Abstract
Context: The effects of GH replacement therapy in patients who develop GH deficiency (GHD) after cure of acromegaly have not been established in a placebo-controlled study.
Objective: The objective of the study was to determine whether GH replacement improves body composition, cardiovascular risk markers and quality of life in patients with GHD and prior acromegaly.
Design: This was a 6-month, randomized, placebo-controlled study.
Setting: The study was conducted at a clinical translational science center.
Study Participants: Participants included 30 subjects with prior acromegaly and current GHD.
Intervention: Interventions included GH or placebo.
Main Outcome Measures: Body composition (dual-energy x-ray absorptiometry and cross-sectional computed tomography at L4), cardiovascular risk markers (high-sensitivity C-reactive protein (hsCRP), total, high-density lipoprotein and low-density lipoprotein cholesterol, fibrinogen, and carotid intimal-medial thickness), and quality of life were measured.
Results: The mean GH dose at 6 months was 0.58 ± 0.26 mg/d. Total fat mass, visceral adipose tissue (−15.3 ± 18.6 vs. 1.3 ± 12.5%, P = 0.01), and total abdominal fat decreased, and fat-free mass increased, in the GH vs. placebo group. Mean hsCRP levels decreased, but there was no GH effect on other cardiovascular risk markers. There was no change in glycosylated hemoglobin or homeostasis model assessment insulin resistance index. Quality of life improved with GH. Side effects were minimal.
Conclusions: This is the first randomized, placebo-controlled study of the effects of GH replacement therapy on body composition and cardiovascular end points in patients who have developed GH deficiency after treatment for acromegaly, a disease complicated by metabolic and body composition alterations and increased cardiovascular risk. GH replacement decreased visceral adipose tissue, increased fat-free mass, decreased hsCRP, and improved quality of life in patients with GHD after cure of acromegaly, with minimal side effects and without an increase in insulin resistance.
In a randomized, placebo-controlled study, data indicate that growth hormone administration reduces visceral adiposity and improves quality of life in patients who have developed GH deficiency after cure of acromegaly.
Patients with acromegaly may develop GH deficiency (GHD) after successful treatment of the disease, due to the effects of surgery or radiation. GHD secondary to nonsomatotroph tumors is associated with abnormalities in body composition and cardiovascular risk markers, including an increase in visceral adipose tissue and high-sensitivity C-reactive protein (hsCRP) (1,2,3,4,5,6,7,8). In addition, GH replacement therapy has been shown in randomized, placebo-controlled studies to reverse such abnormalities in hypopituitary men and women (9,10,11,12). GHD in patients with a history of acromegaly treated with surgery and/or radiation therapy is common. Ronchi et al. (13) reported that severe GHD is present in 61% of such patients, many of whom had been treated with surgery alone. However, little is known about the effects of GH replacement therapy in men and women who develop GHD after cure of acromegaly, many of whom have experienced prolonged exposure to elevated GH levels during the period of active disease. Three open-label studies of GH replacement in patients with GHD after cure of acromegaly have been published (14,15,16). These studies demonstrated no significant improvements in body composition, lipid profiles, or quality of life, except for an increase in body cell mass and high-density lipoprotein (HDL) cholesterol in the study by Norrman et al. (15) and no deterioration in glucose homeostasis (14,15,16).
In this manuscript, we report the first randomized, placebo-controlled study of patients with GHD after cure of acromegaly. This study was designed to determine whether GH administration improves body composition, cardiovascular risk markers, and/or quality of life in such patients.
Subjects and Methods
Subjects
Sixty-four patients with a history of acromegaly were screened for GHD. Thirty study subjects (13 males, 17 females) met criteria (see below) and were enrolled in the study. Subjects were recruited from the Massachusetts General Hospital Neuroendocrine Clinical Center (n = 43), through institutional review board-approved advertising (n = 14), and from referring endocrinologists (n = 7). Not all study subjects received their primary treatment for acromegaly at the Massachusetts General Hospital. GHD was defined as a peak GH level of less than 5 ng/ml after stimulation with GHRH-arginine (n = 27) or an IGF-I level more than 2 sd below the age-specific normal range and at least three concomitant anterior pituitary deficiencies (n = 3), The GHRH-arginine criterion was based on the American Association of Clinical Endocrinologists 2003 guidelines (17), which specified a cutoff of 5 μg/liter regardless of the stimulation test used, and the data by Biller et al. (18) in which 95% of patients with adult-onset hypothalamic-pituitary disease and multiple additional pituitary hormone deficiencies stimulated to a GH of less than 5 ng/ml to GHRH and arginine. The IGF-I criterion was based on the data by Hartman et al. (19) in which three pituitary hormone deficiencies predicted severe GHD on stimulation testing in 96% of patients. GHRH-arginine testing was performed per standard protocol (GHRH 1 μg/kg plus arginine 30 g administered iv, with measurement of GH levels at baseline and every 30 min thereafter for 2 h) (18). Exclusion criteria included pharmacological therapy for acromegaly; untreated adrenal, thyroid, or gonadal steroid hormone (in men and women <50 yr) deficiency; unstable cardiovascular disease; congestive heart failure (New York Heart Association class II-IV); uncontrolled hypertension or diabetes mellitus; pregnancy or breast-feeding within 1 yr before study enrollment; history of malignancy (except for nonmelanoma skin cancer); hemoglobin less than 11.0 g/dl; alanine aminotransferase or aspartate aminotransferase greater than 3 times the upper limit of normal or creatinine level greater than 2.5 mg/dl; and active carpal tunnel syndrome. All subjects were required to have had a colonoscopy, and women aged 40 yr and older were required to have had screening mammograms within 1 yr before the baseline visit. As an entry criterion, all subjects were receiving stable doses of all pituitary hormone replacements for at least 3 months before study participation. The study was approved by the Partners Healthcare, Inc. and Massachusetts Institute of Technology Institutional Review Boards, and written informed consent was obtained from all subjects. Baseline clinical characteristics and baseline quality of life scores, but not body composition or cardiovascular risk marker results, have been reported on a subset of these study subjects (20). This is the first report of the effects of GH replacement in any of these subjects.
Protocol
The study was a 6-month, single-blind, randomized, placebo-controlled protocol performed on the Massachusetts Institute of Technology and Massachusetts General Hospital General Clinical Research Centers and the Harvard Medical School Clinical Translational Science Center. Baseline bloods for IGF-I and cardiovascular risk markers were drawn in the early morning, after an overnight fast, followed by a 75-g, 2-h oral glucose tolerance test. Dual-energy x-ray absorptiometry (DXA) and cross-sectional computed tomography (CT) at the level of L4 were performed to assess baseline body composition, and bioelectric impedance analysis was performed to assess total body water (see Body composition evaluation). After baseline evaluation, subjects were randomized to receive daily sc recombinant human GH (Genotropin; Pfizer, Inc., New York, NY) or placebo (Pfizer), which was identical in appearance to the GH, for 6 months. Randomization was stratified for sex and use of oral estrogen. Starting GH dose was determined based on age, sex, and use of oral estrogen therapy, as follows. Three micrograms per kilogram per day was the starting dose in men 50 yr or older and women 50 yr or older not receiving oral estrogen. A starting dose of 4 μg/kg · d was used in men younger than 50 yr. The starting dose was 5 μg/kg · d in women younger than 50 yr not receiving oral estrogens and 6 μg/kg · d in women younger than 50 yr receiving oral estrogens.
Follow-up visits were performed at 1, 3, 4.5, and 6 months after baseline testing. GH doses were adjusted based on IGF-I levels at these visits to target the midnormal range for age. Participants in the placebo group were sham dose adjusted to maintain study-subject blinding to randomization assignment. Body composition and cardiovascular end points were measured at 6 months. One study participant did not complete the study due to discomfort at injection sites. Data from an additional patient were excluded from analysis due to initiation of appetite suppressants to induce weight loss during the study, as prescribed by a physician at a bariatric clinic.
Body composition evaluation
Fat mass and fat-free mass were measured by DXA using a Hologic QDR-4500 densitometer (Hologic Inc., Waltham, MA), with a percent accuracy error for body fat mass of 1.7% and for fat-free mass of 2.4% (21). Abdominal adipose tissue, including cross-sectional total abdominal fat, sc fat, and intraabdominal fat compartments, was determined in duplicate using single-slice quantitative CT scans at the level of L4 using 10-mm-thick axial images (RP high speed helical computed tomography scanner; General Electric, Milwaukee, WI) and graphical analysis software (Advantage Windows work station version 2.0; General Electric). Technical factors for the scanning were as follows: 80 kVp, 70 mA, and a 2 sec scan time. All scans were read by personnel who were blinded as to the randomization assignments. Total body water was measured using bioimpedance analysis (Quantum II, bioelectric impedance analysis instrument with Cyprus 2.7 software; RJL Systems, Clinton Township, MI). Resting energy expenditure was calculated from substrate oxidation rates obtained by indirect calorimetry (Vmax 29N Sensor Medics; Vyasis Healthcare, Loma Linda, CA) after a 12-h fast (22).
Cardiovascular risk factor analysis
Serum and plasma samples were collected and stored at −80 C. Serum IGF-I levels were measured using the Immulite 2000 automated immunoanalyzer (Siemens Healthcare Diagnostics, Deerfield, IL), a solid-phase, enzyme-labeled, chemiluminescent immunometric assay, with an interassay coefficient of variation (cv) of 3.7–4.2%. Before July 2005, serum GH levels were measured with a chemiluminescent immunometric assay (Nichols Institute Diagnostics, San Juan Capistrano, CA), with an intraassay cv of 5.4% or less and a sensitivity of 0.02 ng/ml. As of July 2005, this assay was no longer available, and therefore, serum GH levels were measured using an immunoradiometric assay kit (Diagnostic Systems Laboratories, Inc., Webster, TX), with a minimum detection limit of 0.01 ng/ml, an intraassay cv of 3.1–5.4% and an interassay cv of 5.9–11.5% [R2 = 0.98, slope 1.083, intercept 0.4457, and average bias of 8.4% for comparison between the two assays (our unpublished data)]. hsCRP was measured by latex particle-enhanced immunoturbidimetric assay (on the Hitachi 917) with reagents manufactured by Equal Diagnostics (Exton, PA), with an interassay cv of less than 5.08%. Insulin was measured by RIA (Millipore, Billerica, MA) with an intraassay cv of 2.2–4.4% and an interassay cv of 2.9-6.0%. Homeostasis model assessment-insulin resistance (HOMA-IR) was calculated as insulin (microinternational units per milliliter)glucose (millimoles per liter)/22.5 as a validated measure of insulin resistance (23,24,25).
Carotid intimal-medial thickness (IMT) was analyzed by a single cardiologist (L.H.), blinded to treatment randomization assignment as follows: long-axis B-mode images of the far wall of the distal right and left common carotid arteries of each subject were obtained using a variable frequency 5- to 12-MHz ultrasound transducer on an HDI 5000 ultrasound system (Philips Medical Systems, Bothell, WA). Average carotid IMT over the distal 1-cm segment was measured using semiautomated edge detection software on the direct digital captures. The average cv for IMT measurement using this system by this cardiologist (L.H.) is 4.7%.
Quality-of-life assessment
Quality of life was assessed with the following three self-administered questionnaires. The Quality of Life Assessment of Growth Hormone Deficiency in Adults (QOL-AGHDA) is a GHD disease-generated questionnaire (26,27) based on interviews with GH-deficient patients, and assesses energy, physical and mental drive, concentration/memory, personal relationships, social life, emotions, and cognition. Higher scores are indicative of a poorer quality of life. The Symptom Questionnaire is a validated questionnaire composed of four subscales: 1) anxiety, 2) depression, 3) somatic symptoms, and 4) anger/hostility (28,29,30,31,32,33). Higher scores are indicative of greater symptom severity, with scores between 1 and 2 sd above the mean for healthy controls suggesting moderate distress and scores more than 2 sd above the mean suggesting severe distress or psychopathology (33). The Short-Form Health Survey (SF-36) is a validated questionnaire that assesses well-being during the preceding 4 wk in the following domains: 1) physical functioning, 2) role limitations due to physical functioning, 3) general health perception, 4) pain, 5) vitality, 6) mental health, 7) role limitations due to emotional health, and 8) social functioning (34,35). Higher scores indicate a better quality of life.
Statistical analysis
JMP statistical discoveries (version 4.0.2; SAS Institute, Inc., Cary, NC) software was used for statistical analysis. The study was powered based on our expectation that GH administration would result in a decrease in hsCRP (primary end point) and an improvement in body composition (secondary end point) in study subjects with GHD after treatment for acromegaly, as has been shown in randomized placebo-controlled studies of GH administration in patients with GHD after treatment of hypothalamic and pituitary disorders other than acromegaly. For the powering of the primary end point, based on the study by Sesmilo et al. (11) with 15 subjects in each study arm, we had an 84% probability of detecting a difference of 1.99 U/liter in hsCRP between the groups with a significance level of 0.05 using a two-sided two-sample t test. For the powering of the secondary end point, with 15 subjects in each study arm, we had a 96% probability of detecting a difference of 4.4% in body fat and a 98% probability of detecting a difference of 4.3 kg in fat-free mass by DXA, as shown in the study by Hoffman et al. (36) with a significance level of 0.05 using a two-sided two-sample t test. Baseline means and mean 6-month change (6 month value minus baseline value) were compared with ANOVA. Univariate regression models were constructed to determine whether baseline IGF-I sd score (SDS), peak GH levels, body mass index (BMI), or change in IGF-I levels predicted changes in the end points studied, and Spearman coefficients are reported. Analysis of covariance was performed to determine the effect of sex. Due to the small number of nonoverweight or obese subjects (n = 5 who had BMIs <25 kg/m2), we did not have the power to compare response to GH in the lean vs. overweight/obese group. Statistical significance was defined as a two-tailed P ≤ 0.05. Data are presented as mean ± sd.
Results
Baseline clinical characteristics and GH dosing
Baseline clinical characteristics are presented in Table 1. Fifteen subjects were randomized to GH and 15 to placebo. At baseline, the two groups were of comparable age, gender, BMI, number of pituitary hormone deficiencies, and IGF-I SDS. The range in age of subjects was 23–74 yr. BMI ranged from 23.2 to 45 kg/m2. Baseline IGF-I SDS ranged from −2.90 to −0.43. Body composition parameters (Table 2), cardiovascular risk markers (Table 2), and quality-of-life scores (Table 3) were also comparable between the GH and placebo groups at baseline, except for mean triglyceride concentrations, which were higher in the group randomized to placebo. Four patients in the GH group and two in the placebo group were receiving antihypertensive medications at baseline, and two subjects in the placebo group had diabetes mellitus.
Table 1.
Baseline clinical characteristics
| GH group (n = 15) | Placebo group (n = 15) | |
|---|---|---|
| Male/female | 6/9 | 7/8 |
| History of surgery | 13 | 14 |
| History of radiation therapy | 12 | 11 |
| Time since acromegaly diagnosis (months) | 136.4 ± 100.1 | 177.6 ± 141.1 |
| Time since cure (months) | 67.6 ± 58.6 | 109.4 ± 115.6 |
| Hypoadrenal | 7 | 7 |
| Hypothyroid | 10 | 12 |
| Hypogonadal (male) | 4 | 6 |
| Testosterone use | 4 | 6 |
| Hypogonadal (female) | 7 | 6 |
| Oral estrogen use | 4 | 4 |
| Transdermal estrogen use | 1 | 1 |
| Current tobacco use | 2 | 2 |
| Age (yr) | 46.1 ± 13.1 | 47.5 ± 11.4 |
| BMI (kg/m2) | 30.9 ± 6.1 | 31.8 ± 7.0 |
| Weight (kg) | 90.0 ± 19.0 | 92.5 ± 25.6 |
| IGF-I (ng/ml) | 92.9 ± 38.9 | 93.1 ± 58.7 |
| IGF-I SDS | −1.98 ± 0.60 | −1.99 ± 0.74 |
| Free T4 (ng/dl) | 1.15 ± 0.39 | 1.14 ± 0.23 |
Values are reported as mean ± sd. Free T4 normal range is 0.9–1.8 ng/dl; there was no significant difference between the groups in any parameter.
Table 2.
Change in body composition and cardiovascular risk marker variables with treatment
| Placebo
|
GH
|
P value comparing baseline between groups | P value comparing change at 6 months between groups | |||
|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | |||
| Total body fat (kg) | 32.3 ± 12.0 | 0.7 ± 2.2 | 29.9 ± 9.2 | −1.6 ± 3.2 | 0.54 | 0.04 |
| Total abdominal fat (mm2) | 46949 ± 16674 | 1164 ± 4319 | 47654 ± 12725 | −4434 ± 7684 | 0.91 | 0.04 |
| Subcutaneous adipose tissue (mm2) | 30778 ± 13304 | 1288 ± 4257 | 33447 ± 11047 | −2430 ± 5247 | 0.57 | 0.06 |
| Visceral adipose tissue (mm2) | 19006 ± 9922 | 540 ± 2401 | 14207 ± 6730 | −2004 ± 3515 | 0.14 | 0.03 |
| Total fat-free mass (kg) | 59.9 ± 17.1 | −0.1 ± 1.9 | 58.7 ± 13.4 | 1.5 ± 1.4 | 0.84 | 0.02 |
| Total body water (kg) | 47.5 ± 16.5 | −0.8 ± 4.4 | 43.85 ± 9.7 | 1.4 ± 2.1 | 0.49 | 0.13 |
| Resting energy expenditure (kcal) | 1665.6 ± 351.9 | −24.9 ± 229.5 | 1513.1 ± 256.9 | 146.9 ± 163.5 | 0.21 | 0.04 |
| Mean arterial pressure (mm Hg) | 91.1 ± 8.0 | −2.2 ± 7.8 | 89.1 ± 7.7 | −1.4 ± 7.9 | 0.50 | 0.80 |
| Total cholesterol (mg/dl) | 196 ± 28 | −9 ± 22 | 189 ± 44 | −8 ± 18 | 0.61 | 0.93 |
| HDL (mg/dl) | 46 ± 14 | −8 ± 11 | 53 ± 13 | −8 ± 9 | 0.15 | 0.91 |
| LDL (mg/dl) | 125 ± 27 | −4 ± 20 | 119 ± 37 | −3 ± 18 | 0.62 | 0.90 |
| Triglycerides (mg/dl) | 127 ± 39 | 17 ± 53 | 86 ± 32 | 17 ± 29 | 0.004 | 0.98 |
| hsCRP (mg/liter) | 3.23 ± 2.42 | 1.64 ± 3.36 | 2.93 ± 3.32 | −0.36 ± 1.52 | 0.78 | 0.05 |
| Mean IMT (mm) | 0.65 ± 0.12 | −0.008 ± 0.03 | 0.66 ± 0.16 | 0.008 ± 0.05 | 0.81 | 0.32 |
| Fibrinogen (mg/dl) | 306 ± 60 | 13 ± 74 | 339 ± 68 | −19 ± 62 | 0.91 | 0.24 |
| Fasting glucose (mg/dl) | 87 ± 11 | 1 ± 10 | 83 ± 7 | 2 ± 10 | 0.26 | 0.91 |
| Fasting insulin (μIU/ml) | 9.70 ± 6.54 | 2.08 ± 2.57 | 10.28 ± 4.67 | 3.47 ± 10.17 | 0.79 | 0.62 |
| HOMA-IR | 2.16 ± 1.61 | 0.49 ± 0.63 | 2.23 ± 0.97 | 0.89 ± 2.68 | 0.89 | 0.59 |
| Hemoglobin A1c (%) | 5.79 ± 0.50 | 0.03 ± 0.35 | 5.49 ± 0.35 | 0.19 ± 0.36 | 0.07 | 0.23 |
Data are presented mean ± sd. Mean arterial pressure = diastolic blood pressure + 1/3 (systolic blood pressure − diastolic blood pressure). Significant P values are bolded.
Table 3.
Quality of life
| Placebo
|
Growth Hormone
|
P value comparing change at 6-months between groups P value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Scoring ≤25% of normal (%) | 6 months | Scoring ≤25% of normal (%) | Baseline | Scoring ≤25% of normal (%) | 6 months | Scoring ≤5% of normal (%) | ||
| Tests in which higher scores reflect poorer quality of life | |||||||||
| QOL-AGHDA | 9.8 ± 6.2 | 9.3 ± 7.7 | 9.2 ± 6.2 | 4.0 ± 3.9 | 0.05 | ||||
| Symptom Questionnaire | |||||||||
| Anxiety | 8.2 ± 7.2 | 7.2 ± 8.4 | 5.2 ± 4.5 | 2.5 ± 3.4 | NS | ||||
| Depression | 6.8 ± 6.2 | 7.2 ± 7.8 | 4.1 ± 5.4 | 1.2 ± 1.5 | NS | ||||
| Anger/hostility | 3.6 ± 5.1 | 3.8 ± 4.5 | 2.8 ± 5.1 | 1.6 ± 2.0 | NS | ||||
| Somatic | 8.6 ± 6.0 | 10.0 ± 6.2 | 7.1 ± 4.3 | 4.2 ± 2.6 | 0.008 | ||||
| Tests in which higher scores reflect better quality of life | |||||||||
| SF-36 | |||||||||
| Physical functioning | 66.1 ± 23.4 | 47 | 63.9 ± 30.8 | 57 | 76.9 ± 17.4 | 43 | 85.4 ± 18.6 | 14 | NS |
| Role limitations due to physical health | 73.2 ± 39.8 | 33 | 62.5 ± 41.3 | 43 | 59.6 ± 40.2 | 36 | 98.1 ± 6.9 | 0 | 0.0008 |
| Bodily pain | 59.6 ± 34.4 | 53 | 61.1 ± 27.9 | 50 | 71.9 ± 22.1 | 43 | 78.1 ± 11.9 | 14 | NS |
| General health | 48.2 ± 22.8 | 47 | 48.6 ± 20.7 | 64 | 63.8 ± 18.6 | 43 | 76.8 ± 11.7 | 14 | 0.05 |
| Vitality | 39.3 ± 20.9 | 60 | 46.1 ± 26.4 | 50 | 35.8 ± 23.2 | 71 | 61.5 ± 18.9 | 21 | 0.01 |
| Social functioning | 71.4 ± 23.7 | 67 | 67.0 ± 28.0 | 64 | 74.0 ± 23.6 | 57 | 96.2 ± 7.9 | 7 | 0.008 |
| Role limitations due to emotional health | 59.5 ± 41.7 | 53 | 57.1 ± 44.2 | 57 | 82.1 ± 32.2 | 29 | 92.3 ± 14.6 | 21 | NS |
| Mental health | 64.3 ± 22.8 | 60 | 66.3 ± 24.4 | 43 | 69.8 ± 18.7 | 29 | 85.2 ± 11.2 | 7 | 0.03 |
Values are reported as mean ± sd. NS, Not significant.
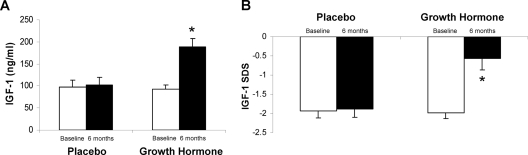
The mean GH dose at 6 months in the treatment group was 0.58 ± 0.26 (sd) mg/d (7.0 ± 3.8 μg/kg · d), resulting in a mean IGF-I increase of 93.0 ± 49.0 ng/ml (P < 0.0001 vs. placebo) to a mean IGF-I level of 189.3 ± 70.5 ng/ml (P = 0.001 vs. placebo) (Fig. 1A). Mean IGF-I SDS increased from −1.98 ± 0.60 at baseline to −0.6 ± 1.1 (P = 0.0007 vs. placebo) at 6 months in the GH group (Fig. 1B).
Figure 1.
Mean (±sem) IGF-I (A) and IGF-I SDS (B) in the GH-treated and placebo-treated study participants at baseline and 6 months. *, P < 0.05 vs. placebo-treated subjects.
Effects of GH administration on body composition and cardiovascular risk markers
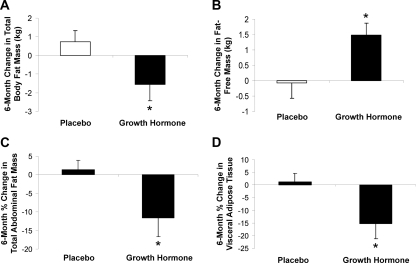
There was no significant change in weight (P = 0.43) or BMI (P = 0.92) in the GH treatment group compared with placebo. Total fat mass decreased in the treatment group [−1.6 ± 3.2 (GH) vs. 0.7 ± 2.2 (placebo) kg, P = 0.04] (Table 2 and Fig. 2A). Total abdominal fat (−11.6 ± 18.9 vs. 1.4 ± 8.7%, P = 0.04) decreased in study subjects receiving GH compared with placebo (Fig. 2C), as did visceral adipose tissue (−15.3 ± 18.6 vs. 1.3 ± 12.5%, P = 0.01) (Fig. 2D), and the percent decrease in sc fat mass approached significance (−10.1 ± 19.8 vs. 2.3 ± 11.5%, P = 0.06). Fat-free mass increased in patients receiving GH [1.5 ± 1.4 (GH) vs. −0.1 ± 1.9 (placebo) kg, P = 0.02] (Fig. 2B), without a change in total body water (Table 2).
Figure 2.
Mean (±sem) change in total body fat, measured by DXA (A), fat-free mass, measured by DXA (B), total abdominal fat, measured by cross-sectional CT (C), and visceral adipose tissue, measured by cross-sectional CT (D) over 6 months in the GH-treated and placebo-treated study participants. *, P < 0.05 vs. placebo-treated study subjects.
Mean hsCRP levels decreased in the treatment group compared with placebo (Table 2) from 2.9 ± 3.3 to 2.1 ± 2.5 mg/liter (P = 0.05). GH administration did not result in a significant change in carotid IMT, total cholesterol, HDL, low-density lipoprotein (LDL), triglycerides, or fibrinogen. Glycosylated hemoglobin and HOMA-IR were unchanged (Table 2).
Effects of GH administration on quality of life
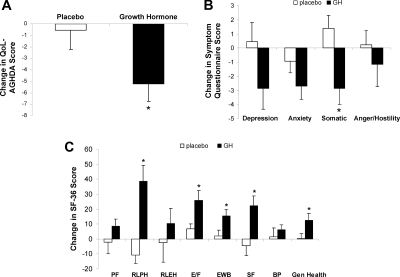
Quality of life, as measured by the AGHDA, improved with GH (P = 0.05), as did five of eight SF-36 subscales [role limitation due to physical health (P = 0.0008), vitality (P = 0.01), mental health (P = 0.03), social functioning (P = 0.008), and general health (P = 0.05)] and one of four Symptom Questionnaire subscales [somatic symptoms (P = 0.008)] (Fig. 3).
Figure 3.
Mean (±sem) change in the GH and placebo groups over 6 months in quality of life, as measured by the QOL-AGHDA (A), Symptom Questionnaire (B), and SF-36 (C). PF, Physical functioning; RLPH, role limitations due to physical health; RLEH, role limitations due to emotional health; MH, mental health; SF, social functioning; BP, bodily pain; Gen Health, general health. Higher scores reflect a poorer quality of life on the QOL-AGHDA and Symptom Questionnaire and a better quality of life on the SF-36. *, P < 0.05 vs. placebo-treated study subjects.
Predictors of response
In the group as a whole, change in IGF-I levels predicted the change at 6 months in visceral adipose tissue (R = −0.51, P = 0.006), fat-free mass (R = 0.59, P = 0.001), hsCRP (R = −0.42, P = 0.02), SF-36 emotional well-being scale (R = 0. 39, P < 0.05), SF-36 social functioning (R = 0.38, P < 0.05), and the Symptom Questionnaire somatic symptoms scale (R = −0.42, P = 0.03).
In the GH group alone, lower peak GH to GHRH-arginine stimulation at baseline predicted a greater reduction in total fat by DXA (R = 0.59, P = 0.04) but less of a response in severity of depression on the depression subscale of the Symptom Questionnaire (R = −0.61, P < 0.05). Lower IGF-I SDS at baseline predicted a greater decrease in sc abdominal fat (R = 0.55, P = 0.04) and a greater improvement in the somatic symptoms scale of the Symptom Questionnaire (R = 0.58, P = 0.04). A higher GH dose predicted a greater decrease in total fat by DXA (R = −0.58, P = 0.03), a greater decrease in visceral adipose tissue (R = −0.57, P = 0.03), and a greater decrease in sc fat (R = −0.55, P = 0.04). Baseline BMI was a significant predictor of response to the Rand SF-36, role limitation due to emotional health subscale (R = −0.63, P = 0.02), such that, in general, subjects with higher BMIs experienced less of an improvement. Baseline BMI was not a predictor of response to GH for any other end point.
Neither age nor sex was a predictor of response of any variable to GH administration.
Side effects
Side effects were minimal, without higher rates of edema, joint pain, or carpal tunnel syndrome symptoms in the GH vs. placebo group. Specifically, five subjects in the GH group and eight in the placebo group reported arthralgias during the study, five subjects in the GH group and five in the placebo group experienced mild edema, and one subject in the GH group and four subjects in the placebo group reported mild carpal tunnel symptoms. One study participant randomized to the GH group dropped out of the study after her 3-month visit due to discomfort at the injection sites. All other study participants completed the 6-month protocol.
Discussion
This is the first randomized, placebo-controlled study of the effects of GH replacement therapy on body composition and cardiovascular end points in patients who developed GHD after treatment for acromegaly, a disease complicated by metabolic and body composition alterations and increased cardiovascular risk. In this 6-month study, we demonstrate decreases in visceral fat mass and total fat mass, an increase in fat-free mass, a decrease in hsCRP, and an improvement in quality of life with GH administration in patients with GHD after cure of acromegaly. Of importance, these positive effects occurred in the absence of significant side effects and without an increase in insulin resistance. Improvements in body composition, particularly visceral adiposity, may have important cardiovascular clinical consequences for patients because visceral adiposity is clearly associated with a marked increase in coronary heart disease (37,38) as well as strongly associated with metabolic, atherothrombotic and inflammatory cardiovascular risk marker abnormalities (39,40,41). Further studies are warranted to determine the long-term effects of GH replacement in patients with prior acromegaly who develop GHD.
GHD after definitive treatment of acromegaly is common. Studies have shown that 30–50% of patients develop GHD after radiation therapy for acromegaly (42,43,44). A recent report demonstrated a 70% prevalence of severe GHD in patients with acromegaly treated with surgery followed by radiation therapy (13). Moreover, 55% of patients treated for acromegaly with surgery alone, i.e. in the absence of a history of radiation therapy, also developed severe GHD (13). Of importance, GHD in such patients appears to be associated with abnormalities in body composition (14,15) and a diminished quality of life (20), as in patients who develop GHD after treatment of nonsomatotroph tumors (2,3,8,45,46). However, no randomized, placebo-controlled studies have addressed the question of whether GH replacement therapy reverses these abnormalities in patients with GHD after definitive therapy for acromegaly.
Our findings of improvements in body composition and hsCRP with GH administration in patients with prior acromegaly are comparable with those of randomized, placebo-controlled studies of GH administration in men and women with GH due to nonsomatotroph tumors (9,10,11,12). The results of this randomized, placebo-controlled study differ in important ways from the three open-label trials in patients with GHD and prior acromegaly reported in the literature (14,15,16). Only one of the open-label trials reported any effects of GH administration on body composition. Specifically, Norrman et al. (15) demonstrated a significant increase compared with baseline in body cell mass with GH administration. Neither of the other two open-label studies showed significant improvements in body composition, although none used cross-sectional CT scans to assess visceral and sc fat. It also should be noted that there was a significant increase in HDL cholesterol and a trend toward a decrease in waist to hip ratio, total cholesterol, and LDL cholesterol compared with baseline in the smallest of these studies, by Norrman et al. (15), the power of which was limited due to its size (n = 10).
Previously published placebo-controlled studies in patients with nonsomatotroph tumors reported that GH replacement reduces visceral adipose stores and hsCRP and increases lean body or muscle mass (9,10,11,12). However, cardiovascular disease is common in patients with acromegaly, with more than two thirds manifesting concentric cardiac hypertrophy with diastolic dysfunction at diagnosis (47). Hypertension is present in close to 50% of patients with acromegaly (48). In addition, other cardiovascular abnormalities, including valvular regurgitation and rhythm disturbances are common in patients with acromegaly (47). Because this cardiovascular background differs from that of most patients with hypopituitarism, it was not clear whether results of studies performed in patients with nonsomatroph tumor-related hypopituitarism could be generalized to patients who had prior prolonged exposure to elevated GH levels. The results of the current study suggest that effects on body composition and cardiovascular risk markers are comparable in the two populations. We did not observe a decrease in IMT with GH therapy in contrast to results from published studies in patients with hypopituitarism from other causes. These studies suggest, but do not definitively demonstrate, that such decreases may occur with short courses of GH replacement therapy in hypopituitary patients (49,50,51). Increased IMT, without an increase in atherosclerotic plaques, has been demonstrated in patients with active acromegaly and ascribed to the increased prevalence of hypertension in this group (52). Therefore, the difference between our results and those in hypopituitary patients without a history of acromegaly may reflect true differences between patients with a history of somatotroph tumors and patients with other causes of hypopituitarism. It is also possible that a longer study duration or larger number of study subjects would have been necessary to detect the GH effect size.
We demonstrate significant improvements in several aspects of quality of life in patients with GHD and prior acromegaly in contrast to results from two open-label trials (14,16). We have recently shown that patients with prior acromegaly and GHD have a diminished quality of life compared with patients who have been cured of acromegaly but who have peak GH levels within the normal range (20). Therefore, GHD may be a contributing factor to the well-documented diminished quality of life in patients despite cure of acromegaly (53,54,55,56). Most (57,58,59,60,61), although not all (62), randomized, placebo-controlled studies demonstrated an improvement in quality of life with GH administration in patients with GHD after treatment of nonsomatatroph tumors. Our data suggest that GH replacement therapy may improve quality of life in patients who develop GHD after definitive therapy for acromegaly.
Of note, side effects were infrequent, mild, and comparable between the GH and placebo groups. Whether patients who have experienced an increased exposure to GH experience comparable or lower side effect rates than their counterparts with GHD but without a history of increased GH exposure is not clear. Our results are in contrast to those of Norrman et al. (15), who reported that one of 10 GH-deficient patients with a history of acromegaly studied experienced a myocardial infarction (5 months after initiation of GH therapy) and two, both of whom had received cranial radiation therapy, experienced cerebral vascular accidents (one at 2 wk and one at 6 wk after initiation of GH therapy) with low-dose (mean 0.36 mg/d) GH therapy. However, of the 10 patients in that study, three had hypertension, one diabetes mellitus, and one hyperlipidemia. The contribution, if any, of the role of GH administration in these events, which occurred soon after initiation of GH, is unclear because similar events were not observed in the other two open-label studies or our placebo-controlled study. It should be noted that in the Pfizer International Metabolic Study database study, Feldt-Rasmussen et al. (14) reported an increased prevalence of cerebral vascular events at baseline, i.e. before GH administration, in patients with GHD and prior acromegaly compared with patients with GHD of other etiologies. GH administration was not associated with a relative increased incidence of serious events in such patients (14). It is possible that the risk of vascular events may be conferred by the history of acromegaly or radiation therapy, rather than the effects of GH administration. The number of patients in this and previous trials is too small to draw definitive conclusions regarding side effects. However, one cannot rule out an increased risk of vascular events with GH replacement in GH-deficient patients with prior acromegaly, and therefore, caution seems prudent in prescribing GH to such patients, especially those with particularly elevated cardiovascular risk, until further, long-term data are available.
In summary, we demonstrate improvements in body composition, hsCRP, and quality of life with 6 months of low-dose GH administration in patients with GHD after cure of acromegaly in the first such randomized, placebo-controlled trial. Of note, our findings include an approximately 15% decrease in visceral adipose tissue, a reduction in hsCRP levels, and an increase in lean body mass. Our data suggest that such patients may benefit from a trial of GH replacement therapy. These positive effects were accompanied by a very low side effect rate, including no deterioration in glucose tolerance. However, it is important to note that the number of patients in this and previous trials and the length of administration of GH are too small to draw definitive conclusions regarding side effects. Because an open-label trial of GH administration in patients with prior acromegaly reported an elevated incidence of cardiovascular events (15), further studies are warranted to examine the safety and advisability of GH administration in patients with prior acromegaly, particularly those with elevated cardiovascular risk.
Acknowledgments
We thank the nurses and bionutritionists of the Massachusetts General Hospital General Clinical Research Center and the Harvard Catalyst Clinical Translational Science Center and the patients who participated in the study.
Footnotes
This work was supported by National Institutes of Health Grants MO1 RR01066 and ULI RR0257801, an investigator-initiated grant from Pfizer Inc., and the Guthart Family Foundation.
Disclosure Summary: A.K. received funding for this investigator-initiated study from Pfizer Inc. B.M.K.B. consults for and has received research funding from Pfizer, Inc. No other authors have any conflicts to declare.
First Published Online January 8, 2010
Abbreviations: BMI, Body mass index; CT, computed tomography; cv, coefficient of variation; DXA, dual-energy x-ray absorptiometry; GHD, GH deficiency; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment-insulin resistance; hsCRP, high-sensitivity C-reactive protein; IMT, intimal-medial thickness; LDL, low-density lipoprotein; QOL-AGHDA, Quality of Life Assessment of Growth Hormone Deficiency in Adults; SDS, sd score; SF-36, Short-Form Health Survey.
References
- Barreto-Filho JA, Alcântara MR, Salvatori R, Barreto MA, Sousa AC, Bastos V, Souza AH, Pereira RM, Clayton PE, Gill MS, Aguiar-Oliveira MH 2002 Familial isolated growth hormone deficiency is associated with increased systolic blood pressure, central obesity, and dyslipidemia. J Clin Endocrinol Metab 87:2018–2023 [DOI] [PubMed] [Google Scholar]
- Binnerts A, Deurenberg P, Swart GR, Wilson JH, Lamberts SW 1992 Body composition in growth hormone-deficient adults. Am J Clin Nutr 55:918–923 [DOI] [PubMed] [Google Scholar]
- De Boer H, Blok GJ, Voerman HJ, De Vries PM, van der Veen EA 1992 Body composition in adult growth hormone-deficient men, assessed by anthropometry and bioimpedance analysis. J Clin Endocrinol Metab 75:833–837 [DOI] [PubMed] [Google Scholar]
- Devin JK, Blevins Jr LS, Verity DK, Chen Q, Bloodworth Jr JR, Covington J, Vaughan DE 2007 Markedly impaired fibrinolytic balance contributes to cardiovascular risk in adults with growth hormone deficiency. J Clin Endocrinol Metab 92:3633–3639 [DOI] [PubMed] [Google Scholar]
- Gomez JM, Sahun M, Vila R, Domenech P, Catalina P, Soler J, Badimon L 2006 Peripheral fibrinolytic markers, soluble adhesion molecules, inflammatory cytokines and endothelial function in hypopituitary adults with growth hormone deficiency. Clin Endocrinol (Oxf) 64:632–639 [DOI] [PubMed] [Google Scholar]
- Rosen T, Eden S, Larson G, Wilhelmsen L, Bengtsson BA 1993 Cardiovascular risk factors in adult patients with growth hormone deficiency. Acta Endocrinol (Copenh) 129:195–200 [DOI] [PubMed] [Google Scholar]
- Sesmilo G, Miller KK, Hayden D, Klibanski A 2001 Inflammatory cardiovascular risk markers in women with hypopituitarism. J Clin Endocrinol Metab 86:5774–5781 [DOI] [PubMed] [Google Scholar]
- Weaver JU, Monson JP, Noonan K, John WG, Edwards A, Evans KA, Cunningham J 1995 The effect of low dose recombinant human growth hormone replacement on regional fat distribution, insulin sensitivity, and cardiovascular risk factors in hypopituitary adults. J Clin Endocrinol Metab 80:153–159 [DOI] [PubMed] [Google Scholar]
- Baum HB, Biller BM, Finkelstein JS, Cannistraro KB, Oppenhein DS, Schoenfeld DA, Michel TH, Wittink H, Klibanski A 1996 Effects of physiologic growth hormone therapy on bone density and body composition in patients with adult-onset growth hormone deficiency. A randomized, placebo-controlled trial. Ann Intern Med 125:883–890 [DOI] [PubMed] [Google Scholar]
- Beauregard C, Utz AL, Schaub AE, Nachtigall L, Biller BM, Miller KK, Klibanski A 2008 Growth hormone decreases visceral fat and improves cardiovascular risk markers in women with hypopituitarism: a randomized, placebo-controlled study. J Clin Endocrinol Metab 93:2063–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesmilo G, Biller BM, Llevadot J, Hayden D, Hanson G, Rifai N, Klibanski A 2000 Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med 133:111–122 [DOI] [PubMed] [Google Scholar]
- Maison P, Griffin S, Nicoue-Beglah M, Haddad N, Balkau B, Chanson P 2004 Impact of growth hormone (GH) treatment on cardiovascular risk factors in GH-deficient adults: a metaanalysis of blinded, randomized, placebo-controlled trials. J Clin Endocrinol Metab 89:2192–2199 [DOI] [PubMed] [Google Scholar]
- Ronchi CL, Giavoli C, Ferrante E, Verrua E, Bergamaschi S, Ferrari DI, Corbetta S, Montefusco L, Arosio M, Ambrosi B, Spada A, Beck-Peccoz P 2009 Prevalence of GH deficiency in cured acromegalic patients: impact of different previous treatments. Eur J Endocrinol 161:37–42 [DOI] [PubMed] [Google Scholar]
- Feldt-Rasmussen U, Abs R, Bengtsson BA, Bennmarker H, Bramnert M, Hernberg-Ståhl E, Monson JP, Westberg B, Wilton P, Wüster C 2002 Growth hormone deficiency and replacement in hypopituitary patients previously treated for acromegaly or Cushing’s disease. Eur J Endocrinol 146:67–74 [DOI] [PubMed] [Google Scholar]
- Norrman LL, Johannsson G, Sunnerhagen KS, Svensson J 2008 Baseline characteristics and the effects of two years of growth hormone (GH) replacement therapy in adults with GH deficiency previously treated for acromegaly. J Clin Endocrinol Metab 93:2531–2538 [DOI] [PubMed] [Google Scholar]
- van der Klaauw AA, Bax JJ, Roelfsema F, Stokkel MP, Bleeker GB, Biermasz NR, Smit JW, Romijn JA, Pereira AM 2009 Limited effects of growth hormone replacement in patients with GH deficiency during long-term cure of acromegaly. Pituitary 12:339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharib H, Cook DM, Saenger PH, Bengtsson BA, Feld S, Nippoldt TB, Rodbard HW, Seibel JA, Vance ML, Zimmerman D 2003 American Association of Clinical Endocrinologists medical guidelines for clinical practice for growth hormone use in adults and children—2003 update. Endocr Pract 9:64–76 [DOI] [PubMed] [Google Scholar]
- Biller BM, Samuels MH, Zagar A, Cook DM, Arafah BM, Bonert V, Stavrou S, Kleinberg DL, Chipman JJ, Hartman ML 2002 Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab 87:2067–2079 [DOI] [PubMed] [Google Scholar]
- Hartman ML, Crowe BJ, Biller BM, Ho KK, Clemmons DR, Chipman JJ 2002 Which patients do not require a GH stimulation test for the diagnosis of adult GH deficiency? J Clin Endocrinol Metab 87:477–485 [DOI] [PubMed] [Google Scholar]
- Wexler T, Gunnell L, Omer Z, Kuhlthau K, Beauregard C, Graham G, Utz AL, Biller B, Nachtigall L, Loeffler J, Swearingen B, Klibanski A, Miller KK 2009 Growth hormone deficiency is associated with decreased quality of life in patients with prior acromegaly. J Clin Endocrinol Metab 94:2471–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutkia P, Canavan B, Breu J, Torriani M, Kissko J, Grinspoon S 2004 Growth hormone-releasing hormone in HIV-infected men with lipodystrophy. JAMA 292:210–218 [DOI] [PubMed] [Google Scholar]
- Cunningham JJ 1990 Calculation of energy expenditure from indirect calorimetry: assessment of the Weir equation. Nutrition 6:222–223 [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M 2000 Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23:57–63 [DOI] [PubMed] [Google Scholar]
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ 2000 Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85:2402–2410 [DOI] [PubMed] [Google Scholar]
- McKenna SP, Doward LC, Alonso J, Kohlmann T, Niero M, Prieto L, Wíren L 1999 The QoL-AGHDA: an instrument for the assessment of quality of life in adults with growth hormone deficiency. Qual Life Res 8:373–383 [DOI] [PubMed] [Google Scholar]
- Holmes SJ, McKenna SP, Doward LC, Hunt SM, Shalet SM 1995 Development of a questionnaire to access the quality of life of adults with growth hormone deficiency. Endocrinol Metab 2:63–69 [Google Scholar]
- Perahia DG, Gilaberte I, Wang F, Wiltse CG, Huckins SA, Clemens JW, Montgomery SA, Montejo AL, Detke MJ 2006 Duloxetine in the prevention of relapse of major depressive disorder: double-blind placebo-controlled study. Br J Psychiatry 188:346–353 [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV 2006 Adverse psychological and coronary risk profiles in young patients with coronary artery disease and benefits of formal cardiac rehabilitation. Arch Intern Med 166:1878–1883 [DOI] [PubMed] [Google Scholar]
- Denninger JW, Papakostas GI, Mahal Y, Merens W, Alpert JE, Nierenberg AA, Yeung A, Fava M 2006 Somatic symptoms in outpatients with major depressive disorder treated with fluoxetine. Psychosomatics 47:348–352 [DOI] [PubMed] [Google Scholar]
- Fava M, Rosenbaum JF, Pava JA, McCarthy MK, Steingard RJ, Bouffides E 1993 Anger attacks in unipolar depression, part 1: clinical correlates and response to fluoxetine treatment. Am J Psychiatry 150:1158–1163 [DOI] [PubMed] [Google Scholar]
- Fava GA, Kellner R, Perini GI, Fava M, Michelacci L, Munari F, Evangelisti LP, Grandi S, Bernardi M, Mastrogiacomo I 1983 Italian validation of the Symptom Rating Test (SRT) and Symptom Questionnaire (SQ). Can J Psychiatry 28:117–123 [DOI] [PubMed] [Google Scholar]
- Kellner R 1987 A symptom questionnaire. J Clin Psychiatry 48:268–274 [PubMed] [Google Scholar]
- Ware JE, Snow KK, Kosinski M, Gandek B 1993 SF-36 health survey manual and interpretation guide. Boston: The Health Institute, New England Medical Center [Google Scholar]
- McHorney CA, Ware Jr JE, Raczek AE 1993 The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 31:247–263 [DOI] [PubMed] [Google Scholar]
- Hoffman AR, Kuntze JE, Baptista J, Baum HB, Baumann GP, Biller BM, Clark RV, Cook D, Inzucchi SE, Kleinberg D, Klibanski A, Phillips LS, Ridgway EC, Robbins RJ, Schlechte J, Sharma M, Thorner MO, Vance ML 2004 Growth hormone (GH) replacement therapy in adult-onset GH deficiency: effects on body composition in men and women in a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab 89:2048–2056 [DOI] [PubMed] [Google Scholar]
- Després JP, Lemieux I, Prud'homme D 2001 Treatment of obesity: need to focus on high risk abdominally obese patients. BMJ 322:716–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche B 1998 Abdominal obesity and its metabolic complications: implications for the risk of ischaemic heart disease. Coron Artery Dis 9:473–481 [DOI] [PubMed] [Google Scholar]
- Després JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C 1990 Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis 10:497–511 [DOI] [PubMed] [Google Scholar]
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino Sr RB, O'Donnell CJ 2007 Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116:39–48 [DOI] [PubMed] [Google Scholar]
- Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S 1987 Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 36:54–59 [DOI] [PubMed] [Google Scholar]
- Biermasz NR, van Dulken H, Roelfsema F 2000 Long-term follow-up results of postoperative radiotherapy in 36 patients with acromegaly. J Clin Endocrinol Metab 85:2476–2482 [DOI] [PubMed] [Google Scholar]
- Murray RD, Peacey SR, Rahim A, Toogood AA, Thorner MO, Shalet SM 2001 The diagnosis of growth hormone deficiency (GHD) in successfully treated acromegalic patients. Clin Endocrinol (Oxf) 54:37–44 [DOI] [PubMed] [Google Scholar]
- van der Klaauw AA, Pereira AM, van Thiel SW, Smit JW, Corssmit EP, Biermasz NR, Frolich M, Iranmanesh A, Veldhuis JD, Roelfsema F, Romijn JA 2006 GH deficiency in patients irradiated for acromegaly: significance of GH stimulatory tests in relation to the 24 h GH secretion. Eur J Endocrinol 154:851–858 [DOI] [PubMed] [Google Scholar]
- Bjork S, Jonsson B, Westphal O, Levin JE 1989 Quality of life of adults with growth hormone deficiency: a controlled study. Acta Paediatr Scand Suppl 356:55–59; discussion 60, 73–74 [DOI] [PubMed] [Google Scholar]
- McGauley GA 1989 Quality of life assessment before and after growth hormone treatment in adults with growth hormone deficiency. Acta Paediatr Scand Suppl 356:70–72; discussion 73–74 [DOI] [PubMed] [Google Scholar]
- Colao A 2008 The GH/IGF axis and the cardiovascular system: clinical implications. Clin Endocrinol (Oxf) 69:347–358 [DOI] [PubMed] [Google Scholar]
- Vitale G, Pivonello R, Auriemmo RS, Guerra E, Milone F, Savastano S, Lombardi G, Colao A 2005 Hypertension in acomegaly and in the normal population: prevalence and determinants. Clin Endocrinol (Oxf) 63:470–476 [DOI] [PubMed] [Google Scholar]
- Borson-Chazot F, Serusclat A, Kalfallah Y, Ducottet X, Sassolas G, Bernard S, Labrousse F, Pastene J, Sassolas A, Roux Y, Berthezène F 1999 Decrease in carotid intima-media thickness after one year growth hormone (GH) treatment in adults with GH deficiency. J Clin Endocrinol Metab 84:1329–1333 [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Verhovec R, Zizek B, Prezelj J, Poredos P, Clayton RN 1999 Growth hormone (GH) treatment reverses early atherosclerotic changes in GH-deficient adults. J Clin Endocrinol Metab 84:453–457 [DOI] [PubMed] [Google Scholar]
- Colao A, Di Somma C, Rota F, Pivonello R, Savanelli MC, Spiezia S, Lombardi G 2005 Short-term effects of growth hormone (GH) treatment or deprivation on cardiovascular risk parameters and intima-media thickness at carotid arteries in patients with severe GH deficiency. J Clin Endocrinol Metab 90:2056–2062 [DOI] [PubMed] [Google Scholar]
- Colao A, Spiezia S, Cerbone G, Pivonello R, Marzullo P, Ferone D, Di Somma C, Assanti AP, Lombardi G 2001 Increased arterial intima-media thickness by B-M mode echodoppler ultrasonography in acromegaly. Clin Endocrinol (Oxf) 54:515–524 [DOI] [PubMed] [Google Scholar]
- Biermasz NR, Pereira AM, Smit JW, Romijn JA, Roelfsema F 2005 Morbidity after long-term remission for acromegaly: persisting joint-related complaints cause reduced quality of life. J Clin Endocrinol Metab 90:2731–2739 [DOI] [PubMed] [Google Scholar]
- Biermasz NR, van Thiel SW, Pereira AM, Hoftijzer HC, van Hemert AM, Smit JW, Romijn JA, Roelfsema F 2004 Decreased quality of life in patients with acromegaly despite long-term cure of growth hormone excess. J Clin Endocrinol Metab 89:5369–5376 [DOI] [PubMed] [Google Scholar]
- Kauppinen-Mäkelin R, Sane T, Sintonen H, Markkanen H, Välimäki MJ, Löyttyniemi E, Niskanen L, Reunanen A, Stenman UH 2006 Quality of life in treated patients with acromegaly. J Clin Endocrinol Metab 91:3891–3896 [DOI] [PubMed] [Google Scholar]
- van der Klaauw AA, Kars M, Biermasz NR, Roelfsema F, Dekkers OM, Corssmit EP, van Aken MO, Havekes B, Pereira AM, Pijl H, Smit JW, Romijn JA 2008 Disease-specific impairments in quality of life during long-term follow-up of patients with different pituitary adenomas. Clin Endocrinol (Oxf) 69:775–784 [DOI] [PubMed] [Google Scholar]
- Attanasio AF, Shavrikova EP, Blum WF, Shalet SM 2005 Quality of life in childhood onset growth hormone-deficient patients in the transition phase from childhood to adulthood. J Clin Endocrinol Metab 90:4525–4529 [DOI] [PubMed] [Google Scholar]
- Cuneo RC, Judd S, Wallace JD, Perry-Keene D, Burger H, Lim-Tio S, Strauss B, Stockigt J, Topliss D, Alford F, Hew L, Bode H, Conway A, Handelsman D, Dunn S, Boyages S, Cheung NW, Hurley D 1998 The Australian Multicenter Trial of Growth Hormone (GH) Treatment in GH-Deficient Adults. J Clin Endocrinol Metab 83:107–116 [DOI] [PubMed] [Google Scholar]
- Holmes SJ, Shalet SM 1995 Factors influencing the desire for long-term growth hormone replacement in adults. Clin Endocrinol (Oxf) 43:151–157 [DOI] [PubMed] [Google Scholar]
- McGauley GA, Cuneo RC, Salomon F, Sonksen PH 1990 Psychological well-being before and after growth hormone treatment in adults with growth hormone deficiency. Horm Res 33(Suppl 4):52–54 [DOI] [PubMed] [Google Scholar]
- Burman P, Broman JE, Hetta J, Wiklund I, Erfurth EM, Hagg E, Karlsson FA 1995 Quality of life in adults with growth hormone (GH) deficiency: response to treatment with recombinant human GH in a placebo-controlled 21-month trial. J Clin Endocrinol Metab 80:3585–3590 [DOI] [PubMed] [Google Scholar]
- Baum HB, Katznelson L, Sherman JC, Biller BM, Hayden DL, Schoenfeld DA, Cannistraro KE, Klibanski A 1998 Effects of physiological growth hormone (GH) therapy on cognition and quality of life in patients with adult-onset GH deficiency. J Clin Endocrinol Metab 83:3184–3189 [DOI] [PubMed] [Google Scholar]