Abstract
Context: Adjuvant chemotherapy is associated with significant reductions in bone mineral density (BMD) in premenopausal women with breast cancer (BC) that is prevented with zoledronic acid (ZA) every 3 months for 1 yr.
Objective: The aim of the study was to examine the effect on BMD of discontinuing ZA during the subsequent year.
Design: We conducted a randomized, double-blind trial.
Patients: Premenopausal women (mean age, 42 yr) undergoing adjuvant chemotherapy for BC participated in the study.
Intervention: ZA (4 mg iv every 3 months) vs. placebo was administered for 12 months.
Outcome Measures: We measured percentage change in BMD and bone turnover markers at 12 and 24 months (1 yr after last infusion).
Results: Of 101 women randomized, 85 completed 12-month and 62 completed 24-month evaluations. In the placebo group, serum C-telopeptide (CTX) increased progressively over the first 12 months, returned toward baseline but remained significantly above baseline by 24 months. Lumbar spine BMD decreased from baseline by 5.5% at 12 and 6.3% at 24 months. Similarly, by 24 months, total hip and femoral neck BMD declined by 2.6 and 2.4%, respectively. In ZA patients, BMD remained stable (P < 0.0001 compared to placebo). Serum CTX declined significantly by 6 months, but returned to baseline by 12 months, remaining there at 24 months.
Conclusions: Premenopausal women receiving chemotherapy for BC sustained significant bone loss during the first year, without recovery during the second year. ZA effectively prevented bone loss during the first year of chemotherapy. BMD remained stable 1 yr after completion of ZA. Serum CTX increased significantly by 12 and 24 months. More frequent administration may be required to suppress bone resorption in this patient population.
One year of zoledronic acid prevents bone loss during the first year of chemotherapy as well as during the second year after completion of therapy.
Premenopausal women undergoing adjuvant chemotherapy for early stage breast cancer (BC) experience significant bone loss over the course of the first year after the initiation of treatment (1,2). The loss of bone mineral density (BMD) is greatest in the lumbar spine (LS), with declines ranging from 3–6% depending on the age of the population studied and the duration or permanence of chemotherapy-induced amenorrhea. This high rate of BMD loss may translate into an increased risk of postmenopausal fractures. Data from the Women’s Health Initiative reported that postmenopausal survivors of BC have a 15% higher fracture risk than women without a history of BC (3,4). Several randomized controlled trials have investigated bisphosphonates for the prevention of chemotherapy-induced BMD loss. Although some protection is observed with oral bisphosphonates, such as clodronate (5,6,7), their use is commonly limited by gastrointestinal side effects (8), an issue of particular concern in women receiving chemotherapy.
Two randomized, controlled trials have established that iv zoledronic acid (ZA), a potent bisphosphonate, prevents bone loss in premenopausal women undergoing adjuvant chemotherapy (1,2). In the two adjuvant trials, ZA was initiated at the start of chemotherapy, both trials used a dose of 4 mg every 3 months, and BMD results were reported at 6 months and 1 yr. However, data to inform the optimal administration schedule and long-term effects of ZA are lacking.
Simultaneously, several large randomized trials have established the efficacy of yearly administration of ZA in women with postmenopausal osteoporosis (9,10,11). Annual ZA has been shown to be equally effective at preventing bone loss as daily oral bisphosphonate therapy (9). Subsequent trials have shown that 5 mg annually for 3 yr reduces fractures in this population by up to 70% (10).
The primary objective of this investigation was to determine whether ZA, given every 3 months for 1 yr to premenopausal women with BC undergoing chemotherapy, prevented a reduction in BMD at 6 and 12 months (1). These results were previously reported. We now present the secondary analysis to determine whether 1 yr of therapy prevented reductions in BMD during the year after the last administered dose, to evaluate the duration of the effect of ZA on bone turnover markers, and to characterize further the natural history of bone loss over a 2-yr period in women randomized to placebo. Based upon the long duration of effect of ZA in postmenopausal women (9), we hypothesized that BMD would remain stable and bone turnover markers would be suppressed in the subjects who had been randomized to ZA during the first year.
Patients and Methods
Participants
Subjects were premenopausal women with newly diagnosed, histologically proven, nonmetastatic BC. Premenopausal status was defined as last menstruation no more than 6 months earlier or FSH below 20 mIU/liter. Patients were enrolled after surgery, but before initiating chemotherapy. The chemotherapeutic regimens were not dictated by study investigators. Exclusion criteria included a T score of less than −2.0 at any site, fragility fracture, prior therapy with a bisphosphonate, LS anatomy precluding accurate BMD measurement of at least three lumbar vertebrae, serum creatinine of at least 2 mg/dl, or pregnancy.
Protocol
After signing informed consent, patients were randomized to placebo or ZA 4 mg iv over 15 min every 3 months for 12 months. Treatment assignment was stratified by tumor hormone receptor status. Upon enrollment, information on tumor stage, history of fractures, reproductive and menstrual history, tobacco exposure, alcohol intake, physical activity, and medications was collected. The baseline evaluation included a chemistry panel; intact PTH; 25-hydroxyvitamin D; bone-specific alkaline phosphatase (BSAP), a marker of bone formation; and serum C-telopeptide of type I collagen (CTX), a marker of bone resorption.
A separate restricted randomization list was prepared for each stratum at each recruitment site using random permuted blocks. When a new patient was enrolled, the research pharmacy distributed study drug/placebo in unmarked blister packs. All patients were provided with oral supplements containing calcium (1000 mg) and vitamin D (400 IU).
The protocol was initially limited to Columbia University Medical Center (CUMC) and then was opened at eight additional sites to increase enrollment. Four of the eight additional sites contributed a total of 10 patients. Due to incomplete 2-yr data, only the patients at CUMC were included in this analysis. The CUMC Institutional Review Board approved the study, and all patients gave written informed consent.
Outcome measurements
BMD
BMD of the LS (L1–L4), total hip (TH), and femoral neck (FN) was measured by dual-energy x-ray absorptiometry at randomization, 6, 12, and 24 months using Hologic QDR 4500 bone densitometers (Hologic Inc., Bedford, MA) in the array mode by radiology technicians certified by the International Society for Clinical Densitometry. An established program of instrument calibration and ongoing quality control to allow for accurate comparisons of BMD data were used. All instruments were calibrated before beginning the study with reference phantoms to read BMD within 1%. The subsequent calibration strategy included rescanning of the reference phantoms at 6-month intervals. Patients were assessed on the same machine for each follow-up visit. All densitometry results were reviewed by a third party.
Assays
Blood was obtained before initiation of chemotherapy and at 6, 12, 24, 52, and 100 weeks and after an overnight fast when possible. Serum was aliquoted, frozen immediately, and stored at −70 C until batch analyses were undertaken. BSAP was measured using an immunoassay kit (Metra BAP; Quidel Corp., San Diego, CA), with low cross-reactivity with the liver form of alkaline phosphatase (3–8%). Interassay variability was 8.6% at 13.7 U/liter. Normal range in premenopausal women is 11.6–29.6 U/liter. CTX was measured using a sandwich ELISA (Serum Crosslaps; IDS Ltd., Fountain Hills, AZ) with an interassay variability of 11.1% at 0.408 ng/ml. Plasma 25-hydroxyvitamin D was analyzed by RIA as previously described (Diasorin, Inc., Stillwater, MN) (12). Interassay precision at 15 ng/ml was 14%. Specimens were thawed, processed batch wise, and identified by a study number without reference to treatment assignment.
Statistical analysis
The primary efficacy endpoint was percentage change in LS BMD at 6 months after initiation of chemotherapy. This study was designed to detect a between-group difference of 3% change in LS BMD at 6 months. The primary analysis was previously reported (1). Secondary endpoints included percentage change in LS, TH, and FN BMD at 12 and 24 months. The data were held by the investigators and analyzed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Baseline group differences were assessed with independent t tests for continuous measures and Fisher’s exact test for categorical data. The effects of ZA/placebo on changes in BMD and biochemical measures were estimated with separate linear mixed models. In each model, group, time, and group by time interaction were entered as fixed effects; subject and error were entered as random effects; and an autoregressive covariance structure was used for the within-subject correlation. Biochemical measures were log10 transformed before statistical analysis. Means and sd values are presented for baseline measures, and means and se values from model estimates are reported. P values less than 0.05 were interpreted as statistically significant.
Results
Baseline characteristics of the study population
Of 103 patients who completed the baseline evaluation, 85 who were enrolled at CUMC completed all evaluations through the 12 month visit. For the 24-month visit, 62 completed serum assessments and 57 completed both serum and BMD assessments. The majority of the remaining 23 patients (n = 18) had the 24-month BMD performed more than 30 months after randomization and were not included in the analysis. Other reasons for discontinuation included disease progression (n = 3) and loss to follow-up (n = 2). Baseline characteristics of the participants included in this analysis are shown in Table 1. The mean age (42 ± 6 yr) and mean body mass index (26 ± 5 kg/m2) did not differ between groups. The groups were similar with regard to stage, treatment duration, and race. The population was racially/ethnically diverse, with 51% non-Hispanic White, 35% Hispanic, 12% African-American, and 2% Asian. The groups were balanced with respect to menstrual history and risk factors for osteoporosis. Subjects in the placebo group reported slightly more calcium supplement use. Baseline LS, FN, and TH BMD, expressed both as grams per square centimeter and Z scores, were within the normal range and comparable in both groups (Table 2). By 24 months, 61% had not regained their menses, 35% were taking tamoxifen, and 40% were taking an aromatase inhibitor.
Table 1.
Baseline characteristics of original study participants compared to those who had complete 2-yr data
| Study population at 12 months | ZA completers | Placebo completers | |
|---|---|---|---|
| n | 85 | 27 | 30 |
| Age (yr) | 44 ± 6 | 44 ± 5 | 42 ± 5 |
| Body mass index (kg/m2) | 26.0 ± 5.0 | 27.2 ± 4.5 | 25.7 ± 5.0 |
| Race/ethnicity | |||
| White | 34 (41) | 14 (52) | 10 (34) |
| Hispanic | 36 (43) | 10 (37) | 13 (45) |
| Black | 10 (18) | 3 (11) | 6 (21) |
| Asian | 3 (4) | 0 (0) | 0 (0) |
| Stage | |||
| 1 | 27 (32) | 9 (33) | 13 (43) |
| 2 | 54 (64) | 17 (63) | 16 (53) |
| 3 | 4 (4) | 1 (4) | 1 (3) |
| Hormone receptor positive | 56 (66) | 23 (85) | 20 (67) |
| Tamoxifen (n) | 44 | 7 | 9 |
| Aromatase inhibitor (n) | 12 | 16 | 11 |
| Chemotherapy | |||
| 4 cycles | 17 (20) | 6 (22) | 7 (23) |
| 6–8 cycles | 68 (80) | 21 (78) | 23 (77) |
| Menses during treatment | |||
| Stopped | 58 (68) | 21 (77) | 23 (76) |
| Stopped and returned | 27 (32) | 6 (23) | 7 (24) |
| Ever oral contraceptives | 28 (33) | 11 (41) | 12 (40) |
| Ever tobacco use | 28 (33) | 10 (37) | 10 (33) |
| Pack years | 8.1 ± 6.0 | 8.3 (2.1) | 7.6 (1.8) |
| Family history of osteoporosis | 18 (21) | 8 (30) | 7 (23) |
| Current exercise | 26 (31) | 11 (41) | 9 (30) |
| Past exercise | 45 (53) | 13 (48) | 17 (57) |
| Calcium supplements | 37 (44) | 8 (30) | 16 (53) |
| Vitamin D | 34 (40) | 8 (30) | 15 (50) |
| Multivitamin | 26 (31) | 8 (30) | 9 (30) |
Data are presented as mean ± sd or number (percentage).
Table 2.
Absolute BMD and percentage change from baseline in patients randomized to ZA or placebo at 24 and 52 wk
| BMD in g/cm2 (sd)
|
% Change in BMD (sem)
|
||||||
|---|---|---|---|---|---|---|---|
| Baseline | 1 yr | 2 yr | P value | 1 yr | 2 yr | P value | |
| LS | |||||||
| Placebo | 1.071 (0.12) | 1.020 (0.13) | 1.011 (0.14) | <0.001a | −5.4 (0.55) | −6.3 (0.83) | <0.001a |
| ZA | 1.036 (0.12) | 1.048 (0.13) | 1.028 (0.12) | <0.001b | 1.3 (0.58) | −0.6 (0.84) | NSb |
| FN | |||||||
| Placebo | 0.864 (0.13) | 0.860 (0.13) | 0.854 (0.14) | 0.09a | −1.5 (0.61) | −2.4 (0.71) | 0.05a |
| ZA | 0.830 (0.13) | 0.843 (0.13) | 0.842 (0.13) | 0.10b | 1.1 (0.42) | 0.04 (0.84) | NSb |
| TH | |||||||
| Placebo | 0.988 (0.11) | 0.984 (0.14) | 0.978 (0.14) | 0.002a | −1.9 (0.66) | −2.6 (0.84) | <0.001a |
| ZA | 0.946 (0.11) | 0.955 (0.11) | 0.962 (0.10) | <0.001b | 0.7 (1.06) | 0.8 (1.07) | NSb |
NS, Not significant.
P value for the comparison of the difference between treatment group means ignoring time.
P value for the comparison of treatment group differences in their pattern of change over time.
Change in BMD
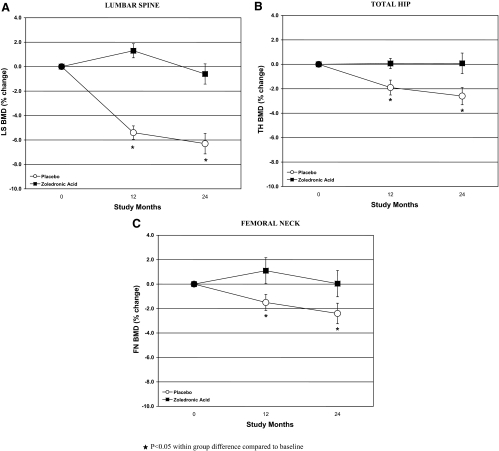
As shown in Table 2, LS, FN, and TH BMD remained stable in the ZA group at 12 and 24 months. For women randomized to placebo, however, there was a substantial and consistent decrease in LS BMD. By 12 months, LS BMD was −5.4% below baseline, and by 24 months, it was −6.3% below baseline (Fig. 1). At 24 months, TH and FN BMD were also statistically significantly lower compared with baseline by −2.6% and −2.4%, respectively. In the ZA arm, however, there was a slight increase in BMD at each site at 12 months, with return to baseline at 24 months. The change in BMD between 12 and 24 months was not significant at any site in either the ZA or placebo groups. The percentage of patients with Z scores below −1.0 changed from 23% at baseline to 18% at 12 months and to 29% at 24 months in the ZA group, and from 21% to 37% to 43% in the placebo group. In the placebo group, change in BMD was not correlated with type of chemotherapy, baseline BMD, hormonal therapy, or permanence of menstrual cessation.
Figure 1.
Percentage change in BMD at the LS (A), TH (B), and FN (C) 12 and 24 months from baseline in women treated with ZA and those treated with placebo.
Markers of bone turnover
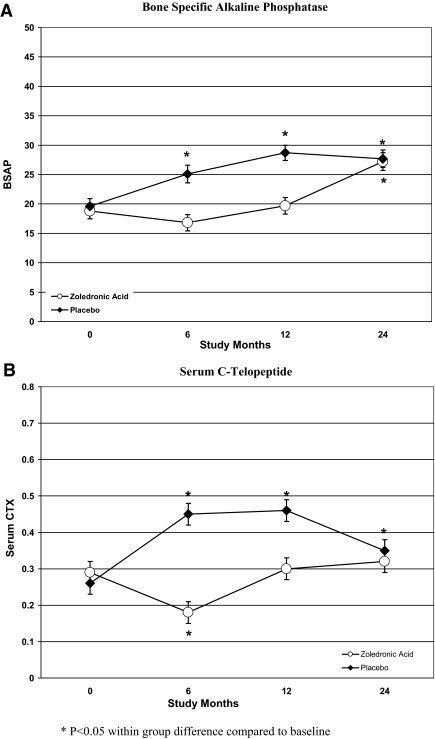
In the placebo group, markers of bone formation (BSAP) and resorption (CTX) increased progressively and significantly during the first year (Fig. 2). Although serum CTX declined during the second year, both serum BSAP and CTX were significantly above baseline at 12 and 24 months. BSAP and CTX differed between groups at 12 months, but not at 24 months. In the ZA group, serum BSAP and CTX were significantly below baseline at 6 months but returned to baseline levels at 12 months; at 24 months, serum BSAP was significantly above baseline, and CTX was similar to the placebo group. PTH and albumin-corrected calcium were stable at 12 and 24 months in both groups. Mean serum 25-hydroxyvitamin D levels increased similarly with supplementation over the 2-yr period in both groups, from 23 to 36 ng/ml.
Figure 2.
Change in bone turnover markers at the BSAP (A) and serum CTX (B) at 12 and 24 months from baseline in women treated with ZA and those treated with placebo.
Discussion
This study shows that premenopausal women with BC experience significant bone loss during the first year after initiating chemotherapy and continued, although nonsignificant, loss the second year. The amount of bone loss was greatest at the LS, with a decrease of 5.3% by 1 yr and 6.3% by 2 yr. Significant, although less severe, losses were also observed at the FN and TH. The iv administration of 4 mg of ZA every 3 months for 1 yr prevented significant bone loss at all sites. In fact, bone density actually increased during the first year in the ZA group, followed by a nonsignificant decrease during the second year.
The amount of bone loss we observed during the first year after initiating adjuvant chemotherapy is consistent with other studies of premenopausal women with BC on chemotherapy that have reported declines ranging from 3.5%–5%, depending on the proportion of women who enter menopause (1,2,6,13). This loss is similar to that seen in medically and surgically induced menopause, which is also prevented by ZA (14). Although iv bisphosphonates (ZA and pamidronate) have been shown to prevent this bone loss, the oral bisphosphonate, risedronate, did not (13). The lack of efficacy observed with oral risedronate may stem from its lower potency or from poorer patient compliance.
Although BMD declined in the placebo group during the second year at all sites measured, the decrease was not significant. Thus, our study confirms that bone loss sustained during the first year on chemotherapy exceeds that in subsequent years. This observation may support a strategy of using iv bisphosphonates to maintain bone mass and prevent deterioration of bone microarchitecture during the initial period of rapid bone loss. Although the optimal dosing schedule to accomplish this goal is unknown, subsequent studies have suggested equal benefit from every 6-month administration (14).
Although BSAP and CTX levels were significantly lower in the ZA than the placebo group at 12 months, it is noteworthy that they were not suppressed relative to baseline, despite a dose of 4 mg every 3 months. The increase in bone turnover markers in the ZA group between 6 and 12 months and of BSAP during the second year speak to the larger dose of ZA required and the shorter duration of its antiresorptive effect in this clinical situation than in the setting of postmenopausal osteoporosis. Moreover, by the 12- and 24-month points, markers of both bone formation (BSAP) and resorption (CTX) were comparable to the placebo group, indicating that the antiresorptive effects of ZA had dissipated. This differs from findings in postmenopausal women with low bone density, in whom a single infusion of ZA suppressed bone turnover markers for a full year (15).
One could argue that the lack of suppression of bone turnover markers at 12 months and their rise by 24 months and the slight, albeit insignificant, decreases in BMD during the second year in the ZA group favor intervening with ZA during the first year, with continued dosing perhaps at less frequent intervals thereafter. This may be a prudent approach, particularly if the goal is to prevent bone loss completely during the period when it is most likely to decline rapidly. Moreover, once the ZA effect dissipates completely, bone loss may accelerate, particularly if amenorrhea persists or aromatase inhibitor therapy is instituted, because these drugs further increase bone turnover. Conversely, one could also argue that close monitoring is sufficient after the first year, because most women start chemotherapy with normal BMD, and there was no significant change from baseline during the year after quarterly ZA administration. In this scenario, only patients with low baseline BMD or who sustained significant bone loss during the second year would be considered for additional antiresorptive treatment at 2 yr.
When considering these issues, both cost and toxicity become important features for the risk-benefit decision-making process. There may be risks associated with suppressing bone remodeling profoundly and/or for long periods of time. Recent reports of spontaneous fractures in patients on long-term bisphosphonate therapy are of concern, particularly because histomorphometric evidence of complete suppression of bone remodeling activity has been documented in some of these cases (16,17,18). Oversuppression of remodeling may also contribute to the development of osteonecrosis of the jaw (ONJ) (19). Our finding that after 1 yr of ZA, bone turnover markers were not suppressed suggests that the dosing regimen we used did not suppress remodeling completely, but largely prevented the increase in remodeling seen in the placebo group. The risk of ONJ with an annual schedule is currently being investigated but is likely to be low. Notably, in the pivotal phase III clinical trial of ZA for postmenopausal osteoporosis, one case of ONJ was found in both the placebo and the ZA groups (10).
Decisions regarding the use of bisphosphonates for cancer therapy-induced bone loss alone will be less difficult if these drugs prove to decrease the risk of BC recurrence. The skeleton is the initial site of recurrence in 35–40% of patients, and up to 60–80% of patients with metastatic BC eventually develop bone metastases (20). BC cells present in bone marrow release factors that activate osteoclasts to resorb bone (21). Several studies have reported a survival benefit for patients treated with adjuvant bisphosphonates (22,23). Interestingly, in June 2008, the results of the Austrian Breast and Colorectal Cancer Study Group study were reported. The study included 1803 premenopausal women with endocrine-responsive BC who were randomized to goserelin and tamoxifen ± ZA (4 mg every 6 months) or goserelin and anastrazole ± ZA for 3 yr. With median follow-up of 60 months, endocrine therapy plus ZA significantly reduced the risk of recurrence by 36% compared with endocrine therapy alone. For overall survival, there was a nonsignificant trend favoring ZA (22). However, further follow-up is required to confirm these results.
The current study has several limitations. First, there was considerable dropout from registration to the 2-yr follow-up, and only patients enrolled at CUMC were included in the 2-yr analysis. However, the dropout was similar in both the ZA and placebo groups, and the subset in the analysis did not differ from the larger study cohort. Other studies with a sample size of only 40 subjects found a statistically significant difference at 1 yr. In addition, there were slightly more patients in the ZA group on aromatase inhibitors, which if anything, would bias our results toward the null. Our results are consistent with other studies that have shown significant dropout from baseline to annual follow-up because young patients who have completed cancer therapy often do not want to return for study visits. Secondly, due to the small numbers in the placebo group, we were unable to stratify the analysis by type of hormonal treatment or menopausal status.
In summary, we have shown that premenopausal women receiving chemotherapy and hormone therapy for BC sustained significant bone loss in the first year that persisted in the second year. ZA 4 mg every 3 months effectively prevented bone loss during the first year of chemotherapy. The protective effect of ZA on BMD persisted 1 yr after completion of ZA treatment. However, by this time point, bone turnover markers, especially BSAP, were significantly higher than at 12 months and were indistinguishable from the placebo group. This observation provides support for close monitoring and, if necessitated by rising rates of bone loss, annual dosing in this patient population. Questions remain regarding the appropriate dose and duration of therapy. In addition, given the uncertain risks of long-term bisphosphonate administration, it is important to consider whether preemptive intervention to reduce fractures that may occur far in the future is appropriate in such a young population. Given the number of women who experience significant bone loss and the epidemiological data supporting higher fracture incidence in postmenopausal BC survivors, close monitoring and initiation of ZA, if appropriate, may be a reasonable approach.
Footnotes
D.L.H. received research support for this study from a K07 Award from the National Cancer Institute (CA95597) and a career development award from the American Society of Clinical Oncology. E.S. is the recipient of a K24 Award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR 052665). Additional support for the trial was provided by Novartis Pharmaceuticals Corporation.
Disclosure Summary: The authors have no additional conflicts to disclose.
First Published Online December 18, 2009
Abbreviations: BC, Breast cancer; BMD, bone mineral density; BSAP, bone-specific alkaline phosphatase; CTX, C-telopeptide of type I collagen; FN, femoral neck; LS, lumbar spine; ONJ, osteonecrosis of the jaw; TH, total hip; ZA, zoledronic acid.
References
- Hershman DL, McMahon DJ, Crew KD, Cremers S, Irani D, Cucchiara G, Brafman L, Shane E 2008 Zoledronic acid prevents bone loss in premenopausal women undergoing adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol 26:4739–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro C, Halabi S, Gibson G, Weckstein D, Kirshner J, Sikov W, Winer E, Hudis C, Isaacs C, Weckstein D, Schilsky R, Paskett E 2008 Effect of zoledronic acid (ZA) on bone mineral density (BMD) in premenopausal women who develop ovarian failure (OF) due to adjuvant chemotherapy (AdC). J Clin Oncol 26:Abstract 512 [Google Scholar]
- Chen Z, Maricic M, Bassford TL, Pettinger M, Ritenbaugh C, Lopez AM, Barad DH, Gass M, Leboff MS 2005 Fracture risk among breast cancer survivors: results from the Women’s Health Initiative Observational Study. Arch Intern Med 165:552–558 [DOI] [PubMed] [Google Scholar]
- Kanis JA, McCloskey EV, Powles T, Paterson AH, Ashley S, Spector T 1999 A high incidence of vertebral fracture in women with breast cancer. Br J Cancer 79:1179–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarto T, Blomqvist C, Välimäki M, Mäkelä P, Sarna S, Elomaa I 1997 Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: a randomized study in premenopausal breast cancer patients. J Clin Oncol 15:1341–1347 [DOI] [PubMed] [Google Scholar]
- Fuleihan Gel-H, Salamoun M, Mourad YA, Chehal A, Salem Z, Mahfoud Z, Shamseddine A 2005 Pamidronate in the prevention of chemotherapy-induced bone loss in premenopausal women with breast cancer: a randomized controlled trial. J Clin Endocrinol Metab 90:3209–3214 [DOI] [PubMed] [Google Scholar]
- Saarto T, Blomqvist C, Välimäki M, Mäkelä P, Sarna S, Elomaa I 1997 Clodronate improves bone mineral density in post-menopausal breast cancer patients treated with adjuvant antioestrogens. Br J Cancer 75:602–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnick S, Saag KG, Kiel DP, McClung M, Hochberg M, Burnett SM, Sebba A, Kagan R, Chen E, Thompson DE, de Papp AE 2006 Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 91:2631–2637 [DOI] [PubMed] [Google Scholar]
- Reid IR, Miller P, Lyles K, Fraser W, Brown JP, Saidi Y, Mesenbrink P, Su G, Pak J, Zelenakas K, Luchi M, Richardson P, Hosking D 2005 Comparison of a single infusion of zoledronic acid with risedronate for Paget’s disease. N Engl J Med 353:898–908 [DOI] [PubMed] [Google Scholar]
- Lyles KW, Colón-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S 2007 Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR 2007 Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822 [DOI] [PubMed] [Google Scholar]
- Hollis BW 1997 Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol 282:174–186 [DOI] [PubMed] [Google Scholar]
- Hines SL, Mincey BA, Sloan JA, Thomas SP, Chottiner E, Loprinzi CL, Carlson MD, Atherton PJ, Salim M, Perez EA 2009 Phase III randomized, placebo-controlled, double-blind trial of risedronate for the prevention of bone loss in premenopausal women undergoing chemotherapy for primary breast cancer. J Clin Oncol 27:1047–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Kainberger F, Kässmann H, Piswanger-Sölkner JC, Seifert M, Ploner F, Menzel C, Dubsky P, Fitzal F, Bjelic-Radisic V, Steger G, Greil R, Marth C, Kubista E, Samonigg H, Wohlmuth P, Mittlböck M, Jakesz R 2008 Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol 9:840–849 [DOI] [PubMed] [Google Scholar]
- Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, Widmer A, Devogelaer JP, Kaufman JM, Jaeger P, Body JJ, Brandi ML, Broell J, Di Micco R, Genazzani AR, Felsenberg D, Happ J, Hooper MJ, Ittner J, Leb G, Mallmin H, Murray T, Ortolani S, Rubinacci A, Saaf M, Samsioe G, Verbruggen L, Meunier PJ 2002 Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346:653–661 [DOI] [PubMed] [Google Scholar]
- Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY 2005 Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90:1294–1301 [DOI] [PubMed] [Google Scholar]
- Ott SM 2001 Fractures after long-term alendronate therapy. J Clin Endocrinol Metab 86:1835–1836 [DOI] [PubMed] [Google Scholar]
- Ott SM 2005 Long-term safety of bisphosphonates. J Clin Endocrinol Metab 90:1897–1899 [DOI] [PubMed] [Google Scholar]
- Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E 2007 Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 22:1479–1491 [DOI] [PubMed] [Google Scholar]
- Coleman RE 2006 Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12:6243s–6249s [DOI] [PubMed] [Google Scholar]
- Guise TA 2000 Molecular mechanisms of osteolytic bone metastases. Cancer 88:2892–2898 [DOI] [PubMed] [Google Scholar]
- Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rücklinger E, Greil R, Marth C 2009 Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 360:679–691 [DOI] [PubMed] [Google Scholar]
- Powles T, Paterson S, Kanis JA, McCloskey E, Ashley S, Tidy A, Rosenqvist K, Smith I, Ottestad L, Legault S, Pajunen M, Nevantaus A, Männistö E, Suovuori A, Atula S, Nevalainen J, Pylkkänen L 2002 Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol 20:3219–3224 [DOI] [PubMed] [Google Scholar]