Abstract
Context: Low bone mineral density (BMD) is commonly reported in young men and women with HIV infection, and fracture rates may be higher. With effective antiretroviral therapy (ART), the HIV population is aging. However, little is known about the skeletal status of postmenopausal women.
Objective: We aimed to assess the effects of HIV infection and ART on BMD and bone turnover in postmenopausal minority women.
Design, Setting, and Patients: A prospective cohort study was performed in 92 HIV+ and 95 HIV− postmenopausal Hispanic and African-American women.
Main Outcome Measures: We measured BMD by dual-energy x-ray absorptiometry, fracture prevalence, serum levels of inflammatory cytokines (TNFα, IL-6), bone turnover markers, calciotropic hormones, and estrone.
Results: HIV+ women were younger (56 ± 1 vs. 60 ± 1 yr; P < 0.01) and had lower BMI (28 ± 1 vs. 30 ± 1 kg/m2; P < 0.01) and estrone levels. Prevalence of T scores below −1.0 was greater in HIV+ women at the spine (78 vs. 64%; P < 0.05), total hip (45 vs. 29%; P < 0.05), and femoral neck (64 vs. 46%; P < 0.05), and Z scores adjusted for BMI were lower in HIV+ women at the same sites. Serum TNFα, N-telopeptide, and C-telopeptide were significantly higher in HIV+ than HIV− women, particularly those receiving ART. HIV+ status was independently and negatively associated with spine and hip BMD after adjustment for age, ethnicity, BMI, and alcohol.
Conclusion: The lower BMD, higher prevalence of low BMD, and higher levels of bone turnover markers detected in HIV+ postmenopausal minority women could place them at high risk for future fractures.
Bone density is lower and bone turnover markers higher in postmenopausal minority HIV+ women than HIV− controls.
Antiretroviral therapy (ART) has dramatically increased life expectancy of HIV+ individuals. By 2015, more than half of HIV+ individuals in the United States will be over age 50 (1). As these patients live longer, they develop common diseases of aging, such as cardiovascular and renal disease and osteoporosis (2), possibly at a younger age than the general population (3).
Low bone mineral density (BMD) has been reported in many cross-sectional studies of younger HIV+ individuals (4,5,6,7,8,9,10), but relatively few studies of middle-aged and older men and women (11,12,13). Lower BMD measurements are strongly related to the generally lower body weight of HIV+ individuals (5,14,15,16). In addition, some studies suggest that HIV infection and ART affect the skeleton directly. BMD declines by 2–6% within the first 2 yr of initiating various ART regimens (17,18,19) but is relatively stable in patients on established ART (16,20). Of concern, a recent study suggests that fractures occur more commonly in HIV+ men and women, especially in older individuals (21).
Although numbers of HIV-infected postmenopausal women are increasing and postmenopausal women are at highest risk for osteoporotic fractures, few studies have evaluated skeletal status in this group. Hypothesizing that postmenopausal women might be particularly vulnerable to any adverse effects of HIV infection or ART on the skeleton, we initiated a longitudinal study to assess the prevalence of osteoporosis/osteopenia, determinants and etiology of low BMD, and rates of bone loss in HIV+ postmenopausal Hispanic and African-American women, who constitute the majority of new infections in women in the United States (22). Here, we summarize cross-sectional analyses of the baseline visit of the study.
Subjects and Methods
Subjects
Between 2002 and 2007, we screened 108 HIV− and 110 HIV+ postmenopausal women from Columbia University Medical Center (CUMC) and Bronx-Lebanon Hospital Center (BLHC) in New York City. HIV− controls were volunteers who were attending the General Internal Medicine Clinics of CUMC. They were recruited through their providers who were informed about the study and asked to refer postmenopausal women under their care. Our intention was to recruit HIV− controls similar to HIV+ subjects with regard to other chronic medical illnesses and comorbidities that are highly prevalent in our inner-city population of minority women. Eligible subjects were over age 40, Hispanic or African-American, and postmenopausal as defined by more than 1 yr of amenorrhea and FSH above 30 mIU/ml, or FSH above 20 mIU/ml and estradiol below 30 pg/ml, or age above 55 regardless of FSH and estrogen. Women were excluded with metabolic bone disease, multiple myeloma, cancer, endocrinopathies, serum creatinine above 1.5 mg/dl, celiac or inflammatory bowel disease, current glucocorticoid or anticonvulsant use, current hormone replacement therapy, and current or past treatment of osteoporosis. Thirteen HIV− and 18 HIV+ women were ineligible; 95 HIV− and 92 HIV+ women were enrolled. The first 40 HIV+ women were reported previously (23).
Medical, surgical, and reproductive history, osteoporosis risk factors, current and past medication history, and HIV and ART history were obtained by interview and chart review. For hysterectomized women, time since menopause was estimated as years since onset of menopausal symptoms; if none, menopausal age was set at 50. Control subjects met the same criteria; ELISA testing verified HIV-negative status.
All subjects gave written informed consent. The Institutional Review Boards of CUMC and BLHC approved the study.
BMD
BMD of the lumbar spine (LS; L1–L4), femoral neck (FN), total hip (TH), non-dominant 1/3 radius, and body composition were measured by dual-energy x-ray absorptiometry (DXA) on a QDR 4500 bone densitometer (Hologic, Inc., Bedford, MA) at CUMC. Short-term in vivo reproducibility is 0.68% for the LS, 1.36% for the TH, and 0.70% for the radius.
T scores, which compare subjects to young individuals of the same sex and race, were derived from the National Health and Nutrition Examination Survey (NHANES III). Osteoporosis and osteopenia were defined by World Health Organization criteria for postmenopausal Caucasian women: T scores of −2.5 or less represent osteoporosis; T scores between −1.0 and −2.49 represent osteopenia (24). Z scores, comparing BMD to an age-, sex-, and ethnicity-matched reference population, were also calculated (12). Height and weight were measured by Harpenden stadiometer and balance beam scale, respectively.
Fracture ascertainment
Spine radiographs were performed at CUMC according to the Study of Osteoporotic Fractures protocol (25). The semiquantitative method, which uses visual inspection and characterizes vertebrae as having no, mild, moderate, or severe deformity, was employed to diagnose vertebral fractures. The validity of this method compares favorably with morphometric assessment (25).
Dietary calcium
Dietary calcium intake was estimated by a dietary rapid assessment questionnaire (26). Calcium supplementation was determined separately.
Laboratory methods
Serum calcium was measured by autoanalyzer (normal, 8.4–10.2 mg/dl) and albumin by colorimetry (Roche Diagnostics, Indianapolis, IN) in CUMC Laboratories; albumin-adjusted calcium levels are reported. Fasting morning serum, stored at −80 C, was batch-analyzed in research laboratories for: PTH (RIA; Corning-Nichols Laboratory, San Clemente, CA); serum 25-hydroxyvitamin D (25-OHD) (RIA; Diasorin, Stillwater, MN); serum 1,25 dihydroxyvitamin D (RIA; Diasorin); estrone (RIA; Diagnostic Systems Laboratories, Inc., Webster, TX); bone-specific alkaline phosphatase (BAP) (ELISA; Quidel Corp., San Diego, CA); osteocalcin (OC) (RIA; Immutopics, San Clemente, CA); and cross-linked N-telopeptide of type I bone collagen (NTx) by competitive-inhibition ELISA (Inverness Medical, Princeton, NJ). C-telopeptide of type 1 collagen (CTx) was measured in 146 subjects by sandwich ELISA (Serum Crosslaps; IDS Ltd., Fountain Hills, AZ). IL-6 was measured by RIA (Diasorin). TNFα was measured by ELISA (Quantikine; R&D Systems, Minneapolis, MN).
CD4 counts were measured by flow cytometry (FACS Calibur; Becton Dickinson, San Jose, CA). HIV-1 RNA was quantified by PCR using the AMPLICOR HIV-1 MONITOR Ultrasensitive Test (version 1.5) with a linear range of 50–100,000 copies/milliliter (Roche Diagnostics).
Statistical analysis
The primary outcome was BMD at four sites between HIV+ and HIV− groups without adjustment for testing multiple outcomes. Comparisons between HIV treatment subgroups were exploratory. Continuous variables were examined for normality with the Kolmogorov-Smirnov univariate normality test; none met criteria for transformation (D-statistics P values <0.05). Between-group differences in categorical measures were assessed with Fisher’s exact test. Estimation of between-group differences in continuous measures and comparisons involving covariate adjustment used one-way ANOVA and analyses of covariance, respectively.
Univariate associations of demographic, anthropometric, reproductive, medical, and lifestyle variables with areal BMD at each site were examined to identify variables associated with low BMD. These variables entered a stepwise multiple regression (P of 0.20 to enter and 0.05 to be retained in the model; SAS Proc REG; SAS Institute, Cary, NC) and partial correlation coefficients were used to identify variables independently related to BMD. Collinearity was assessed, and model-selected nonoverlapping covariates were retained for inclusion in final inferential models. Regression models with these covariates were then created to assess the contribution of HIV status to the prediction of BMD over and above that accounted for by known risk factors for low BMD. Subsequently, bone turnover markers (BTMs) and cytokine levels were forced into the regression model to examine their role as modifiers of the association of HIV status with BMD. Serum CTx and estrone were not entered into the multivariate model because results were available for 73 and 85% of the study participants, respectively.
With the sample size of 95 HIV− and 92 HIV+ women, the study had 80% power and 5% two-tailed α level to detect a 0.42 standardized difference between groups. Data are presented as means ± se values unless otherwise noted. P values of <0.05 were considered significant.
Results
Characteristics of the study population
HIV+ women were approximately 4 yr younger, weighed less, and had lower body mass index (BMI) than HIV− women (Table 1). HIV+ women were similar with respect to years since menopause; were less likely to have had surgical menopause, hypertension, hyperlipidemia, and to take statins and calcium supplements; and were more likely to be hepatitis C seropositive and to take multivitamins. HIV+ women on ART (HIV+ART+) had comparable BMI to women currently not on ART (HIV+ART−) but had lower body fat, were less likely to consume alcohol, and had lower HIV-1 RNA levels.
Table 1.
Characteristics of study population
| HIV− | HIV+ | P | HIV+ART− | HIV+ART+ | P | |
|---|---|---|---|---|---|---|
| n | 95 | 92 | 19 | 73 | ||
| Demographic and anthropomorphic features | ||||||
| Age | 59.6 ± 0.7 | 55.9 ± 0.7 | <0.01 | 55.6 ± 1.3 | 56.0 ± 0.8 | 0.80 |
| Race/ethnicity (%) | 0.16 | 0.60 | ||||
| Hispanic | 73 | 63 | 58 | 64 | ||
| African-American | 27 | 37 | 42 | 36 | ||
| Weight (kg) | 76.6 ± 1.8 | 68.6 ± 1.6 | <0.01 | 74.9 ± 4.7 | 67.0 ± 1.6 | 0.13 |
| Lowest adult weight (kg) | 57.6 ± 1.3 | 57.2 ± 1.2 | 0.84 | 58.5 ± 3.9 | 56.9 ± 1.2 | 0.68 |
| BMI (kg/m2) | 30.4 ± 0.7 | 27.6 ± 0.6 | <0.01 | 29.0 ± 1.9 | 27.2 ± 0.6 | 0.38 |
| Body fat (%) | 40.7 ± 0.6 | 34.7 ± 0.9 | <0.0001 | 39.7 ± 1.8 | 33.5 ± 0.9 | <0.01 |
| Truncal fat (%) | 40.9 ± 0.7 | 35.4 ± 0.9 | <0.0001 | 38.3 ± 1.6 | 34.6 ± 1.0 | 0.09 |
| Reproductive/gynecological history | ||||||
| Age at menarche (yr) | 12.8 ± 0.2 | 12.9 ± 0.2 | 0.80 | 12.1 ± 0.4 | 13.0 ± 0.2 | 0.08 |
| Premenopausal oligomenorrhea (%) | 6 | 12 | 0.18 | 16 | 11 | 0.69 |
| Years since menopause | 12.1 ± 0.8 | 9.8 ± 1.2 | 0.11 | 7.8 ± 1.4 | 10.2 ± 1.5 | 0.24 |
| TAH/BSO (%) | 22 | 4 | <0.001 | 11 | 3 | 0.19 |
| Osteoporosis risk factors | ||||||
| FH of osteoporosis (%) | 24 | 21 | 0.56 | 37 | 16 | 0.06 |
| Current smoker (%) | 19 | 29 | 0.10 | 21 | 32 | 0.57 |
| Cigarette pack-years | 7.2 ± 1.6 | 9.8 ± 1.8 | 0.27 | 10.4 ± 3.1 | 9.6 ± 2.1 | 0.86 |
| Alcohol (>1 drink/day) (%) | 3 | 12 | 0.03 | 26 | 8 | 0.046 |
| Intravenous drug use (ever) (%) | 6 | 14 | 0.09 | 21 | 12 | 0.46 |
| Chronic medical conditions (%) | ||||||
| Hyperlipidemia | 4 | 22 | <0.001 | 21 | 22 | 1.00 |
| Asthma | 17 | 24 | 0.23 | 16 | 26 | 0.55 |
| Hypertension | 53 | 36 | 0.02 | 37 | 36 | 1.00 |
| Cardiovascular disease | 4 | 8 | 0.32 | 11 | 7 | 0.63 |
| Type 2 diabetes | 24 | 23 | 0.82 | 16 | 25 | 0.55 |
| Hepatitis C seropositive | 6 | 22 | <0.01 | 37 | 18 | 0.11 |
| Medications (%) | ||||||
| Levothyroxine | 4 | 2 | 0.68 | 0 | 3 | 1.00 |
| Oral hypoglycemics | 24 | 18 | 0.34 | 16 | 19 | 1.00 |
| Insulin | 8 | 8 | 0.84 | 11 | 7 | 0.63 |
| Statins | 38 | 14 | <0.001 | 16 | 14 | 0.73 |
| Steroids (ever) | 7 | 16 | 0.07 | 5 | 19 | 0.18 |
| Calcium supplements | 38 | 23 | 0.02 | 32 | 21 | 0.36 |
| Multivitamins | 33 | 51 | 0.01 | 47 | 52 | 0.72 |
| HIV and ART characteristics | ||||||
| Years since HIV diagnosis | 8.5 ± 0.4 | 8.8 ± 1.0 | 8.5 ± 0.5 | 0.73 | ||
| AIDS criteria (ever) (%) | 53 | 37 | 58 | 0.12 | ||
| CD4 at baseline (cells/mm3) | 493 ± 31 | 456 ± 71 | 503 ± 34 | 0.53 | ||
| CD4 nadir (cells/mm3) | 213 ± 21 | 337 ± 77 | 182 ± 15 | 0.07 | ||
| HIV-1 RNA (log10 copies/ml) | 2.38 ± 0.10 | 3.48 ± 0.23 | 2.08 ± 0.08 | <0.0001 | ||
| Current ART (%) | 79 | NA | 100 | |||
| Current NNRTI-based ART (%) | 28 | NA | 36 | |||
| Current PI-based ART (%) | 39 | NA | 49 | |||
| Current NRTI-only ART (%) | 9 | NA | 11 | |||
| Current PI and NNRTI (%) | 3 | NA | 4 |
Data are presented as mean ± sem. FH, Family history; TAH/BSO, total abdominal hysterectomy with bilateral salpingo-oophorectomy; NRTI, nucleoside reverse transcriptase inhibitor.
Among HIV+ART− women, 11 of 19 (58%) were ART-naïve, and eight had previous antiretroviral exposure. Most women on ART were receiving protease inhibitor (PI)-based regimens either with (22 of 36) or without (14 of 36) ritonavir boosting. Mean duration of PI exposure did not differ between ritonavir-boosted and unboosted groups (3.3 ± 0.7 vs. 4.8 ± 0.7 yr, respectively; P = 0.15). Twenty percent of HIV+ART+ women had tenofovir exposure with mean duration of 1.2 ± 0.2 yr.
BMD
Unadjusted BMD (grams per square centimeter) was 5.9% lower in HIV+ compared with the HIV− women at the TH (Table 2), but did not differ significantly at the spine, FN, or forearm. We performed analysis of covariance to address HIV group differences in age, race/ethnicity, and BMI, important predictors of BMD. After adjustment for these parameters, BMD became lower (by 4.5%) in HIV+ women at the LS, and there was a trend toward lower BMD (by 3–4%) at the non-dominant 1/3 radius and TH (Fig. 1A).
Table 2.
Unadjusted absolute BMD and biochemistries
| HIV− | HIV+ | P | HIV+ART− | HIV+ART+ | P | |
|---|---|---|---|---|---|---|
| n | 95 | 92 | 19 | 73 | ||
| BMD (g/cm2) | ||||||
| LS | 0.923 ± 0.016 | 0.887 ± 0.014 | 0.09 | 0.914 ± 0.041 | 0.880 ± 0.014 | 0.44 |
| TH | 0.914 ± 0.014 | 0.863 ± 0.015 | 0.01 | 0.860 ± 0.041 | 0.864 ± 0.015 | 0.92 |
| FN | 0.780 ± 0.015 | 0.748 ± 0.014 | 0.12 | 0.761 ± 0.046 | 0.744 ± 0.013 | 0.73 |
| Radius (1/3) | 0.665 ± 0.009 | 0.661 ± 0.009 | 0.78 | 0.668 ± 0.022 | 0.659 ± 0.010 | 0.70 |
| Gonadal and calciotropic hormones | ||||||
| Estrone (pg/ml) | 29.6 ± 1.4 | 24.0 ± 1.2 | <0.01 | 24.5 ± 2.7 | 23.9 ± 1.4 | 0.84 |
| FSH (mIU/ml) | 67.4 ± 3.1 | 64.1 ± 2.7 | 0.43 | 56.9 ± 6.6 | 66.0 ± 3.0 | 0.17 |
| Calcium (mg/dl)a | 9.09 ± 0.03 | 9.11 ± 0.05 | 0.73 | 9.13 ± 0.10 | 9.10 ± 0.05 | 0.80 |
| PTH (pg/ml) | 42.9 ± 2.0 | 36.4 ± 1.6 | 0.01 | 41.3 ± 4.2 | 35.1 ± 1.7 | 0.12 |
| 25-OHD (ng/ml) | 22.8 ± 1.3 | 25.0 ± 2.1 | 0.35 | 25.9 ± 5.3 | 24.8 ± 2.2 | 0.82 |
| 1,25-dihydroxyvitamin D (pg/ml) | 41.8 ± 1.6 | 41.9 ± 1.8 | 0.99 | 44.1 ± 4.8 | 41.2 ± 1.9 | 0.51 |
| BTMs | ||||||
| OC (ng/ml) | 6.0 ± 0.2 | 6.8 ± 0.3 | 0.06 | 5.0 ± 0.6 | 7.2 ± 0.4 | <0.01 |
| BAP (U/liter) | 31.7 ± 1.3 | 34.8 ± 1.7 | 0.16 | 28.7 ± 2.4 | 36.4 ± 2.0 | 0.02 |
| NTx (nmol/BCE/liter) | 16.6 ± 0.6 | 19.4 ± 1.0 | 0.02 | 16.6 ± 1.5 | 20.1 ± 1.2 | 0.15 |
| CTx (ng/ml) | 0.46 ± 0.02 | 0.65 ± 0.05 | <0.001 | 0.38 ± 0.06 | 0.72 ± 0.06 | <0.01 |
| Proresorptive cytokines | ||||||
| IL-6 (pg/ml) | 2.4 ± 0.3 | 2.5 ± 0.3 | 0.81 | 3.9 ± 1.2 | 2.1 ± 0.3 | 0.16 |
| TNF-α (pg/ml) | 30.5 ± 1.6 | 43.1 ± 2.9 | <0.001 | 40.1 ± 4.3 | 44.0 ± 3.5 | 0.57 |
Data are expressed as mean ± sem.
Calcium corrected for albumin level.
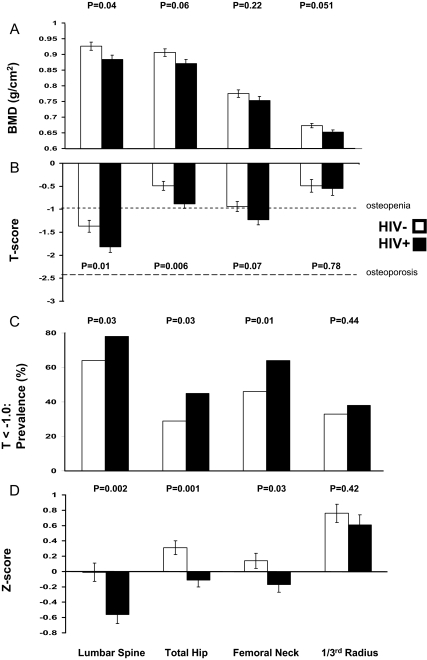
Figure 1.
A, Absolute BMD (grams per square centimeter) by DXA adjusted for age, race/ethnicity, and BMI in HIV− and HIV+ women. B, T scores by DXA in HIV− and HIV+ women. C, Prevalence of low BMD (T < −1.0) in HIV− and HIV+ women. D, Z scores adjusted for BMI in HIV− and HIV+ women. Adjusted comparisons of BMD at different sites are presented for HIV− (n = 95) and HIV+ (n = 92) postmenopausal minority women. In panel B, the dotted gray lines at T score = −1.0 and T score = −2.5 denote World Health Organization criteria for osteopenia and osteoporosis, respectively.
Mean T scores were 0.4 sd lower in HIV+ women (Fig. 1B) at the LS and TH. In both HIV+ and HIV− groups, T scores were lowest at the spine, where they were in the osteopenic range. The prevalence of T scores below −1.0 was significantly higher in HIV+ women (Fig. 1C): 78 vs. 64% at the LS, 45 vs. 29% at the TH, and 64 vs. 46% at the FN. Prevalence of osteoporosis, however, did not differ between HIV+ and HIV− groups.
We also evaluated Z scores, which compare BMD to age- and race/ethnicity-matched normal populations, after adjustment for the lower BMI in HIV+ women. Adjusted Z scores were within the expected range for age (−2.0 to +2.0) in both groups but were significantly lower in HIV+ women at the spine (−0.56 ± 0.1 vs. −0.01 ± 0.1; P < 0.05), TH (−0.10 ± 0.1 vs. +0.31 ± 0.1; P < 0.01), and FN (−0.17 ± 0.1 vs. +0.14 ± 0.1; P < 0.05), but not the forearm (Fig. 1D). Z scores were similarly lower in HIV+ than HIV− Hispanics and in HIV+ than HIV− African-Americans (data not shown).
Despite higher BTMs in HIV+ART+ women, BMD (Table 2) and T and Z scores (data not shown) did not differ at any site in HIV+ women by ART status. Similarly, in HIV+ART+ women, there were no differences in BMD in those on non-nucleoside reverse transcriptase inhibitors (NNRTIs) or PIs. However, statistical power was low for these analyses.
Fractures
The proportion of women with a history of fracture did not differ between HIV+ and HIV− women at the spine (0 vs. 1%), hip (1 vs. 0%), or forearm (3 vs. 3%). However, HIV+ women reported more fractures at other sites (ribs, ankles, shoulders, pelvis; 7 vs. 0%; P < 0.05). Radiographs revealed mild thoracic vertebral fractures in 4.1% of HIV+ and 6.5% of HIV− women (P = 0.72), with one severe fracture per group.
Calciotropic and gonadal hormones
Serum calcium, 25-OHD, and 1,25 dihydroxyvitamin D levels were similar, whereas PTH was lower in HIV+ women (Table 2). The majority of HIV+ and HIV− women had suboptimal serum 25-OHD (<32 ng/ml; 74 vs. 81%; P = 0.24). Prevalence of severe vitamin D deficiency (<10 ng/ml) was also similar in HIV+ and HIV− groups (15 vs. 13%; P = 0.61). Serum 25-OHD and PTH correlated inversely (r = −0.30; P < 0.01) in both HIV+ and HIV− groups; neither correlated with BMD.
Serum estrone was lower in HIV+ than HIV− women. In HIV− women, estrone was positively associated with BMD at the TH, FN, and forearm (r = 0.21 to 0.25; P < 0.05), but not with BTMs. In contrast, estrone was not associated with BMD or BTMs in HIV+ women. Estrone levels did not correlate with BMI or percentage fat and did not differ by ART status.
BTMs and cytokines
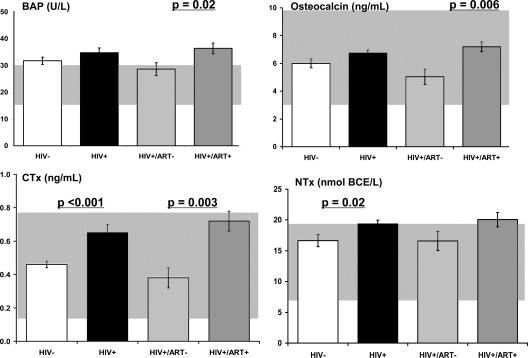
Formation markers, BAP, and OC were slightly but not significantly higher in HIV+ women (Table 2 and Fig. 2). Resorption markers, NTx and CTx, were significantly higher in HIV+ women. Serum BAP, OC, and CTx were significantly higher in HIV+ART+ than HIV+ART− women. Serum TNFα was higher in HIV+ than HIV− women, whereas IL-6 levels were similar. OC and CTx differences by ART status remained significant after adjusting for CD4 count or cytokine levels or weight.
Figure 2.
BTM levels in HIV− women (open) and HIV+ women (shaded) by current status of ART. Background shaded areas represent the range of normal serum levels of BAP, OC, NTx, and CTx in premenopausal women.
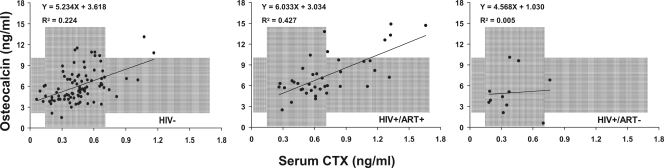
Bone formation and resorption markers correlated moderately in HIV− women (r = 0.36 to 0.56; P < 0.001) and HIV+ women on ART (r = 0.56 to 0.69; P < 0.001) (Fig. 3). In contrast, formation and resorption markers were not correlated in HIV+ART− women, suggesting uncoupling of remodeling.
Figure 3.
Regression of OC by CTx levels in HIV− women and HIV+ women by current ART status. Background shaded areas represent the range of normal serum levels of OC and CTx in premenopausal women.
There was a negative correlation between TNFα and NTx (r = −0.29; P < 0.01) in HIV− women and a positive, although nonsignificant, correlation between TNFα and NTx (r = 0.22; P = 0.06) in HIV+ women. Of note, the between-group difference for the correlation of TNFα with NTx was significant (P < 0.05).
The most consistent association between BTMs and BMD was with OC, which correlated negatively with BMD at all sites (r = −0.21 to −0.29; P < 0.01).
Determinants of BMD
We used regression analysis to assess whether HIV status was an independent predictor of BMD after adjustment for between-group differences. Younger age, higher BMI, and African-American race were associated with higher BMD at all sites (Table 3). Alcohol use was associated with higher TH BMD, and higher BTMs were associated with lower BMD at various sites. Estrone and years since menopause were not independent predictors of BMD. When added to models containing age, BMI, race, and alcohol use, HIV status remained an independent predictor of BMD at the spine and TH with a trend at the forearm.
Table 3.
Linear regression of factors independently associated with BMD
| Characteristics | Univariate model
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LS (0.01 g/cm2)
|
TH (0.01 g/cm2)
|
FN (0.01 g/cm2)
|
1/3 Radius (0.01 g/cm2)
|
|||||||||
| β | r | P | β | r | P | β | r | P | β | Partial r | P | |
| Age (10 yr) | −4.19 | 0.19 | <0.01 | −3.68 | 0.18 | 0.02 | −4.97 | 0.23 | <0.01 | −5.91 | 0.48 | <0.0001 |
| BMI (5 kg/m2) | 4.18 | 0.37 | <0.0001 | 5.93 | 0.54 | <0.0001 | 5.94 | 0.53 | <0.0001 | 1.89 | 0.29 | <0.0001 |
| AA:Hispanic | 12.35 | 0.4 | <0.0001 | 6.08 | 0.2 | <0.01 | 8.67 | 0.28 | <0.0001 | 5.42 | 0.3 | <0.0001 |
| Alcohol use | 4.85 | 0.21 | <0.01 | 3.22 | 0.14 | 0.051 | 2.81 | 0.12 | 0.1 | 1.41 | 0.11 | 0.15 |
| OC (ng/ml) | −1.15 | 0.21 | <0.01 | −1.54 | 0.3 | <0.0001 | −1.17 | 0.22 | <0.01 | −0.72 | 0.23 | <0.01 |
| BAP (10 U/liter) | −1.14 | 0.12 | 0.12 | −2.22 | 0.23 | <0.01 | −1.57 | 0.16 | 0.03 | −1.24 | 0.22 | <0.01 |
| NTx (10 nmol/BCE/liter) | −2.19 | 0.12 | 0.1 | −3.63 | 0.2 | <0.01 | −2.60 | 0.14 | 0.05 | −1.74 | 0.16 | 0.02 |
| TNF-α (pg/ml) | −0.10 | 0.14 | 0.08 | −0.09 | 0.14 | 0.08 | −0.08 | 0.12 | 0.13 | −0.06 | 0.14 | 0.08 |
| IL-6 (pg/ml) | 0.92 | 0.18 | 0.03 | 0.54 | 0.11 | 0.17 | 0.76 | 0.15 | 0.06 | −0.06 | 0.02 | 0.81 |
| HIV status +/− | −3.64 | 0.13 | 0.09 | −5.02 | 0.18 | 0.01 | −3.25 | 0.11 | 0.12 | −0.34 | 0.02 | 0.78 |
|
Full model
|
||||||||||||
|
LS: R2 = 0.296, P < 0.0001
|
TH: R2 = 0.345, P < 0.0001
|
FN: R2 = 0.353, P < 0.0001
|
1/3 Radius: R2 = 0.343, P < 0.0001
|
|||||||||
|
|
β
|
Partial r
|
P
|
β
|
Partial r
|
P
|
β
|
Partial r
|
P
|
β
|
Partial r
|
P
|
| Age (10 yr) | −3.20 | 0.16 | 0.03 | −2.90 | 0.16 | 0.03 | −3.88 | 0.21 | <0.01 | −5.72 | 0.47 | <0.0001 |
| BMI (5 kg/m2) | 3.04 | 0.29 | <0.0001 | 5.28 | 0.49 | <0.0001 | 5.21 | 0.48 | <0.0001 | 1.19 | 0.21 | <0.01 |
| AA:Hispanic | 9.86 | 0.33 | <0.0001 | 2.37 | 0.10 | 0.16 | 5.84 | 0.22 | <0.01 | 3.91 | 0.25 | <0.001 |
| Alcohol use | 2.64 | 0.13 | 0.09 | 2.71 | 0.12 | 0.10 | ||||||
| HIV status +/− | −4.46 | 0.17 | 0.02 | −3.72 | 0.15 | 0.04 | −2.27 | 0.09 | 0.22 | −2.14 | 0.14 | 0.05 |
Simple linear regression parameter estimates and correlation coefficients for covariates found to be significant in univariate screening against BMD, ignoring HIV-status, are presented on the top half of the table for each BMD site. The full model at each BMD site started with race/ethnicity (Hispanic/African-American), corrected calcium, hypertension, history of prior fracture, age at menarche, calcium supplements, alcohol, statin or multivitamin use, and age and BMI forced into the model; partial, semipartial correlations and colinearity were examined, and redundant predictors were removed. Remaining predictors entered a stepwise selection model with criteria of P value of 0.20 to enter and 0.05 to stay in the final model. The parameter estimates and partial-r coefficients reported come from the selected reduced model. The partial-r is the correlation of the target regressor with the dependent variable after the influence of the other predictors in the model has been removed from both the dependent variable and the target predictor. AA, African-American.
Addition of BTMs to the model attenuated the effect of HIV status, suggesting that the effects of HIV on BMD are mediated at least in part by higher bone turnover (Table 3). TNFα and IL-6 levels were not independent predictors of BMD at any site in the multivariate models; however, addition of either variable also attenuated the effect of HIV on the model, suggesting that the effects of HIV on BMD could be mediated in part by elevated proresorptive cytokines.
Among HIV+ women, age, BMI, alcohol use, and BTMs correlated with BMD at various sites, whereas CD4 count, HIV-1 plasma RNA levels, AIDS criteria, duration of ART, or class of ART did not. Negative correlations between cumulative exposure to ritonavir-boosted PI regimens and TH BMD (r = −0.20; P = 0.067) approached statistical significance after adjustment for age, BMI, and ethnicity. In multivariate analyses, no specific ART regimen significantly predicted BMD after inclusion of traditional covariates.
Discussion
Despite their younger age, HIV+ postmenopausal minority women had lower unadjusted BMD than HIV− women at the TH, and after adjustment for group differences in age, race/ethnicity, and BMI, HIV+ women had lower BMD at both the spine and hip. The prevalence of low spine and hip BMD was higher, and HIV status remained an independent predictor of spine and hip BMD in multivariate models. Bone turnover was higher in HIV+ than HIV− women and independently associated with lower BMD. Among HIV+ women, high BTMs were found mainly in those on ART. Factors traditionally associated with BMD (age, BMI, race/ethnicity, alcohol use) predicted BMD in HIV+ women, whereas HIV- and ART-specific factors did not. Spine, hip, and forearm fractures did not differ between HIV+ and HIV− women. Although HIV+ women did report more fractures of other sites, this observation must be interpreted with caution given the small size of the study.
The BMD differences are generally consistent, although perhaps slightly higher than other studies of older HIV+ subjects. The 5.9% unadjusted difference in TH BMD between our HIV+ and HIV− postmenopausal women is slightly greater than the 3% difference between HIV+ and HIV− men of median age 55 reported elsewhere (11). Similarly, prevalence of LS T score below −1.0 may be higher in our subjects (78%) than HIV+ men and women (67%; median age, 61) (13), possibly because there were relatively fewer African-Americans in our study. The differences in BMD we observed between HIV+ and HIV− postmenopausal women were larger than reported by Arnsten et al. (12) in middle-aged, predominantly premenopausal women (mean age, 44 yr; BMD, 2.4 to 3.8% lower) and comparable to those reported by Dolan et al. (5) (mean age, 41 yr; BMD, 4.0 to 5.6% lower). Although Dolan et al. (5) reported that 48% had low BMD at all sites combined, Arnsten et al. (12) reported low spine BMD in only 21% and low FN BMD in 12% of younger HIV+ women. The higher prevalence of low BMD in our subjects may reflect older age and postmenopausal status. In addition, T scores reported by Arnsten et al. were based upon Caucasian women, whereas those reported by Dolan et al. and us were race/ethnicity specific (27).
Another goal of our study was to investigate the pathogenesis of low BMD. Lower body weight is a powerful determinant of BMD. In a recent meta-analysis, Bolland et al. (14) argue that differences in BMD between HIV+ and HIV− are largely related to lower body weight of HIV+ subjects and are clinically insignificant (2.2–2.8%) after adjustment for group differences in weight. In our study, spine BMD remained 4.5% lower in HIV+ than HIV− women after adjusting for age, race/ethnicity, and BMI. In multivariate models, HIV positivity accounted for 2% of the 28% variance at the spine and 2% of the 35% variance at the TH, which is similar in magnitude to the 3% contribution to both models from age. Thus, our data suggest that whereas the effects of HIV or ART on BMD are modest, they are in part independent of weight. Importantly, low body weight is a powerful risk factor for osteoporotic fracture. Thus, the fact that BMD is lower in HIV+ individuals is of clinical relevance, whether or not the mechanism by which it is lower is attributable directly to HIV or is mediated indirectly by effects of HIV on weight or other parameters. That being said, it is noteworthy that the BMI of the HIV+ group was in the overweight range (>25–30 kg/m2), whereas BMI of the HIV− women was in the obese range (>30 kg/m2). Thus, it is possible that a contributing factor to our findings is that the control group had “high” bone density related to obesity rather than the HIV group having low bone density.
Several biochemical differences may contribute to lower BMD in our HIV+ subjects. In postmenopausal women, estrone, the major circulating estrogen, is directly associated with weight and BMD (28). The etiology for lower estrone levels in HIV+ women is unclear; we detected no relationship with weight, BMI, or total body or truncal fat. In addition, we did not observe the expected direct associations between estrone and BMD and inverse associations between estrone and BTMs found in HIV− women. However, lower estrone may still place these women at risk for more rapid bone loss than observed in premenopausal HIV+ women (20).
In older studies of untreated HIV+ subjects with advanced disease, resorption markers were elevated and OC levels suppressed, suggesting that normally tightly regulated processes of formation and resorption were “uncoupled” (29). After ART initiation, markers of bone formation and resorption become correlated (29), although resorption markers are typically elevated (7,30,31). Our cross-sectional results are generally consistent with these data. Serum CTx, BAP, and OC were all higher in HIV+ART+ than HIV+ART− women, in whom BTM levels resembled HIV− women. Formation and resorption markers were correlated in HIV− and HIV+ART+ women, but not in HIV+ART− women, suggesting that bone remodeling is elevated but coupled in HIV+ women on ART and uncoupled in untreated HIV+ women. Although this could be related to immunological or nutritional differences between ART+ and ART− groups (20), BTMs remained elevated in the ART+ group after adjustment for current CD4 count, cytokine levels, or BMI.
In the multivariate model, addition of BTMs attenuated the effect of HIV on BMD in a model that included age, ethnicity, BMI, and alcohol use. This observation suggests that the association between HIV and low BMD is mediated by increased bone turnover. Whether or not increased levels of proresorptive cytokines contribute to increased bone turnover is less clear. TNFα was higher in HIV+ than HIV− women, although IL-6 did not differ. In HIV+ women, there was a positive correlation between TNFα levels and NTx, whereas in HIV− women, the correlation was negative. Although cytokine levels were not significant predictors of BMD in the multivariate model, the addition of cytokine levels to the model attenuated the association between HIV and BMD. Although serum cytokine levels may not reflect those within the bone microenvironment, higher TNFα concentrations in HIV+ women and their differential association with BTMs suggests that HIV infection affects BMD through TNFα-mediated effects on osteoclastic bone resorption.
The strengths of this study include its focus on postmenopausal women, a population at high risk of bone loss and fractures; on minority women, a rapidly growing segment of the HIV+ population; and on enrollment of a clinic-based control population. Recruitment of a completely healthy control group could have biased our results toward finding larger BMD differences between HIV+ and HIV− women because completely healthy women might be expected to have higher BMD. To our knowledge, this is the only study of older HIV+ individuals to include radiographic assessment of vertebral fractures and measures of estrone, calciotropic hormones, BTMs, and proresorptive cytokines.
Study limitations include the significantly lower age of the HIV+ group; however, this difference would bias the results toward finding a higher rather than a lower BMD in the HIV+ group. Other limitations include the relatively modest sample size, large enough to permit group comparisons between HIV+ and HIV− women, but small for detailed explorations of determinants among HIV+ women, especially with regard to specific ART regimens. This is of importance because in vitro data suggest that antiretrovirals may have differential effects on osteoclasts and osteoblasts (32,33,34) that are not consistent across class. These cross-sectional baseline analyses preclude assessments of causal relationships between HIV status, ART exposure, bone turnover, cytokine levels, and BMD. Additionally, we did not include computed tomography imaging to measure visceral adipose tissue.
In conclusion, low BMD was more common in postmenopausal HIV+ than HIV− minority women. HIV infection was independently associated with lower BMD after adjusting for BMI and traditional osteoporosis risk factors. BTMs and TNFα were higher in HIV+ART+ women, and multivariate modeling suggested that they may mediate the effect of HIV on BMD. Completion of the ongoing longitudinal study should permit assessment of whether increased bone turnover associated with ART translates into accelerated bone loss and increased fracture rates in aging postmenopausal women.
Acknowledgments
We thank the staff and participants of Columbia University Medical Center and Bronx-Lebanon Hospital Center.
Footnotes
This work was supported by National Institutes of Health Grants AI059884 (to M.T.Y.), AI1065200 (to E.S.), AR 052665 (to E.S.), and DK65511 (to J.L.).
Disclosure Summary: The authors have nothing to declare.
First Published Online December 4, 2009
Abbreviations: ART, Antiretroviral therapy; ART−, currently not receiving ART; ART+, currently receiving ART; BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; BMI, body mass index; BTM, bone turnover marker; CTx, C-telopeptide; DXA, dual-energy x-ray absorptiometry; FN, femoral neck; LS, lumbar spine; NNRTI, non-nucleoside reverse transcriptase inhibitor; NTx, N-telopeptide; OC, osteocalcin; 25-OHD, 25-hydroxyvitamin D; PI, protease inhibitor; TH, total hip.
References
- Luther VP, Wilkin AM 2007 HIV infection in older adults. Clin Geriatr Med 23:567–583, vii [DOI] [PubMed] [Google Scholar]
- Goulet JL, Fultz SL, Rimland D, Butt A, Gibert C, Rodriguez-Barradas M, Bryant K, Justice AC 2007 Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis 45:1593–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, Huebner RE, Janoff EN, Justice AC, Kuritzkes D, Nayfield SG, Plaeger SF, Schmader KE, Ashworth JR, Campanelli C, Clayton CP, Rada B, Woolard NF, High KP 2008 Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis 47:542–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr A, Miller J, Eisman JA, Cooper DA 2001 Osteopenia in HIV-infected men: association with asymptomatic lactic acidemia and lower weight pre-antiretroviral therapy. AIDS 15:703–709 [DOI] [PubMed] [Google Scholar]
- Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S 2004 Reduced bone density in HIV-infected women. AIDS 18:475–483 [DOI] [PubMed] [Google Scholar]
- Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, Yarasheski KE 2000 Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS 14:F63–F67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann J, Stephan E, Lange U, Discher T, Friese G, Lohmeyer J, Stracke H, Bretzel RG 2003 Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. J Infect 46:221–227 [DOI] [PubMed] [Google Scholar]
- Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J 2003 Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS 17:1917–1923 [DOI] [PubMed] [Google Scholar]
- Knobel H, Guelar A, Vallecillo G, Nogués X, Díez A 2001 Osteopenia in HIV-infected patients: is it the disease or is it the treatment? AIDS 15:807–808 [DOI] [PubMed] [Google Scholar]
- Brown TT, Qaqish RB 2006 Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 20:2165–2174 [DOI] [PubMed] [Google Scholar]
- Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS 2007 Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS 21:617–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Santoro N, Schoenbaum EE 2006 HIV infection and bone mineral density in middle-aged women. Clin Infect Dis 42:1014–1020 [DOI] [PubMed] [Google Scholar]
- Jones S, Restrepo D, Kasowitz A, Korenstein D, Wallenstein S, Schneider A, Keller MJ 2008 Risk factors for decreased bone density and effects of HIV on bone in the elderly. Osteoporos Int 19:913–918 [DOI] [PubMed] [Google Scholar]
- Bolland MJ, Grey AB, Gamble GD, Reid IR 2007 Clinical review: low body weight mediates the relationship between HIV infection and low bone mineral density: a meta-analysis. J Clin Endocrinol Metab 92:4522–4528 [DOI] [PubMed] [Google Scholar]
- Bolland MJ, Grey AB, Horne AM, Briggs SE, Thomas MG, Ellis-Pegler RB, Woodhouse AF, Gamble GD, Reid IR 2006 Bone mineral density is not reduced in HIV-infected Caucasian men treated with highly active antiretroviral therapy. Clin Endocrinol (Oxf) 65:191–197 [DOI] [PubMed] [Google Scholar]
- Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, Hoffmann M, Tebas P 2003 Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis 36:482–490 [DOI] [PubMed] [Google Scholar]
- Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA 2009 Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral Regimen. J Acquir Immune Defic Syndr 51:554–561 [DOI] [PubMed] [Google Scholar]
- Cassetti I, Madruga JV, Suleiman JM, Etzel A, Zhong L, Cheng AK, Enejosa J 2007 The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1-infected patients. HIV Clin Trials 8:164–172 [DOI] [PubMed] [Google Scholar]
- Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy RL, Katlama C, Costagliola D 2009 Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS 23:817–824 [DOI] [PubMed] [Google Scholar]
- Dolan SE, Kanter JR, Grinspoon S 2006 Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metab 91:2938–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Brown TT, Lee H, Grinspoon SK 2008 Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 93:3499–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2009 HIV/AIDS Surveillance Report, 2007. 19:1–63 [Google Scholar]
- Yin M, Dobkin J, Brudney K, Becker C, Zadel JL, Manandhar M, Addesso V, Shane E 2005 Bone mass and mineral metabolism in HIV+ postmenopausal women. Osteoporos Int 16:1345–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanis JA 2002 Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936 [DOI] [PubMed] [Google Scholar]
- Black DM, Palermo L, Nevitt MC, Genant HK, Epstein R, San Valentin R, Cummings SR 1995 Comparison of methods for defining prevalent vertebral deformities: the Study of Osteoporotic Fractures. J Bone Miner Res 10:890–902 [DOI] [PubMed] [Google Scholar]
- Hertzler AA, Frary RB 1994 A dietary calcium rapid assessment method (RAM). Top Clin Nutr 9:76–85 [Google Scholar]
- Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, Kalkwarf HJ, Langman CB, Plotkin H, Rauch F, Zemel BS, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Silverman S 2008 International Society for Clinical Densitometry 2007 Adult and Pediatric Official Positions. Bone 43:1115–1121 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Yano T, Nakazawa N, Yoshikawa H, Taketani Y 1995 A possible role of estrone produced in adipose tissues in modulating postmenopausal bone density. Maturitas 22:9–12 [DOI] [PubMed] [Google Scholar]
- Aukrust P, Haug CJ, Ueland T, Lien E, Müller F, Espevik T, Bollerslev J, Frøland SS 1999 Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone-remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab 84:145–150 [DOI] [PubMed] [Google Scholar]
- Fairfield WP, Finkelstein JS, Klibanski A, Grinspoon SK 2001 Osteopenia in eugonadal men with acquired immune deficiency syndrome wasting syndrome. J Clin Endocrinol Metab 86:2020–2026 [DOI] [PubMed] [Google Scholar]
- Zamboni G, Antoniazzi F, Bertoldo F, Lauriola S, Antozzi L, Tatò L 2003 Altered bone metabolism in children infected with human immunodeficiency virus. Acta Paediatrica 92:12–16 [DOI] [PubMed] [Google Scholar]
- Fakruddin JM, Laurence J 2003 HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor κB ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-γ/RANKL cross-talk. J Biol Chem 278:48251–48258 [DOI] [PubMed] [Google Scholar]
- Jain RG, Lenhard JM 2002 Select HIV protease inhibitors alter bone and fat metabolism ex vivo. J Biol Chem 277:19247–19250 [DOI] [PubMed] [Google Scholar]
- Wang MW, Wei S, Faccio R, Takeshita S, Tebas P, Powderly WG, Teitelbaum SL, Ross FP 2004 The HIV protease inhibitor ritonavir blocks osteoclastogenesis and function by impairing RANKL-induced signaling. J Clin Invest 114:206–213 [DOI] [PMC free article] [PubMed] [Google Scholar]