Abstract
Context: Several endocrine diseases that share resistance to PTH are grouped under the term pseudohypoparathyroidism (PHP). Patients with PHP type Ia show additional hormone resistance, defective erythrocyte Gsα activity, and dysmorphic features termed Albright’s hereditary osteodystrophy (AHO). Patients with PHP-Ib show less diverse hormone resistance and normal Gsα activity; AHO features are typically absent in PHP-Ib. Mutations affecting Gsα coding exons of GNAS and epigenetic alterations in the same gene are associated with PHP-Ia and -Ib, respectively. The epigenetic GNAS changes in familial PHP-Ib are caused by microdeletions near or within GNAS but without involving Gsα coding exons.
Objective: We sought to identify the molecular defect in a patient who was diagnosed with PHP-Ia based on clinical presentation (hormone resistance and AHO) but displayed the molecular features typically associated with PHP-Ib (loss of methylation at exon A/B) without previously described genetic mutations.
Methods: Microsatellite typing, comparative genome hybridization, and allelic dosage were performed for proband and her parents.
Results: Comparative genome hybridization revealed a deletion of 30,431 bp extending from the intronic region between exons XL and A/B to intron 5. The same mutation was also demonstrated, by PCR, in the patient’s mother, but polymorphism and allele dosage analyses indicated that she had this mutation in a mosaic manner.
Conclusion: We discovered a novel multiexonic GNAS deletion transmitted to our patient from her mother who is mosaic for this mutation. The deletion led to different phenotypic manifestations in the two generation and appeared, in the patient, as loss of GNAS imprinting.
Data show a loss of GNAS imprinting due to a multiexonic GNAS deletion transmitted to the patient from her mother who is mosaic for this mutation.
Pseudohypoparathyroidism (PHP; MIM 103580) includes a heterogeneous group of endocrine diseases that are characterized by the presence of hypocalcemia, hyperphosphatemia, and tissue resistance to PTH (1). Genetic defects associated with different forms of PHP involve the α-subunit of the stimulatory G protein (Gsα), a signaling protein essential for the actions of PTH and many other hormones. Gsα is encoded by the GNAS gene (guanine nucleotide-binding protein, α stimulating), located on the long arm of chromosome 20 (20q13.32) (2). GNAS is a highly complex locus, because it generates different paternal, maternal, or biallelic transcripts that use alternative promoters and first exons and share most of the exons encoding Gsα (3).
Patients with PHP type Ia (PHP-Ia; MIM 103580) have multihormonal resistance (4) and Albright’s hereditary osteodystrophy (AHO) characterized by round face, short neck, obesity, sc calcifications, brachydactyly, and/or mental retardation (1). These patients have maternally inherited heterozygous inactivating mutations in one of the 13 GNAS exons (5,6), including missense mutations, nonsense mutations, insertions, and deletions. Moreover, few genomic rearrangements have been described at GNAS locus, such as terminal deletions (7) or an inversion (8). Patients who exhibit AHO features without evidence for hormone resistance are said to have pseudopseudohypoparathyroidism (PPHP; MIM 612463). Those patients also carry heterozygous inactivating Gsα mutations, but the mutations are inherited paternally (9,10).
Patients with PHP type Ib (PHP-Ib; MIM 603233) are defined by renal resistance to PTH and, in some cases, mild resistance to TSH in the absence of the AHO phenotype and resistance to other hormones (11). PHP-Ib patients lack Gsα coding mutations within GNAS, and this PHP subtype is associated with imprinting defects within GNAS. The most common defect is a loss of methylation in alternative exon A/B (also referred to as 1A) on the maternal allele, leading to biallelic expression of the A/B transcript (12,13). In familial cases, loss of imprinting of exon A/B is associated with heterozygous deletions within the gene encoding syntaxin 16 (STX16), which is located more than 220 kb upstream of GNAS (14,15). Some familial and nearly all sporadic PHP-Ib cases also show broad loss of imprinting at GNAS, which results, at least in some cases, from the deletion of the NESP55 differentially methylated region (16).
Here we report the first description of an intragenic GNAS deletion that involves not only Gsα coding exons but also one of the upstream differentially methylated exons (A/B). This deletion causes PHP-Ia but is also associated with an apparent GNAS imprinting defect. Our studies also show that the deletion is inherited from the mother who is gonosomal mosaic for this genetic aberration.
Patients and Methods
Case reports
Clinical features of the patient were described previously (17). Briefly, a 5-month-old girl born from healthy nonconsanguineous parents was referred to us because of a peculiar phenotype and morbid obesity. Physical examination revealed obesity with short neck and a round face. She weighed 12.730 kg (>95th percentile) and stood 66 cm tall (94th percentile); head circumference was 43.5 cm (75–90th percentile). Body mass index was 29.2 (>97th percentile). Both hands appeared short and stubby, with markedly short fourth and fifth metacarpals. Serum ionized calcium (4.88 mg/dl; normal, 4.6–5.16 mg/dl) and serum phosphorus (7.3 mg/dl; normal, 3.9–8.0 mg/dl) were normal with mildly elevated intact PTH (48 pg/ml; normal, 10–40 pg/ml); free T4 was 0.97 ng/dl (normal, 0.89–1.8 ng/dl), and TSH was 11.9 μU/ml (normal, 0.35–5.5 μU/ml); TRH test showed basal TSH of 11 μU/ml with a peak at 30 min of 50 μU/ml (normal response <30 μU/ml). Neurological examination was unremarkable. Levothyroxine (50 μg/d) was started. A skin biopsy, performed at age 8 months due to sc nodular lesions, revealed calcifications containing newly formed bone. At that time, calcium, phosphorous, and PTH levels were normal. At 3 yr of age, ionized calcium was low (4.16 mg/dl) with high PTH levels (264 pg/ml), and therefore, treatment with 1,25-dihydroxyvitamin D3 (0.25 μg/d) was initiated. On her latest physical examination at age 13 yr 2 months (skeletal age 14 yr using Greulich and Pyle standards) (18), she weighed 42.2 kg (25–50th percentile) without dietary restrictions, she was 142 cm tall (10–25th percentile) (mother’s height, 150 cm; father’s height, 158 cm), and her body mass index was 20.93 (50–75th percentile). Her puberty showed normal timing and onset (nowadays she is on Tanner V, with menarche at 12 yr 7 months). New sc calcifications are found in her legs and the right fifth toe. On treatment, she had normal serum calcium (9.6 mg/dl; normal, 8.1–10.4 mg/dl), phosphorous (4.8 mg/dl), and TSH levels (4.18 μU/ml), although intact PTH was elevated (198 pg/ml). At the molecular level, the patient exhibited an isolated loss of exon A/B methylation, but she lacked the previously described STX16 deletions.
Molecular studies
Genetic analyses were performed after informed consent of the parents upon approval of the study by the Institutional Review Board. Genomic DNA was extracted from peripheral blood leukocytes using a commercial kit (QIAamp® Blood Midi; QIAGEN, Düren, Germany).
Microsatellite typing
Several microsatellite markers centromeric (D20S832, D20S102, D20S1063, D20S496, and D20S459) and telomeric (D20S443, D20S171, and D20S173) of GNAS, intragenic polymorphisms (SNPs) (exon A/B pentanucleotide repeat, exon 5 and intron 12 single-nucleotide polymorphisms), and nine additional polymorphisms (rs481019, rs6128453, rs62203846, rs6026577, rs4812042, rs6026578, rs6026579, rs9679845, and rs6123837) located flanking exon A/B were genotyped as previously described (17). The eight microsatellite markers were typed by fluorescent PCR.
Comparative genome hybridization (CGH) array
Reference DNA was obtained from a healthy donor’s whole peripheral blood.
A customized Human 44k CGH microarray spanning the human chromosome 20q13–qter region (Agilent Technologies, Palo Alto, CA) with an average resolution <0.5 Kb was used. The array was designed using Agilent′s e-array tool (https://earray.chem.agilent.com/earray/) and the probes were selected from nucleotide 47,897,125 to 62,435,965 on chromosome 20q.
ArrayCGH (aCGH) assays were performed as previously described (19) with minor modifications. Briefly, 1 μg sample and reference DNA was digested with AluI and RsaI restriction enzymes (Promega Corp., Madison, WI) and were differentially labeled with Cy5-dUTP and Cy3-dUTP, respectively, using Agilent Genomic DNA Labeling Kit Plus (Agilent Technologies, Palo Alto, CA). After purification, the probe was prepared and hybridized on to a 20q-focused aCGH platform using Agilent Oligo aCGH kit (Agilent). Hybridization was performed at 65 C for 24 h in a rotator hybridization oven. After washing, slides were scanned using an Agilent G2505B DNA microarray scanner.
Microarray images were transformed to fluorescence intensities by means of Feature Extraction software, version 9.5.1 (Agilent). Data were visualized and analyzed using Agilent CGH Analytics version 3.4.40 software. As quality criteria, a derivative log ratio spread lower than 0.2 log units and a signal to noise ratio for each channel greater than 30 were used. To establish gained and lost regions, a 20-kb weighted moving-average window and a Z-score threshold of ±7.5 were used. Additionally, data were analyzed by means of InSilicoArray CGH tool (http://isacgh.bioinfo.cipf.es/) (20) and ADaCGH v.20070605 (analysis of data from aCGH, http://adacgh.bioinfo.cnio.es/) (21).
To confirm the results of the aCGH, specific PCR and sequencing were designed to determine the boundaries of the deletion. The primers selected were 5′-CAGTCCTCCATCTTAGATCCTGAGC-3′ and 5′-GTGGCGGTTACTTACTACTGGGCA-3′. Five microliters of DNA were used for the amplification with BioTaq polymerase (Bioline, Taunton, MA), according to the manufacturer’s instructions, in a 50-μl final volume. Amplification of a control exon in the same tube was used to determine whether the absence of amplification was due to contaminants inhibiting the reaction. Amplification conditions consisted of an initial denaturation step of 5 min at 95 C, followed by 40 cycles at 95 C for 45 sec, 65 C for 45 sec, and 72 C for 45 sec, and a final extension of 10 min at 72 C.
Real-time PCR
Allele dosage analyses were performed by real-time PCR on an ABI PRISM 7900HT sequence detection system (Applied Biosystems, Foster City, CA) with TaqMan chemistry, for the rs7121 SNP, which is located at GNAS exon 5. Primers and probes for rs7121 expression was a predeveloped assay reagents mix (Applied Biosystems; catalog item 4351379). The probes are labeled with VIC for allele C and with FAM for allele T.
Template volume of 2 μl containing approximately 20 ng/μl DNA for each type of artificial heterozygous, normal heterozygous, and the patient’s mother were mixed with Takara premix Ex Taq reagents (catalog item RR039A; Takara Bio Inc., Shiga, Japan) for the real-time quantitative PCR (qPCR) assay, according to the manufacturer’s instructions in a final volume of 20 μl.
To validate the qPCR assay, we performed standard curve analysis with 18 artificial heterozygous samples prepared by mixing different concentrations of real homozygous for each allele from 1:10 to 10:1 (data not shown). The correlation between crossing point and input template (r2) was 0.96 for allele 1 and 0.95 for allele 2, demonstrating that change in cycle threshold (ΔCt) was constant relative to DNA quantity over the dynamic range of the experiment and satisfying criteria for the comparative Ct method of quantification.
The expression of each allele was simultaneously quantified in each experiment. Relative expression of each allele was calculated using the accurate Ct method (22). The accurate Ct represents the threshold cycle at which the exponential phase of the PCR amplification begins, and thus, amplification is proportional to the amount of input-specific DNA. We used allele 2 as an endogenous control. First, we calculated the difference between the allele 2 Ct and allele 1 Ct (ΔCt) for each patient. Second, we normalized the ΔCt to a control, in this case the dilution 6:4 (ΔΔCt), because this dilution works as expected heterozygous (1:1). Finally, the ratio sample to control equals 2−ΔΔCt.
Semiquantitative amplification of exon A/B
To assess the mosaicism of the deletion in the mother, a semiquantitative amplification of exon A/B was performed. The exon A/B pentanucleotide repeat was amplified in mother’s and father’s DNA in a nonsaturated PCR with only 25 cycles. The ratio among allele 2 (two pentanucleotide repeats) and 3 was established in both the father (as control) and the mother (as suspected mosaicism). The experiment was run twice and in triplicate.
Additionally, to ascertain the parental origin of the deletion, this study was simultaneously performed on HpaII-digested DNA, thus revealing the methylation status of the deleted allele.
Results
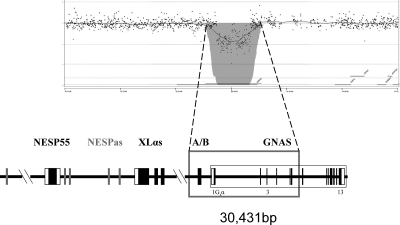
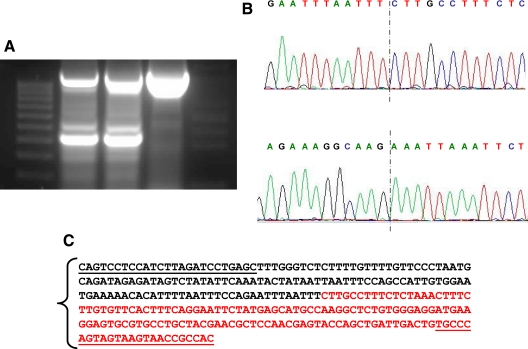
The patient exhibited severe AHO features and an apparent isolated loss of exon A/B methylation without the previously described STX16 deletions. As reported previously, a putative deletion or uniparental interstitial disomy involving exon A/B, which could explain the observed AHO phenotype and the loss of methylation, was investigated, but the genotype analysis of the patient and parents for several markers within GNAS failed to yield informative data (Table 1) (17). Moreover, Southern blot analyses using probes adjacent to the exon A/B differentially methylated region had not revealed evidence for a deletion (no additional hybridizing bands were detected) (17). However, to investigate this possibility further, we performed aCGH analysis focusing on the chromosomal region 20q13. Comparison of genomic DNA from the patient with that from a healthy control individual showed that the patient has a deletion extending from the intronic region between exons XL and A/B to intron 5 (Fig. 1). Raw data are available on request. Specific PCR and subsequent nucleotide sequence analysis designed to determine the boundaries of the deletion revealed that it encompassed nucleotides 56,883,365–56,913,796 according to Ensembl sequence ENSG00000087460. The analysis of the entire family by PCR indicated that the deletion had been inherited from the mother (Fig. 2).
Table 1.
Genotyping of the polymorphic markers at the GNAS region in the patient and her parents
| Father | Mother | Index | ||||
|---|---|---|---|---|---|---|
| D20S832 | 212 | 212 | 212 | 212 | 212 | 212 |
| D20S102 | 177 | 177 | 179 | 177 | 177 | 177 |
| D20S1063 | 199 | 199 | 201 | 201 | 199 | 201 |
| D20S496 | 202 | 204 | 204 | 202 | 204 | 202 |
| D20S459 | 227 | 227 | 227 | 227 | 227 | 227 |
| rs4810149 | C | T | C | T | T | T |
| rs6128453 | G | G | G | G | G | G |
| rs62203846 | T | C | T | C | C | C |
| rs6026577 | C | C | C | C | C | C |
| rs4812042 | G | A | G | A | A | A |
| rs6026578 | C | G | C | G | G | G |
| rs6026579 | C | T | C | T | T | T |
| A/B | 2 | 3 | 2 | 3 | 3 | 3 |
| rs9679845 | G | G | G | G | G | G |
| rs6123837 | G | G | G | G | G | G |
| E5 (rs7121) | C | C | T | C | C | C |
| I12 | C | T | C | T | T | T |
| D20S443 | 135 | 141 | 141 | 141 | 141 | 141 |
| D20S171 | 138 | 132 | 134 | 134 | 132 | 134 |
| D20S173 | 183 | 183 | 183 | 135 | 183 | 135 |
Figure 1.
aCGH profile at chromosome and gene level. The figure shows chromosome 20 ideogram and the aCGH profile of the patient, with the lost probes in the gray area; also under the ideogram is a gene view of the deleted region.
Figure 2.
A, Agarose electrophoresis showing the PCR of the deleted region (294 bp) and the internal PCR control. The first lane corresponds to the weight marker; lane 2, PCR amplification of genomic DNA of the patient; lane 3, PCR amplification of genomic DNA of the mother; lane 4, PCR amplification of genomic DNA of the father. The loss of the region at GNAS locus in the patient and her mother is evident. B, Sequencing of the deletion, with the sense sequence on the top and the reverse on the bottom with the dotted line marking the point where deletion occurred. C, Plain text of the sequence with the used primers underlined. In black is represented the upstream sequence and in red the downstream one.
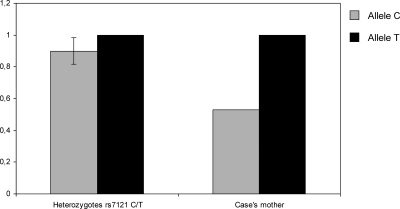
Although the deletion was present in the mother, our previous data had shown that she was heterozygous for several SNPs in the deleted region (17). To reconcile these findings, we hypothesized that the mother might be mosaic for this mutation. A quantitative real-time PCR analysis of a SNP within the deleted region showed a difference in the relative quantities of the alleles upon comparison of the lymphocyte DNA of the control individuals and the mother (0.9 vs. 0.53, respectively), strongly indicating that the mother carried the deletion in a mosaic manner (Fig. 3).
Figure 3.
Results of allele dosage analyses for the rs7121 SNP. The height of the columns represents the dosage of the respective allele in the genomic DNA for the patient and the mean of the controls (sd is included for allele C because data were normalized for allele T). Diminished dosage is observed for the patient’s allele C. Data are representative of two independent experiments with similar results.
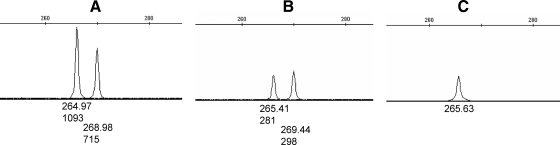
To study the parental origin of the mosaicism, semiquantitative PCR was performed to amplify the exon A/B pentanucleotide polymorphism, revealing that the mother carried the deletion in the short allele (allele with two repeats). The PCR amplification of the HpaII-digested DNA revealed that this allele was present on the methylated (thus protected) allele. Given that the maternal allele is methylated in this GNAS region (3), these results indicated that the patient’s mother had the deletion on the maternal allele in a mosaic manner (Fig. 4).
Figure 4.
Electropherogram of the semiquantitative amplification of exon A/B comprising the pentanucleotide repeat polymorphism. A, PCR amplification of the heterozygous father; B, PCR amplification of the heterozygous mother; C, PCR amplification of the mother’s genomic DNA after the overnight digestion with HpaII enzyme. Note that the amplification of the short allele (allele with two repeats) relative to the long allele (allele with three repeats) is less intense in the mother than the father. This short allele is the only one amplified after HpaII digestion, indicating that the deletion is present in the maternally inherited chromosome in the mother. The numbers under peaks represent the size of the amplicon (top) and the height of the peak (bottom).
To understand the formation of the deletion, we searched Low Copy Repeats within the sequences near the breakpoints by using UCSC Genome Browser (http://genome.uscsc.edu) and RepeatMasker (http://www.repeatmasker.org/cgi-bin/WEBRepeatMasker). An AluSp sequence 3271 bp upstream of the 5′ breakpoint and an AluSg sequence 1196 bp downstream of the 3′ breakpoint were identified. Although there are described nonallelic homologous recombination (NAHR) between different Alu families (23), the distance between Alu sequences and the breakpoints is too large to be the cause of this deletion (24). We also employed BLAST search (www.ncbi.nlm.nih.gov/blast/bl2seq/wblast2.cgi) to identify any homologies between the two ends of the deletion. However, our efforts did not reveal any direct or indirect repetitions, or identical sequences flanking the breakpoints of the deletion. However, the Human DNA Palindrome Database (HPALDB) (http://vhp.ntu.edu.sg/hpaldb) identified a palindromic sequence (AATTAATT) on both sides of the deletion. In the region of the 5′ breakpoint, this palindromic sequence is located 59 bp upstream, whereas at the 3′ breakpoint, it is located 219 bp downstream.
Discussion
We describe, for the first time, a PHP-Ia case with a multiexonic deletion within the GNAS locus. Remarkably, because the deletion involved a differentially methylated region frequently affected in patients with PHP-Ib, this patient appeared, at the molecular level, to have the latter subtype of PHP. The identification of this large deletion now explains both the loss of methylation at exon A/B and the AHO phenotype as well as the reduced erythrocyte Gsα activity demonstrated for this patient (17). The boundaries of the identified deletion also provide an explanation as to why our earlier study could not reveal this deletion (17). The probes used for the Southern blot analysis were located within the deleted genomic region, thus hybridizing exclusively to the intact allele.
No apparent Alu sequences were identified to explain a possible NAHR as cause of the deletion (24,25). However, our in silico researches showed the presence of a palindromic AATTAATT sequence located 59 bp upstream of the 5′ breakpoint and 219 bp downstream of the 3′ breakpoint. It is possible, as previously described (26), that these palindromic regions underlie the NAHR responsible for the multiexonic deletion originated in the mother’s DNA.
The index case inherited the deletion from her mother who presumably carries the deletion as a gonosomal mosaicism. Mosaicism is a condition in which genetically different cells coexist in tissues of the same individual and can modulate the resulting phenotype and has been recognized, over the past few years, as an important and relatively frequent mechanism at the origin of genetic disorders. Mosaicism for single gene mutations results from events occurring after fertilization, and it is referred to as somatic mosaicism. The earlier the mutational event occurs, the more likely it is that the individual will express the condition to a significant degree and that the gonadal tissue will be involved (gonosomal mosaicism), thus conferring a risk of transmission to the offspring. Mosaicism can also occur exclusively in gonadal tissue; this is referred to as gonadal mosaicism, which also poses a risk to the offspring (27). The data presented here suggest that the index case’s mother is a somatic and germline mosaic. Although germinal tissue was unavailable for examination, germline involvement is proven by the segregation of the mutation in her daughter. Identification of the mutation in leukocyte-derived DNA demonstrates somatic involvement.
Depending on various factors, such as the gene involved, the degree of mosaicism and/or the imprinting status, the carrier of a somatic mosaicism may be asymptomatic or may present with various symptoms of the disease. It is not easy to define the origin of a mosaicism; nevertheless, it allows for a better insight into the mechanisms involved and pathogenesis (28). The real-time qPCR analysis of rs7121 as well as the semiquantitative amplification of exon A/B suggested that approximately 60% of the tested leukocyte DNA had the normal genotype, and approximately 40% had the intragenic deletion (0.53/0.9 for rs7121 and 0.89/1.41 for exon A/B). Thus, the mutation must have arisen at a relatively early stage of embryogenesis, before the commitment of the germinal cells.
The index case has AHO features and PTH resistance, but her mother, despite carrying the deletion in the allele inherited from her mother, does not display any features of PHP (except for short stature). The apparent absence of phenotype in the mother may be due to a lower level of mosaicism for the deletion in other tissues such as the renal cortex, pituitary, or thyroid. However, taking into account the high degree of mosaicism detected in blood cells, originated from the same embryological layer as the renal cortex, and the presence of mosaicism in germinal cells, it appears likely that the nonmutant cells allow for a physiological complementation. This might be an important issue, because (epi)genetic alterations in mosaicism could explain those symptomatic cases without detectable genetic or epigenetic alteration in peripheral blood, the most investigated tissue (i.e. McCune-Albright syndrome). However, the findings in the mother suggest that mosaic alterations of inactivating mutations lead to very mild manifestations of PHP.
In summary, we identified a heterozygous intragenic GNAS deletion that removes not only some of the exons encoding Gsα but also the upstream differentially methylated exon A/B, thus explaining both the AHO features and the apparent loss of imprinting observed in our patient. Our findings indicate that somatic and germline mosaicism can increase the difficulty of a correct diagnosis of PHP and that mosaicism should be considered in rare diseases. We recommend that a careful genotype analysis is performed to confirm carrier status, because this would provide the fullest information possible for genetic counseling.
Acknowledgments
We thank all members of the affected family for their collaborative participation in this study.
Footnotes
G.P.d.N. is a Fondo de Investigacion Sanitaria researcher supported by the Instituto de Salud Carlos III of the Spanish Ministry of Health (Grant CP03/0064). I.G. has received a grant from the Basque Department of Education (BFI06.266). This work was partially supported by Grant GV2008/111035 from the Basque Department of Health and BIO08/ER/001. This group is founded by the Centro de Investigacion Biomedica en Red de Enfermedades Raras (CIBERER), Instituto de Salud Carlos III. This work was also funded, in part, by Grant R01DK073911 from the National Institute of Diabetes and Digestive and Kidney Diseases (to M.B.).
Current address for E.F.-R.: Endocrine Unit, Department of Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 11, 2009
Abbreviations: aCGH, ArrayCGH; AHO, Albright’s hereditary osteodystrophy; CGH, Comparative genome hybridization; ΔCt, change in cycle threshold; Gsα, α-subunit of the stimulatory G protein; NAHR, nonallelic homologous recombination; PHP, pseudohypoparathyroidism; qPCR, quantitative PCR; SNP, single-nucleotide polymorphism.
References
- Albright F, Burnett CH, Smith PH, Parson W 1942 Pseudohypoparathyroidsm: an example of “Seabright syndrome.” Endocrinology 30:922–932 [Google Scholar]
- Kozasa T, Itoh H, Tsukamoto T, Kaziro Y 1988 Isolation and characterization of the human Gs alpha gene. Proc Natl Acad Sci USA 85:2081–2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein LS, Yu S, Warner DR, Liu J 2001 Endocrine manifestations of stimulatory G protein α-subunit mutations and the role of genomic imprinting. Endocr Rev 22:675–705 [DOI] [PubMed] [Google Scholar]
- Wemeau JL, Balavoine AS, Ladsous M, Velayoudom-Cephise FL, Vlaeminck-Guillem V 2006 Multihormonal resistance to parathyroid hormone, thyroid stimulating hormone, and other hormonal and neurosensory stimuli in patients with pseudohypoparathyroidism. J Pediatr Endocrinol Metab 19(Suppl 2):653–661 [DOI] [PubMed] [Google Scholar]
- Weinstein LS, Gejman PV, Friedman E, Kadowaki T, Collins RM, Gershon ES, Spiegel AM 1990 Mutations of the Gs α-subunit gene in Albright hereditary osteodystrophy detected by denaturing gradient gel electrophoresis. Proc Natl Acad Sci USA 87:8287–8290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten JL, Johns DR, Valle D, Eil C, Gruppuso PA, Steele G, Smallwood PM, Levine MA 1990 Mutation in the gene encoding the stimulatory G protein of adenylate cyclase in Albright’s hereditary osteodystrophy. N Engl J Med 322:1412–1419 [DOI] [PubMed] [Google Scholar]
- Aldred MA, Aftimos S, Hall C, Waters KS, Thakker RV, Trembath RC, Brueton L 2002 Constitutional deletion of chromosome 20q in two patients affected with Albright hereditary osteodystrophy. Am J Med Genet 113:167–172 [DOI] [PubMed] [Google Scholar]
- Fernandez-Rebollo E, Barrio R, Perez-Nanclares G, Carcavilla A, Garin I, Castano L, de Nanclares GP 2008 New mutation type in pseudohypoparathyroidism type Ia. Clin Endocrinol (Oxf) 69:705–712 [DOI] [PubMed] [Google Scholar]
- Davies SJ, Hughes HE 1993 Imprinting in Albright’s hereditary osteodystrophy. J Med Genet 30:101–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LC, Oude Luttikhuis ME, Clayton PT, Fraser WD, Trembath RC 1994 Parental origin of Gsα gene mutations in Albright’s hereditary osteodystrophy. J Med Genet 31:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MA, Downs Jr RW, Moses AM, Breslau NA, Marx SJ, Lasker RD, Rizzoli RE, Aurbach GD, Spiegel AM 1983 Resistance to multiple hormones in patients with pseudohypoparathyroidism. Association with deficient activity of guanine nucleotide regulatory protein. Am J Med 74:545–556 [DOI] [PubMed] [Google Scholar]
- Liu J, Litman D, Rosenberg MJ, Yu S, Biesecker LG, Weinstein LS 2000 A GNAS1 imprinting defect in pseudohypoparathyroidism type IB. J Clin Invest 106:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastepe M, Pincus JE, Sugimoto T, Tojo K, Kanatani M, Azuma Y, Kruse K, Rosenbloom AL, Koshiyama H, Jüppner H 2001 Positional dissociation between the genetic mutation responsible for pseudohypoparathyroidism type Ib and the associated methylation defect at exon A/B: evidence for a long-range regulatory element within the imprinted GNAS1 locus. Hum Mol Genet 10:1231–1241 [DOI] [PubMed] [Google Scholar]
- Bastepe M, Fröhlich LF, Hendy GN, Indridason OS, Josse RG, Koshiyama H, Körkkö J, Nakamoto JM, Rosenbloom AL, Slyper AH, Sugimoto T, Tsatsoulis A, Crawford JD, Jüppner H 2003 Autosomal dominant pseudohypoparathyroidism type Ib is associated with a heterozygous microdeletion that likely disrupts a putative imprinting control element of GNAS. J Clin Invest 112:1255–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linglart A, Gensure RC, Olney RC, Jüppner H, Bastepe M 2005 A novel STX16 deletion in autosomal dominant pseudohypoparathyroidism type Ib redefines the boundaries of a cis-acting imprinting control element of GNAS. Am J Hum Genet 76:804–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastepe M, Fröhlich LF, Linglart A, Abu-Zahra HS, Tojo K, Ward LM, Jüppner H 2005 Deletion of the NESP55 differentially methylated region causes loss of maternal GNAS imprints and pseudohypoparathyroidism type Ib. Nat Genet 37:25–27 [DOI] [PubMed] [Google Scholar]
- de Nanclares GP, Fernández-Rebollo E, Santin I, García-Cuartero B, Gaztambide S, Menéndez E, Morales MJ, Pombo M, Bilbao JR, Barros F, Zazo N, Ahrens W, Jüppner H, Hiort O, Castaño L, Bastepe M 2007 Epigenetic defects of GNAS in patients with pseudohypoparathyroidism and mild features of Albright’s hereditary osteodystrophy. J Clin Endocrinol Metab 92:2370–2373 [DOI] [PubMed] [Google Scholar]
- Greulich WW, Pyle SI 1959 Radiographic atlas of skeletal development of the hand and wrist. 2nd ed. Stanford, CA: Stanford University Press; 125–183 [Google Scholar]
- Barrett MT, Scheffer A, Ben-Dor A, Sampas N, Lipson D, Kincaid R, Tsang P, Curry B, Baird K, Meltzer PS, Yakhini Z, Bruhn L, Laderman S 2004 Comparative genomic hybridization using oligonucleotide microarrays and total genomic DNA. Proc Natl Acad Sci USA 101:17765–17770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde L, Montaner D, Burguet-Castell J, Tárraga J, Medina I, Al-Shahrour F, Dopazo J 2007 ISACGH: a web-based environment for the analysis of Array CGH and gene expression which includes functional profiling. Nucleic Acids Res 35:W81–W85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Uriarte R, Rueda OM 2007 ADaCGH: a parallelized web-based application and R package for the analysis of aCGH data. PLoS ONE 2:e737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzinger DG 2002 Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol 30:503–512 [DOI] [PubMed] [Google Scholar]
- Yatsenko SA, Brundage EK, Roney EK, Cheung SW, Chinault AC, Lupski JR 2009 Molecular mechanisms for subtelomeric rearrangements associated with the 9q34.3 microdeletion syndrome. Hum Mol Genet 18:1924–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger PL, Batzer MA 1999 Alu repeats and human disease. Mol Genet Metab 67:183–193 [DOI] [PubMed] [Google Scholar]
- Shaw CJ, Lupski JR 2004 Implications of human genome architecture for rearrangement-based disorders: the genomic basis of disease. Hum Mol Genet 13(Spec 1):R57–R64 [DOI] [PubMed] [Google Scholar]
- Emanuel BS 2008 Molecular mechanisms and diagnosis of chromosome 22q11.2 rearrangements. Dev Disabil Res Rev 14:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel DH, Sybert VP 2006 Mosaicism in genetic skin disorders. Pediatr Dermatol 23:87–92 [DOI] [PubMed] [Google Scholar]
- Bernards A, Gusella JF 1994 The importance of genetic mosaicism in human disease. N Engl J Med 331:1447–1449 [DOI] [PubMed] [Google Scholar]