Abstract
The past decade, particularly the last 18 months, witnessed a vigorous increase in interest in vitamin D from both the lay and biomedical worlds. Much of the growing interest in vitamin D is powered by new data being extracted from the National Health and Nutrition Examination Survey (NHANES). The newest statistics demonstrate that more than 90% of the pigmented populace of the United States (Blacks, Hispanics, and Asians) now suffer from vitamin D insufficiency (25-hydroxyvitamin D <30 ng/ml), with nearly three fourths of the white population in this country also being vitamin D insufficient. This represents a near doubling of the prevalence of vitamin D insufficiency seen just 10 yr ago in the same population. This review attempts to provide some explanation for: 1) the rapid decline in vitamin D status in the United States; 2) the adverse impact of vitamin D insufficiency on skeletal, infectious/inflammatory, and metabolic health in humans; and 3) the therapeutic rationale and reliable means for vigorous supplementation of our diets with vitamin D.
The majority of Americans are now vitamin D insufficient, the consequences of which are being observed beyond just the musculoskeletal system.
There is renewed interest in vitamin D synthesis, metabolism, and action. The two principal driving forces for heightened interest can be traced to: 1) the worsening, worldwide trend to nutritional vitamin D insufficiency (1,2); and 2) new knowledge regarding the nonhormonal, intracrine, and paracrine actions of 1-hydroxylated vitamin D metabolites in man (3). Annual citations in the PubMed database on vitamin D were approximately 4800 from May 2008 to May 2009. This represents a doubling in the last decade and a 15% increase in the last year.
The major endogenous, synthetic source of vitamin Da for humans is the epidermis. Vitamin D3 is produced in the skin by a UVB-mediated, photolytic, nonenzymatic reaction that converts 7-dehydrocholesterol to previtamin D3 (4). Previtamin D3 undergoes a subsequent nonenzymatic, thermal isomerization conversion to vitamin D3, also in the skin. From the skin, vitamin D3 finds its way into the general circulation. In the hepatic parenchyma, vitamin D3 is converted by one of several, high-capacity cytochrome P450s to 25-hydroxyvitamin D3 (25OHD3); the microsomal CYP2R1 appears to have the highest affinity for substrate vitamin D (5). 25OHD (see Footnote 1) is the most plentiful and stable metabolite of vitamin D in human serum, qualities determined by the heightened affinity by which 25OHD is bound by the serum vitamin D binding protein and other members of the albumin superfamily of proteins found in the blood (6). As such, the 25OHD level in the serum is the best indicator of vitamin D (see Footnote 1) entering the host, either by cutaneous synthesis or by ingestion in the diet. In cross-sectional studies, especially those performed in populations living at relatively elevated latitudes in North America, Europe, and Asia, serum levels of the 25OHD metabolite are maximal some 30–60 d after peak sunlight exposure in the summer months. 25OHD is a prohormone or immediate precursor metabolite to the active form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D) (see Footnote 1). 1,25(OH)2D is the product of a single enzyme, the mitochondrial CYP27B1-hydroxylase, and it serves as a high-affinity ligand for the vitamin D receptor (VDR) in target tissues where it acts to modulate expression of vitamin D-directed genes. 1,25(OH)2D circulates in the serum at concentrations that are roughly 0.1% that of the prohormone 25OHD.
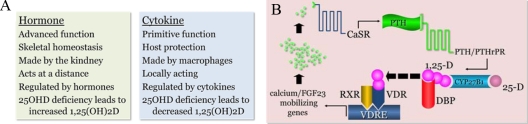
Figure 1A defines the evolutionarily distinct, but preserved, functions of vitamin D. The more evolutionarily advanced function of vitamin D is that of a hormone. This function is reserved for species bearing an endoskeleton where the 1,25(OH)2D hormone serves as a circulating regulator of both mineral and skeletal homeostasis in the host. The only recognized source of the hormone in man is the CYP27B1-hydroxylase; this enzyme is confined principally but not entirely to the proximal tubular epithelial cell of the kidney. 1,25(OH)2D synthesis in the kidney is regulated by other hormones. It is stimulated primarily by PTH and inhibited by circulating fibroblast growth factor 23 (FGF23) made by osteocytes (7).
Figure 1.
A, Summary of distinctions between the two, phylogentically discrete functions of vitamin D (see Footnote 1), one as a circulating hormone (left) and the other as a locally produced, locally active cytokine made by monocyte-macrophages (right). B, Schematic tracing the serial endocrine responses to a diminishment in vitamin D-directed intestinal calcium absorption as might be seen in humans with acquired vitamin D insufficiency/deficiency; the final step in the process is 1,25-dihydroxyvitamin D (1,25-D) stimulation of genes controlling calcium absorption from the gut, mobilization of calcium from the skeleton and, finally, synthesis and release of FGF23, which acts to return previously enhanced PTH and CYP27B1-hydroxylase gene expression to normal. DBP, Vitamin D binding protein; RXR, retinoid X receptor.
The more evolutionarily primitive function of vitamin D is that of a cytokine generated to protect the inside environment of the host (single cell organisms to man) from microbial invaders in the external environment (8). The 1,25(OH)2D cytokine is synthesized primarily by monocyte-macrophages and acts in an intracrine mode via interaction with the VDR to modulate the innate immune response to invading microbial agents (9). When produced in sufficient quantities, 1,25(OH)2D can escape the confines of the monocyte-macrophage to interact with and control the cytokine profiles of activated, VDR-expressing T- and B-lymphocytes in the local, inflammatory microenvironment (8). A key distinction between the 1,25(OH)2D hormone and cytokine systems is that an inadequate supply of substrate 25OHD will stimulate the renal CYP27B1-hydroxylase to maintain or increase production of the active, 1,25(OH)2D metabolite via secondary hyperparathyroidism, whereas a deficiency of substrate for the extrarenal CYP27B1-hydroxylase leads to a decrease in product 1,25(OH)2D.
The Problem of Vitamin D Insufficiency (see Footnote 1)
Changes in hormonal vitamin D metabolism and action occur quickly when the human host is required to defend the state of normocalcemia in the face of vitamin D insufficiency/deficiency and a reduction in the efficiency of intestinal calcium absorption. These events are depicted in Fig. 1B. A drop in intestinal calcium absorption results in a small but real decrease in the serum calcium concentration. This decrease is detected by the calcium-sensing receptor embedded in the plasma membrane of the parathyroid cell. This is a signal to release PTH from the gland and to increase PTH gene expression. Interaction of PTH with the PTH/PTHrP receptor in the plasma membrane of the proximal tubular epithelial cell of the kidney signals an increase in CYP27B1 gene expression and conversion of available substrate 25OHD to 1,25(OH)2D. 1,25(OH)2D finds its way back into the serum and onto a serum vitamin D binding protein, whereby it gains access to control regions (vitamin D responsive elements) of VDR-regulated genes in the gut and bone to: 1) promote intestinal calcium and phosphate absorption; and 2) liberate calcium and phosphate from the mineral phase of bone, respectively. When the deficit in the serum calcium concentration is corrected by such means, the activated 1,25(OH)2D:PTH axis is subsequently down-regulated by FGF23 released from bone (7).
An understanding of the above-stated physiology led Chapuy et al. (10) and subsequently others to define endocrine vitamin D (i.e. 25OHD) insufficiency/deficiency in terms of a significant increase in serum immunoreactive PTH (iPTH) levels. It is now generally agreed that the serum iPTH will start to rise significantly once the serum 25OHD drops to less than 30 ng/ml or 75 nmol/liter (2); values of 25OHD between 30 ng/ml or 75 nmol/liter and 20 ng/ml or 50 nmol/liter are considered to represent vitamin D insufficiency, whereas those less than 20 ng/ml or 50 nmol/liter fall into the frankly vitamin D-deficient range; it is usually only in the latter situation where one is likely to observe clinically apparent skeletal effects of the deficient state. In the United States, as well as in other parts of the world, the mean populational serum 25OHD levels have plummeted in the last decade. The best example of this drop comes from the National Health and Nutrition Examination Survey (NHANES) population studied in 1994 and again in 2004 (11). Although these analyses were carried out by the same group using the same assay technology, there was some variation in the 25OHD antiserum employed. Nevertheless, these minor variations in methodology cannot explain the near doubling in the number of subjects in the American population from 1994 to 2004 with 25OHD levels less than 30 ng/ml. Currently, only 20–25% of the assayed NHANES population has a serum 25OHD level of at least 30 ng/ml, whereas 25–35% of the population has frank vitamin D deficiency (i.e. 25OHD < 20 ng/ml) (12). This finding holds across all age groups, ages 12 to more than 60 yr, males and females alike. In pigmented Black and Latino segments of the population, the percentage of vitamin D-sufficient subjects is less than 10%. In the American Black population, less than 3% of mothers are vitamin D sufficient (11), and the mean cord blood level of 25OHD in the offspring of African-American mothers (10 ± 6 ng/ml) is well into the vitamin D-deficient range (13).
What underlies this dramatic decrease in vitamin D sufficiency? According to Looker et al. (14), the downward trend in 25OHD levels in the NHANES population is associated with a significant trend: 1) downward in milk consumption (in the U.S. milk products are artificially fortified with vitamin D); 2) upward in sun protection and sun avoidance; and 3) upward in body mass index (BMI). There is no doubt that more liberal use of sunscreens, sun avoidance, and milk intake have had a role to play in the descent of the serum 25OHD levels in the last decade in the United States, but this does not really explain the more exaggerated fall in serum 25OHD of 77% and 69% in pigmented African-American and Latino subpopulations, respectively, in the NHANES. This suggests that obesity, as will be discussed in more detail below, may be an especially important contributor to the vitamin D insufficient/deficient state in this subgroup of subjects.
Adverse Outcomes Associated with Vitamin D Insufficiency
Musculoskeletal system
We have known for almost two centuries now that increased sunlight exposure was a critical factor in the correction of most rachitic syndromes (soft bones and proximal muscle weakness) in man (15). Hence, it is not surprising that in 2008–2009 we observe a low 25OHD level in the serum to be significantly associated with low bone mineral density (16) and increased risk of nonvertebral (17) and hip fracture (18). For example, results from NHANES III (16) have disclosed that low bone density in the hip, a surrogate measure of fracture risk at that site, in both men and women is directly related to the serum 25OHD level but unrelated to the dietary calcium intake of the host. Furthermore, data from the Women’s Health Initiative (18) indicate that for every 10 ng/ml decrease in the serum 25OHD level there is a significant trend upward in the odds ratio of risk for hip fracture (unadjusted and adjusted), with a near doubling of that odds ratio if one has a serum 25OHD in the frankly vitamin D-deficient range (<20 ng/ml). It is assumed that the decrease in 25OHD leads to a state of persistent secondary hyperparathyroidism with enhanced osteoclastogenesis and consequent increased bone resorption outstripping osteoblast-directed bone formation (Fig. 1B). In fact, the consequences of vitamin D deficiency in the mother can be observed in the fetal skeleton as early as 19 wk of human gestation. Mahon et al. (19) have recently demonstrated a rachitic phenotype in the fetal femur, the severity of which is directly associated with a decreasing 25OHD level in the maternal circulation.
Microbial disease
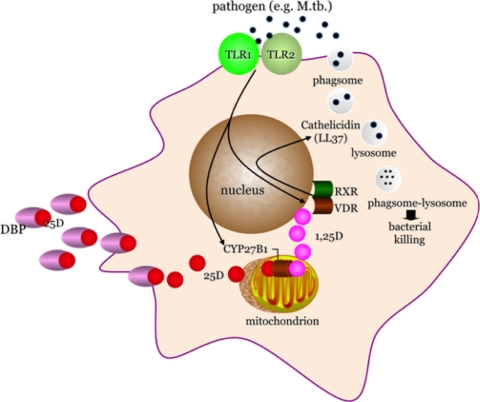
Recent studies in humans have emphasized a potential role for the vitamin D monokine system as a necessary intermediate in the generation of antimicrobial peptides by monocyte-macrophages (9). In these and subsequent experiments with human monocyte-macrophages (8,20,21,22), it is now clear that activation of the Toll-like receptor (TLR) pathway in the human monocyte-macrophage by pathogen-associated membrane patterns, or PAMPs, shed by microbial agents like Mycobacterium tuberculosis initiate expression of the CYP27B1-hydroxylase and VDR genes in that cell (Fig. 2). If insufficient substrate 25OHD is available to the macrophage CYP27B1-hydroxylase, as is the case in vitamin D insufficiency/deficiency (23), then insufficient product 1,25(OH)2D will be generated locally. This, in turn, will diminish binding of 1,25(OH)2D to the macrophage VDR, thereby limiting the activation of 1,25-(OH)2D-VDR-directed antimicrobial genes (i.e. cathelicidin) and associated killing of ingested microbe. These events appear to be borne out in a metaanalysis of M. tuberculosis infection rates (effect sizes) in humans; compared with matched control subjects from the same population, patients with active tuberculosis possessed significantly lower serum 25OHD levels (24). These kinds of data with tuberculosis have prompted investigators to look at the effect of low 25OHD levels on other microbial infections. For example, Ginde et al. (25) recently demonstrated that the prevalence of upper respiratory tract infections in the NHANES III population 1) increased significantly, regardless of season of the year, as the serum 25OHD dropped; and 2) was greatest during winter months when 25OHD levels were at their lowest. Other recent studies have shown that 1,25-(OH)2D-directed production of cathelicidin is not restricted to monocyte-macrophages. Indeed, this appears to involve an antimicrobial mechanism also employed by epithelial cells from a variety of tissue “barrier” sites, like the gut (26), lung (27), placenta (28), and skin (29,30,31,32). In contrast to the stimulation of antibacterial responses by vitamin D, its impact on viral infection is much less clear. However, the ability of viruses to activate TLR-induced pathways similar to those activated by mycobacteria and bacteria suggests that induction of intracrine immune responses to vitamin D may also promote antiviral activity.
Figure 2.
Shown is a schematic of a human macrophage responding to pathogen-associated membrane patterns (PAMPs), shed from wall of M. tuberculosis via the pattern recognition, TLR dimmer pair, TLR1–TLR2. Signaling through the TLRs leads to induction of expression of the endogenous CYP27B1-hydroxylase and VDR. Provided that adequate amounts of substrate 25-hydroxyvitamin D (25D), circulated to the monocyte-macrophage bound to the serum vitamin D binding protein (DBP), are available to the mitochondrial CYP27B1 for intracrine conversion to 1,25-dihydroxyvitamin D (1,25D), the 1,25D ligand activates the VDR; instigates its dimerization with retinoid X receptor (RXR) and nuclear localization of the VDR-RXR; and promotes expression of the cathelicidin antimicrobial gene. In turn, the cathelicidin gene product, LL37, supports the phagolysosomal killing of ingested mycobacterium.
All-cause and cardiovascular mortality
Although low 25OHD levels have been known to be associated for some time now with colon cancer mortality (33), only recently have data emerged demonstrating that vitamin D insufficiency/deficiency is significantly associated with all-cause mortality, at least in the American population (Table 1). The NHANES III database clearly shows: 1) an increase in adjusted all-cause mortality as the serum 25OHD level falls to less than 30 ng/ml, especially in women; and 2) peak protection from death with a 25OHD level in the 35–40 ng/ml range. In fact, most of the increase in all-cause mortality can be attributed to cardiovascular disease deaths in this population (Table 1). The prevalence of coronary artery disease, heart failure, and peripheral artery disease is significantly increased in a stepwise fashion as the serum 25OHD level drops to less than 30 and then 20 ng/ml (34).
Table 1.
Recent association studies demonstrating a significant inverse correlation between the serum 25D level and an increase in components of the human metabolic syndrome
| Mortality causes | First author | Year | Ref. |
|---|---|---|---|
| All causes | Melamed | 2008 | Arch Intern Med 168:1629 |
| Dobnig | 2008 | Arch Intern Med 168:1340 | |
| Cardiovascular disease | Kim | 2008 | Am J Cardiol 102:1540 (34) |
| Wang | 2008 | Circulation 117:503 | |
| Kendrick | 2009 | Atherosclerosis 205:255 | |
| Hypertension | Judd | 2008 | Am J Clin Nutr 87:136 |
| BMI | Looker | 2008 | Am J Clin Nutr 88:1519 (14) |
| Insulin resistance | Liu | 2009 | J Nutr 139:329 |
| Wu | 2009 | J Nutr 139:547 |
Metabolic disease
Inspection of the recent vitamin D insufficiency/deficiency epidemiological literature has brought to light the striking inverse correlation of the 25OHD level not only to cardiovascular disease but also to essentially all of the elements of the human metabolic syndrome (Table 1): 1) hypertension; 2) obesity; 3) insulin resistance and glucose intolerance. In fact, the only age-adjusted trend in the NHANES population that matches the magnitude of the rate of decrease in the serum 25OHD level over time is the rate of increase in the BMI in that period (National Center for Health Statistics, 2009). If one looks at the NHANES III data set, there is a significant stepwise quartile drop in the serum 25OHD level with the reverse quartile trend upward in both percentage body weight and BMI in that population (12). A similar trend can be observed in smaller studies of adolescents, particularly postpubertal females of Hispanic ethnicity (35).
Is There a Vitamin D:Fat:Bone Axis in Humans?
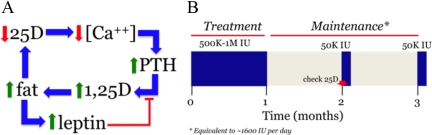
The preceding discussion with regard to the “association” of increasing adiposity and its metabolic consequences (e.g. cardiovascular disease) with vitamin D insufficiency/deficiency begs the question of whether there is a causal link between the two. For example, it has recently been shown that the serum 25OHD level rises significantly in extremely obese patients after undergoing intestinal bypass surgery (36) that induces a rapid reduction in fat mass in the host. These observations suggest that low 25OHD levels observed in association with obesity may be due in part to: 1) the increased volume of distribution to fat of lipid soluble vitamin D as it leaves the general circulation after being synthesized in the skin or obtained through the diet; and 2) preferential retention of vitamin D in those fat stores. Although not yet performed in vivo in humans, studies with mice also indicate that: 1) vitamin D deficiency, in and of itself, increases body weight (Hewison, M., unpublished data); and 2) null mutations in the VDR or the CYP27B1α-hydroxylase have the opposite effect, to prevent abdominal fat mass accumulation and weight gain in mice on a normal chow (37) and as well as on a high-fat diet (38). As depicted in Fig. 3A, these data suggest that systemic 25OHD deficiency, which is known to result in secondary hyperparathyroidism and stimulation of the renal CYP27B1-hydroxylase, is associated with maintenance of the serum 1,25(OH)2D in the normal range (7) until the substrate 25OHD level in blood drops to less than 10 nmol/liter or 4 ng/ml (39). Acting through the VDR, these events result in an increase in fat mass by yet unknown means. It is hypothesized that this increase in body fat mass acts in a “feed-forward” fashion to further increase the volume of distribution for vitamin D, deepen the state of vitamin D deficiency, and amplify the consequences of secondary hyperparathyroidism on the skeleton (Fig. 1A) and body fat. There are some data now (40) to suggest that there exists a leptin-mediated “negative feedback” loop to interrupt this cycle of fat accumulation. It is well known that an increase in fat mass is associated with increased fat-derived, serum leptin. Leptin may act as a brake on this cycle by providing a means of static inhibition of CYP27B1 gene expression and 1-hydroxylase enzyme activity in the kidney and/or adipose tissue itself (41).
Figure 3.
A, Proposed schema linking low serum 25D levels and secondary hyperparathyroidism with 1,25-dihydroxyvitamin D (1,25D)-driven fat accumulation amplifying the state of vitamin D depletion, while at the same time generating an increase in the serum leptin concentration, which exerts feedback inhibition on the synthesis of 1,25D. B, Schematic of the regimen for vitamin D replacement and maintenance therapy in subjects with serum 25D levels less than 30 ng/ml. The regimen calls for: 1) delivery of 500,000 to 1,000,000 IU vitamin D (see Footnote 1) orally over 4–5 wk; 2) a similarly timed period of no vitamin D to permit ascertainment and confirmation of steady-state 25D levels; and 3) maintenance therapy of 50,000 IU vitamin D orally every month thereafter to maintain a vitamin D sufficiency.
Vitamin D Replacement
Restoration of serum 25OHD to normal
Although cause-and-effect data from human studies are still lacking, there is reason to believe from the above presented population association and small animal analyses that there is sound rationale for avoiding vitamin D depletion. That being the case and because vitamin D (i.e. 25OHD) insufficiency/deficiency is rampant worldwide, how does one go about: 1) replenishing vitamin D stores, and 2) maintaining normal 25OHD levels thereafter? Figure 3B depicts the therapeutic replacement strategy that we employ for our clinical studies (23,42). After first measuring the serum calcium, 25OHD, and iPTH level to establish the presence or absence of vitamin D insufficiency/deficiency and degree of secondary hyperparathyroidism if present, and second, ruling out underlying hypercalcemia, hypercalciuria, and primary hyperparathyroidism, the subject receives 500,000 IU vitamin D (see Footnote 1) orally in divided 50,000 IU doses over the course of 4 to 5 wk. After a second 4- to 5-wk period of equilibration without drug to permit stabilization of the serum 25OHD concentration, the serum 25OHD level is obtained to determine whether levels are greater than 30 ng/ml. If so, the subject is given 50,000 IU ergocalciferol (vitamin D2) (43) or cholecalciferol (vitamin D3) orally on a monthly basis to maintain vitamin D sufficiency. The serum 25OHD is monitored annually to ensure sufficiency. The serum iPTH is also redetermined at this interval to ensure that it has not increased from baseline, which may be indicative of coexistent primary hyperparathyroidism, a not uncommon occurrence (44). If the 25OHD does not normalize at the 10- to 12-wk mark, the replacement and monitoring regimen is repeated. If the serum 25OHD is still subnormal after two rounds of vitamin D replacement and compliance with the treatment regimen is documented, then a search for and treatment of intestinal fat malabsorption is undertaken. Considering that parenteral delivery of vitamin D is no longer an option in the United States, a program of more vigorous oral vitamin D supplementation or carefully graded whole body UVB radiation exposure (45) is a therapeutic option.
Controversy exists as to whether replacement therapy should be achieved with ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). A study performed early in the decade showed that a 50,000-IU oral dose of vitamin D2, compared with the same oral dose of vitamin D3, first seemed to be associated with a higher volume of distribution and/or less effective conversion to 25OHD2 (e.g. lower peak blood levels of 25OHD after dosing); and second, that there was more rapid clearance of converted 25OHD2 from the serum (46). There was no mention in this study as to whether subjects taking vitamin D2 and vitamin D3 were matched for basal 25OHD concentration or BMI. A more recent study by Holick et al. (47) of vitamin D-insufficient subjects given much smaller daily doses (1000 IU) of vitamin D2, vitamin D3, a combination of vitamin D2 and vitamin D3 (500 IU each), or placebo for 11 wk showed no difference in the incremental increase in total 25OHD levels among the vitamin D treatment regimens; all significantly increased the serum 25OHD, albeit not to the 25OHD-sufficient range (>30 ng/ml). There was no assessment of a vitamin D-specific bioresponse in either of these studies. As a consequence of the above, all of our currently active, prospective vitamin D replacement trials compare the oral administration vitamin D2 (Swanson Health Products; not FDA-approved) and vitamin D3 preparations (Calciferol, Schwartz Pharma; FDA-approved) each at a concentration of 1000 IU (in vegetable oil) per drop and use a relevant biomarker of vitamin D action (e.g. serum iPTH for skeletal and ex vivo host macrophage cathelicidin gene expression (23) (see Fig. 2 for innate immunity outcomes).
Maintenance of 25OHD sufficiency
Maintenance of vitamin D sufficiency has become a substantial problem because of: 1) self-imposed limitations on sunlight exposure; 2) the current underestimates for recommended daily intake of vitamin D (100–800 IU daily depending on age and disease); 3) the relative scarcity of vitamin D in our diets (see below); and perhaps iv) increasing body fat mass in populations around the world. The most plentiful natural sources of vitamin D3 and vitamin D2 in our diet are derived from wild-caught, not farm-raised, fatty fishes like salmon (∼1,000 IU per 3.5 ounces; Ref. 48) and UVB-exposed fungoids (i.e. mushrooms; up to 100,000 IU per 3.5 ounces; Ref. 49), respectively. However, because of expense and lack of quality control over vitamin D content of cultivated products, neither fish nor irradiated mushroom ingestion is currently recommended as a reliable means of vitamin D supplementation, especially if one considers the fact that the new recommended daily intake for vitamin D intake to maintain vitamin sufficiency is likely to increase significantly in the near future (www.iom.edu). As such, in the absence of sufficient cutaneous vitamin D3 production, maintenance of vitamin D sufficiency is routinely achieved by the daily consumption of dietary supplements containing synthetic vitamin D3 or vitamin D2.
Measuring 25OHD
There is also substantial controversy regarding the best commercial methodology for measuring 25OHD in the serum of humans (50). Immunoassay remains the “gold standard.” It has been employed by most of the large-scale population studies performed in the recent past in the United States (e.g. NHANES and the Women’s Health Initiative). This assay technology measures total 25OHD after solid-phase extraction of the serum. It measures 25OHD2 and 25OHD3 equally well and does not discriminate between the two. Liquid chromatography-tandem mass spectrometric analysis requires lipid extraction of the serum before assay and measures 25OHD2 and 25OHD3 separately. It is the fastest growing method; however, normative standards for commercially available assays are not yet published. Finally, HPLC is employed by a few commercial labs. Like liquid chromatography-tandem mass spectrometric analysis, it requires lipid extraction of the serum and measures 25OHD2 and 25OHD3 separately. Normative standards exist, but the technology is hampered by the fact that it is time-consuming and expensive.
Conclusions
The last year in clinical vitamin D research has confirmed the presence of a worldwide problem with vitamin D depletion, a problem that appears to be worsening. Large-scale population studies bear out long-held concerns that low serum 25OHD levels are associated with a number of adverse outcomes in the human musculoskeletal, innate immune, and cardiovascular systems; in fact, low vitamin D levels are significantly associated with all-cause mortality in the U.S. population. It is hypothesized that the rise in global obesity contributes to the worsening of the problem of vitamin D insufficiency/deficiency, amplifying adverse impacts on the host skeleton, immunoreactivity to microbes, and metabolic status. Because of its frequency, its ease of detection, its associated adverse outcomes, and the straightforward, inexpensive and effective means by which is can be treated, vitamin D insufficiency should be sought especially when evaluating and treating osteoporotic, otherwise immuno-compromised, and obese subjects. Finally, it should be remembered that treatment of vitamin D insufficiency/deficiency has two phases: 1) restoration of 25OHD levels to more than 30 ng/ml; and 2) maintenance of the serum 25OHD in that range.
Footnotes
This work was supported by Grants RO1 AR037399-22, RO1 AR050626-05, RO1 AI073539-02, and RO1 EB009306-02 from National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Expression of vitamin D or vitamin D metabolites without a subscript refers to the total compliment of both vitamin D2 and vitamin D3 or total compliment of vitamin D2 and vitamin D3 metabolites, respectively, in the sample.
Abbreviations: BMI, Body mass index; FGF23, fibroblast growth factor 23; iPTH, immunoreactive PTH; 1,25(OH)2D, 1,25-dihydroxyvitamin D; OHD, 25-hydroxyvitamin D; TLR, Toll-like receptor; VDR, vitamin D receptor.
References
- Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J 2009 Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 20:1807–1820 [DOI] [PubMed] [Google Scholar]
- Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P 2009 Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res 24:693–701 [DOI] [PubMed] [Google Scholar]
- Hewison M 2008 Vitamin D and innate immunity. Curr Opin Investig Drugs 9:485–490 [PubMed] [Google Scholar]
- Holick MF 2008 Vitamin D: a D-lightful health perspective. Nutr Rev 66:S182–194 [DOI] [PubMed] [Google Scholar]
- Strushkevich N, Usanov SA, Plotnikov AN, Jones G, Park HW 2008 Structural analysis of CYP2R1 in complex with vitamin D3. J Mol Biol 380:95–106 [DOI] [PubMed] [Google Scholar]
- Chun RF, Adams JS, Hewison M 2008 Back to the future: a new look at ‘old’ vitamin D. J Endocrinol 198:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles LD 2008 Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118:3820–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JS, Hewison M 2008 Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 4:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL 2006 Activation of human TLR2/1 triggers a vitamin D receptor-dependent antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ 1997 Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439–443 [DOI] [PubMed] [Google Scholar]
- Ginde AA, Liu MC, Camargo Jr CA 2009 Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 169:626–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetley EA 2008 Assessing the vitamin D status of the US population. Am J Clin Nutr 88:558S–564S [DOI] [PubMed] [Google Scholar]
- Greer FR 2008 25-Hydroxyvitamin D: functional outcomes in infants and young children. Am J Clin Nutr 88:529S–533S [DOI] [PubMed] [Google Scholar]
- Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA 2008 Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr 88:1519–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozolowski W 1939 Jedrzej Sniadecki (1768–1838) on the cure of rickets. Nature 143:121 [Google Scholar]
- Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B, Orav JE, Li R, Spiegelman D, Dietrich T, Willett WC 2009 Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res 24:935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J 2009 Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med 169:551–561 [DOI] [PubMed] [Google Scholar]
- Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, Lee JS, Jackson RD, Robbins JA, Wu C, Stanczyk FZ, LeBoff MS, Wactawski-Wende J, Sarto G, Ockene J, Cummings SR 2008 Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med 149:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, Swaminathan R, Cooper C, Godfrey K 2009 Low maternal vitamin D status and fetal bone development: a cohort study. J Bone Miner Res doi: 10.1359/jbmr.090701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Tang DH, Modlin RL 2007 Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 179:2060–2063 [DOI] [PubMed] [Google Scholar]
- Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL 2008 IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol 181:7115–7120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zügel U, Hollis BW, Cheng G, Modlin RL 2009 Convergence of IL-1ß and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One 4:e5810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M 2009 Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 182:4289–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnoaham KE, Clarke A 2008 Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol 37:113–119 [DOI] [PubMed] [Google Scholar]
- Ginde AA, Mansbach JM, Camargo Jr CA 2009 Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 169:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, Hollis B, DeLuca HF, Adams JS, Hewison M 2008 Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology 149:4799–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW 2008 Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol 181:7090–7099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, Hewison M 2009 Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod 80:398–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Oda Y, Büchau AS, Yun QC, Steinmeyer A, Zügel U, Bikle DD, Gallo RL 2008 Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol 128:816–824 [DOI] [PubMed] [Google Scholar]
- Segaert S 2008 Vitamin D regulation of cathelicidin in the skin: toward a renaissance of vitamin D in dermatology? J Invest Dermatol 128:773–775 [DOI] [PubMed] [Google Scholar]
- Peric M, Koglin S, Kim SM, Morizane S, Besch R, Prinz JC, Ruzicka T, Gallo RL, Schauber J 2008 IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol 181:8504–8512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Büchau A, Steinmeyer A, Zügel U, Ruzicka T, Schauber J 2009 Vitamin D analogs differentially control antimicrobial peptide “alarmin” expression in psoriasis. PLoS One 4:e6340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Feskanich D, Fuchs CS, Willett WC, Hollis BW, Giovannucci EL 2007 A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst 99:1120–1129 [DOI] [PubMed] [Google Scholar]
- Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ 2008 Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol 102:1540–1544 [DOI] [PubMed] [Google Scholar]
- Kremer R, Campbell PP, Reinhardt T, Gilsanz V 2009 Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab 94:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luis DA, Pacheco D, Izaola O, Terroba MC, Cuellar L, Martin T 2008 Clinical results and nutritional consequences of biliopancreatic diversion: three years of follow-up. Ann Nutr Metab 53:234–239 [DOI] [PubMed] [Google Scholar]
- Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J 2009 Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology 150:651–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KE, Szeto FL, Zhang W, Ye H, Kong J, Zhang Z, Sun XJ, Li YC 2009 Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am J Physiol Endocrinol Metab 296:E820–E828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need AG, O'Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE 2008 Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res 23:1859–1863 [DOI] [PubMed] [Google Scholar]
- Matsunuma A, Horiuchi N 2007 Leptin attenuates gene expression for renal 25-hydroxyvitamin D3–1α-hydroxylase in mice via the long form of the leptin receptor. Arch Biochem Biophys 463:118–127 [DOI] [PubMed] [Google Scholar]
- Li J, Byrne ME, Chang E, Jiang Y, Donkin SS, Buhman KK, Burgess JR, Teegarden D 2008 1α,25-Dihydroxyvitamin D hydroxylase in adipocytes. J Steroid Biochem Mol Biol 112:122–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller JL, Hu B, Reed S, Mirocha J, Adams JS 2008 Increase in bone mass after correction of vitamin D insufficiency in bisphosphonate-treated patients. Endocr Pract 14:293–297 [DOI] [PubMed] [Google Scholar]
- Pepper KJ, Judd SE, Nanes MS, Tangpricha V 2009 Evaluation of vitamin D repletion regimens to correct vitamin D status in adults. Endocr Pract 15:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastell R, Arnold A, Brandi ML, Brown EM, D'Amour P, Hanley DA, Rao DS, Rubin MR, Goltzman D, Silverberg SJ, Marx SJ, Peacock M, Mosekilde L, Bouillon R, Lewiecki EM 2009 Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab 94:340–350 [DOI] [PubMed] [Google Scholar]
- Khazai NB, Judd SE, Jeng L, Wolfenden LL, Stecenko A, Ziegler TR, Tangpricha V 2009 Treatment and prevention of vitamin D insufficiency in cystic fibrosis patients: comparative efficacy of ergocalciferol, cholecalciferol, and UV light. J Clin Endocrinol Metab 94:2037–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armas LA, Hollis BW, Heaney RP 2004 Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89:5387–5391 [DOI] [PubMed] [Google Scholar]
- Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD 2008 Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93:677–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A, Kohn N, Martinello S, Berkowitz R, Holick MF 2007 Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys 460:213–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JS, Teichert A, McHugh TH 2008 Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J Agric Food Chem 56:4541–4544 [DOI] [PubMed] [Google Scholar]
- Hollis BW 2008 Measuring 25-hydroxyvitamin D in a clinical environment: challenges and needs. Am J Clin Nutr 88:507S–510S [DOI] [PubMed] [Google Scholar]