Abstract
Context: Fenofibrate is a peroxisome proliferator-activated receptor α agonist widely used in clinical practice, but its mechanism of action is incompletely understood.
Objective: The aim of the study was to assess whether improvement in subclinical inflammation or glucose metabolism contributes to its antiatherogenic effects in insulin-resistant subjects with the metabolic syndrome (MetS).
Design and Setting: We conducted a randomized, double-blind, placebo-controlled study in the research unit at an academic center.
Patients: We studied 25 nondiabetic insulin-resistant MetS subjects.
Intervention(s): We administered fenofibrate (200 mg/d) and placebo for 12 wk.
Main Outcome Measures: Before and after treatment, we measured plasma lipids/apolipoproteins, inflammatory markers (high-sensitivity C-reactive protein, IL-6, intercellular adhesion molecule/vascular cell adhesion molecule), adipocytokines (adiponectin, TNFα, leptin), and insulin secretion (oral glucose tolerance test). We also assessed adipose tissue, hepatic and peripheral (muscle) insulin resistance fasting and during a euglycemic insulin clamp with 3H glucose and 14C palmitate infusion combined with indirect calorimetry.
Results: Subjects displayed severe insulin resistance and systemic inflammation. Fenofibrate significantly reduced plasma triglyceride, apolipoprotein (apo) CII, apo CIII, and apo E (all P < 0.01), with a modest increase in high-density lipoprotein-cholesterol (+12%; P = 0.06). Fenofibrate markedly decreased plasma high-sensitivity C-reactive protein by 49.5 ± 8% (P = 0.005) and IL-6 by 29.8 ± 7% (P = 0.03) vs. placebo. However, neither insulin secretion nor adipose tissue, hepatic or muscle insulin sensitivity or glucose/lipid oxidation improved with treatment. Adiponectin and TNF-α levels were also unchanged. Improvement in plasma markers of vascular/systemic inflammation was dissociated from changes in triglyceride/high-density lipoprotein-cholesterol, apo CII/CIII, or free fatty acid concentrations or insulin secretion/insulin sensitivity.
Conclusions: In subjects with the MetS, fenofibrate reduces systemic inflammation independent of improvements in lipoprotein metabolism and without changing insulin sensitivity. This suggests a direct peroxisome proliferator-activated receptor α-mediated effect of fenofibrate on inflammatory pathways, which may be important for the prevention of CVD in high-risk patients.
Fenofibrate reduces hsCRP and IL-6 independent of improvements in lipoprotein metabolism or insulin sensitivity, suggesting a direct PPARαλπηα-mediated effect on inflammatory pathways in insulin-resistant metabolic syndrome patients.
Insulin resistance and subclinical inflammation are prominent features of patients with the metabolic syndrome (MetS) and may contribute significantly to their increased cardiovascular risk (1,2). The presence of insulin resistance appears to identify subjects with the MetS who are at the greatest risk of cardiovascular (CV) disease (CVD). Clinical trials have demonstrated that fibrates are effective for primary (3,4) and secondary (5) prevention of CVD. However, the precise mechanism for the reduction in cardiovascular events by fibric acid derivatives may not be exclusively linked to lipid changes (6).
In the FIELD trial (4), fenofibrate reduced the primary endpoint of coronary heart disease nonsignificantly by 11% (5.9% in placebo vs. 5.2% with fenofibrate; P = 0.16), perhaps due to the overall low coronary heart disease event rate and the much higher use of statins in the placebo arm. In contrast, a number of secondary macrovascular endpoints were improved by fenofibrate treatment, including total CVD events (−11%; P = 0.035), nonfatal myocardial infarction (−24%; P = 0.01), coronary revascularizations (−21%; P = 0.03), angina (−21%; P = 0.04), and amputations (−38%; P = 0.01). These positive results occurred although the effects on plasma triglyceride (TG), low-density lipoprotein (LDL)-cholesterol (LDL-C), and high-density lipoprotein (HDL)-cholesterol (HDL-C) concentration were rather modest. In the VA-HIT study (5), only 26% of the CV risk reduction attributable to gemfibrozil could be explained by changes in plasma lipids. The observation of larger benefits in fibrate trials in patients with elevated TG and/or low HDL-C (5,7), characteristic of MetS patients with underlying insulin resistance and systemic inflammation, opens the intriguing possibility that improvement in CV outcomes could be related to effects beyond lipid metabolism, such as a reduction in insulin resistance and/or amelioration of subclinical inflammation.
Studies in patients with the MetS or type 2 diabetes mellitus (T2DM) have met mixed results on whether fibrates may improve insulin sensitivity, with some (8,9,10), but not others (11,12,13), showing an improvement in insulin action. Insulin resistance in the MetS is also frequently associated with an elevation of plasma high-sensitivity C-reactive protein (hsCRP), IL-6, and other adipocytokines as well as biomarkers of endothelial dysfunction. In particular, hsCRP has been reported to be a strong predictor for the development of CVD and T2DM (14). Fibrates may ameliorate systemic inflammation and improve vascular reactivity (15,16,17), but as with investigations related to insulin resistance and glucose metabolism, studies have been generally small, uncontrolled and/or failed to carefully assess insulin sensitivity. Although the role in clinical practice of hsCRP and other biomarkers of inflammation to identify and treat subjects at higher CV risk is currently subject to intense debate (18,19), understanding the impact of fibrate therapy on subclinical inflammation has potential clinical implications for the management of a large number of patients with the MetS.
The aim of this study was to understand the mechanism(s) by which fenofibrate may ameliorate cardiovascular risk beyond its lipid-lowering properties in subjects with the MetS. To this end, we assessed its impact on lipoprotein metabolism in relation to its effects on subclinical inflammation and insulin sensitivity at the level of the liver, skeletal muscle, and adipose tissue.
Subjects and Methods
Subjects
Twenty-five healthy nondiabetic subjects with the MetS (as defined by the National Cholesterol Education Program Adult Treatment Panel III) participated in the study. Four subjects in the fenofibrate arm and two in the placebo arm had impaired glucose tolerance, whereas all other participants had normal glucose tolerance based on an oral glucose tolerance test (OGTT) performed on the initial visit. Patients were not allowed to participate if they were on any medication that altered glucose/lipid metabolism. Body weight was stable for at least 3 months before and throughout the study. Each subject gave written informed consent. The study was approved by the University of Texas Health Science Center at San Antonio Institutional Review Board.
Study design
Subjects who qualified participated in a 4-wk run-in and were given placebo tablets. Compliance was assessed by pill count on follow-up visits. A research dietician educated them on a eucaloric diet. During the run-in, the following measurements were performed at the research unit: 1) total body fat [dual-energy x-ray absorptiometry (DXA)]; 2) markers of systemic/vascular inflammation: hsCRP, IL-6, human soluble vascular cell adhesion molecule-1 (VCAM-1)/intercellular adhesion molecule-1 (ICAM-1), and adipocytokines (plasma adiponectin, leptin, and TNF-α); 3) plasma lipid/apolipoprotein concentrations; 4) insulin secretion [OGTT with samples every 30 min for plasma glucose/insulin/free fatty acid (FFA) levels]; and 5) insulin sensitivity (euglycemic insulin clamp with 3H glucose and 14C palmitate infusion and indirect calorimetry).
After baseline metabolic measurements, volunteers were randomized in a double-blind fashion to receive either fenofibrate (200 mg Tricor; Abbott Laboratories, Abbott Park, IL) or placebo once daily in a computer-generated 2:1 randomization. Volunteers were seen every 2 wk for potential adverse events and medication compliance (being ≥90% in all patients). All measurements were repeated after 12 wk of treatment.
Euglycemic insulin clamp
At 0700 h, a primed (25 μCi/min × fasting plasma glucose/100)-continuous (0.25 μCi/min) infusion of 3-3H glucose was started and continued throughout the study to measure glucose turnover. A second catheter was inserted retrogradely into a vein on the dorsum of the hand (placed in a thermoregulated box at 65 C) for collection of arterialized blood samples. After a 2-h isotopic equilibration period, a 2-h euglycemic insulin clamp (80 mU/m2 · min) was initiated, and the plasma glucose was maintained constant by the adjustment of a 20% dextrose infusion as previously described by our group. Indirect calorimetry was used to measure glucose/lipid oxidation. Blood was obtained every 10–15 min for plasma insulin, FFA, and 3H glucose and 14C palmitate radioactivity.
Analytical determinations
Plasma glucose concentration was determined by the glucose oxidase method (Beckman Glucose Analyzer II; Beckman Instruments Inc., Fullerton, CA). Plasma insulin was measured by RIA, and FFA by standard colorimetric methods. Plasma glucose radioactivity was determined on barium hydroxide/zinc sulfate-precipitated plasma extracts. Plasma hsCRP was measured by RIA (LipoScience, Raleigh, NC); IL-6, VCAM-1/ICAM-1, and TNF-α by ELISA (R&D Systems, Minneapolis, MN); and adiponectin by RIA (Linco Research Inc., St. Charles, MI). Apolipoprotein analysis was performed at Linco Research, Inc. Whole body fat was measured by DXA (Hologic Inc., Waltham, MA).
Calculations
Endogenous glucose production (EGP) and insulin-stimulated glucose disposal (Rd)
Both EGP and Rd were calculated as previously reported by our group, as well as an index of hepatic insulin resistance estimated as the product of the fasting EGP and fasting plasma insulin concentration [EGP × fasting plasma insulin (mg/kgLBM (lean body mass)−1 · min−1 · μU/ml)] as previously described (20).
Statistical analysis
All values represent the mean ± se. Normality was checked before any analysis, and we applied a zero-skew log transformation to normalize positively skewed measures before analysis. Differences between basal and insulin clamp periods and between groups were tested by two-way ANOVA for repeated measures. An analysis of covariance model was used to evaluate treatment difference before/after treatment change on the lipid, apolipoprotein, hsCRP, IL-6, and soluble adhesion molecule concentrations. The pretreatment value was used as a covariate in these analyses. Because the percentage change values are also fairly skewed and prone to outliers, Spearman rank order correlations were used between hsCRP and all lipid measures. Robust linear regression was used to compute fitted values (with 95% prediction intervals) for each lipid measure (21). Robust regression uses an iteratively reweighted least-squares algorithm that assigns weights to each observation based on residuals. The larger the residual, the smaller the weight assigned for an observation at each iteration. Descriptive statistics, t tests on baseline differences, and repeated measures ANOVA results were calculated using JMP for Windows, version 5.0 (SAS Institute Inc., Cary, NC). Stata 10.1 was used to compute the analysis of covariance and robust regression models (Stata Corp., College Station, TX).
Results
Three subjects did not complete the study: two were terminated due to adverse reactions potentially related to fenofibrate use (one with >2.5-fold elevation in liver transaminases, and another with a mild allergic skin reaction in hands), whereas one subject in the placebo arm dropped out soon after randomization for personal reasons. Detailed questionnaires on diet and physical activity were performed by our research dietician and study staff during the entire study (because changes could alter glucose/lipid metabolism or markers of systemic inflammation), and we observed no changes in any subject.
Baseline clinical characteristics (Table 1)
Table 1.
Baseline clinical and laboratorial characteristics
| Treatment group
|
|||
|---|---|---|---|
| Fenofibrate | Placebo | Controls | |
| n (males/females) | 16 (11/5) | 9 (6/3) | 15 (9/6) |
| Age (yr) | 46 ± 2 | 46 ± 3 | 34 ± 4 |
| Ethnicity (Caucasian/Hispanic/African-American/Asian) | 8/6/1/1 | 5/3/1/0 | 5/10/0/0 |
| Body mass index (kg/m2) | 31.6 ± 1 | 31.5 ± 1 | 28.2 ± 1b |
| Total body fat (%)a | 29 ± 2 | 30 ± 2 | 26.8 ± 1b |
| Fasting plasma glucose (mg/dl) | 102 ± 2 | 105 ± 2 | 88 ± 2b |
| 2-h glucose OGTT (mg/dl) | 142 ± 10 | 142 ± 10 | 92 ± 5b |
| A1c (%) | 5.2 ± 0.1 | 5.1 ± 0.1 | 5.0 ± 0.1b |
| Fasting plasma insulin (μU/ml) | 9 ± 1 | 12 ± 3 | 7 ± 1b |
| Hepatic glucose production (mg/kgLBM−1 · min−1) | 2.6 ± 0.1 | 2.6 ± 0.1 | 2.1 ± 0.2b |
| Hepatic insulin resistance index (mg/kgLBM−1 · min−1 · μU/ml) | 23 ± 3 | 29 ± 6 | 14 ± 3b |
| Insulin-stimulated glucose disposal (mg/kgLBM−1 · min−1 · μU/ml) | 5.2 ± 0.4 | 4.8 ± 0.5 | 8.1 ± 0.3 |
| Adipose tissue insulin resistance index (mmol/liter · μU/ml) | 7.7 ± 2.3 | 12.6 ± 2.7 | 3.6 ± 1b |
| TG (mg/dl) | 500 ± 71 | 343 ± 42 | 87 ± 15b |
| Total cholesterol (mg/dl) | 228 ± 18 | 219 ± 11 | 158 ± 10b |
| HDL-C (mg/dl) | 34 ± 2 | 34 ± 3 | 49 ± 2b |
| LDL-C (mg/dl) | 109 ± 14 | 117 ± 10 | 94 ± 9b |
| FFA (μmol/liter) | 700 ± 48 | 744 ± 59 | 482 ± 55b |
| hsCRP (mg/liter) | 6.5 ± 1.5 | 4.9 ± 1.2 | 1.4 ± 1.8b |
| IL-6 (pg/ml) | 2.2 ± 0.3 | 2.1 ± 0.3 | 1.7 ± 0.1b |
| ICAM (ng/ml) | 237 ± 12 | 250 ± 21 | 190 ± 13b |
| VCAM (ng/ml) | 410 ± 15 | 405 ± 31 | 377 ± 19b |
| Adiponectin (ng/ml) | 5.1 ± 0.7 | 4.8 ± 0.9 | 15 ± 3b |
| TNF-α (pg/ml) | 3.3 ± 0.8 | 4.5 ± 1.6 | 1.3 ± 0.2b |
Data are expressed as mean ± se. A1c, Glycosylated hemoglobin A1c.
Total body fat (%) measured by DXA.
P < 0.001 between control and fenofibrate or placebo group.
As shown in Table 1, the fenofibrate and placebo groups were well matched for all clinical variables. Plasma TG showed a trend to be higher in the fenofibrate group but was not statistically significant (P = 0.13). Subjects with the MetS had severe insulin resistance at the level of liver, adipose tissue, and muscle (see Effect of fenofibrate on liver and muscle insulin resistance). In the fasting state, MetS patients exhibited severe hepatic insulin resistance compared with non-obese healthy controls (hepatic insulin resistance index, 25 ± 3 vs. 14 ± 3 mg/kgLBM−1 · min−1 · μU/ml; P < 0.001). Peripheral (muscle) insulin resistance was also evident because insulin-stimulated glucose disposal (Rd) during the insulin clamp was 40% lower in obese MetS patients compared with controls (P < 0.001). Markers of systemic inflammation and adipocytokines were significantly higher in obese insulin-resistant subjects with the MetS compared with controls (all P < 0.001). Non-obese healthy controls had a fasting plasma FFA level that was ∼35% lower vs. MetS patients, consistent with a much larger suppression of plasma FFA during the euglycemic insulin clamp (lean controls vs. MetS patients, −87 ± 3 vs. −68 ± 6%; P < 0.001), indicating severe adipose insulin resistance in obese subjects with the MetS.
Effect of fenofibrate on lipid and apolipoprotein concentrations (Table 2)
Table 2.
Effect of fenofibrate on plasma lipid and lipoprotein concentrations
| Plasma lipid concentrations | Fenofibrate
|
P | Placebo
|
P | Fenofibrate vs. placeboa | ||
|---|---|---|---|---|---|---|---|
| Pretreatment | Posttreatment | Pretreatment | Posttreatment | ||||
| TG (mg/dl) | 500 ± 71 | 207 ± 24 | <0.001 | 343 ± 42 | 310 ± 44 | 0.12 | <0.001 |
| Total cholesterol (mg/dl) | 228 ± 18 | 211 ± 16 | 0.11 | 219 ± 11 | 215 ± 14 | 0.71 | 0.54 |
| HDL-C (mg/dl) | 33 ± 2 | 36 ± 2 | 0.06 | 34 ± 3 | 32 ± 3 | 0.07 | 0.06 |
| LDL-C (mg/dl) | 114 ± 16 | 122 ± 18 | 0.17 | 115 ± 7 | 120 ± 6 | 0.44 | 0.92 |
| Non-HDL-C (mg/dl) | 194 ± 18 | 175 ± 15 | 0.07 | 185 ± 12 | 183 ± 13 | 0.86 | 0.27 |
| apo Al (μg/ml) | 2194 ± 211 | 2755 ± 403 | 0.11 | 1731 ± 230 | 1672 ± 127 | 0.82 | 0.25 |
| apo AII (μg/ml) | 690 ± 74 | 931 ± 109 | 0.03 | 549 ± 99 | 573 ± 77 | 0.82 | 0.28 |
| apo B (μg/ml) | 1847 ± 366 | 1208 ± 303 | 0.10 | 1638 ± 648 | 1621 ± 553 | 0.97 | 0.25 |
| apo CIII (μg/ml) | 527 ± 58 | 334 ± 37 | <0.001 | 355 ± 41 | 374 ± 34 | 0.72 | 0.004 |
| apo CIII/CII ratio | 2.9 | 2.9 | 0.94 | 2.4 | 2.6 | 0.69 | 0.59 |
All data are presented in milligrams per deciliter and as mean ± se. apo, Apolipoprotein.
Comparison between groups for the effect of treatment (change from baseline).
Placebo led to no significant changes in plasma lipid levels. In contrast, fenofibrate decreased plasma TG by 60 ± 3% (P < 0.001). Fenofibrate significantly decreased plasma apolipoproteins CIII and CII, leaving the ratio unchanged (CIII/CII = 2.9 before and after treatment). This was associated with a trend toward an increase in plasma HDL-C (+12%; P = 0.06) and a reduction in non-HDL-C (−11%; P = 0.07).
Effect of fenofibrate on plasma hsCRP, IL-6, soluble adhesion molecule, and adipocytokine concentrations
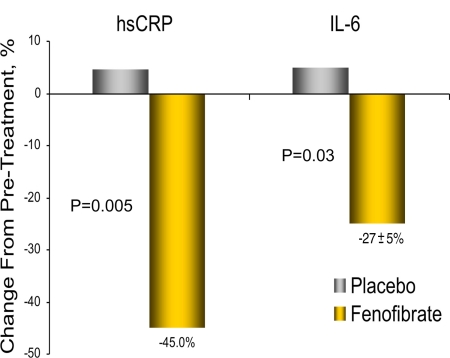
Fenofibrate significantly reduced subclinical inflammation as represented by a 45 ± 11% decrease in hsCRP from baseline (6.5 ± 1.5 vs. 3.6 ± 0.9 mg/liter; P < 0.01) and 49.5% vs. placebo, which was overall unchanged (from 4.9 ± 1.2 to 5.5 ± 1.4 mg/liter; P = not significant; P = 0.005 vs. fenofibrate). Plasma IL-6 concentration was also significantly reduced by 27 ± 5% from baseline (2.2 ± 0.3 vs. 1.4 ± 0.2 pg/ml; P < 0.01) and 31.8% vs. placebo, which slightly increased from 2.0 ± 0.3 to 2.2 ± 0.4 pg/ml (P = 0.03 vs. fenofibrate), suggesting a beneficial effect of fenofibrate to ameliorate subclinical systemic inflammation. Plasma ICAM was reduced 5 ± 2% compared with baseline (P = 0.05), but this did not reach statistical significance vs. placebo (P = 0.19), whereas VCAM, adiponectin, leptin, and TNFα were unchanged. Placebo had no effect on any of these parameters.
Effect of fenofibrate on liver and skeletal muscle insulin resistance and adipose tissue lipolysis in patients with the MetS
Effect of fenofibrate on adipose tissue insulin resistance
Compared with baseline, neither placebo nor fenofibrate treatment altered glycemic control (i.e. glycosylated hemoglobin A1c) or the plasma glucose/insulin concentration either fasting or during the OGTT. The fasting plasma FFA concentration was similar before treatment in both groups [fenofibrate vs. placebo, 700 ± 48 vs. 744 ± 59 μmol/liter; P = not significant (NS)]; it decreased significantly to 464 ± 35 μmol/liter only in the fenofibrate group (P = 0.002), but not in the subjects that received placebo (to 648 ± 56 μmol/liter; P = NS). However, plasma FFA concentrations during the OGTT followed the same suppression curve compared with pretreatment, indicating unchanged postprandial suppression of lipolysis (i.e. adipose tissue insulin resistance) in obese subjects after fenofibrate treatment. Consistent with a lack of effect of fenofibrate on adipose tissue, insulin suppression of plasma FFA levels during the euglycemic insulin clamp was unchanged by fenofibrate or placebo (before vs. after: fenofibrate, −68 ± 4 vs. −67 ± 5%; and placebo, −68 ± 6 vs. −72 ± 7% of the basal values, respectively; P = NS). FFA turnover (measured by the infusion of 14C palmitate) and glucose/lipid oxidation (indirect calorimetry) were unchanged (data not shown), as well as plasma adiponectin concentration (5.1 ± 0.7 vs. 4.8 ± 0.6 ng/ml; P = NS), an indicator of dysfunctional insulin-resistant fat, which was approximately one-third of that of healthy controls, as shown in Table 1.
Effect of fenofibrate on hepatic insulin resistance
Fenofibrate did not elicit an improvement in hepatic insulin sensitivity, as the fasting plasma insulin and EGP were unchanged (2.6 ± 0.1 vs. 2.7 ± 0.1 mg · kg lean body mass−1 · min−1; P = 0.60). Because the rate of EGP is a major determinant of the fasting plasma glucose, it was not unexpected that the fasting plasma glucose was also unchanged by treatment (98 ± 2 vs. 98 ± 2 mg/dl). During the last hour of the insulin infusion period, EGP was more than 90% suppressed in both groups before and after treatment (data not shown).
Effect of fenofibrate on muscle insulin resistance
During the clamp studies, the insulin increase above baseline was similar between groups before vs. after treatment (fenofibrate, +122 ± 12 vs. +131 ± 14 μU/ml; placebo, +119 ± 9 vs. +123 ± 12 μU/ml; for both, P = NS). Insulin sensitivity (Rd) did not improve significantly after 12 wk of fenofibrate treatment (5.2 ± 0.4 vs. 5.8 ± 0.5 mg · kg lean body mass−1 · min−1; P = 0.11).
Correlations
Given the large impact of fenofibrate on plasma lipid/apolipoprotein levels, we examined whether changes in inflammatory markers (i.e. hsCRP, IL-6) could be attributable to these lipid improvements. However, none of the lipid variables examined correlated significantly with the large reduction in hsCRP and IL-6. There was no relation between the marked reduction in plasma hsCRP by fenofibrate and change in LDL-C (r = 0.02), HDL-C (r = −0.22), TG (r = 0.06), or FFA (r = 0.31). A similar lack of correlation was found for these lipid parameters and reduction in plasma IL-6 concentration. There was a significant although modest correlation between plasma hsCRP and IL-6 (r = 0.36; P = 0.05).
Discussion
Although previous work in the field has focused on the lipid improvements associated with fenofibrate use, this study examined the impact of peroxisome proliferator-activated receptor (PPAR)-α activation in relation to plasma biomarkers of subclinical inflammation and liver/muscle/adipose tissue insulin sensitivity and insulin secretion using gold standard metabolism techniques. Fenofibrate markedly reduced plasma hsCRP (∼50%) and IL-6 (∼30%) levels (Fig. 1) in the absence of a significant change in insulin secretion or insulin action, and having no correlation with changes in lipid or plasma FFA/adipose tissue insulin sensitivity. Taken together, these data suggest that the reduction of these biomarkers was a direct effect of fenofibrate on inflammatory pathways and not an indirect effect from an improvement in lipid or glucose metabolism.
Figure 1.
Plasma hsCRP and IL-6 in patients with the MetS before and after 12 wk of treatment with fenofibrate or placebo. Fenofibrate significantly reduced plasma hsCRP (−49.5%; P = 0.005) and IL-6 (−31.8%; P = 0.03) compared to placebo after 12 wk of treatment in patients with the MetS.
In rodents, fenofibrate has been reported to improve liver and muscle insulin sensitivity (22,23). In humans, data are limited to small, uncontrolled trials (8,9,10,24) or large, long-term clinical trials in which the glucose-lowering medication is being changed over time (3,4,25), both types of studies limiting our ability to gain insights on the issue. Of note, no previous study has used insulin clamp studies with tracer turnover measurements and indirect calorimetry to examine liver/muscle insulin action during fenofibrate therapy in patients with the MetS. However, comparable (negative) results were reported by Abbasi et al. (13) measuring insulin action by a modified version of the insulin suppression test. Lack of a significant change in muscle insulin sensitivity, as in the present study, is also in agreement with previous insulin clamp studies with gemfibrozil (11) and with fenofibrate (12) in subjects with T2DM. Hepatic insulin sensitivity was also unaltered. This highlights a significant species difference between humans and rodents. In the latter, PPARα agonism has profound effects on hepatic glucose and lipid metabolism, enhancing hepatic insulin sensitivity, FFA oxidation, and resolution of hepatic steatosis (22,26). The significant lipoprotein improvement in the face of a lack of change in hepatic insulin resistance indicates that PPARα activation plays a minor role, if any, in determining the rate of hepatic glucose production in humans.
Although liver fat content was not examined, the lack of changes in hepatic transaminases is consistent with minimal changes reported in large controlled trials (4) and likely reflects a lack of efficacy of fibrates to reverse steatosis in humans. Two recent preliminary reports showed a lack of effect of fenofibrate to alter hepatic steatosis measured either by magnetic resonance imaging and spectroscopy (27) or by liver biopsy (28), consistent with the view that fibrates do not have a significant impact in nonalcoholic fatty liver disease in humans. This failure may be due in part to the fewer PPARα receptors and/or lower plasma fibrate concentrations during treatment in humans. It may also reflect the inability of fenofibrate to refrain FFA flux from dysfunctional adipose tissue because insulin induced suppression of plasma FFA during the OGTT, insulin sensitivity studies were unaffected by fenofibrate, and FFA turnover was also unchanged. Adiponectin and leptin levels, both hormones that originate in adipose tissue, were also abnormal and did not improve with treatment. Taken together, they are a frank contrast to the hepatic and adipose tissue-sensitizing effects of PPARγ agonists we have previously reported in humans (20).
Fenofibrate reduced the fasting plasma FFA concentration by 34%, a finding reported previously in obese subjects (10,16). However, there was no improvement in FFA turnover or insulin suppression of plasma FFA, consistent with a previous report (29). Reductions in plasma FFA have typically been observed when plasma TG levels are markedly increased (>300 mg/dl), as in this study. Given the approximately 60% decrease in plasma TG concentration, we speculate that the reduction of plasma FFA during fenofibrate administration is not due to enhanced adipose tissue insulin sensitivity, but rather is secondary to a decrease in the plasma FFA “spillover” from peripheral intravascular clearance of TG in very low-density lipoproteins. This has been demonstrated experimentally in obese subjects when plasma TG-rich lipoproteins are increased experimentally by the infusion of a lipid emulsion (30). The decrease in plasma FFA by fenofibrate is in contrast to the effects of niacin that are characterized by a rebound in plasma FFA concentration between doses that may exacerbate hepatic/skeletal muscle insulin resistance (31) and promote glucose intolerance, depending on the dose of nicotinic acid and the degree of β-cell reserve in subjects with MetS and impaired glucose tolerance or T2DM. Fenofibrate did not alter insulin secretion or glucose tolerance in the present study. From a clinical perspective, the neutral effect of fenofibrate on insulin sensitivity is reassuring regarding its use in patients with the MetS.
Our study confirms that subjects with the MetS display a profile of marked systemic inflammation, abnormal lipoprotein metabolism, and severe insulin resistance (2). The reduction of hsCRP and IL-6 may have significant clinical implications. The use of hsCRP as a biomarker of vascular inflammation and cardiovascular risk is now gaining momentum (18), although its exact place in clinical practice remains controversial (19). C-reactive protein (CRP) not only is a biomarker of subclinical inflammation but also may directly promote endothelial activation/dysfunction, cause smooth muscle cell proliferation and neointimal damage, and alter monocyte/macrophage and matrix metalloproteinase function (32). This effect has been linked, at least in part, to the up-regulation of angiotensin II type 1 receptors, the major target of angiotensin II, a well-known proinflammatory peptide. Fenofibrate antagonizes in vitro and in vivo the deleterious effects of CRP to induce activation of human aortic smooth muscle cells (15). CRP is an acute-phase response protein synthesized primarily by the liver in response to adipocytokines such as IL-6 and TNFα. Such an effect of fenofibrate on CRP is also in agreement with another recent report in subjects with the MetS (17). Whether fenofibrate reduced hsCRP by a direct effect on the liver, on the vascular bed, or by other mechanisms remains unclear. Of note, IL-6 drives hepatic CRP production, and the lower plasma hsCRP levels could have been secondary to a PPARα-induced reduction in IL-6 production (Fig. 1), although the correlation between CRP and IL-6 was modest. A decrease of IL-6 (which may originate T and B cells, endothelial cells, adipocytes, and skeletal muscle), and to a lesser extent of ICAM (largely from activated endothelial cells) plasma concentrations, suggests that fenofibrate may reduce systemic inflammation by actions on multiple tissues. PPARα receptors are abundant in the vascular bed, and recent studies in humans have reported that fenofibrate may improve endothelial function and vascular reactivity as well as reduce plasma levels of ICAM/VCAMs (16,33,34), consistent with a modest reduction in ICAM in our fenofibrate-treated subjects.
In summary, the current study demonstrates that in patients with the MetS, the antiinflammatory action of fenofibrate is not dependent on changes in plasma glucose, insulin, FFA, or lipoprotein concentrations. Although the clinical implications remain to be fully understood in the context of our evolving view of the role of subclinical inflammation in CVD, we believe that this work expands our horizons on the complexity of PPARα signaling and the role of fibrates in human disease.
Acknowledgments
The authors thank our study volunteers, the nursing staff, and the nutrition and laboratory staff for their assistance in performing the described studies.
Footnotes
This work was supported by a Veterans Administration Research Career Development Award (to K.C.), a Howard Hughes Medical Institute Grant (to K.C.), the Veterans Affairs Medical Research Fund, and Award no. UL 1RR025767 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources of the National Institutes of Health.
Disclosure Summary: K.C. is a member of the Speakers Bureau of Abbott, BMS/AstraZeneca, and Merck/Schering Plough. R.Bel., R.Ber., and J.C. have no relevant conflict of interest to disclose.
First Published Online January 8, 2010
Abbreviations: CRP, C-Reactive protein; CV, cardiovascular; CVD, CV disease; DXA, dual-energy x-ray absorptiometry; EGP, endogenous glucose production; FFA, free fatty acid; HDL, high-density lipoprotein; HDL-C, HDL-cholesterol; hsCRP, high-sensitivity CRP; ICAM, intercellular adhesion molecule; LBM, lean body mass; LDL, low-density lipoprotein; LDL-C, LDL-cholesterol; MetS, metabolic syndrome; NS, not significant; OGTT, oral glucose tolerance test; PPAR, peroxisome proliferator-activated receptor; Rd, insulin-stimulated glucose uptake; T2DM, type 2 diabetes mellitus; TG, triglyceride; VCAM, vascular cell adhesion molecule.
References
- Cusi K 2001 Cardiovascular risk management in type 2 diabetes: from clinical trials to clinical practice. The Endocrinologist 11:474–490 [Google Scholar]
- Caballero AE 2004 Endothelial dysfunction, inflammation, and insulin resistance: a focus on subjects at risk for type 2 diabetes. Curr Diab Rep 4:237–246 [DOI] [PubMed] [Google Scholar]
- Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V. Helsinki Heart Study 1987 Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 317:1237–1245 [DOI] [PubMed] [Google Scholar]
- Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M 2005 Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 366:1849–1861 [DOI] [PubMed] [Google Scholar]
- Rubins HB, Robins SJ, Collins D, Nelson DB, Elam MB, Schaefer EJ, Faas FH, Anderson JW for the VA-HIT Study Group 2002 Diabetes, plasma insulin and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs High-Density Lipoprotein Intervention Trial (VA-HIT). Arch Intern Med 162:2597–2604 [DOI] [PubMed] [Google Scholar]
- Plutzky J 2007 Preventing type 2 diabetes and cardiovascular disease in metabolic syndrome: the role of PPARα. Diab Vasc Dis Res 4(Suppl 3):S12–S14 [DOI] [PubMed] [Google Scholar]
- Scott R, O'Brien R, Fulcher G, Pardy C, D'Emden M, Tse D, Taskinen MR, Ehnholm C, Keech A 2009 Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care 32:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DC, Fuh MM, Shieh SM, Chen YD, Reaven GM 1991 Effect of gemfibrozil treatment in sulfonylurea-treated patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 73:503–510 [DOI] [PubMed] [Google Scholar]
- Avogaro A, Miola M, Favaro A, Gottardo L, Pacini G, Manzato E, Zambon S, Sacerdoti D, de Kreutzenberg S, Piliego T, Tiengo A, Del Prato S 2001 Gemfibrozil improves insulin sensitivity and flow-mediated vasodilatation in type 2 diabetic patients. Eur J Clin Invest 31:603–609 [DOI] [PubMed] [Google Scholar]
- Damci T, Tatliagac S, Osar Z, Ilkova H 2003 Fenofibrate treatment is associated with better glycemic control and lower serum leptin and insulin levels in type 2 diabetic patients with hypertriglyceridemia. Eur J Intern Med 14:357–360 [DOI] [PubMed] [Google Scholar]
- Vuorinen-Markkola H, Yki-Järvinen H, Taskinen MR 1993 Lowering of triglycerides by gemfibrozil affects neither the glucoregulatory nor antilipolytic effect of insulin in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36:161–169 [DOI] [PubMed] [Google Scholar]
- Anderlová K, Dolezalová R, Housová J, Bosanská L, Haluzíková D, Kremen J, Skrha J, Haluzík M 2007 Influence of PPAR-α agonist fenofibrate on insulin sensitivity and selected adipose tissue-derived hormones in obese women with type 2 diabetes. Physiol Res 56:579–586 [DOI] [PubMed] [Google Scholar]
- Abbasi F, Chen YD, Farin HM, Lamendola C, Reaven GM 2008 Comparison of three treatment approaches to decreasing cardiovascular disease risk in nondiabetic insulin-resistant dyslipidemic subjects. Am J Cardiol 102:64–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM 2001 High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 103:1813–1818 [DOI] [PubMed] [Google Scholar]
- Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A 1998 Activation of human aortic smooth-muscle cells is inhibited by PPARa but not by PPARg activators. Nature 393:790–793 [DOI] [PubMed] [Google Scholar]
- Capell WH, DeSouza CA, Poirier P, Bell ML, Stauffer BL, Weil KM, Hernandez TL, Eckel RH 2003 Short-term triglyceride lowering with fenofibrate improves vasodilator function in subjects with hypertriglyceridemia. Arterioscler Thromb Vasc Biol 23:307–313 [DOI] [PubMed] [Google Scholar]
- Rosenson RS 2009 Effect of fenofibrate on adiponectin and inflammatory biomarkers in metabolic syndrome patients. Obesity 17:504–509 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto Jr AM, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ 2008 Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 359:2195–2207 [DOI] [PubMed] [Google Scholar]
- Després JP 2009 Bringing JUPITER down to earth. Lancet 373:1147–1148 [DOI] [PubMed] [Google Scholar]
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K 2006 A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 355:2297–2307 [DOI] [PubMed] [Google Scholar]
- Rousseeuw PJ, Leroy AM 2003 Robust regression and outlier detection. New York: Wiley [Google Scholar]
- Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF 2005 “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab 1:309–322 [DOI] [PubMed] [Google Scholar]
- Haluzik MM, Lacinova Z, Dolinkova M, Haluzikova D, Housa D, Horinek A, Vernerova Z, Kumstyrova T, Haluzik M 2006 Improvement of insulin sensitivity after peroxisome proliferator-activated receptor-α agonist treatment is accompanied by paradoxical increase of circulating resistin levels. Endocrinology 147:4517–4524 [DOI] [PubMed] [Google Scholar]
- Jones IR, Swai A, Taylor R, Miller M, Laker MF, Alberti KG 1990 Lowering of plasma glucose concentrations with bezafibrate in patients with moderately controlled NIDDM. Diabetes Care 13:855–863 [DOI] [PubMed] [Google Scholar]
- Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J 1999 Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 341:410–418 [DOI] [PubMed] [Google Scholar]
- Kim H, Haluzik M, Asghar Z, Yau D, Joseph JW, Fernandez AM, Reitman ML, Yakar S, Stannard B, Heron-Milhavet L, Wheeler MB, LeRoith D 2003 Peroxisome proliferator-activated receptor-α agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Diabetes 52:1770–1778 [DOI] [PubMed] [Google Scholar]
- Korenblat K, Fabbrini E, Mohammed BS, Patterson B, Klein S, Effects of fenofibrate and long-acting nicotinic acid on intrahepatic triglyceride content and adipose tissue insulin sensitivity in obese human subjects. Proc of the 44th Annual Meeting of the European Association for the Study of the Liver, Copenhagen, 2009 (Abstract 57) [Google Scholar]
- Conjeevaram HS, McKenna BJ, Kang H, Oral EA, Omo J, White D, Burant CF 2009 A randomized, placebo-controlled study of PPAR-α agonist fenofibrate in patients with nonalcoholic steatohepatitis (NASH). Hepatology 50(Suppl 4):774A [Google Scholar]
- Vega GL, Cater NB, Hadizadeh 3rd DR, Meguro S, Grundy SM 2003 Free fatty acid metabolism during fenofibrate treatment of the metabolic syndrome. Clin Pharmacol Ther 74:236–244 [DOI] [PubMed] [Google Scholar]
- Nelson RH, Basu R, Johnson CM, Rizza RA, Miles JM 2007 Splanchnic spillover of extracellular lipase-generated fatty acids in overweight and obese humans. Diabetes 56:2878–2884 [DOI] [PubMed] [Google Scholar]
- Vega GL, Cater NB, Meguro S, Grundy SM 2005 Influence of extended-release nicotinic acid on nonesterified fatty acid flux in the metabolic syndrome with atherogenic dyslipidemia. Am J Cardiol 95:1309–1313 [DOI] [PubMed] [Google Scholar]
- Ridker PM 2007 C-reactive protein and the prediction of cardiovascular disease events among those at intermediate risk. J Am Coll Cardiol 49:2129–2138 [DOI] [PubMed] [Google Scholar]
- Marchesi S, Lupattelli G, Lombardini R, Roscini AR, Siepi D, Vaudo G, Pirro M, Sinzinger H, Schillaci G, Mannarino E 2003 Effects of fenofibrate on endothelial function and cell adhesion molecules during post-prandial lipemia in hypertriglyceridemia. J Clin Pharm Ther 28:419–424 [DOI] [PubMed] [Google Scholar]
- Rosenson RS, Wolff DA, Huskin AL, Helenowski IB, Rademaker AW 2007 Fenofibrate therapy ameliorates fasting and postprandial lipoproteinemia, oxidative stress, and the inflammatory response in subjects with hypertriglyceridemia and the metabolic syndrome. Diabetes Care 30:1945–1951 [DOI] [PubMed] [Google Scholar]