Abstract
Background: Transsphenoidal surgery (TSS) is the treatment of choice for Cushing’s disease (CD). Postoperative hypercortisolemia mandates further therapy.
Objective: The aim of the study was to characterize patients without immediate postoperative remission who have a delayed decrease to normal or low cortisol levels without further therapy.
Design and Setting: A retrospective case series was conducted at three tertiary care centers.
Patients and Intervention: We reviewed the records of 620 patients (512 females, 108 males; mean age, 38 ± 13 yr) who underwent transsphenoidal pituitary surgery for CD between 1982 and 2007.
Results: Outcomes were classified into the following three groups based upon the postoperative pattern of cortisol testing: group IC (immediate control) included 437 of the 620 patients (70.5%) with hypocortisolism and/or cortisol normalization throughout the postoperative follow-up; group NC (no control) included 148 of 620 patients (23.9%) with persistent hypercortisolism; and group DC (delayed control) included 35 of 620 patients (5.6%) who had early elevated or normal UFC levels and developed a delayed and persistent cortisol decrease after an average of 38 ± 50 postoperative days. The total rate of recurrence was 13% at a median follow-up time of 66 months after TSS; the cumulative rate of recurrence at 4.5 yr was significantly higher in group DC vs. group IC (43 vs. 14%; P = 0.02).
Conclusions: Hormonal assessment in the immediate postoperative period after TSS for CD may be misleading because delayed remission can occur in a subset of patients. Expectant management and retesting may spare some patients from unnecessary further treatment. Optimal timing to determine the need for further therapy after TSS remains to be determined.
A subset of patients with Cushing’s disease who are not hypoadrenal within the first 2-3 days postoperatively undergoes a spontaneous late remission.
Transsphenoidal pituitary surgery is the first-line treatment for patients with Cushing’s disease (CD), and remission rates vary from 60 to 90% (1,2,3,4,5,6,7,8,9,10,11,12). This variability relates to the size and location of tumors, neurosurgical expertise, length of follow-up, and the definition of remission. Indeed, the latter is a controversial aspect in the management of CD, with no consensus on the most accurate postoperative assessment of remission and its predictive value for long-term prognosis (13).
Hypoadrenalism within 2 wk after adenomectomy is considered the most reliable criterion for defining remission in many (9,14,15), but not all series (1,3,4,5,6,16). There is agreement that persistent hypercortisolism in the immediate postoperative period indicates surgical failure (13). Patients who only normalize serum and/or urinary cortisol have a significantly higher risk of hypercortisolemia on long-term follow-up, compared with those with low/undetectable serum cortisol levels in the immediate postoperative period (2,8,17). It has been suggested, and in many centers is common practice, that any patient who is not hypoadrenal in the immediate postoperative period should receive further treatment (15,18). However, in some patients, serum cortisol levels may undergo a delayed decline after pituitary resection, suggesting that a longer follow-up over 6–12 wk after surgery may be more accurate (12,19,20).
The aim of the present study was to analyze retrospectively the outcome in 620 patients with CD from three referral centers (one in Boston and two in Milan) who underwent transsphenoidal surgery (TSS) between 1982 and 2007 to characterize subjects who experienced a delayed decline of cortisol levels after TSS, leading to reclassification of the surgical outcome. Such patients are important to identify so that they may be spared unnecessary additional therapy.
Subjects and Methods
Subjects
The study was a retrospective chart review of 620 cases of CD after TSS performed at tertiary care centers in the United States and Italy. A total of 219 patients were evaluated for CD in the United States (Massachusetts General Hospital, Boston, MA) from 1998 to 2007, and 401 patients were evaluated at two clinical centers in Milan, Italy (Ospedale San Luca, Istituto Auxologico Italiano, and Istituto Scientifico San Raffaele) from 1982 to 2007. Of the 401 patients in Italy, 262 (214 females and 48 males; mean age, 38.4 ± 0.8 yr) were described in a previous study investigating TSS efficacy and safety in a large series of patients with all types of pituitary adenomas. In that paper (21), no evaluation of the endpoint of this study, the possible delayed decline in cortisol levels, was performed. Recurrence data from the U.S. series have been previously reported without analysis of delayed cures (22).
Mean follow-up for the Italian series was 56 ± 48 months (range, 1–300) vs. 25 ± 30 months (range, 1–131) in the U.S. cohort (P < 0.001). The diagnosis of CD was made after clinical, biochemical, and radiological evaluation. Although patients were referred to tertiary centers based upon different local testing protocols, all had abnormal values on at least two of the following tests as described in recent consensus guidelines: 24-h urinary free cortisol (UFC), late-night salivary or serum cortisol, 1-mg overnight dexamethasone suppression test (ODST), 2-d low-dose dexamethasone suppression test, and the combination of low-dose dexamethasone suppression test and ovine or human CRH stimulation test (23).
A pituitary source of Cushing’s syndrome was determined using standard criteria including plasma ACTH levels, CRH test, high-dose dexamethasone suppression test, and combined low-dose and high-dose dexamethasone suppression tests.
Imaging studies included either pituitary gland magnetic resonance imaging (MRI) or computed tomography, the latter performed in earlier patients and in those patients unable to undergo an MRI. Bilateral inferior petrosal sinus sampling was performed in the U.S. series for pituitary lesions less than 10 mm in diameter and in Italy when the MRI was negative or when it showed a microadenoma but hormone testing was indicative of a potential ectopic source. A single pituitary surgeon performed all operative procedures in the United States, whereas in the Italian series, TSS was performed by two pituitary surgeons. Histopathology to confirm the diagnosis of ACTH-staining adenoma was performed when tissue was available (98% of cases).
Postoperative testing varied with individual center practice; it was performed within 3 d after the operation in almost all patients and on the fourth or fifth day in a few patients. Institutional Review Board approval was obtained at each institution (Partners Health Care Institutional Review Board, Boston, MA; Istituto Auxologico Italiano Ethical Committee, Milan, Italy; and Istituto Scientifico San Raffaele, Ethical Committee, Milan, Italy).
Postsurgical evaluation included: 1) morning serum cortisol; 2) 24-h UFC collection; 3) plasma ACTH (only in the Italian series); and 4) ODST (only in the Italian series).
Postoperative classification of surgical outcomes
Classification into three groups was made a posteriori (i.e. in retrospect) based on eventually stabilized cortisol levels.
We defined three groups of patients based upon the timing of the postoperative nadir cortisol levels: controlled, not controlled, or delayed control. The word “control” is used for all patients who are no longer hypercortisolemic and have cortisol levels within or below the normal range immediately after surgery. This group includes both patients who would be considered in remission/cured based on profound hypoadrenalism as well as patients whose immediate postoperative cortisol levels have decreased into the normal range. Traditionally, patients who are adrenally insufficient after surgery for CD are considered cured/in remission, whereas patients with “normal” cortisol levels are not. However, in this analysis, patients with cortisol levels within or below the normal range are grouped together because all such patients were no longer hypercortisolemic and did not receive further therapy for CD.
Immediate control (IC) group
Patients in this group had UFC below 70 μg/24 h (193 nmol/d) on d 2–3 after TSS for the U.S. cohort or below 80 μg/24 h (221 nmol/d) on d 3 for the Italian cohort; and/or, when UFC measurements were not available, they had serum cortisol levels within the normal range and/or suppression of serum cortisol in response to an ODST [<5 μg/dl (138 nmol/liter)]. All patients in this group maintained this testing pattern at subsequent evaluation(s).
No control (NC) group
Patients in this group had UFC above 70 μg/24 h (193 nmol/d) on d 2–3 after TSS for the U.S. cohort or above 80 μg/24 h (221 nmol/d) on d 3 for the Italian cohort; and/or, when UFC measurements were not available, they had normal serum cortisol concentrations and/or failure to suppress serum cortisol in response to an ODST. All patients in this group maintained this testing pattern at subsequent follow-up.
Delayed control (DC) group
This group includes patients who were either hypercortisolemic in the immediate postoperative period but whose UFC levels subsequently decreased to eu- or hypocortisolemia, or patients who were eucortisolemic in the immediate postoperative period who became hypocortisolemic at subsequent evaluation(s).
Recurrence criteria
Criteria for recurrence included at least two of the following: 1) elevated serum cortisol; 2) elevated 24-h UFC; 3) failed ODST [>5 μg/dl (138 nmol/liter)]; or 4) abnormal serum cortisol during the combination of low-dose dexamethasone suppression test and ovine or human CRH stimulation test.
Exclusion criteria
Six patients were excluded because adequate postoperative testing was not available. The total number of patients included in the analysis was therefore 620 of a possible 626.
Glucocorticoid replacement therapy
Patients in the U.S. cohort were discharged within 48 h postoperatively before adequate cortisol testing could be completed. Therefore, a low dose of dexamethasone (1–2 mg/d) was administered for patient safety in the outpatient setting given the high rate of adrenal insufficiency after adenomectomy for CD. In the Italian cohort, glucocorticoids (cortisone acetate) were withheld for at least 24 h while biochemical testing was performed on inpatients in the immediate postoperative period.
Hormone assays
In the U.S. series, plasma ACTH was measured using a commercial immunoluminescent kit (Immulite 2000; Siemens Medical Solutions Diagnostic, Los Angeles, CA). The intraassay variability was 6.7–9.5%, and interassay variability was 6.1–10%. Serum and UFC were measured using a chemiluminescent microparticle immunoassay (Abbott Diagnostics, Chicago, IL), with a total coefficient of variation (CV) of 2.5–7.7% and an intraassay CV range of 2.1–6.1%. Normal ranges are: ACTH, 6–76 pg/ml (1.3–16.7 pmol/liter); UFC, 20–70 μg/24 h (55–193 nmol/24 h); and serum cortisol, 5–25 μg/dl (138–690 nmol/liter). In one of the Italian centers, plasma ACTH and serum cortisol were measured by immunometric assay (Liason; Nichols Institute, San Juan Capistrano, CA). UFC was measured by RIA after urine extraction with dichloromethane (Byk-Sangtec Diagnostica, Dietzenbach, Germany). Intra- and interassay CVs were 3.2 and 8.2% for ACTH, 3.0 and 4.7% for serum cortisol, and 3.5 and 6.2% for UFC, respectively. Normal ranges are: ACTH, 10–80 pg/ml (2.2–17.6 pmol/liter); serum cortisol, 2–25 μg/dl (55–690 nmol/liter); and UFC, 20–80 μg/24 h (55–221 nmol/24 h). In the other Italian center, plasma ACTH level was measured by chemiluminescence immunoassay (Nichols Advantage ACTH assay; Nichols Institute Diagnostics, San Juan Capistrano, CA). The intraassay CV is 8.4, 6.4, and 6.9% at ACTH concentrations of 6 pg/ml (1.3 pmol/liter), 12.9 pg/ml (2.8 pmol/liter), and 214.6 pg/ml (46.5 pmol/liter), respectively. Serum cortisol was measured by competitive enzyme immunoassay (AIA-PACK CORT; Tosoh, Tokyo, Japan). The intraassay CV is 5.9–3.8% for cortisol values of 1.8 and 13 μg/dl (50 and 360 nmol/liter), respectively. In the early years of the series, measurements were performed by RIA, whose characteristics have been previously described (24).
Statistical analysis
Data are presented as mean ± sd for descriptive statistics. To compare groups IC, NC, and DC, a closed testing procedure was used for multiple comparisons (25). ANOVA was used for continuous variables, and Fisher’s exact or χ2 test was used for nominal variables. If the three-way comparison was found to be significant, then the three pairwise comparisons were also tested. Stepwise logistic regression was used to identify clinical variables (age, sex, biochemical testing before surgery, pattern of cortisol change after surgery, MRI results, pathology findings, and previous TSS for CD) that might predict remission. Stepwise logistic regression was performed using SAS PROC LOGISTIC. All categorical predictors were included in a model that pooled the U.S. and Italian data. Because the assays were different in the two countries, we standardized hormone levels to put values on the same scale. Computing Z-score was done separately in the two countries, subtracting the mean from each value and dividing by the sd. Stepwise proportional hazards regression was used to identify predictors of relapse using SAS PROC PHREG. A P value threshold of 0.05 was used for variable entry and inclusion in the final model. Kaplan-Meier survival estimates were used to compare relapse rates. Difference in the survival curves between group IC and group DC was calculated by log-rank test. All analyses were done using SAS version 9.1 (SAS Institute Inc., Cary, NC), and two-sided P values ≤0.05 were considered evidence of statistical significance.
Results
Establishing surgical outcomes
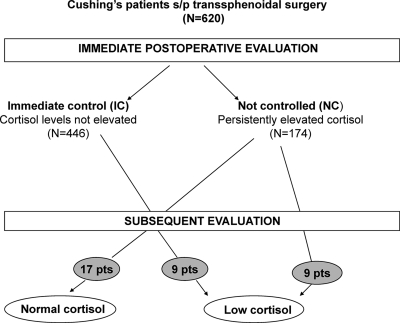
The outcome after surgery was classified as shown in Fig. 1.
Figure 1.
Postoperative outcomes in our series of 620 TSS interventions. Grey circles represent patients with delayed control of cortisol on subsequent evaluation, including 18 patients who became hypoadrenal and 17 who became eucortisolemic.
Group IC
Of the 620 patients with adequate follow-up data, 446 were hypocortisolemic (n = 327) or eucortisolemic (n = 119) in the immediate postoperative period (postoperative d 1–3).
Group NC
Of the 620 patients, there were 174 patients with early postoperative hypercortisolemia. A total of 148 patients had persistent hypercortisolism, even at late evaluation.
Group DC
This group includes 35 patients who experienced a delayed decline in UFC levels. Nine of the 119 patients described above with initial eucortisolism became hypoadrenal (7.5%) (Fig. 2, patient 18), and of 174 patients with initial hypercortisolism, nine (5%) became hypoadrenal (Fig. 2, patient 22) and 17 (9.8%) became and remained euadrenal (Fig. 2, patient 15). The delayed decrease to low/normal UFC levels was documented at a mean of 38 ± 50 d (median, 9 d; range, 4–180 d). This timing may be related to the different times of follow-up visits. Fourteen patients had two collections over a period of 31 ± 25 postoperative days (range, 4–60 d), 15 had three collections over a period of 44 ± 66 d (range, 4–180), and six patients had four or five collections over a period of 39 ± 46 d (range, 6–127 d) (Table 1). Specifically, delayed UFC normalization was documented after a mean of 41 ± 51 d (median, 25 d; range, 4–180 d), and delayed UFC hypocortisolism was documented after 36 ± 52 postoperative days (median, 8; range, 4–150).
Figure 2.
Examples of individual time courses of UFC after TSS. UFC is scaled. Data were standardized so that the horizontal reference line at −1 represents the lowest limit of the normal range and the horizontal reference line at +1 represents the upper limit of the normal range. Note that the vertical axes of the three panels do not span the same region of the normal range.
Table 1.
Trend of UFC levels over the postoperative period in patients who experienced UFC normalization from early elevated levels (patients 1–17) and hypocortisolism (low UFC) from early elevated/normal levels (patients 18–35)
| Patient ID | Follow-up (1)
|
Follow-up (2)
|
Follow-up (3)
|
Follow-up (4)
|
Follow-up (5)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| POD | UFC | POD | UFC | POD | UFC | POD | UFC | POD | UFC | |
| 1 | 4 | 95a | 6 | 60a | 18 | 10a | 25 | 21a | ||
| 2 | 4 | 1763c | 5 | 630c | 6 | 558c | 16 | 11c | ||
| 3 | 2 | 76a | 5 | 24a | 8 | 32a | ||||
| 4 | 2 | 181a | 5 | 60a | 6 | 40a | 7 | 29a | ||
| 5 | 2 | 76a | 4 | 27a | ||||||
| 6 | 5 | 95a | 6 | 60a | 7 | 59a | ||||
| 7 | 3 | 94a | 4 | 42a | 5 | 29a | ||||
| 8 | 3 | 259a | 4 | 53a | 5 | 69a | ||||
| 9 | 4 | 403a | 11 | 79a | 37 | 62a | ||||
| 10 | 2 | 93a | 4 | 41a | ||||||
| 11 | 3 | 182b | 60 | 21b | ||||||
| 12 | 3 | 101b | 60 | 38.8b | ||||||
| 13 | 3 | 141b | 40 | 43b | ||||||
| 14 | 3 | 102b | 60 | 113d | ||||||
| 15 | 3 | 300b | 60 | 228e | 150 | 124e | ||||
| 16 | 3 | 178b | 30 | 54b | ||||||
| 17 | 3 | 177b | 5 | 33b | 180 | 23.9b | ||||
| 18 | 3 | 31a | 4 | 49a | 5 | 30a | 43 | 25a | 127 | 10a |
| 19 | 3 | 93a | 5 | 16a | 7 | 10a | ||||
| 20 | 2 | 48a | 3 | 43a | 4 | 25a | 6 | 19a | ||
| 21 | 2 | 285a | 5 | 78a | 14 | <1a | ||||
| 22 | 4 | 220a | 11 | 116a | 38 | 4a | 51 | 16a | ||
| 23 | 2 | 37a | 4 | <1a | 5 | 15a | ||||
| 24 | 2 | 26a | 5 | <1a | 6 | 15a | ||||
| 25 | 2 | 27.8a | 9 | <1a | ||||||
| 26 | 2 | 61a | 9 | <1a | ||||||
| 27 | 2 | 176a | 4 | 49a | 8 | <1a | ||||
| 28 | 2 | 506a | 3 | <1a | 5 | <1a | ||||
| 29 | 2 | 56a | 6 | <1a | ||||||
| 30 | 3 | 168a | 6 | <1a | ||||||
| 31 | 2 | 24a | 4 | <1a | ||||||
| 32 | 3 | 157b | 45 | 56d | 150 | 46d | ||||
| 33 | 3 | 368b | 30 | 25b | 150 | 17b | ||||
| 34 | 3 | 184b | 30 | 13b | ||||||
| 35 | 3 | 51b | 60 | 5b | ||||||
POD, Postoperative day; <1, undetectable.
UFC normal range:
20–70 μg/24 h;
20–80 μg/24 h;
10–64 μg/24 h;
70–320 μg/24 h;
75–270 μg/24 h.
Long-term follow-up data were available in 118 of 148 patients in group NC. In these patients with persistent hypercortisolism, a second intervention (medical therapy, radiation, adrenalectomy, transcranial surgery, TSS) was performed 9 to 2700 d after TSS (mean, 262 ± 340 d). Of those, a number of patients with persistent hypercortisolemia underwent a second intervention within 43 d after TSS (mean, 23 ± 8 d; range, 9–43 d). This range overlaps with the period in which spontaneous delayed UFC normalization was documented in several group DC patients.
Characterization of DC and comparison of variables between groups
Tables 2 and 3 display clinical variables and demographic information for group DC.
Table 2.
Clinical and biochemical features of the entire cohort
| Variables | IC | DC | NC | P value |
|---|---|---|---|---|
| Age (yr) | 37 (13)b | 44 (12) | 40 (15) | 0.004 |
| Female:male ratio | 5:1 (365/72) | 11:1 (32/3) | 3:1 (115/33)a | 0.08 |
| ACTH before surgery (pg/ml) | ||||
| Overall (Z-score) | −0.071 (0.8) | −0.15 (0.77) | 0.25 (1.4) | <0.01 |
| Italy | 69 (41) | 48 (26) | 93 (99) | <0.01 |
| United States | 78 (90) | 79 (73) | 91 (65) | 0.76 |
| UFC before surgery (μg/24 h) | ||||
| Overall (Z-score) | −0.05 (0.53)b | 0.37 (2.2) | 0.04 (1.4) | 0.049 |
| Italy | 497 (449) | 638 (1151) | 619 (1403) | 0.42 |
| United States | 266 (390)a | 651 (1840) | 225 (116) | 0.04 |
| Serum cortisol before surgery (μg/dl) | 22 (7) | 21 (5) | 26 (20) | 0.017 |
| MRI (positive findings) | 66% (288) | 63% (22) | 52% (77) | 0.011 |
| Micro vs. macro | 86% (375) vs. 14% (62) | 77% (27) vs. 23% (8) | 76% (112) vs. 24% (36) | 0.015 |
| Adenoma at pathology | 85% (370)a | 68% (24) | 49% (73) | <0.001 |
| Previous surgery | 14% (61) | 14% (5) | 22% (32) | 0.09 |
| Serum cortisol after surgery | ||||
| Overall (Z-score) | −0.23 (0.9)b | 0.5 (1) | 0.6 (0.8) | <0.001 |
| Italy | 5 (31) | 14 (5) | 20 (18) | <0.001 |
| United States | 5 (6)b | 19 (17) | 28 (16) | <0.001 |
| UFC after surgery (μg/24 h) | ||||
| Overall (Z-score) | −0.35 (0.08)b | 0.30 (0.9) | 0.94 (1.6)a | <0.001 |
| Italy | 18 (14)b | 176 (90) | 378 (381) | <0.001 |
| United States | 8 (12)b | 204 (354) | 348 (617) | <0.001 |
| ACTH after surgery (pg/ml) | 12 (9)b | 25 (14) | 57 (49)a | <0.001 |
Data are presented as mean (sd) or as percentage (absolute number of patients). Normal ranges for Italian series are: 10-80 pg/ml for ACTH, 2–25 μg/dl for serum cortisol, and 20–80 μg/24 h for UFC. Normal ranges for U.S. series are: 6–76 pg/ml for ACTH, 20–70 μg/24 h for UFC, and 5–25 μg/dl for serum cortisol. P values are presented for three comparison groups.
Comparison between DC vs. IC or NC:
P < 0.05;
P < 0.010.
Table 3.
Preoperative characteristics and incidence of relapse in group DC
| ID | Age (yr) | Sex | ACTH before TSS (pg/ml) | UFC before TSS (μg/24 h) | Previous surgery | MRI size (mm) | Path | FU (months) | R |
|---|---|---|---|---|---|---|---|---|---|
| 1a | 49 | F | 81 | 147 | No | Micro | + | 46 | No |
| 2a | 38 | F | 193 | 670 | Yes | Micro | − | 71 | Yes |
| 3a | 47 | F | 60 | 96 | No | Micro | + | 66 | Yes |
| 4a | 55 | F | 27 | 122 | No | Micro | + | 48 | Yes |
| 5a | 53 | F | 35 | 204 | Yes | Macro | + | 64 | Yes |
| 6a | 49 | F | 77 | 467 | No | Micro | + | 30 | No |
| 7a | 19 | F | 38 | 102 | No | Macro | + | 35 | No |
| 8a | 45 | F | 130 | 1300 | No | Micro | + | 38 | Yes |
| 9a | 48 | F | 19 | 125.5 | Yes | Macro | + | 59 | No |
| 10a | 39 | M | 128 | 9186 | No | Micro | + | 6 | No |
| 11b | 32 | F | 51 | 189 | No | Micro | − | 38 | No |
| 12b | 26 | F | 39 | 186 | No | Micro | + | 108 | No |
| 13b | 61 | M | 21 | 508 | No | Micro | + | 46 | No |
| 14b | 65 | F | 71 | 145 | No | Macro | + | 46 | No |
| 15b | 34 | F | 95 | 4080 | No | Macro | + | 24 | No |
| 16b | 56 | F | 37.5 | 158 | No | Macro | + | 30 | No |
| 17b | 45 | F | 37 | 578 | No | Micro | − | 123 | No |
| 18a | 34 | F | 353 | 136 | No | Micro | − | 48 | No |
| 19a | 55 | F | 31 | 76 | No | Micro | + | 1 | No |
| 20a | 19 | F | 27 | 140 | Yes | Micro | − | 92 | Yes |
| 21a | 38 | F | 90 | 129 | No | Macro | + | 1 | No |
| 22a | 59 | F | 77 | 754 | No | Micro | + | 55 | Yes |
| 23a | 48 | F | 65 | 556 | No | Micro | + | 4 | No |
| 24a | 43 | F | 48 | 130 | No | Micro | + | 51 | Yes |
| 25a | 49 | F | 56 | Yes | Micro | + | 1 | No | |
| 26a | 41 | F | 55 | 126 | No | Micro | − | 2 | No |
| 27a | 56 | F | 15 | 176 | No | Micro | − | 1 | No |
| 28a | 55 | F | 37 | 240 | No | Micro | + | 1 | No |
| 29a | 60 | F | 97 | 103 | No | Micro | − | 1 | No |
| 30a | 20 | F | 48 | 273 | No | Micro | − | 12 | No |
| 31a | 29 | M | 86 | 312 | No | Micro | − | 12 | No |
| 32b | 40 | F | 93 | 211 | No | Macro | + | 104 | No |
| 33b | 51 | F | 29 | 322 | No | Micro | + | 63 | No |
| 34b | 41 | F | 36 | 408 | No | Micro | + | 77 | No |
| 35b | 53 | F | 22 | 235 | No | Micro | − | 8 | No |
F, Female; M, male; −, negative; +, positive; Path, pathology; FU, follow-up; R, relapse.
Normal ranges for these patients are: 6–76 pg/ml for ACTH, and 20–70 μg/24 h for UFC.
Normal ranges for these patients are: 10–80 pg/ml for ACTH, and 20–80 μg/24 h for UFC. To convert UFC (μg/24 h) to Systeme International (SI) units (nmol/24 h), multiply by 2.759; to convert values for ACTH (pg/ml) to SI units (picomoles per liter), multiply by 0.2202.
The female:male ratio was significantly higher in group DC compared with group NC (P = 0.047). Patients in group DC were significantly older than those in group IC (44 ± 12 vs. 37 ± 13 yr; P = 0.002).
The results of biochemical testing related to the initial diagnosis of CD are presented in Tables 2 and 3. Before surgery, there was a significant difference in ACTH Z-score among the three groups in the overall cohort (P = 0.003), but when group IC or NC was compared separately with group DC, the difference was no longer significant (Table 2). Similarly, preoperative serum cortisol, which was measured only in the Italian series, was significantly different only in the three-way comparison (P = 0.017). Preoperative UFC Z-score was significantly higher in group DC as compared with group IC (P = 0.0036).
Imaging studies of the pituitary gland were obtained in all patients. As shown in Table 2, almost two thirds of patients with imaging of the pituitary gland had a positive study. Patients in group IC were more likely to have a microadenoma than a macroadenoma (86 vs. 14%) when compared with those in group DC (77 vs. 23%) or NC (76 vs. 24%) (P = 0.015). No significant difference in the ratio of microadenoma to macroadenoma was found when either group IC or group NC alone was compared with group DC.
Histopathology reports were available in 609 (98%) of the 620 patients. Patients in group IC were more likely to have pathological confirmation of CD (85%) compared with either group DC (68%) or group NC (49%) (P < 0.001). When groups were analyzed separately in relation to group DC, positive pathology remained significantly more prevalent in group IC (P = 0.017).
Patients in group IC had significantly lower postoperative ACTH levels (available in 383 patients) compared with those in the other two groups (P < 0.001). ACTH levels in group DC (25 ± 14 pg/ml) were significantly lower than in group NC (57 ± 49 pg/ml; P = 0.03) and higher than group IC (12 ± 9 pg/ml; P < 0.001). Postoperative plasma ACTH levels were correlated with serum cortisol (r = 0.25; P < 0.001) and with UFC (r = 0.37; P < 0.001) concentrations after surgery.
Predictors of persistent hypercortisolism
The potential correlation between clinical and biochemical characteristics and persistent hypercortisolism (group NC) was assessed using a logistic regression model. When preoperative variables (age, gender, visualization of an adenoma at MRI, histological confirmation of an adenoma, previous surgery, ACTH, and preoperative UFC Z-scores) were included in the model, the only statistically significant positive predictors of persistent hypercortisolism were the lack of histological confirmation of an adenoma [odds ratio (OR), 8.36; 95% confidence interval (CI), 5–13.9; P < 0.001] and an elevated ACTH Z-score (OR, 1.5; 95% CI, 1.16–2; P < 0.0022). Patients with a microadenoma (OR, 0.36; 95% CI, 0.2–0.6; P < 0.001) and those without a history of previous TSS (OR, 0.52; 95% CI, 0.28–1; P < 0.05) were less likely to demonstrate persistent hypercortisolism.
Relapse rate
Biochemical recurrence of CD was documented in 62 patients (13%) after initially successful TSS over the median follow-up period of 66 months. Mean duration of follow-up after surgery for patients in group IC (48 ± 46 months; median, 35, range, 1–300) was similar to those in group DC (40 ± 34 months; median, 38, range, 1–123; P = 0.31).
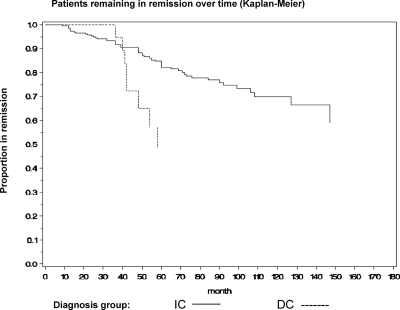
The relapse curves of the two groups were significantly different (P = 0.018) (Fig. 3).
Figure 3.
Disease-free survival by Kaplan-Meier curve in group IC and group DC.
At 1 yr, surveillance data for recurrent CD were available in 334 patients [311 in group IC (71%) and 23 in group DC (65.7%)]. The Kaplan-Meier analysis showed that the cumulative relapse rate 1 yr after TSS was 1.5% for group IC and 0% in group DC. At 4.5 yr, surveillance data for recurrent disease were available in 191 patients [177 in group IC (43.7%), and 14 in group DC (40%)]. Survival analysis 4.5 yr after TSS showed that the cumulative rate of recurrence was significantly higher in group DC compared with group IC (43 vs. 14%; P = 0.02).
Preoperative characteristics of the eight patients in group DC who developed recurrent CD are shown in Table 3. Five of them had developed delayed normalization of their UFCs from initially elevated levels, whereas three had developed delayed hypocortisolism from early normal (two of three) and elevated UFC (one of three), respectively.
Discussion
These data show that, in a small but significant number of patients with CD (5.6% overall) after TSS, the immediate postoperative biochemical testing may be misleading. In our study, 18% of patients with initial postoperative hypercortisolism developed delayed low or normal cortisol levels after an average of 45 ± 53 d. In addition, 8% of patients with initial postoperative eucortisolemia subsequently became hypocortisolemic after an average of 25 ± 42 d. Overall, these data demonstrate that cortisol levels may further decline after the initial postoperative period in some patients, which has important implications for decisions regarding the timing of additional therapy. Hormonal measurements several weeks after surgery in those patients who are not immediately hypoadrenal may better reflect the true surgical outcome.
Because patients with persistent hypercortisolism in the immediate postsurgical period are often referred for additional treatment (15,18), this group of patients is important to recognize. A few previous studies have shown that cortisol levels 1–3 months after surgery may be, on average, significantly lower than those measured within the initial 2 wk, raising the possibility that early hormonal assessment might not accurately reflect the ultimate outcome (5,19). Toms et al. (19) found that serum cortisol concentrations reached a delayed nadir (<1.3 μg/dl) at 6–12 wk, and this value better correlated with sustained remission. McDonald et al. (26) reported two patients who showed restored suppression with dexamethasone after 30 and 180 d, respectively. In the report of Rollin et al. (27), two patients who were not on glucocorticoid replacement showed a progressive decline of cortisol from normal levels 1 d after surgery to the hypoadrenal range (2.2 and 1.3 μg/dl) after 90 and 30 d, respectively. Similarly, Pereira et al. (20) found that six of 30 (20%) patients with serum cortisol levels higher than 5 μg/dl 2 wk after surgery had a nadir serum cortisol below 1.8 μg/dl when assessed after 6–12 wk and showed permanent remission. A recent study of 426 patients, which focused mainly on the long-term results of pituitary surgery as compared with other therapeutic procedures, included 17 patients with early failure to suppress to 2 mg-ODST and who suppressed normally after 3 months (12).
In our series, the largest ever studied, 35 patients of the total cohort of 620 (5.6%) experienced a delayed decline in cortisol levels. This included 26 patients with elevated and nine with normal UFC in the immediate postoperative period who later attained eu- or hypoadrenalism between 4 and 180 d after surgery, and this remission persisted in 77% at 40 ± 34 months of follow-up. Of note, in one patient with normal UFC 6 wk (43 d) after TSS, hypoadrenalism was documented 12 wk later, suggesting that a longer follow-up may be needed in some cases. Perhaps some of our 10 patients who were thought to have persistent hypercortisolism and underwent early reoperation (within 1 month) might have spontaneously developed hypocortisolism or normalization on later testing if further treatment had not been administered.
This suggests that waiting until cortisol levels reach a nadir before considering further treatment may be advisable to spare some patients a second unnecessary intervention. Indeed, repeat surgery is associated with a lower remission rate and a higher prevalence of complications, such as pituitary deficiency (5,6,7,8,9,10,11,12,13). The long-term safety of alternative treatments, such as radiotherapy, is still under debate (28,29). Moreover, the risk associated with persistent hypercortisolism during the follow-up period is another important issue to consider. In some cases, medical management of hypercortisolemia with reevaluation may be considered. However, delaying reintervention may further decrease the surgical success rate, although this is controversial in the neurosurgery literature (15). Thus, although prospective studies are needed to establish the optimal strategy for the management of apparently uncured CD, a careful evaluation of the individual risk to benefit ratio in each patient should be performed by clinicians and surgeons, before opting for a waiting approach.
Patients with delayed hypocortisolism/normalization showed mean UFC levels early after surgery that were significantly lower than those of patients in the group with persistent hypercortisolism. Further studies are needed to determine the optimal postoperative test and the timing that best predicts ultimate outcome. It is important to note that UFC measurement is not an accurate test for the diagnosis of hypoadrenalism, but it was used in this study as a postoperative tool for classifying patients into three groups and to demonstrate a declining postoperative cortisol trend. A recent study suggests that salivary cortisol levels, rather than UFC, may be more reliable (30).
The mechanisms of a late decline in cortisol after TSS are unclear. There may be adrenal hyperplasia with partial autonomy from chronic exposure to elevated ACTH levels during the active phase of the disease that gradually resolves. We cannot assess this possibility because adrenal scans are not routinely performed in patients whose biochemical testing confirms ACTH-dependent Cushing’s syndrome. However, patients in the Italian series who experienced delayed hypocortisolism or normalization had significantly higher ACTH levels in the immediate postoperative period compared with those with confirmed hypocortisolism, arguing against adrenal autonomy as the cause. Pereira et al. (20) did not find any differences in ACTH levels between delayed and early cured patients. Alternatively, the delayed decline of cortisol may be explained by late necrosis of residual corticotroph adenoma cells after surgical manipulation. Indirectly supporting this is that lack of histological confirmation of an adenoma was more common in those patients with delayed hypocortisolism or normalization, suggesting that these cells may have been damaged in situ as opposed to surgically removed.
Indeed, as mentioned above, early postoperative ACTH levels in the Italian patients with delayed hypocortisolism or normalization fell between the two extremes of low and very high levels observed in group IC and group NC, respectively, and correlated with serum cortisol levels. This suggests that some residual adenomatous cells were still actively secreting ACTH during the early postoperative testing period. It is also possible that cyclical cortisol secretion may play a role in variable postoperative levels (31). The hypothesis of cortisol cyclicity may be further supported by the finding of an increased risk of recurrence in those patients with delayed remission (43% in group DC vs. 14% in group IC at 4.5 yr). However, cyclic cortisol would not explain the 18 patients who exhibited late hypoadrenalism. In addition, visualization of an adenoma on MRI and UFC Z-score were the only independent predictors of recurrence in our series, in accordance with others (8,17), suggesting that classification of patients in the IC and DC groups did not have an independent association with the risk of recurrence. Recent observations suggest that cyclical cortisol secretion may be present in up to 15% of patients with CD and may be associated with a trend of lower cure rate (32). Alexandraki et al. (32) showed that about 20% of patients with cyclicity recurred after TSS. Interestingly, serum cortisol levels were higher than 1.8 μg/dl (50 nmol/liter) early after surgery in four of the six patients with cyclicity who were cured, and then gradually decreased over 3–7 d.
Patients in the DC group were more likely to have had macroadenomas, perhaps because larger tumors were more likely to be incompletely resected, with delayed death of residual cells. The potential presence of residual adenoma cells is another finding that may account for the increased risk of recurrence in the DC group. The retrospective and three-center design of this study are limitations because there were different diagnostic approaches, analysis techniques, and pituitary surgeons. It is unlikely, however, that the large numbers of patients required to demonstrate the frequency of delayed remission could be attained without a multicenter study. The widely variable time points of postoperative assessment are another important limitation because it precludes a definitive suggestion regarding the timing of follow-up frequency and duration. Although our data do not provide conclusive clinical guidelines, they suggest that close follow-up of patients until hormonal parameters stabilize may be the best monitoring strategy to establish a reliable classification of surgical outcomes and avoid unnecessary surgery or other treatments.
Postoperative cortisol assessment in U.S. patients while on dexamethasone treatment represents another limitation. However, patients were prescribed a low dose (1–2 mg/d) of dexamethasone because this is the dose used in the initial diagnosis of CD that does not suppress cortisol.
In conclusion, we show that a subset of patients with CD who are not hypoadrenal within the first 2–3 d postoperatively may undergo a spontaneous late remission. The finding of such wide variability of cortisol dynamics in the postoperative period argues against immediate retreatment for these patients and suggests that longer follow-up and retesting may be advisable to avoid unnecessary treatment.
Acknowledgments
Dalia Batista, MD is acknowledged for her dedicated effort and contributions to the manuscript.
Footnotes
This work was supported by Grant UL1 RR025758 from National Institute of Health (NIH). E.V. was supported in part by the “L’OREAL Italia Per le Donne e la Scienza” Award 2008.
Disclosure Summary: The authors have nothing to disclose.
First Published Online January 15, 2010
Abbreviations: CD, Cushing’s disease; CI, confidence interval; CV, coefficient of variation; DC, delayed control; IC, immediate control; MRI, magnetic resonance imaging; NC, no control; ODST, overnight dexamethasone suppression test; OR, odds ratio; TSS, transsphenoidal surgery; UFC, urinary free cortisol.
References
- Atkinson AB, Kennedy A, Wiggam MI, McCance DR, Sheridan B 2005 Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clin Endocrinol (Oxf) 63:549–559 [DOI] [PubMed] [Google Scholar]
- Patil CG, Prevedello DM, Lad SP, Vance ML, Thorner MO, Katznelson L, Laws Jr ER 2008 Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab 93:358–362 [DOI] [PubMed] [Google Scholar]
- Rees DA, Hanna FW, Davies JS, Mills RG, Vafidis J, Scanlon MF 2002 Long-term follow-up results of transsphenoidal surgery for Cushing’s disease in a single centre using strict criteria for remission. Clin Endocrinol (Oxf) 56:541–551 [DOI] [PubMed] [Google Scholar]
- Yap LB, Turner HE, Adams CB, Wass JA 2002 Undetectable postoperative cortisol does not always predict long-term remission in Cushing’s disease: a single centre audit. Clin Endocrinol (Oxf) 56:25–31 [DOI] [PubMed] [Google Scholar]
- Chee GH, Mathias DB, James RA, Kendall-Taylor P 2001 Transsphenoidal pituitary surgery in Cushing’s disease: can we predict outcome? Clin Endocrinol (Oxf) 54:617–626 [DOI] [PubMed] [Google Scholar]
- Invitti C, Pecori Giraldi F, de Martin M, Cavagnini F 1999 Diagnosis and management of Cushing’s syndrome: results of an Italian multicenter study. Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal axis. J Clin Endocrinol Metab 84:440–448 [DOI] [PubMed] [Google Scholar]
- Swearingen B, Biller BM, Barker 2nd FG, Katznelson L, Grinspoon S, Klibanski A, Zervas NT 1999 Long-term mortality after transsphenoidal surgery for Cushing’s disease. Ann Intern Med 130:821–824 [DOI] [PubMed] [Google Scholar]
- Bochicchio D, Losa M, Buchfelder M 1995 Factors influencing the immediate and late outcome of Cushing’s disease treated by transsphenoidal surgery: a retrospective study by the European Cushing’s Disease Survey Group. J Clin Endocrinol Metab 80:3114–3120 [DOI] [PubMed] [Google Scholar]
- Pieters GF, Hermus AR, Meijer E, Smals AG, Kloppenborg PW 1989 Predictive factors for initial cure and relapse rate after pituitary surgery for Cushing’s disease. J Clin Endocrinol Metab 69:1122–1126 [DOI] [PubMed] [Google Scholar]
- Guilhaume B, Bertagna X, Thomsen M, Bricaire C, Vila-Porcile E, Olivier L, Racadot J, Derome P, Laudat MH, Girard F 1988 Transsphenoidal pituitary surgery for the treatment of Cushing’s disease: results in 64 patients and long-term follow up studies. J Clin Endocrinol Metab 66:1056–1064 [DOI] [PubMed] [Google Scholar]
- Mampalam TJ, Tyrrell JB, Wilson CB 1988 Transsphenoidal microsurgery for Cushing disease. Ann Intern Med 109:487–493 [DOI] [PubMed] [Google Scholar]
- Hofmann BM, Hlavac M, Martinez R, Buchfelder M, Müller OA, Fahlbusch R 2008 Long-term results after microsurgery for Cushing’s disease: experience with 426 primary operations over 35 years. J Neurosurg 108:9–18 [DOI] [PubMed] [Google Scholar]
- Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M 2008 Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93:2454–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance DR, Gordon DS, Fannin TF, Hadden DR, Kennedy L, Sheridan B, Atkinson AB 1993 Assessment of endocrine function after transsphenoidal surgery for Cushing’s disease. Clin Endocrinol (Oxf) 38:79–86 [DOI] [PubMed] [Google Scholar]
- Trainer PJ, Lawrie HS, Verhelst J, Howlett TA, Lowe DG, Grossman AB, Savage MO, Afshar F, Besser GM 1993 Transsphenoidal resection in Cushing’s disease: undetectable serum cortisol as the definition of successful treatment. Clin Endocrinol (Oxf) 38:73–78 [DOI] [PubMed] [Google Scholar]
- Estrada J, García-Uría J, Lamas C, Alfaro J, Lucas T, Diez S, Salto L, Barceló B 2001 The complete normalization of the adrenocortical function as the criterion of cure after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab 86:5695–5699 [DOI] [PubMed] [Google Scholar]
- Sonino N, Zielezny M, Fava GA, Fallo F, Boscaro M 1996 Risk factors and long-term outcome in pituitary-dependent Cushing’s disease. J Clin Endocrinol Metab 81:2647–2652 [DOI] [PubMed] [Google Scholar]
- Locatelli M, Vance ML, Laws ER 2005 Clinical review: the strategy of immediate reoperation for transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab 90:5478–5482 [DOI] [PubMed] [Google Scholar]
- Toms GC, McCarthy MI, Niven MJ, Orteu CH, King TT, Monson JP 1993 Predicting relapse after transsphenoidal surgery for Cushing’s disease. J Clin Endocrinol Metab 76:291–294 [DOI] [PubMed] [Google Scholar]
- Pereira AM, van Aken MO, van Dulken H, Schutte PJ, Biermasz NR, Smit JW, Roelfsema F, Romijn JA 2003 Long-term predictive value of postsurgical cortisol concentrations for cure and risk of recurrence in Cushing’s disease. J Clin Endocrinol Metab 88:5858–5864 [DOI] [PubMed] [Google Scholar]
- Mortini P, Losa M, Barzaghi R, Boari N, Giovanelli M 2005 Results of transsphenoidal surgery in a large series with pituitary adenoma. Neurosurgery 56:1222–1233 [DOI] [PubMed] [Google Scholar]
- Aghi MK, Petit J, Chapman P, Loeffler J, Klibanski A, Biller BM, Swearingen B 2008 Management of recurrent and refractory Cushing’s disease with reoperation and/or proton beam radiosurgery. Clin Neurosurg 55:141–144 [PubMed] [Google Scholar]
- Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM 2008 The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93:1526–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller BM, Federoff HJ, Koenig JI, Klibanski A 1990 Abnormal cortisol secretion and responses to corticotropin-releasing hormone in women with hypothalamic amenorrhea. J Clin Endocrinol Metab 70:311–317 [DOI] [PubMed] [Google Scholar]
- Marcus R, Peritz E, Gabriel KR 1976 On closed testing procedures with special reference to ordered analysis of variance. Biometrika 63:655–660 [Google Scholar]
- McDonald SD, Von Hofe SE, Dorfman SG, Jordan RM, LaMorgese JR, Young RL 1978 Delayed cure of Cushing’s disease after transsphenoidal surgery of pituitary adenomas. Report of two cases. J Neurosurg 49:593–596 [DOI] [PubMed] [Google Scholar]
- Rollin GA, Ferreira NP, Junges M, Gross JL, Czepielewski MA 2004 Dynamics of serum cortisol levels after transsphenoidal surgery in a cohort of patients with Cushing’s disease. J Clin Endocrinol Metab 89:1131–1139 [DOI] [PubMed] [Google Scholar]
- Estrada J, Boronat M, Mielgo M, Magallón R, Millan I, Díez S, Lucas T, Barceló B 1997 The long-term outcome of pituitary irradiation after unsuccessful transsphenoidal surgery in Cushing’s disease. N Engl J Med 336:172–177 [DOI] [PubMed] [Google Scholar]
- Petit JH, Biller BM, Yock TI, Swearingen B, Coen JJ, Chapman P, Ancukiewicz M, Bussiere M, Klibanski A, Loeffler JS 2008 Proton stereotactic radiotherapy for persistent adrenocorticotropin-producing adenomas. J Clin Endocrinol Metab 93:393–399 [DOI] [PubMed] [Google Scholar]
- Carrasco CA, Coste J, Guignat L, Groussin L, Dugué MA, Gaillard S, Bertagna X, Bertherat J 2008 Midnight salivary cortisol determination for assessing the outcome of transsphenoidal surgery in Cushing’s disease. J Clin Endocrinol Metab 93:4728–4734 [DOI] [PubMed] [Google Scholar]
- Atkinson AB, McCance DR, Kennedy L, Sheridan B 1992 Cyclical Cushing’s syndrome first diagnosed after pituitary surgery: a trap for the unwary. Clin Endocrinol (Oxf) 36:297–299 [DOI] [PubMed] [Google Scholar]
- Alexandraki KI, Kaltsas GA, Isidori AM, Akker SA, Drake WM, Chew SL, Monson JP, Besser GM, Grossman AB 2009 The prevalence and characteristic features of cyclicity and variability in Cushing’s disease. Eur J Endocrinol 160:1011–1018 [DOI] [PubMed] [Google Scholar]