbreviations: CDC, Cinnamyl-3, 4-dihydroxy-α-cyanocinnamate; HBSS, Hanks’ balanced salt solution; HETE, hydroxyeicosatetraenoic acid; HPETE, hydroperoxyeicosatetraenoic acid; IFN, interferon; JNK, c-Jun N-terminal kinase; LO, lipoxygenase; LSF, lisofylline; PI, propidium iodide; p38, pp38, phosphorylated p38-MAPK; ppJNK1 and -2, phosphorylated JNK; siRNA, small interfering RNA.
References
- Yamamoto S 1992 Mammalian lipoxygenase: molecular structures and functions. Biochim Biophys Acta 1128:117–131 [DOI] [PubMed] [Google Scholar]
- Funk CD 1996 The molecular biology of mammalian lipoxygenase and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim Biophys Acta 1304:65–84 [DOI] [PubMed] [Google Scholar]
- Bleich D, Chen S, Gu JL, Nadler JL 1998 The role of 12-lipoxygenase in pancreatic-cells (review). Int J Mol Med 1:265–272 [PubMed] [Google Scholar]
- McDuffie M, Maybee NA, Keller SR, Stevens BK, Garmey JC, Morris MA, Kropf E, Rival C, Ma K, Carter JD, Tersey SA, Nunemaker CS, Nadler JL 2008 Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes 57:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleich D, Chen S, Zipser B, Sun D, Funk CD, Nadler JL 1999 Resistance to type 1 diabetes induction in 12-lipoxygenase knockout mice. J Clin Invest 103:1431–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleich D, Chen S, Wen Y, Nadler JL 1997 The stress- activated c-Jun protein kinase (JNK) is stimulated by lipoxygenase pathway product 12-HETE in RIN m5F cells. Biochem Biophys Res Commun 230:448–451 [DOI] [PubMed] [Google Scholar]
- Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA 2008 Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care 31(Suppl 2):S161–S164 [DOI] [PubMed] [Google Scholar]
- Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL 2005 Activation of 12-lipoxygenase in proinflammatory cytokine-mediated β-cell toxicity. Diabetologia 48:486–495 [DOI] [PubMed] [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A 2006 The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA 103:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZD, Chen M, Wu R, McDuffie M, Nadler JL 2002 The anti-inflammatory compound lisofylline prevents type I diabetes in non-obese diabetic mice. Diabetologia 45:1307–1314 [DOI] [PubMed] [Google Scholar]
- Jahanshahi P, Wu R, Carter JD, Nunemaker CS 2009 Evidence of diminished glucose stimulation and endoplasmic reticulum function in nonoscillatory pancreatic islets. Endocrinology 150:607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrup-Poulsen T, Bendtzen K, Dinarello CA, Nerup J 1987 Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic β-cell cytotoxicity. J Immunol 139:4077–4082 [PubMed] [Google Scholar]
- Campbell IL, Iscaro A, Harrison LC 1988 IFN-γ and tumor necrosis factor-α. Cytotoxicity to murine islets of Langerhans. J Immunol 141:2325–2329 [PubMed] [Google Scholar]
- Rabinovitch A, Suarez-Pinzon WL, Strynadka K, Schulz R, Lakey JR, Warnock GL, Rajotte RV 1994 Human pancreatic islet β-cell destruction by cytokines is independent of nitric oxide production. J Clin Endocrinol Metab 79:1058–1062 [DOI] [PubMed] [Google Scholar]
- Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL 2005 Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic β-cells. Diabetes 54:452–461 [DOI] [PubMed] [Google Scholar]
- Deering TG, Ogihara T, Trace AP, Maier B, Mirmira RG 2009 Methyltransferase Set7/9 maintains transcription and euchromatin structure at islet-enriched genes. Diabetes 58:185–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chen M, Carter JD, Nunemaker CS, Garmey JC, Kimble SD, Nadler JL 2006 Combined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. Biochem Biophys Res Commun 344:1017–1022 [DOI] [PubMed] [Google Scholar]
- Bleich D, Chen S, Gu JL, Thomas L, Scott S, Gonzales N, Natarajan R, Nadler JL 1995 Interleukin-1β regulates the expression of a leukocyte type of 12-lipoxygenase in rat islets and RIN m5F cells. Endocrinology 136:5736–5744 [DOI] [PubMed] [Google Scholar]
- Moreno JJ 2009 New aspects of the role of hydroxyeicosatetraenoic acids in cell growth and cancer development. Biochem Pharmacol 77:1–10 [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman EM, Abadir PM, Siragy HM 2008 Regulation of renal 12(S)-hydroxyeicosatetraenoic acid in diabetes by angiotensin AT1 and AT2 receptors. Am J Physiol Regul Integr Comp Physiol 295:R1473–R1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Yang Z, Wu R, Nadler JL 2002 Lisofylline, a novel anti-inflammatory agent, protects pancreatic β-cells from proinflammatory cytokine damage by promoting mitochondrial metabolism. Endocrinology 143:2341–2348 [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T 2001 β-Cell apoptosis: stimuli and signaling. Diabetes 50(Suppl 1):S58–S63 [DOI] [PubMed] [Google Scholar]
- Ammendrup A, Maillard A, Nielsen K, Aabenhus Andersen N, Serup P, Dragsbaek Madsen O, Mandrup-Poulsen T, Bonny C 2000 The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic β-cells. Diabetes 49:1468–1476 [DOI] [PubMed] [Google Scholar]
- Larsen CM, Wadt KA, Juhl LF, Andersen HU, Karlsen AE, Su MS, Seedorf K, Shapiro L, Dinarello CA, Mandrup-Poulsen T 1998 Interleukin-1β-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Biol Chem 273:15294–15300 [DOI] [PubMed] [Google Scholar]
- Paraskevas S, Aikin R, Maysinger D, Lakey JR, Cavanagh TJ, Agapitos D, Wang R, Rosenberg L 2001 Modulation of JNK and p38 stress activated protein kinases in isolated islets of Langerhans: insulin as an autocrine survival signal. Ann Surg 233:124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldeen J, Lee JC, Welsh N 2001 Role of p38 mitogen-activated protein kinase (p38 MAPK) in cytokine-induced rat islet cell apoptosis. Biochem Pharmacol 61:1561–1569 [DOI] [PubMed] [Google Scholar]
- Turk J, Colca JR, Kotagal N, McDaniel ML 1984 Arachidonic acid metabolism in isolated pancreatic islets. I. Identification and quantitation of lipoxygenase and cyclooxygenase products. Biochim Biophys Acta 794:110–124 [DOI] [PubMed] [Google Scholar]
- Wen Y, Gu J, Vandenhoff GE, Liu X, Nadler JL 2008 Role of 12/15-lipoxygenase in the expression of MCP-1 in mouse macrophages. Am J Physiol Heart Circ Physiol 294:H1933–H1938 [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Melino G, Finazzi-Agrò A 2001 Lipoxygenases and their involvement in programmed cell death. Cell Death Differ 8:776–784 [DOI] [PubMed] [Google Scholar]
- Antonipillai I, Nadler J, Vu EJ, Bughi S, Natarajan R, Horton R 1996 A 12-lipoxygenase product, 12-hydroxyeicosatetraenoic acid, is increased in diabetics with incipient and early renal disease. J Clin Endocrinol Metab 81:1940–1945 [DOI] [PubMed] [Google Scholar]
- Kang SW, Adler SG, Nast CC, LaPage J, Gu JL, Nadler JL, Natarajan R 2001 12-Lipoxygenase is increased in glucose-stimulated mesangial cells and in experimental diabetic nephropathy. Kidney Int 59:1354–1362 [DOI] [PubMed] [Google Scholar]
- Patricia MK, Kim JA, Harper CM, Shih PT, Berliner JA, Natarajan R, Nadler JL, Hedrick CC 1999 Lipoxygenase products increase monocyte adhesion to human aortic endothelia cells. Arterioscler Thromb Vasc Biol 19:2615–2622 [DOI] [PubMed] [Google Scholar]
- Reddy MA, Sahar S, Villeneuve LM, Lanting L, Natarajan R 2009 Role of Src tyrosine kinase in the atherogenic effects of the 12/15-lipoxygenase pathway in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 29:387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]