Abstract
Context: Reduced longevity observed in hypopituitarism has been attributed to GH deficiency (GHD). It is, however, unclear whether GHD or other confounding factors cause this early mortality.
Objective: The aim was to study longevity in subjects from a large kindred with untreated, lifetime isolated GHD (IGHD) due to a homozygous mutation in the GHRH receptor gene and in heterozygous carriers of the mutation.
Design, Setting, and Participants: We carried out a retrospective cohort study on three groups. We first compared mortality risk of 65 IGHD individuals and their 128 unaffected siblings from 34 families. We then compared mean age of death of the IGHD to the general population. A transversal study was carried out to compare the rate of heterozygosity for the mutation in two groups of young (20–40 yr old) and old (60–80 yr old) normal-appearing subjects from the same county.
Main Outcome Measure: We measured longevity.
Results: The risk of death of IGHD subjects was not different from their siblings. Life span in IGHD individuals was shorter than the general population. When stratified by sex, this difference persisted only in females, due to a high frequency of IGHD deaths in females aged 4–20. There was no significant difference in life span between IGHD subjects and siblings or the general population when analyzing subjects who reached age 20. The prevalence of heterozygosity did not differ in young and old groups, suggesting no survival advantage or disadvantage.
Conclusions: In a selected genetic background, lifelong untreated IGHD does not affect longevity.
Growth hormone deficiency does not affect longevity.
Human longevity results from a multifactorial interaction between environment and genetics. Genetic factors are thought to be responsible for approximately 20 to 30% of life span (1) with several candidate genes identified over the years. One system involved in longevity is the GH-IGF-I/insulin pathways. Disruption of the insulin signaling is associated with increased life expectancy in nematodes (2), yeast (3), and insects (4). In higher organisms that have a separate GH-IGF-I axis, like mice (5,6,7), dogs (8), and possibly humans (9,10), disruption of this axis at different levels also causes increased life span. Indeed, very recently Suh et al. (11) have shown in centenarians an overrepresentation of heterozygous mutations in the IGF-I receptor gene that cause reduction of intracellular signaling, confirming that genetic alterations in this system can confer increased longevity.
On the other end, aging is associated with a progressive reduction of GH secretion and serum IGF-I, a process often called “somatopause.” Furthermore, GH deficiency (GHD) in young adults causes changes that are strikingly similar to the ones observed during aging: reduction in muscle mass and strength, bone mineral density, and quality of life; increased abdominal adiposity, insulin resistance, and total and low-density lipoprotein (LDL)-cholesterol (12). GH therapy can revert some of the changes associated with aging and many of the features of adult GHD (13). Furthermore, in a landmark study, Rosén et al. (14) reported increased vascular mortality in hypopituitary patients with likely but nonproven GHD.
Hence, there is a paradox that, whereas some studies show that dampening of the GH-IGF axis increases longevity, GHD is associated with features commonly seen in aging and increased mortality. Because most cases of GHD occur in panhypopituitary patients who have often undergone pituitary surgery and/or radiation, it remains yet unclear whether this increased cardiovascular mortality results from untreated GHD or from these confounding factors. Definitive studies on the effects of GHD should be performed in patients with isolated GHD (IGHD), but this disorder is rare and most often treated. We have identified a large extended pedigree with more than 100 individuals (over several generations) affected by familial IGHD and residing in Itabaianinha County, a rural area in the northeastern Brazilian State of Sergipe. They all carry a homozygous null mutation (IVS1 + 1G→A) in the GHRH receptor gene (GHRH-R) (15). Recently, we have reported that heterozygous carriers of this mutation have normal height but reduction in body weight and increase of insulin sensitivity that may also influence longevity (16).
The purpose of this work is to study the longevity in both homozygous and heterozygous carriers of the Itabaianinha GHRHR mutation.
Subjects and Methods
Longevity in IGHD individuals
Working in this community for 15 yr, we have identified a large number of IGHD subjects. Their adult phenotype is unmistakable, with final stature ranging from 117 to 137 cm in males and from 107 to 126 cm in females, peculiar facial appearance, and characteristically high pitched voice. All the living IGHD individuals were genotyped and proven to be homozygous for the IVS1 + 1G→A GHRH-R mutation. For the deceased subjects who could not be genetically tested, we interviewed family members to rule out the presence of dysmorphic features that may indicate achondroplasia or other chondrodysplasias that may cause non-GH-related dwarfism. We are confident that the high degree of familiarity of the Itabaianinha population with the typical phenotype of untreated IGHD would have allowed us to determine from history whether any atypical feature was present. No subjects with dysmorphic features were identified.
To study longevity in IGHD individuals, we collected the date of birth of all the living IGHD individuals from Itabaianinha and their unaffected siblings, and the date and cause of death (categorized in cancer, cardiovascular, external, infectious, and unknown groups) for those who have died. Children aged less than 4 yr were not included due the difficulty of the diagnosis of IGHD before this age by physical exam (growth failure starts in the first year of life, but it becomes more evident as children age). Data were obtained from birth certificates, identity cards, and death certificates. When such documents were not retrievable, a verbal questionnaire was administered to family members. The cause of death was deemed to be “unknown” unless there was a certificate of death or other medical documents, such as hospital records, medications prescription, or previous reports of diseases. We identified 75 IGHD individuals, 53 alive and 22 deceased, who were born between 1892 and 1997. Because the dwarfism in Itabaianinha has been documented for approximately 200 yr, data of some individuals who died in the remote past could not be retrieved.
For 10 of the deceased IGHD individuals, it was not possible to identify any sibling. Analyses comparing the IGHD to their siblings are from 34 families with 65 IGHD individuals (53 alive and 12 dead) and 129 unaffected siblings (112 alive and 17 dead).
We also collected death certificates of individuals from the general population from 1964 (the year of the first registered IGHD death) to 2006 (the year of the latest IGHD death). In this period, 22 IGHD deaths (the 12 included in the comparison between GHD and siblings, and the 10 excluded from that analysis) and 4266 deaths in the general population were registered. For the comparison between GHD and the general population, we selected individuals matched by sex from the general population who were born in the same years as the 22 deceased IGHD subjects, forming a group of 522 individuals.
Statistical methods
Comparison of GHD subjects and unaffected siblings
A retrospective cohort study was performed to compare Kaplan-Meier survival curves for the GHD and their siblings for all individuals and stratified by sex. Curves were constructed for all ages and separately for those who survived to age 20. Survival curves were compared between groups with a log rank statistic, and mortality risk was quantified with hazard ratios (HRs) and 95% confidence intervals. For this comparison, a robust se was estimated to account for the clustering within families. Life span (defined as age of death) was compared between deceased individuals from both groups for all individuals and stratified by sex with a linear regression model that used generalized estimating equations to account for the correlation among individuals from the same family.
Comparison of GHD subjects and the general population
Age of death was categorized into five groups: 4–20, 21–40, 41–60, 61–80, and over 80 yr. The frequencies of deaths within these five age categories and differences in causes of death were compared between the IGHD individuals and the general population using Fisher’s exact test. Life span was analyzed and compared between all individuals and also stratified by sex with a t test. All analyses were performed with statistical software R v2.9.0 (www.rproject.org).
Longevity in the heterozygous carriers of the mutation
Because heterozygosity is not associated with an easily identifiable phenotype, we needed to use a different approach than the one we used for the IGHD subjects. A transversal study was carried out to compare the rate of heterozygosity for the GHRH-R gene mutation in two groups of normal-appearing subjects of different ages—young (20–40 yr) and old (60 to 80 yr) residing in Itabaianinha County. We reasoned that, if heterozygosis changes life expectancy, the prevalence of the mutation will be different in the two groups. This approach can be used in this population because their mobility is close to zero. To avoid the influence of this allele to be buffered by other genes that may strongly influence life span, we did not include individuals with an extremely long life span (>80 yr). Based on the fact that there are at least 70 live homozygous IGHD-affected subjects in a population of 32,000 (incidence 2.1/1000), the predicted number of heterozygous individuals calculated with the Hardy-Weinberg formula (p2 + 2pq + q2 = 1) is 2852 (8.9%). We calculated that, even if the prevalence of heterozygosity was 7.5%, to detect a 7.5% difference in prevalence of the mutated allele with α = 0.05 and a power of 0.8 we would have needed to genotype 278 subjects in each group. To avoid overrepresentation of heterozygous subjects, we did not include subjects who are first- or second-degree relatives of a GHD subject. Advertising by broadcast and loud speakers, we invited people to volunteer on the day of the October 2008 municipal election. A total of 793 subjects volunteered, 456 young (233 males and 223 females) and 340 old (183 males and 144 females). They were all genotyped from buccal swabs.
Genotyping of IVS1 + 1G→A mutation (rs2302022) was performed using predesigned TaqMan SNP Genotyping Assay C_15757069_10 (Applied Biosystems, Foster City, CA). PCR and endpoint detection of fluorescence was carried out in an ABI Prism 7900HT Sequence Detection System (Applied Biosystems) following the manufacturer’s supplied protocols. Genotyping was successful in all the subjects except three old ones, bringing their number to 337. A χ2 test was used to compare the rates of the IVS1 + 1G→A heterozygous change (A/G) and of the normal homozygous (G/G) in the young and old groups.
Institutional Review Boards of both the Johns Hopkins University and the Federal University of Sergipe approved the protocol. Written informed consent was obtained from all the patients who underwent genotyping.
Results
Longevity in GHD individuals
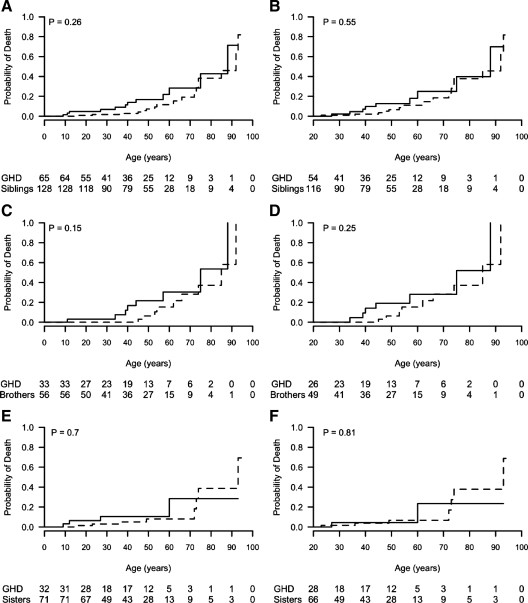
The risk of death was not significantly different between IGHD and their siblings at all ages [HR = 0.61 (95% confidence interval, 0.27, 1.42); log rank P = 0.26]. Kaplan-Meier survival curves comparing IGHD subjects and their siblings (males and females together) at all ages are shown in Fig. 1A (log rank P = 0.26) and from age 20 in Fig 1B (log rank P = 0.55); for male IGHD and brothers at all ages in Fig. 1C (log rank P = 0.15) and from 20 yr in Fig. 1D (log rank P = 0.25); for female IGHD and sisters at all ages in Fig. 1E (log rank P = 0.7) and from 20 yr in Fig. 1F (log rank P = 0.81). The oldest death of an IGHD individual occurred at age 88. As of 2006, there were seven living IGHD subjects in Itabaianinha aged above 70 yr, including an 84-yr-old male and a 93-yr-old female.
Figure 1.
Kaplan-Meier survival curves. Solid lines, IGHD subjects; dotted lines, unaffected siblings. A and B, Both sexes; C and D, males; E and F, females. A, C, and E, Individuals of all ages; B, D, and F, individuals who reached age 20. Values under each graph are the numbers at risk by group.
The comparison of the frequency of deaths in IGHD individuals and the general population, grouped by age of death (Table 1), showed a high frequency of IGHD deaths in females aged 4–20. Table 2 shows life span, defined as age of death among deceased individuals, for the whole group of IGHD individuals, their unaffected siblings, and the general population, stratified by sex, and among all individuals and those who reached age 20. We found that IGHD females have a shorter life span compared with the general population, but not to their unaffected sisters. Male IGHD have a shorter life span than unaffected brothers. However, when we looked at subjects who reached age 20, we observed no difference in life span between IGHD subjects and their siblings or the general population. There were no significant differences in the causes of death between IGHD and the general population (Table 3).
Table 1.
Frequency of death in IGHD individuals and the general population, grouped by age of death
| Age groups (yr)
|
P value | |||||
|---|---|---|---|---|---|---|
| 4–20 | 21–40 | 41–60 | 61–80 | >80 | ||
| All | ||||||
| IGHD | 5 (22.7) | 4 (18.2) | 5 (22.7) | 7 (31.8) | 1 (4.5) | 0.01 |
| General population | 23 (4.4) | 70 (13.4) | 107 (20.5) | 211 (40.4) | 111 (21.3) | |
| Males | ||||||
| IGHD | 1 (7.7) | 3 (23.1) | 4 (30.8) | 4 (30.8) | 1 (7.7) | 0.46 |
| General population | 16 (4.8) | 44 (13.1) | 78 (23.3) | 127 (37.9) | 70 (20.9) | |
| Females | ||||||
| IGHD | 4 (44.4) | 1 (11.1) | 1 (11.1) | 3 (33.3) | 0 (0) | <0.01 |
| General population | 7 (3.7) | 26 (13.9) | 29 (15.5) | 84 (44.9) | 41 (21.9) | |
Frequencies are presented for all individuals, and separately for males and females. Values represent number (percentage). P values are calculated by Fisher’s Exact test.
Table 2.
Life span of IGHD individuals, their siblings and individuals from the general population who were born in the same years as the IGHD
| n | Life span (yr) Mean (median, range) | P | |
|---|---|---|---|
| All individuals | |||
| Affected IGHD (both genders) | 12 | 41.3 (40, 9–88) | |
| Unaffected siblings | 17 | 59.7 (62, 16–93) | 0.08 |
| Affected IGHD (both genders) | 22 | 47.7 (54, 9–88) | |
| Unaffected population | 522 | 63.3 (70, 6–103) | 0.01 |
| Affected IGHD males | 8 | 48.5 (42, 11–88) | |
| Unaffected brothers | 9 | 64.3 (62, 45–92) | <0.01 |
| Affected IGHD males | 13 | 54 (57, 11–88) | |
| Unaffected males (population) | 335 | 62.3 (68, 6–100) | 0.18 |
| Affected IGHD females | 4 | 27 (20, 9–60) | |
| Unaffected sisters | 8 | 54.5 (60, 16–93) | 0.22 |
| Affected IGHD females | 9 | 38.6 (27, 9–71) | |
| Unaffected females (population) | 187 | 65.1 (71, 12–103) | 0.02 |
| Individuals aged 20 and older | |||
| Affected IGHD (both genders) | 9 | 51.6 (44, 27–88) | |
| Unaffected siblings | 16 | 62.4 (64, 23–83) | 0.27 |
| Affected IGHD (both genders) | 17 | 57.6 (60, 27–88) | |
| Unaffected (population) | 499 | 65.5 (71, 21–103) | 0.79 |
| Affected IGHD males | 7 | 53.9 (44, 34–88) | |
| Unaffected brothers | 9 | 64.3 (62, 45–92) | a |
| Affected IGHD males | 12 | 57.6 (57, 34–88) | |
| Unaffected males (population) | 319 | 64.7 (69, 21–100) | 0.18 |
| Affected IGHD females | 2 | 43.5 (44, 27–60) | |
| Unaffected sisters | 7 | 60 (72, 23–93) | a |
| Affected IGHD females | 5 | 57.8 (65, 27–71) | |
| Unaffected females (population) | 180 | 67 (72, 22–103) | 0.31 |
The comparison of life span between IGHD and the population includes all 22 IGHD deaths, whereas the comparison between IGHD and siblings includes 12 IGHD deaths.
Not enough deaths in IGHD subjects to calculate this difference.
Table 3.
Causes of death of the IGHD individuals and the general population
| GHD | General population | |
|---|---|---|
| Cancer | 1 (11) | 8 (10) |
| Cardiovascular | 2 (22) | 29 (37) |
| External | 3 (33) | 33 (41.7) |
| Infectious | 1 (11) | 2 (2.5) |
| Other | 2 (22) | 7 (8.8) |
| Total | 9 (100) | 79 (100) |
Values are expressed as number (percentage). The P value by Fisher’s exact test was not significant.
Longevity in heterozygous carriers
The prevalence of the heterozygosity for the IVS1 + 1G→A GHRH-R mutation was lower than what we had expected by calculation and did not differ in young (4.16%; 10 males and nine females) and old individuals (5.04%; seven males and 10 females). With prevalence of 4.16% in the younger subjects, we had 0.8 power to detect a difference of 5.24%. These results suggest that heterozygosity for this mutation is not associated with changes in longevity.
Discussion
The question of whether GHD is associated with increased or decreased mortality is highly debated. Rosén’s report (14) of increased mortality in hypopituitary patients was interpreted by some investigators as an indication of the detrimental effects of GHD on life expectancy. Subsequently, Besson et al. (17) studied the life span of a family with several subjects with untreated congenital IGHD due to GH-1 gene deletion (causing complete absence of GH) that lived in the 19th and 20th centuries and found that IGHD was associated with reduced life expectancy. However, other studies, including a large epidemiological study of 1014 hypopituitary patients (18), did not find evidence of GHD association with increased mortality, although this may also have been due to the fact that only 115 of the patients had been tested for GHD. In the latter study, independent risk factors for excess mortality were female sex, craniopharyngioma, and untreated gonadotropin deficiency. Furthermore, most animal studies (2,3,4,5,6,7,8) associate GHD or GH resistance with increased longevity, and reduced IGF-I signaling has been shown in human centenarians (11).
The only way to answer the question of whether untreated GHD influences longevity in humans is provided by experiments of nature, as done previously by Besson et al. (17). One of the largest ones is the IGHD kindred of Itabaianinha. The affected individuals represent the human equivalent of animal models of IGHD that scientists have worked hard to develop. Here we demonstrate that the risk of death is not significantly different between the Itabaianinha kindred IGHD subjects and their normal-stature siblings. The main difference between the data reported by us and by Besson et al. (17) and some of those published so far is that the IGHD individuals in Besson’s and our report have severe, lifelong, and untreated IGHD, whereas previously reported epidemiological studies mostly included patients with hypopituitarism after surgery and/or radiotherapy for pituitary tumors, with likely variable degrees of GHD (14,19,20,21). A nationwide Danish study (21) included 1794 hypopituitary individuals, mostly with acquired, adult-onset GHD. They found that mortality was increased in childhood-onset GHD (HR = 8.3 in males and 9.4 in females) due to cancer, and in adult-onset GHD (HR = 1.9 in males and 3.4 in females) due to cancer in both genders and to circulatory diseases in females and in males above 65 yr of age. It is possible that the increased rate of malignancy-related deaths can be explained by factors other than their hypopituitarism, like pituitary surgery and radiation (22), multiple neoplasias (20), higher risk of second cancer (23), and a mitogenic role of GH treatment, especially with older high-dosage regimens (24). The modest albeit significant increase in vascular mortality was restricted to women and older men, and the authors emphasized that a “conclusion on the impact of GHD per se on mortality is not feasible.” As a whole, the previous published literature does not firmly support the concept that GHD reduces longevity, because it is well known that the suboptimal (or excessive) replacement of other hormones (glucocorticoid, thyroid, and sex hormones) can influence several metabolic parameters that can impact vascular risk.
On the other hand, this consideration cannot be applied to the subjects reported by Besson et al. who also had IGHD. The reason for different findings in the two populations can only be speculated. Differently from subjects with large homozygous deletions in the GH-1 gene reported by Besson et al. (17), our subjects have measurable (albeit very low) serum GH levels (15). Furthermore, different genetic backgrounds (possibly associated with modifying genes) and very different environment and food intake (mountains of Switzerland vs. tropical northeast Brazil), and different historical period of observation are all factors likely to interact with the GHD status in determining longevity.
Our data show that a minimal GH secretion since birth can be enough to attain normal longevity. However, the life span of our IGHD individuals is shorter than the general population, but this difference is driven by the high frequency of early deaths (before age 20) in female IGHD. Once IGHD individuals reach adulthood, there is no difference in their life span compared with siblings or the general population, suggesting that IGHD is not a risk factor for cardiovascular mortality in middle or advanced age. It has to be pointed out that we may have missed IGHD deaths that occurred in very young IGHD individuals, before the phenotype of short stature could be clearly evident. Such additional deaths would not alter our conclusion of normal life expectancy once adulthood is reached.
The gender discrepancy in deaths was also reported in IGHD subjects (17), and in two studies of patients with hypopituitarism (18,21). It was hypothesized that simultaneous lack of GH and estrogen could explain the increase in vascular deaths. Because the excess death of our cohort was in females under age 20, and the age of menarche IGHD women of Itabaianinha is delayed (17.6 ± 2.2 yr) (25), one could also advocate a role of the simultaneous lack of GH and estrogen. However, when we looked at the causes of these five early deaths, one official certificate reported an infectious cause, but verbal reports from family members suggested dehydration due to diarrheal diseases in the remainders. Therefore, vascular risk is unlikely to be involved. Although IGHD individuals from Itabaianinha do not present hypoglycemia in infancy (26), one could hypothesize that the lack of GH may make these subjects more vulnerable to the effects of diarrhea-induced dehydration. Furthermore, the GH/IGF-I axis affects the immune system by increasing lymphopoiesis and granulopoiesis and by inhibiting apoptosis of granulocytes (27). Interestingly, body surface area-corrected spleen volume is reduced in these IGHD individuals (28), suggesting that immune function may be influenced by severe lack of GH. Why this excess death occurs more in females than in males remains unclear. Given the excess mortality in young females, the adult female cohort includes survivors of the early death risk.
When we looked at the causes of death, we found no significant difference between IGHD and the general population. Although the accuracy of these data may be suboptimal, it is noteworthy to underline the similar rate of cardiovascular deaths between IGHD and the general population, despite the fact that IGHD individuals are exposed from childhood to cardiovascular risk factors such as increased abdominal adiposity, increased serum LDL cholesterol and C-reactive protein, and elevated systolic blood pressure in adults (29,30,31). The Bogalusa Heart Study showed that childhood obesity and increase in LDL cholesterol are predictors of carotid atherosclerosis and coronary events (32), but this does not seem to apply to IGHD individuals (33).
Although we have only one cancer death in the IGHD group, the rate of cancer death in IGHD individuals was not different from the general population. Several reports have shown an epidemiological association between activity of the GH/IGF-I axis and neoplasms of breast (34), cervix (35), prostate (36), and colon (37). Furthermore, recently no cases of cancer were registered in 222 congenital IGF-I-deficient individuals aged 3 to 78 yr, whereas 9 to 24% of their member families had a malignancy (38), suggesting that congenital IGF-I deficiency can protect against the appearance of cancer. In Rosén’s paper (14), seven hypopituitary patients (three males and four females) died from cancer (expected 10.1 and 4.1; P < 0.02 and P < 0.056), also suggesting that the mortality for cancer in their hypopituitary group was lower than expected. Our report of one confirmed cancer death in an individual with congenital IGHD shows that the protection against cancer by GHD is not absolute.
An inverse correlation between body size and longevity has been reported in several animal species (2,3,4,5,6,7,8,9,10). These findings are difficult to reconcile with the disabling manifestations of somatopause. Possible explanations are related to the divergent physiological roles of GH during different stages of life. Long-term survival presumably confers little or no reproductive, and thus evolutionary, advantage. Robust somatotropic signaling early in life may have been selected to guarantee sexual maturation and reproductive fitness, regardless of the “costs” in terms of longevity and age-related disease (5). Our IGHD patients balance a satisfactory reproductive life, although moderately shortened in females (25), with a normal longevity. They exhibit throughout life severe reduction of serum IGF-I and IGF-I binding protein type 3 (IGFBP-3), such that the ratio of total IGF to IGFBP-3 is higher than controls (39). A similar pattern was found in healthy centenarians (40). A high plasma IGF-I/IGFBP-3 molar ratio might improve insulin action and plasma lipid concentration in centenarians or counteract the adverse lipid profile in IGHD.
Because adult heterozygous have normal height and serum IGF-I levels, their mild phenotype might reflect a reduction in GH secretion not sufficient to impair final height, but sufficient to improve insulin sensitivity (16). Because insulin sensitivity is a hallmark of human centenarians (40), we had hypothesized that heterozygous individuals may live longer than the homozygous normal subjects and that we would find a higher percentage of heterozygous subjects in older individuals. However, heterozygous carriers are equally represented in young and older age groups, suggesting no effect of monoallelic GHRH-R mutation on longevity.
Our study has obvious limitations due to its retrospective nature and to the relatively low number of subjects, particularly when compared with previously published population-based studies of hypopituitary patients (14,18,19). It is therefore possible that we lacked the power to detect a small increase in mortality. Nevertheless, we believe that this unique population provides important additional information on the interaction between IGHD and life expectancy.
In conclusion, differently for a previously published paper (17), our data do not seem to support the concept that IGHD compromises longevity. The multiple differences (age of onset and severity of GHD, unique and selected genetic background, and rural environment) limit the applicability of our findings to subjects with acquired, adult-onset GHD residing in different geographical areas.
Acknowledgments
We thank Mrs. Ivanilde Santana de Sousa for her secretarial assistance and Fapese (“Fundacao de Apoio a Pesquisa e Extensao de Sergipe”) for administrative assistance.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant 1R01 DK065718 (to R.S.). M.H.A.-O. was supported by Grant BEX 4309/08-1 from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, from the Brazilian Government. Neither NIH nor the Brazilian government had any role in the data analysis.
Disclosure Summary: The authors have nothing to disclose.
First Published Online December 4, 2009
Abbreviations: GHD, GH deficiency; HR, hazard ratio; IGFBP-3, IGF binding protein 3; IGHD, isolated GHD; LDL, low-density lipoprotein.
References
- vB Hjelmborg J, Iachine I, Skytthe A, Vaupel JW, McGue M, Koskenvuo M, Kaprio J, Pedersen NL, Christensen K 2006 Genetic influence on human longevity. Hum Genet 119:312–321 [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R 1993 A C. elegans mutant that lives twice as long as wild-type. Nature 366:461–464 [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD 2001 Regulation of longevity and stress resistance by Sch9 in yeast. Science 292:288–290 [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS 2001 A mutant Drosophila insulin receptor homologue that extends life span and impairs neuroendocrine function. Science 292:107–110 [DOI] [PubMed] [Google Scholar]
- Bartke A 2009 The somatotropic axis and aging: mechanisms and persistent questions about practical implications. Exp Gerontol 44:372–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ 2003 Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 144:3799–3810 [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y 2003 IGF-1 receptor regulates life span and resistance to oxidative stress in mice. Nature 421:182–187 [DOI] [PubMed] [Google Scholar]
- Patronek GJ, Waters DJ, Glickman LT 1997 Comparative longevity of pet dogs, and humans: implications for gerontology research. J Gerontol A Biol Sci Med Sci 52:B171–B178 [DOI] [PubMed] [Google Scholar]
- Bonafè M, Barbieri M, Marchegiani F, Olivieri F, Ragno E, Giampieri C, Mugianesi E, Centurelli M, Franceschi C, Paolisso G 2003 Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab 88:3299–3304 [DOI] [PubMed] [Google Scholar]
- van Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, Zwaan BJ, Slagboom PE, Westendorp RG 2005 Reduced insulin/IGF-I signaling and human longevity. Aging Cell 4:79–85 [DOI] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P 2008 Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA 105:3438–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Shalet SM, Vance ML, Stephens PA 2006 Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 91:1621–1634 [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE 1990 Effects of human growth hormone in men over 60 years old. N Engl J Med 323:1–6 [DOI] [PubMed] [Google Scholar]
- Rosén T, Bengtsson BA 1990 Premature mortality due to cardiovascular disease in hypopituitarism. Lancet 336:285–288 [DOI] [PubMed] [Google Scholar]
- Salvatori R, Hayashida CY, Aguiar-Oliveira MH, Phillips 3rd JA, Souza AH, Gondo RG, Toledo SP, Conceicão MM, Prince M, Maheshwari HG, Baumann G, Levine MA 1999 Familial dwarfism due to a novel mutation of the growth hormone-releasing hormone receptor gene. J Clin Endocrinol Metab 84:917–923 [DOI] [PubMed] [Google Scholar]
- Pereira RM, Aguiar-Oliveira MH, Sagazio A, Oliveira CR, Oliveira FT, Campos VC, Farias CT, Vicente TA, Gois Jr MB, Oliveira JL, Marques-Santos C, Rocha IE, Barreto-Filho JA, Salvatori R 2007 Heterozygosity for a mutation in the growth hormone releasing hormone receptor gene does not influence adult stature, but affects body composition. J Clin Endocrinol Metab 92:2353–2357 [DOI] [PubMed] [Google Scholar]
- Besson A, Salemi S, Gallati S, Jenal A, Horn R, Mullis PS, Mullis PE 2003 Reduced longevity in untreated patients with isolated growth hormone deficiency. J Clin Endocrinol Metab 88:3664–3667 [DOI] [PubMed] [Google Scholar]
- Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, Sheppard MC, Stewart PM 2001 Association between premature mortality and hypopituitarism. West Midlands Prospective Pituitary Study Group. Lancet 357:425–431 [DOI] [PubMed] [Google Scholar]
- Bülow B, Hagmar L, Mikoczy Z, Nordström CH, Erfurth EM 1997 Increased cerbrovascular mortality in patients with hypopitutarism. Clin Endocrinol (Oxf) 46:75–81 [DOI] [PubMed] [Google Scholar]
- Svensson J, Bengtsson BA, Rosén T, Odén A, Johannsson G 2004 Malignant disease and cardiovascular morbidity in hypopituitary adults with or without growth hormone replacement therapy. J Clin Endocrinol Metab 89:3306–3312 [DOI] [PubMed] [Google Scholar]
- Stochholm K, Gravholt CH, Laursen T, Laurberg P, Andersen M, Kristensen LØ, Feldt-Rasmussen U, Christiansen JS, Frydenberg M, Green A 2007 Mortality and GH deficiency: a nationwide study. Eur J Endocrinol 157:9–18 [DOI] [PubMed] [Google Scholar]
- Ayuk J, Stewart PM 2009 Mortality following pituitary radiotherapy. Pituitary 12:35–39 [DOI] [PubMed] [Google Scholar]
- Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, Donaldson SS, Meadows AT, Robison LL 2001 Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst 93:618–629 [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Higgins CD, Adlard P, Preece MA 2002 Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959–85: a cohort study. Lancet 360:273–277 [DOI] [PubMed] [Google Scholar]
- Menezes M, Salvatori R, Oliveira CR, Pereira RM, Souza AH, Nobrega LM, Cruz EA, Menezes M, Alves EO, Aguiar-Oliveira MH 2008 Climacteric in untreated isolated growth hormone deficiency. Menopause 15:743–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson H, Barreto ES, Salvatori R, Costa L, Oliveira CR, Pereira RM, Clayton P, Aguiar-Oliveira MH 2007 Metabolic effects of growth hormone (GH) replacement in children and adolescents with severe isolated GH deficiency due to a GHRH receptor mutation. Clin Endocrinol (Oxf) 66:466–474 [DOI] [PubMed] [Google Scholar]
- Kooijman R, Coppens A, Hooghe-Peters E 2002 IGF-I inhibits spontaneous apoptosis in human granulocytes. Endocrinology 143:1206–1212 [DOI] [PubMed] [Google Scholar]
- Oliveira CR, Salvatori R, Nóbrega LM, Carvalho EO, Menezes M, Farias CT, Britto AV, Pereira RM, Aguiar-Oliveira MH 2008 Sizes of abdominal organs in adults with severe short stature due to severe, untreated, congenital GH deficiency caused by a homozygous mutation in the GHRH receptor gene. Clin Endocrinol (Oxf) 69:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de A Barretto ES, Gill MS, De Freitas ME, Magalhães MM, Souza AH, Aguiar-Oliveira MH, Clayton PE 1999 Serum leptin and body composition in children with familial GH deficiency (GHD) due to a mutation in the growth hormone-releasing hormone (GHRH) receptor. Clin Endocrinol (Oxf) 51:559–564 [DOI] [PubMed] [Google Scholar]
- Gleeson HK, Souza AH, Gill MS, Wieringa GE, Barretto ES, Barretto-Filho JA, Shalet SM, Aguiar-Oliveira MH, Clayton PE 2002 Lipid profiles in untreated severe congenital isolated growth hormone deficiency through the lifespan. Clin Endocrinol (Oxf) 57:89–95 [DOI] [PubMed] [Google Scholar]
- Barreto-Filho JA, Alcântara MR, Salvatori R, Barreto MA, Sousa AC, Bastos V, Souza AH, Pereira RM, Clayton PE, Gill MS, Aguiar-Oliveira MH 2002 Familial isolated growth hormone deficiency is associated with increased systolic blood pressure, central obesity, and dyslipidemia. J Clin Endocrinol Metab 87:2018–2023 [DOI] [PubMed] [Google Scholar]
- Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS 2003 Childhood cardiovascular risk factors and carotid vascular changes in adulthood: The Bogalusa Heart Study. JAMA 290:2271–2276 [DOI] [PubMed] [Google Scholar]
- Menezes Oliveira JL, Marques-Santos C, Barreto-Filho JA, Ximenes Filho R, de Oliveira Britto AV, Oliveira Souza AH, Prado CM, Pereira Oliveira CR, Pereira RM, Ribeiro Vicente Tde A, Farias CT, Aguiar-Oliveira MH, Salvatori R 2006 Lack of evidence of premature atherosclerosis in untreated severe isolated growth hormone deficiency due to a GHRH receptor mutation. J Clin Endocrinol Metab 91:2093–2099 [DOI] [PubMed] [Google Scholar]
- Baglietto L, English DR, Hopper JL, Morris HA, Tilley WD, Giles GG 2007 Circulating insulin-like growth factor-I and binding protein-3 and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev 16:763–768 [DOI] [PubMed] [Google Scholar]
- Wu X, Tortolero-Luna G, Zhao H, Phatak D, Spitz MR, Follen M 2003 Serum levels of insulin-like growth factor I and risk of squamous intraepithelial lesions of the cervix. Clin Cancer Res 9:3356–2261 [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M 1998 Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 279:563–566 [DOI] [PubMed] [Google Scholar]
- Weber MM, Fottner C, Liu SB, Jung MC, Engelhardt D, Baretton GB 2002 Overexpression of the insulin-like growth factor I receptor in human colon carcinomas. Cancer 95:2086–2095 [DOI] [PubMed] [Google Scholar]
- Shevah O, Laron Z 2007 Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res 17:54–57 [DOI] [PubMed] [Google Scholar]
- Aguiar-Oliveira MH, Gill MS, de A Barretto ES, Alcântara MR, Miraki-Moud F, Menezes CA, Souza AH, Martinelli CE, Pereira FA, Salvatori R, Levine MA, Shalet SM, Camacho-Hubner C, Clayton PE 1999 Effect of severe growth hormone (GH) deficiency due to a mutation in the GH-releasing hormone receptor on insulin-Like growth factors (IGFs), IGF-binding proteins, and ternary complex formation throughout life. J Clin Endocrinol Metab 84:4118–4126 [DOI] [PubMed] [Google Scholar]
- Paolisso G, Ammendola S, Del Buono A, Gambardella A, Riondino M, Tagliamonte MR, Rizzo MR, Carella C, Varricchio M 1997 Serum levels of insulin-Like growth factor-I (IGF-I) and IGF-binding protein-3 in healthy centenarians: relationship with plasma leptin and lipid concentrations, insulin action, and cognitive function. J Clin Endocrinol Metab 82:2204–2209 [DOI] [PubMed] [Google Scholar]