Abstract
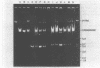
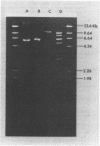
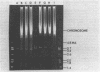
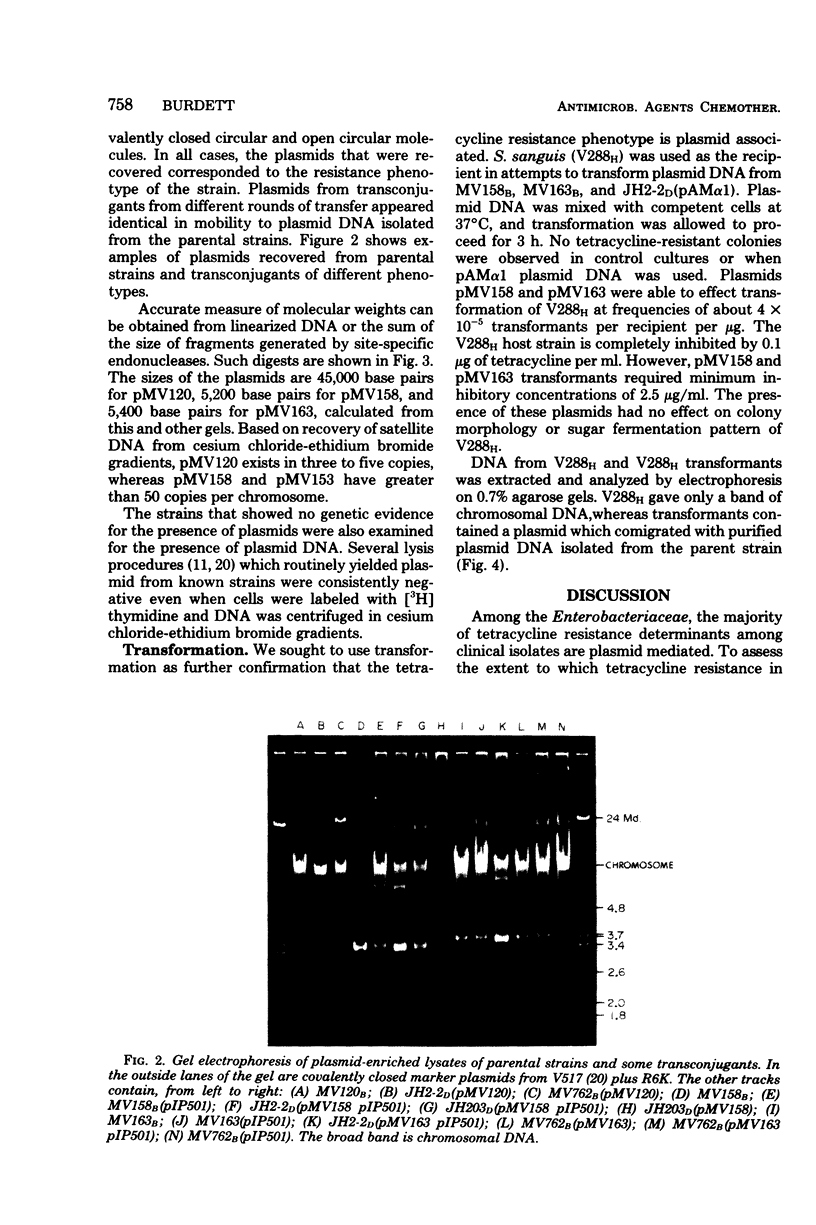
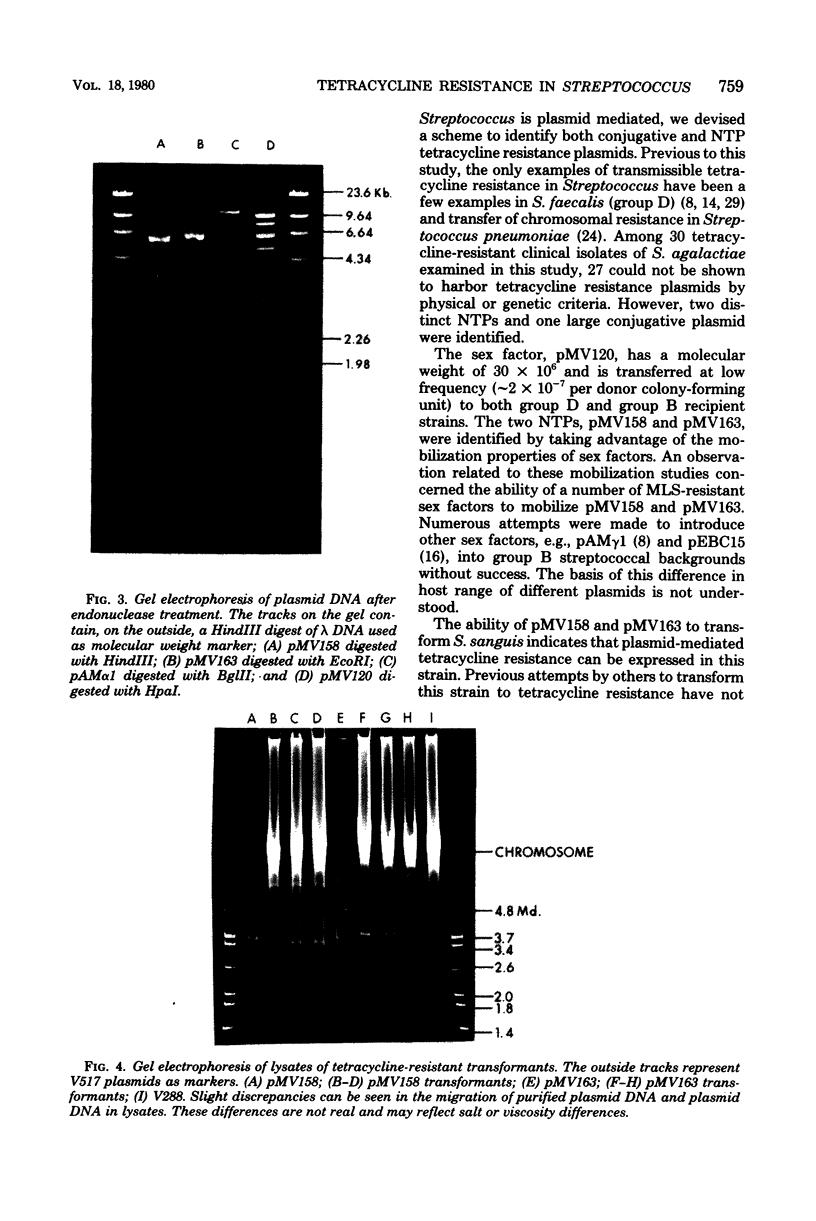
In this report, 30 tetracycline-resistant clinical isolates of group B Streptococcus were examined to assess the extent to which tetracycline resistance is plasmid mediated. Of these, 27 showed no physical or genetic evidence of plasmid-mediated resistance; however, one conjugative and two small (3.5 X 10(6)-dalton) multicopy non-self-transmissible tetracycline resistance plasmids were identified. The conjugative plasmid was transmissible to Streptococcus faecalis as well as to Streptococcus agalactiae (group B). The two nonconjugative plasmids were readily mobilized by a number of sex factors into these same two backgrounds and, in addition, readily transformed Streptococcus sanguis Challis to tetracycline resistance. Due to readily available sites for several site-specific endonuycleases, these small, multicopy plasmids should prove useful as cloning vehicles in this host system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony B. F., Concepcion N. F. Group B streptococcus in a general hospital. J Infect Dis. 1975 Nov;132(5):561–567. doi: 10.1093/infdis/132.5.561. [DOI] [PubMed] [Google Scholar]
- Baker C. J., Webb B. J., Barrett F. F. Antimicrobial susceptibility of group B streptococci isolated from a variety of clinical sources. Antimicrob Agents Chemother. 1976 Jul;10(1):128–131. doi: 10.1128/aac.10.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Croissant O., Blangy D. Identification of two plasmids determining resistance to tetracycline and to erythromycin in group D streptococcus. Mol Gen Genet. 1974;132(3):181–192. doi: 10.1007/BF00269391. [DOI] [PubMed] [Google Scholar]
- Courvalin P. M., Shaw W. V., Jacob A. E. Plasmid-mediated mechanisms of resistance to aminoglycoside-aminocyclitol antibiotics and to chloramphenicol in group D streptococci. Antimicrob Agents Chemother. 1978 May;13(5):716–725. doi: 10.1128/aac.13.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. M., Lipinski A. E. Infections with beta-Hemolytic Streptococcus resistant to lincomycin and erythromycin and observations on zonal-pattern resistance to lincomycin. J Infect Dis. 1974 Oct;130(4):351–356. doi: 10.1093/infdis/130.4.351. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Clewell D. B. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J Bacteriol. 1975 Nov;124(2):784–790. doi: 10.1128/jb.124.2.784-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICKHOFF T. C., KLEIN J. O., DALY A. K., INGALL D., FINLAND M. NEONATAL SEPSIS AND OTHER INFECTIONS DUE TO GROUP B BETA-HEMOLYTIC STREPTOCOCCI. N Engl J Med. 1964 Dec 10;271:1221–1228. doi: 10.1056/NEJM196412102712401. [DOI] [PubMed] [Google Scholar]
- Hershfield V. Plasmids mediating multiple drug resistance in group B streptococcus: transferability and molecular properties. Plasmid. 1979 Jan;2(1):137–149. doi: 10.1016/0147-619x(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bouanchaud D. H., Chabbert Y. A. Conjugative R plasmids in Streptococcus agalactiae (group B). Plasmid. 1979 Apr;2(2):197–206. doi: 10.1016/0147-619x(79)90038-6. [DOI] [PubMed] [Google Scholar]
- Hyder S. L., Streitfeld M. M. Inducible and constitutive resistance to macrolide antibiotics and lincomycin in clinically isolated strains of Streptococcus pyogenes. Antimicrob Agents Chemother. 1973 Sep;4(3):327–331. doi: 10.1128/aac.4.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES W. F., Jr, FELDMAN H. A., FINLAND M. Susceptibility of hemolytic streptococci, other than those of group D, to eleven antibiotics in vitro. Am J Clin Pathol. 1957 Feb;27(2):159–169. doi: 10.1093/ajcp/27.2.159. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Korfhagen T. R., Moellering R. C., Jr, Wennersten C., Swartz M. N. Plasmid-mediated resistance to antibiotic synergism in enterococci. J Clin Invest. 1978 Jun;61(6):1645–1653. doi: 10.1172/JCI109085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson J. W., Gooder H. Growth and development of competence in the group H streptococci. J Bacteriol. 1970 Jun;102(3):820–825. doi: 10.1128/jb.102.3.820-825.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc D. J., Hassell F. P. Transformation of Streptococcus sanguis Challis by plasmid deoxyribonucleic acid from Streptococcus faecalis. J Bacteriol. 1976 Oct;128(1):347–355. doi: 10.1128/jb.128.1.347-355.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Reider J. L., Virgili S. S., Kopecko D. J. Survey of the extrachromosomal gene pool of Streptococcus mutans. Infect Immun. 1977 Jul;17(1):215–226. doi: 10.1128/iai.17.1.215-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y., Takizawa K., Matsushima A., Asai Y., Nakatsuka S. Stepwise acquisition of multiple drug resistance by beta-hemolytic streptococci and difference in resistance pattern by type. Antimicrob Agents Chemother. 1978 Mar;13(3):399–404. doi: 10.1128/aac.13.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955 May;13(2):290–291. [PubMed] [Google Scholar]
- Shoemaker N. B., Smith M. D., Guild W. R. DNase-resistant transfer of chromosomal cat and tet insertions by filter mating in Pneumococcus. Plasmid. 1980 Jan;3(1):80–87. doi: 10.1016/s0147-619x(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Shoemaker N. B., Burdett V., Guild W. R. Transfer of plasmids by conjugation in Streptococcus pneumonias. Plasmid. 1980 Jan;3(1):70–79. doi: 10.1016/s0147-619x(80)90035-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Toala P., McDonald A., Wilcox C., Finland M. Susceptibility of group D streptococcus (enterococcus) to 21 antibiotics in vitro, with special reference to species differences. Am J Med Sci. 1969 Dec;258(6):416–430. doi: 10.1097/00000441-196912000-00006. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Holder S. B., Halling S. M. Deoxyribonucleic acid sequence common to staphylococcal and streptococcal plasmids which specify erythromycin resistance. J Bacteriol. 1979 Jun;138(3):990–998. doi: 10.1128/jb.138.3.990-998.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Embden J. D., Engel H. W., van Klingeren B. Drug resistance in group D streptococci of clinical and nonclinical origin: prevalence, transferability, and plasmid properties. Antimicrob Agents Chemother. 1977 Jun;11(6):925–932. doi: 10.1128/aac.11.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]