Abstract
Food allergies are caused by immunological reactions in individuals sensitized to normal protein components of foods. For any given sensitized individual, the severity of a reaction is generally assumed to be proportional to the dose of allergenic protein. There is substantial clinical evidence that “threshold” doses exist for the elicitation of an allergic reaction; however, the threshold (i.e., lowest dose that elicits a reaction) varies substantially across the sensitized population. Current approaches to protecting sensitized individuals from exposure to food allergens are highly qualitative (i.e., they rely on food avoidance). The Key Events Dose-Response Framework is an analytical approach for refining understanding of the biological basis of the dose-response. Application of this approach to food allergy provides a foundation for a more rigorous quantitative understanding of variability in allergic response. This study reviews the allergic disease process and the current approaches to identifying thresholds for food allergens. The pathway of key biological events occurring between food intake and allergic response is considered, along with factors that may determine the nature and severity of response to food allergens. Data needs, as well as implications for identifying thresholds, and for characterizing variability in thresholds, are also discussed.
Keywords: Low dose dose-response, food allergy, thresholds, minimal eliciting dose, Key Events Dose-Response Framework
INTRODUCTION
Food allergies are caused by immunological responses to specific protein components of foods. True food allergies are distinct from other forms of adverse reactions that are more appropriately termed food intolerances; these do not involve immunological mechanisms. On the basis of the total weight of evidence, the Health Council of The Netherlands estimates the prevalence of food allergies generally in the range of 1–2% for adults and 1–3% for children (Health Council of the Netherlands, 2007). Other studies have found that food allergies may affect up to 4% of adults and 6–8% of children (Nowak-Wegrzyn and Sampson, 2006; Venter et al., 2006). Food allergic reactions are estimated to result in approximately 30,000 emergency room visits and 150 deaths each year in the United States alone (Sampson, 2003).
Worldwide, over 150 different foods have been reported to cause food-allergic reactions (Hefle et al., 1996); however, it appears that a relatively limited number of foods are responsible for most instances of food allergy reactions. The most common allergenic foods in the U.S. are peanuts, tree nuts (e.g. walnuts, almonds), soy, egg, milk, wheat, fish (e.g. salmon, cod), and crustacean shellfish (e.g. shrimp, crab, lobster) as established by the Food Allergen Labeling and Consumer Protection Act of 2004. In Canada, the list of commonly allergenic foods also includes molluscan shellfish (e.g. clam, oyster, squid) and sesame seed. In the European Union, sesame seed, mustard, celery, molluscan shellfish, and lupine are also considered to be common allergenic foods. Other foods are of concern in other parts of the world probably due to different dietary habits. An example would be buckwheat in Japan and Korea, which may be related to the frequent consumption of buckwheat noodles in those countries.
Food allergies can manifest in a wide variety of signs and symptoms (Taylor and Hefle, 2001), ranging from subjective symptoms, such as mild itching or nausea, to severe signs, including extensive urticaria or anaphylactic shock (Table 1). For any given individual patient, the severity of a reaction is generally assumed to be proportional to the level of intake of the offending food (i.e., level of exposure to protein allergens) and that individual's degree of sensitization. This assumption has not been rigorously tested in part due to ethical restrictions. Individual sensitivity may also be affected by a number of factors including stress, exercise, other medical conditions, and medications, although this is well documented only for exercise.
Table 1.
Symptoms of IgE-Mediated Food Allergies
| Gastrointestinal Symptoms | Respiratory Symptoms |
| Nausea | Rhinitis |
| Vomiting | Asthma |
| Diarrhea | Other Symptoms |
| Abdominal Pain | Laryngeal Edema |
| Colic | Anaphylactic Shock |
| Cutaneous Symptoms | Hypotension |
| Urticaria | Cardiac Arrhythmia |
| Eczema or Atopic Dermatitis | |
| Angioedema | |
| Pruritis |
The allergic reaction to food allergens is generally believed to be “thresholded.” Clinical data indicate that the threshold dose level, sometimes referred to as the Minimal Eliciting Dose (MED), can vary from person to person, and can also vary within an individual over time. A wide range of individual threshold doses exists across the food-allergic population (Taylor and Hourihane, 2008). Whether this range represents a continuous distribution of sensitivity within the allergic population or whether discrete highly sensitive subpopulations exist, is not known.
Because of the potentially severe consequences of an allergic food reaction, sensitized individuals are advised to avoid any exposure, no matter how small, of the allergenic food. Moreover, extensive efforts are taken by the food industry to avoid inadvertent introduction of food allergens into processed foods. Meanwhile, there is solid evidence that suggests a small amount of exposure to allergens does not pose a risk in every sensitized individual. But which individuals are at risk, and at what dose levels? Identifying actual threshold levels for specific individuals and identifying protective thresholds for a population of sensitized individuals is a major public health challenge.
Often, the establishment of “safe” doses is approached rather pragmatically with dose-response experiments in laboratory animals or even humans. However, a more mechanistic approach may offer an alternative. The Key Events Dose-Response Framework is an analytical approach for systematically evaluating key biological events that underlie a given dose-response relationship. The goal of this framework is to deepen understanding of the dose-response relationship and thereby improve the scientific basis for identifying safe levels of exposure or intake. This Key Events approach is intended to complement currently available standard approaches to studying low dose dose-response for food allergens and other agents in foods, such as nutrients, additives, and environmental contaminants and is detailed in the introductory paper for this series (Julien et al., 2009).
As described in that introductory paper, the Key Events Dose-Response Framework is based on the premise that for any given bioactive agent, chemical, allergen, pathogen, etc., there are multiple “key events” along the biological pathway from initial intake to the ultimate effect of concern. Moreover, a variety of physiological mechanisms, for example homeostatic feedback mechanisms, immune response mechanisms, repair mechanisms, may operate at these events to maintain a normal biological environment. Various other factors such as life stage, disease state, genetic makeup, etc. can modify the effectiveness of these mechanisms, and thus also play a role in determining outcome. So at each biological event between initial dose/intake and the ultimate effect of concern, multiple factors combine to determine the overall dose-response relationship. An analysis of dose-response at individual key events should provide insight and help to better characterize the overall dose-response relationship.
The remainder of this paper will review the allergic disease process as well as current approaches to identifying thresholds for food allergens. Then, application of the Key Events analytical approach will be discussed along with research needs and potential implications for public health standard setting.
BACKGROUND
Overview of the Allergic Disease Process
The manifestation of a food allergy is a complex two-step process (Taylor and Hefle, 2001). The first step is referred to as “sensitization.” Sensitization requires exposure to protein allergens in the food although oral exposure is not the only route that can induce sensitization. Oral exposure to dietary proteins usually results in oral tolerance (Strobel and Mowat, 2006), which is the normal homeostatic response. Food allergy can be viewed as a breakdown in, or non- or underdevelopment of, oral tolerance in an individual. The reasons for this breakdown or non- or underdevelopment are unclear because even food-allergic individuals are reactive to only one or a few of the many hundreds of thousands of proteins that are ingested with the diet. Moreover, there is a wide range of responses to a particular allergenic food (e.g., individual threshold doses for peanut can range from 0.5 up to 8000–10,000 mg of peanut (0.125 to 2000–2500 mg of total peanut protein) (Taylor et al., 2009)) and this may partially be due to individual differences in the degree of oral tolerance. Thus, sensitization can perhaps be viewed as a spectrum ranging from complete oral tolerance to various degrees of non-tolerance as evidenced by ever decreasing individual threshold doses.
With some allergenic foods (e.g. milk) the allergic state may spontaneously revert over time. Infants who develop milk allergy may outgrow that allergy over a matter of months or years and ultimately become fully tolerant of milk ingestion (Bishop et al., 1990). Some individuals, however, have persistent milk allergy (Skripak et al., 2007). The mechanisms involved in the delayed development of tolerance to a specific food, such as milk, are not known. Recently, clinical research has demonstrated that oral tolerance to peanut can be induced by intentional oral administration of very low, slowly escalating doses of peanut (Jones et al., 2009). And, the likelihood of developing delayed oral tolerance to certain foods such as peanuts is considerably less than for other foods such as milk (Bock, 1986). The biological mechanisms associated with these observed differences are poorly understood.
Both sensitization and oral tolerance to foods are most likely to occur in infancy as new foods are introduced into the diet. However, with respect to sensitization, it is possible to become sensitized to a food at any age. With some food allergies like those to crustacean shellfish, sensitization tends to occur at older ages perhaps because dietary exposure tends to increase with age. However, no clinical evidence exists to indicate that a sizeable number of new food allergies develop in older individuals so oral tolerance remains as the most typical response to the introduction of new foods in older individuals. Other events, including intestinal infections or influenza, may trigger or facilitate sensitization and thus development of a food allergy. The role of infections in enhancing the likelihood of allergic sensitization is better understood with respiratory infections and allergens. While avoidance of commonly allergenic foods has been advocated to prevent sensitization, evidence indicates that this approach has limited effectiveness (Zeiger and Heller, 1995). In fact, some evidence suggests that the early introduction of allergenic foods into the diet of infants may actually increase the development of tolerance (Lack, 2008).
The second step in the allergic disease process is elicitation. Elicitation of an allergic reaction occurs when a sensitized individual is re-exposed to a food allergen under conditions that allow sufficient binding to occur between the allergen or its fragments and allergen-specific IgE antibodies bound to mast cells and basophils such that physiologically active mediators of allergic disease are released from these mast cells and basophils.
There are several potential complications in this sensitization-elicitation process. First, there is the problem of cross-reactivity, whereby sensitization to a given food allergen can occur via exposure to a non-food substance or via exposure to a different food product. For example, sensitization to an allergen via the respiratory route or via skin exposure can convey cross-sensitization to foods that contain proteins with sufficiently similar IgE-reactive epitopes (Aalberse et al., 2001). Well-known examples include cross-sensitization to apples and hazelnuts with birch pollen, cross-sensitization to celery and carrots with mugwort pollen, and cross-sensitization to kiwi and banana with latex allergens. Many times, allergic reactions to these foods are very mild, for example the oral allergy syndrome (itching, angioedema, or urticaria occurring around the mouth). Some sensitized individuals may react to respiratory exposure but not to oral intake of an allergenic food; an example is “baker's asthma” upon occupational exposure to components of dust in bakeries occurring in individuals who can safely consume bakery products (Moneret-Vautrin and Morisset, 2005).
Sensitization to one particular food allergen may also convey cross-reactivity to related foods. Again, this relates to the existence of similar proteins with similar IgE epitopes in related species. For example, tropomyosin is a well-described allergen in crustacean shellfish, and individuals sensitized to shrimp tropomyosin are likely to also react to crab and lobster (Lehrer et al., 2003). But, such cross-reactions do not universally occur. The human diet contains more than 300 edible species of legumes including two commonly allergenic ones, peanuts and soybeans. However, cross-reactions do not often occur in peanut- or soy-allergic individuals to other legumes, although they are occasionally encountered (Bernhisel-Broadbent and Sampson, 1989). Furthermore, recent studies of certain food allergies suggest that IgE antibodies specific to carbohydrate moieties on glycoproteins may be involved in cross sensitization. However, in this particular case, the cross-reactivity is of questionable clinical significance (van Ree, 2004).
Specific IgE antibodies may also recognize more than one protein (Taylor and Lehrer, 1996). Further, allergic individuals may produce several specific IgEs that recognize different proteins from a particular food, although it is not clear if all of these IgEs are of equivalent clinical significance. The spectrum of targeted proteins varies between individuals allergic to a given food, although some proteins from a particular food are more commonly targeted than others. The specific epitopes that are recognized on a given food protein may also vary across individuals, although some epitopes are more commonly targeted than others.
Another complication is the fact that sensitization does not invariably correlate with the development of clinically significant food allergy. Some individuals will have food-specific IgE antibodies in their serum and tissues but will not experience allergic reactions upon exposure to that food. There are numerous potential reasons for this lack of reaction, including inadequate binding of the allergen to the IgE antibody, insufficient dose, and digestive instability of the allergen.
The multiple complications in the sensitization-elicitation process, i.e., cross-reactivity, development of antibodies to multiple antigens in a given food, development of IgE antibodies without subsequent allergic reaction, hinder the identification of the mechanisms underlying food allergy. In turn, this complicates the study of low-dose dose-response.
Current Approaches to Characterizing Low Dose Dose-Response
As explained above, there are two distinct processes involved in the development of a food allergy, sensitization and elicitation. Likewise, there are presumably two distinct dose-response relationships. Both the sensitization and the elicitation processes are probably thresholded although this has not been rigorously demonstrated for sensitization.
A great deal of data regarding the food-allergic response has been gathered in human studies. Unlike studies of other substances in foods (e.g., pesticides, additives), data on food allergens can be obtained via human oral challenge trials. But the study of sensitization in human subjects is complicated by the fact that most allergenic foods are normal components of the human diet; these foods are likely to be consumed in reasonably large quantities until allergic sensitization leads to elicitation of an allergic response. Thus, many, but not all, individuals who become sensitized and allergic to a specific food have been exposed to rather large, but ill-defined, amounts of the allergenic foods or to cross-reacting foods or other materials such as pollens or latex. In contrast to sensitization, the dose-response relationship for elicitation can be explored using sensitized human subjects.
In fact, virtually all data on threshold doses for elicitation have been obtained from human clinical studies. Predictive animal models, able to distinguish potent allergens from nonallergenic or weakly-allergenic proteins on a consistent basis, do not exist for food allergies, either for sensitization or elicitation (Goodman et al., 2008; Knippels et al., 2004; Nelde et al., 2001). Like humans, most animals are prone to become orally tolerant to dietary proteins, thus sensitization usually requires provocation of the immune system by unusual routes of exposure (e.g. intraperitoneal) and the use of adjuvants. So far, none of the existing animal models is able to discriminate between proteins with distinct allergenic potential for humans. Further research on animal models is warranted; however, presently, animal models cannot provide quantitative data related to thresholds for either human sensitization or elicitation.
Published human data do exist on threshold doses for elicitation of allergic reactions and can be found from several types of studies including clinical trials aimed at defining biological thresholds, immunotherapy trials where a baseline threshold dose must be determined to assess the effectiveness of the therapeutic approach, and low dose diagnostic double-blind, placebo-controlled food challenges (DBPCFCs). Of these, the most useful studies are DBPCFCs which are designed to be objective and unbiased (Taylor and Hourihane, 2008). DBPCFCs provide data on individual thresholds for humans allergic to specific foods. A very wide range of individual thresholds is known to exist, e.g. from 0.5 to 10,000 mg for peanut (Taylor et al., 2009; Taylor and Hourihane, 2008; Bindslev-Jensen et al., 2002). For most foods, however, the distribution of individual thresholds within the sensitive population has not yet been well established.
While the acquisition of human data on individual threshold doses offers a practical approach to determining thresholds for populations of food-allergic individuals, the existing clinical data may not be fully satisfactory for several reasons. While DBPCFCs have been widely used for the diagnosis of food allergies, diagnostic DBPCFCs are often not useful for the identification of individual thresholds or characterization of low dose dose-response. The doses of the allergenic foods used for the diagnostic process are often quite high (>250 mg). More than 20% of patients in diagnostic DBPCFCs react to the first dose which prevents identification of NOAELs (Sicherer et al., 2000). In theory, it should be possible to combine data from multiple DBPCFC studies to generate a dose-response curve. But, historically, there has been no standard protocol used with regard to the specific form of the food, the amount and the specific type of protein in the food, the dose range for the challenges, the timing of the challenges, the recording of symptoms (especially subjective responses), and subject selection. Recently, however, a consensus clinical protocol was published for conducting DBPCFC studies towards identifying thresholds (Taylor et al., 2004).
The selection of subjects for clinical trials, in particular, raises questions with regard to the applicability of study findings to the broader food-allergic population. Some clinics may exclude subjects from low dose challenge trials if the subject has a history of severe reactions, even though data do not exist to conclude that severe responders are necessarily more likely to be reactive at low doses. Additionally, most low dose clinical challenges have been conducted at referral centers where the tendency may exist to select patients who may be more sensitive than the overall food-allergic population. It is not known whether, and to what extent, these phenomena affect available threshold information and how these phenomena balance against each other.
In addition to the difficulties noted above (diverse protocols, subject selection, and lack of multiple doses in diagnostic studies), other factors may affect the threshold values observed in a clinical trial, including concomitant exercise or other stresses, other diseases, age, the presence of seasonal pollen allergies, pharmaceutical treatments, etc. In addition, as noted, oral tolerance to food allergens can develop over time, such that individual thresholds change. For example, many infants “outgrow” their allergies to certain allergenic foods (especially milk, egg, soy, and wheat) (Bock, 1986). In other words, their individual thresholds increase over time until they become tolerant of the amounts typically contained in the human diet. All these potentially confounding factors can be controlled in clinical threshold trials, although it is difficult to control for the development of tolerance over time.
Thus, the assessment of thresholds for food-allergic reactions using human clinical data, while quite promising, remains fraught with obstacles. Currently, some data exist on threshold doses for several commonly allergenic foods, including peanut, milk, and eggs (Taylor and Hourihane, 2008). Less data exist for tree nuts, fish, crustacean shellfish, wheat, soybeans, lupine, and mustard. Individual threshold dose data need to be acquired for a larger number of subjects for most allergenic foods. The use of the consensus, low-dose clinical threshold protocol (Taylor et al., 2004) by multiple clinics would facilitate the combined use of data from those clinics. However, approaches are also needed for utilizing clinical data from the multiple clinics that use diverse protocols. Attention needs to be paid to patient selection criteria for clinical trials to assure that the subjects are indeed representative of the overall population of individuals allergic to a certain food.
Current Approaches to Setting Regulatory Standards for Public Health
At this time, there is no cure for a food allergy, and no generally recognized “safe” levels of allergens. Thus, current regulatory approaches for food allergens are purely qualitative, i.e., a product either does, or does not contain allergens, and public health strategies therefore focus on preventing the elicitation of allergic reactions in sensitized individuals. This is done by providing such individuals with notice, via food product labels, of the presence of allergenic foods. This strategy requires allergic individuals to avoid intake of food with the potential to contain even minute amounts of an allergen.
The strategy of relying on product labels and strict food avoidance by allergic consumers poses difficulties for allergic individuals. In addition to the inconvenience, strict food avoidance can deprive allergic individuals of foods with high nutritional value. Food products are labeled as containing allergenic foods even in cases where rather small amounts are present. Because of the uncertainty regarding threshold doses and the resulting qualitative regulatory strategy, food manufacturers tend to declare the presence of trace amounts, or even possible trace amounts on the label. Precautionary labeling (e.g. “may contain”) is in widespread use as a result of this approach. Food-allergic consumers are often confused about the need to avoid ingredients derived from allergenic sources, especially in cases where those ingredients contain very low concentrations of the allergenic proteins; examples would include peanut oil, fish oil, soybean lecithin, lactose, and many others. All of these factors tend to further restrict the diets of food-allergic consumers.
The regulatory uncertainty about thresholds is also problematic for the food industry. For industry, the current regulatory strategy can lead to the need to implement expensive allergen control strategies, sometimes requiring strict segregation of machinery and/or extra steps in food processing and packaging, to prevent inadvertent contamination of unlabeled foods with minute amounts of allergenic protein.
Regulatory consensus does not exist in any country or regulatory jurisdiction regarding how to determine population thresholds for any allergenic food. Several factors may contribute to this situation. First, consensus does not exist regarding approaches for evaluating existing human data, although the U.S. Food & Drug Administration has indicated that they favor the use of risk assessment approaches (US FDA/CFSAN Threshold Working Group, 2008). Furthermore, the existing human data have not been assembled yet for use in risk assessment approaches. However, the potential does exist for progress in this regard (see Taylor et al., 2009).
Risk assessment or modeling approaches have been explored to better and more quantitatively describe the distribution of individual thresholds (Crevel et al., 2007). The selection of the ideal statistical model to use will require further research. However, such approaches have the potential for use to quantitatively describe the risks associated with specific levels of allergenic food residues to segments of the allergic population (Spanjersberg et al., 2007; Kruizinga et al., 2008). Identification of population threshold distributions, coupled with a risk-based approach, presents advantages to industry, government, and food-allergic individuals. It would provide the food industry with data needed to generate more accurate and informative labels. Regulatory agencies would be able to focus regulatory attention, including recalls, on foods and food ingredients that pose the greatest risks. Such risk-based approaches would support more informative and quantitative food labels; in turn, this may also better protect (and lessen restrictions on) food-allergic consumers, who appear to be increasingly ignoring qualitative advisory labels that a product “may contain” an allergen (Hefle et al., 2007).
Thus, advantages exist to using a quantitative risk-based approach to characterizing thresholds; however, to be effective, such quantitative modeling approaches must be consistent with the underlying fundamental biology of the allergic disease process. The Key Events Dose-Response Framework provides an analytical approach for systematically examining the biological components of the elicitation process. Such an analysis helps to generate a coherent picture of the various “drivers” of the allergic response, including drivers that determine the nature or severity of the response. A systematic review of the biology of the elicitation process sheds light on potential sources of inter- and intra-individual variability, which in turn provides a more solid scientific basis for establishing the optimal level of vigilance for an individual or a population.
APPLICATION OF THE KEY EVENTS APPROACH TO FOOD ALLERGENS
The focus of this section will be on the use of a key events analysis to examine dose-response and thresholds for elicitation of allergic reactions in sensitized individuals. As noted previously, clinical evidence demonstrates the existence of thresholds for elicitation; however, the biological basis of such thresholds is not known. A systematic examination of key events leading to elicitation should facilitate the assessment of current knowledge and identify research most needed to refine the understanding of dose-response. Future efforts may examine key events in the development of sensitization; however, much less is known regarding that process. Unlike the elicitation response, there are no substantial collections of data regarding development of sensitization response.
Overview of Key Events
The major biological events in the elicitation of a food allergic reaction are outlined in Fig. 1, along with several factors or aspects of each event that could, in theory, be quantified to reflect the “dose” at the event (Taylor and Hefle, 2001). To summarize the pathway of events, elicitation results when a sensitized individual is re-exposed to a food allergen under conditions that allow binding of an allergen (or its fragments) to allergen-specific IgE antibodies that are bound to mast cells and basophils; if there is sufficient binding, physiologically active mediators are released from these cells. These mediators may be dispersed, or they may interact locally with susceptible tissues. This interaction underlies the signs and symptoms of allergic response.
Figure 1.
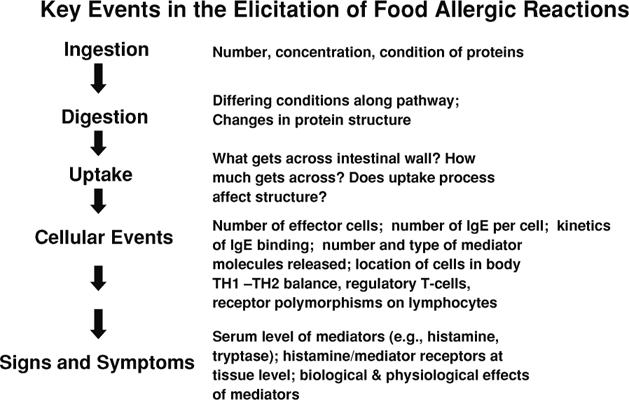
Major biological events and factors in the elicitation of a food allergic reaction.
While these events are listed sequentially in Fig. 1, in certain cases, they may occur almost simultaneously (e.g., signs and symptoms occur immediately after or simultaneously upon ingestion). The pathway as presented, however, outlines the current state of the science with regard to the nature of various events and their likely relationship to each other.
Analysis of Individual Key Events
Ingestion1
Certainly, the initial dose (i.e., ingested amount of allergenic protein or peptide) is a key factor in the likelihood of elicitation of a reaction. But the condition of the allergenic proteins in a food (especially the effects of food processing) can affect their allergenicity. Processing may result in denaturation or degradation of the proteins, chemical modifications, changes in solubility, or aggregation with other food components. Any of these changes can potentially affect the the ability of the protein or peptide to elicit an allergic reaction. The more severe food allergens are considered to be quite stable to food processing, especially heat (Taylor and Lehrer, 1996).
Once allergenic proteins or peptides have been ingested, contact with the oral mucosa may lead directly to a reaction. Some allergens tend to elicit reactions only in the oral cavity (Ortolani et al., 1988). More specifically, they elicit reactions by interacting with mucosal mast cells in the mouth and symptoms start immediately upon consumption. Oral allergy syndrome (OAS) is a constellation of mild and transitory symptoms localized in and around the mouth. As originally described (Ortolani et al., 1988), OAS was thought to be associated with ingestion of unstable allergens from fruits and vegetables that are cross-reactive with pollen allergens, the likely original cause of sensitization. More recently, some foods primarily associated with OAS (e.g., peach and apple) have been found to also contain more stable allergens (lipid transfer proteins primarily) that can trigger more severe, systemic reactions (Ballmer-Weber, 2002).
Digestion
Once allergenic proteins or peptides are ingested by a sensitized individual, several factors related to digestion may affect the dose available to be absorbed, and thus become important in determining the likelihood of reaction. These include:
The stability of the proteins to digestive proteases including pepsin, trypsin, and chymotrypsin,
The digestive capacity of the host, especially acid production in the stomach, which can be affected by physiological conditions (e.g. achlorhydria) or pharmacological treatments (e.g. H2 antihistamines and protein pump inhibitors),
Interactions with the food matrix.
The food matrix containing the food allergen can affect the amount (dose) of allergen available for digestion. Some food allergens, once freed from the matrix and available for digestion, are considered to be comparatively stable to digestion (Taylor and Lehrer, 1996). Certainly, some proteolysis may occur but at a minimum some immunoreactive peptides reach the effector cells in the intestinal tract and beyond. While there is evidence that proteolysis can sometimes generate new allergenic epitopes (Lehmann et al., 2006), resistance to digestion appears to be a key attribute of the major food allergens.
Certain host conditions can also affect stability. For example, some in vitro evidence indicates that acid suppression therapy might increase the digestive stability of certain food allergens (Untersmayr et al., 2003). However, acid suppression therapy and the serious conditions affecting digestion (e.g., achlorhydria) are more common in adults, which as a subpopulation tends to be less sensitive to food allergens than young children and infants.
In summary, the stability of food allergens (i.e., resistance to proteolysis) is variable across food matrices, across proteins, and across individuals. These factors will certainly influence the dose available for uptake.
Uptake and Distribution
Uptake of proteins from the digestive tract is influenced by:
The existence and location of transporter systems
The number of transporters and the kinetics of the transport process, including the affinity of the transport system for the protein, peptides, or aggregates
Interactions with blocking antibodies, IgA and IgG, in the intestinal lumen and mucosa which prevent or slow uptake
Mucosal integrity, and conditions along the digestive tract including any pre-existing intestinal injury
Factors affecting other uptake processes (i.e., those not mediated by transporter systems)
The process of protein uptake into the intestinal mucosa is not well understood, but could be a pivotal process in determining the actual dose that is ultimately presented to effector cells. Specific transporter systems may exist, or processes such as pinocytosis may result in mucosal protein uptake. Active uptake of allergenic proteins, especially those that are digestion-resistant, may also occur via macrophages, and partial proteolysis within such macrophages may result in more reactive peptides being presented to mast cells. That possibility is speculative, however, and it is equally likely that food allergens resist proteolysis within phagocytic cells, or that phagocytic cells are not involved.
Intestinal uptake of food allergens may be hindered by the presence of allergen-specific IgA antibodies in the intestinal lumen, which may block (or partially block) uptake of the allergenic protein (Mayer, 2003). Stimulation of the production of allergen-specific IgA antibodies does not occur in all (or even most) food-allergic subjects (Barnes et al., 1995). This factor could potentially be critical to determining the dose absorbed, and may also explain some of the interindividual variability in response. Allergen-specific IgA antibodies may also play a role in the development of oral tolerance, but that role is as yet undefined.
Following uptake, the allergen or its immunoreactive breakdown product(s) may directly interact with mucosal mast cells (as discussed below). Alternatively, they may first be transported to other tissues, where they may present to, and ultimately trigger, effector cells, i.e., basophils in the blood or mast cells in any other tissue of the body.
Cellular Events—Interaction of Allergens with the Immune System
Once allergenic proteins or peptides have been absorbed from the digestive tract, they can interact directly with the immune system, in particular, mast cells of the mucosal tissues. In fact, the small intestine is considered a primary locale for interactions between mucosal mast cells and food allergens. High numbers of mast cells and basophils are present in all humans whether allergic to foods or not. The distinguishing feature of food-allergic individuals is that their mast cells and basophils (i.e., effector cells) are armed with allergen-specific IgE antibodies attached to the membrane surfaces. For an allergic reaction to occur, a food allergen must cross-link these allergen-specific IgE antibodies on the surface of the effector cells. This cross-linking triggers the release of pre-formed mediators such as histamine, and may also generate de novo mediators such as leukotrienes and prostaglandins.
Note that the release of these mediators into the tissues and bloodstream is dependent upon the cross-linking interaction of the allergenic protein with the bound IgE antibodies. In order to achieve the desired spatial geometry required for cross-linking, the concentration of IgE antibodies attached to the mast cell membranes must be of sufficient number. But the minimum (sufficient) number of binding events that must occur in order to effect mediator release or total mediator release is unknown.
In addition to the number of binding events per cell, there may be a minimum number of armed mast cells and a minimum amount of mediator release required in order for the downstream events (interaction of mediators in tissues) to result in signs and symptoms. This has not been clearly documented in food allergy, however. In fact, estimates of the number of allergen-specific IgE antibodies bound to mast cells in an allergic reaction do not exist. Similarly, the amount of stored mediators generated and released from mast cells and basophils is totally unknown. These numbers are likely to be variable (across individuals and also over time within a given individual) and dynamic.
The levels of both total and allergen-specific serum IgE are readily measureable. Allergen-specific IgE levels vary by more than 100-fold among individuals with food allergies. Quantitatively, the level of specific serum IgE is predictive of allergic reactivity, and serum cutoff values for IgE levels have been established to preclude the need for diagnostic oral challenge tests in patients known to exhibit classical clinical histories for food-allergic reactions (Sampson, 2001). However, the presence of serum allergen-specific IgE can also be demonstrated in individuals who are not reactive to the specific food. Thus, the diagnostic value of serum IgE levels must be coupled to the allergic history of the patient.
Notably, specific serum IgE levels in an allergic individual can be monitored over time to predict with some reliability when oral tolerance has been achieved and the food-allergic state has subsided. Total avoidance of an allergen may lead to a decrease in specific serum IgE levels. This has been clearly documented for penicillin where total avoidance is possible (Celik et al., 2009). The effect of total avoidance is very difficult to evaluate with food allergens; however, due to the presence of very low, subclinical levels of the allergenic food or cross-reactive foods in the diet.
IgG antibodies may also play a role in the elicitation process. Allergen-specific IgG antibodies may block allergen interactions with mast cell-bound or basophil-bound IgE antibodies, thus inhibiting the allergic response (Flicker and Valenta, 2003). Furthermore, allergen-specific IgG antibodies may activate inhibitory receptors on mast cells and thus down-regulate IgE-mediated mast cell activation and the release of mediators (Saxon et al., 2008). Thus, the production of allergen specific IgG antibodies may have the effect of increasing the minimum dose required to provoke an allergic response (i.e., increasing the threshold dose).
Lymphocytes also play a role in the inflammatory process in a manner that could affect thresholds. For example, in the inflammatory process, both at the gut level and elsewhere, antigen elicits not only the immediate activation of mast cells and basophils, but may also stimulate antigen-specific lymphocytes to produce cytokines within several hours to amplify the resulting allergic inflammation (Luccioli et al., 2002; Knight et al., 2007). This process is itself modified by regulatory T-cells and polymorphisms in key receptors, such as the IL-4 receptor.
In summary, multiple factors contribute to determining the outcome of this key event. Note that some factors may increase the likelihood of a response (i.e., decrease the minimum dose required for a response), while others may decrease the likelihood of response (i.e., increase the threshold dose). The ultimate response to a given dose is influenced by a complex combination of the following (sometimes contradictory) factors at this key event:
The number and type of effector cells (mast cells and basophils) in the body
Tissue localization of the mast cells
The number of specific IgE antibodies on the surface of each effector cell
The number/concentration of circulating free IgE molecules
The number of nonspecific IgE antibodies on the surface of each effector cell
The amount of blocking (IgA and IgG) antibodies
For each effector cell, the magnitude of the response to IgE binding of target peptides is determined by:
The types of mediator molecules present in the cell
The concentrations of the mediator molecules in the cell
The percent of each cell's load of mediator molecules released in response to each IgE cross-linking event
The ability of the cell to generate and release unstored mediators
Cellular Events—Interaction of Mediators with Tissues
The interaction of mediator molecules with various cells and tissues in the body results in the signs and symptoms of an allergic reaction. The nature and severity of the reaction are determined by:
The site of mediator release
The catabolism/half-life of the mediator molecules in blood and tissues
The final concentration of the mediator molecules in the affected tissue
The number and distribution of receptors for the mediator in the affected tissue(s)
The cascade of reactions in response to mediators, including:
The ability of cells in affected tissue to respond to mediator-binding events
The presence and capacity of any homeostatic mechanisms
The site of mediator release is a factor worthy of consideration. Empirical evidence indicates that systemic allergic reactions (e.g., onset of hives) progress quickly, suggesting that mediators are rapidly dispersed from the original site of allergen-effector interaction to the various tissues where signs and symptoms result. Allergic reactions can also occur in the absence of systemic release of mediators, however. If sufficient levels of mediators are released within a given tissue, the resulting response can give the appearance of target organ specificity. Accordingly, respiratory symptoms occur more commonly with inhalation allergens than with food allergens. It is also conceivable that certain target organs, for example the lung may contain populations of cells with lower thresholds for activation, although this possibility has not been demonstrated.
In addition to the location of mediator-tissue interactions, variables related to the specific type of mediator and specific receptor polymorphisms may affect the nature or severity of response. Dozens of different mediators have been identified including mediators released from mast cells and basophils, as well as mediators generated in the cascade of events that is activated by those cells (Wasserman, 2008; Gandhi et al., 2009). Given the very large number of possible mediator-receptor combinations, the situation becomes quite complex. Moreover, certain host conditions or behaviors may also affect the outcome (e.g., use of ACE inhibitors) (Sabroe and Black, 1997).
Some mediators, such as the key mediator histamine, have been reasonably well studied.2 Less is known, however, about other mediators. The specific nature of the mediator and its catabolism may be quite critical in determining the mediator dose required to elicit a response (i.e., critical to determining an individual's threshold dose). For example, the half-life of histamine in serum is quite short owing in part to the action of histamine-catabolizing enzymes. Again, host conditions or behaviors, such as the use of certain pharmaceuticals, may affect rates of catabolism and thereby affect threshold doses (Hui and Taylor, 1985). Recently, the role of the platelet-activating factor has been examined in anaphylactic reactions, and data suggest that variability in degradation of this mediator by a circulating degradative enzyme is critical to the expression and severity of anaphylaxis (Vadas et al., 2008).
DISCUSSION
Existing human clinical data on elicitation of the food allergic response very clearly show the existence of threshold doses. But, a hallmark of the food allergic response is the remarkable variability in threshold doses across sensitized individuals (i.e., the relevant population). As noted previously, individual thresholds for elicitation of response to peanut range from 0.5 mg to 8000–10,000 mg (Taylor et al., 2009). Moreover, within a given individual, the threshold dose can vary over time. There is also variability in nature and severity of response to a given dose of a particular food allergen.
But what are the fundamental biological factors that determine the threshold value? What specific underlying differences can explain inter- and intraindividual variability? What is the biological basis of sensitization, which defines the relevant population? What is the role of genetic susceptibility, co-existing disease states, and life-stage in the break-down of tolerance and development of food allergies? Answers to these questions can be expected to substantially advance the scientific basis for public health decisions used to protect food allergic individuals.
Current decision-making relies heavily on data from human clinical challenge trials, and such data will likely remain pivotal in the practical assessment of population thresholds. The Key Events Dose-Response Framework, however, promotes an analytical approach that complements and integrates observational data from clinical trials and the limited mechanistic data gleaned from animal studies. More specifically, this analytical framework:
Facilitates systematic consideration of the wide range of factors that contribute to determining whether a given ingested dose will, or will not, result in signs or symptoms.
Provides deeper insight into potential specific sources of variability in response. This provides a foundation for more quantitatively characterizing population variability.
Helps refine the research agenda for food allergy dose-response assessment.
Helps to generate new hypotheses and pinpoint critically needed data.
Clarifies how new data may be used to refine overall understanding of dose-response –i.e., illustrates where new pieces of information will fit into the overall “puzzle.”
The present study was an initial qualitative attempt to examine key events in the biological pathway underlying elicitation of allergic response to foods. To further this analytical approach, future studies could thoroughly examine the state-of-the-science and all relevant data regarding these key events for a specific food allergen (e.g., peanut). It may also be possible to mine data that were obtained for other purposes from the literature. For example, published clinical studies on the efficacy of various antihistamines may include data from untreated controls that could be used to better characterize the effector molecule-tissue interactions described above.
Another future research strategy could involve compilation of quantitative data on physiological, biochemical, and genetic differences across human subjects for whom threshold doses have already been identified (or at least estimated). This strategy would require a broad collaboration across clinicians, physiologists, molecular biologists, statisticians, and others toward developing a database of biomarkers, genetic polymorphisms, and other relevant variables for a large population of food-allergic individuals. Such an effort would begin a process to quantitatively refine population threshold distributions; this would be immediately useful for practical purposes in public health. Perhaps more importantly, such an effort could be expected to shed much light on the underlying biology of allergic disease. In turn, this would better inform the risk assessment process, and might also inform efforts to develop biomarkers of susceptibility. A compilation of such data would also provide a more robust basis for evaluating whether test populations are representative of the full spectrum of allergic individuals.
ACKNOWLEDGMENTS
This paper is one of the work products of an international group of experts convened by the International Life Sciences Institute (ILSI) Research Foundation. ILSI is a nonprofit, worldwide organization established in 1978 to advance the understanding of scientific issues relating to nutrition, food safety, toxicology, risk assessment, and the environment. The ILSI Research Foundation was established in 1984 to create a vehicle for ILSI to support research. Its risk assessment program sponsors, organizes, and participates in a wide range of activities to develop and disseminate new scientific knowledge, encourage exchange of ideas, and build consensus among scientists from academia, industry, government, and public interest groups in its efforts to improve the scientific basis of risk assessment.
Financial support for this project from the following sources is gratefully acknowledged: ILSI Research Foundation, Health Canada, Ajinomoto, Coca-Cola Company, Groupe Danone, Kellogg Company, Kraft Foods, Inc., Mars, Inc., Mead Johnson Nutritionals, Monsanto Company, Nestlé, PepsiCo, Inc., The Procter & Gamble Company, Syngenta Ltd., Flavor Extract Manufacturers Association, and Grocery Manufacturers Association. Assistance from Ms. Julie Fitzpatrick with coordination of the final preparation of these papers is also gratefully acknowledged.
Declaration of Interests: Dr. Taylor holds a royalty-bearing license with Neogen Corp. for the development of certain immunoassay kits. All other authors declare that they have no relevant interests to disclose.
Footnotes
1 While ingestion is the typical route of exposure of concern for food allergens, symptoms can sometimes also occur via contact of these allergens with skin or with the respiratory mucosa.
2 For histamine, human data on its intravenous toxicity exist (Weiss et al., 1932).
REFERENCES
- Aalberse R. C., Akkerdaas J. H., Van Ree R. Cross-reactivity of IgE antibodies to allergens. Allergy. 2001;56:478–490. doi: 10.1034/j.1398-9995.2001.056006478.x. [DOI] [PubMed] [Google Scholar]
- Ballmer-Weber B. K. Lipid transfer protein as a panallergen? Allergy. 2002;57:873–875. doi: 10.1034/j.1398-9995.2002.23541.x. [DOI] [PubMed] [Google Scholar]
- Barnes R. M. R. IgG and IgA antibodies to dietary antigens in food allergy and intolerance. Clin. Exp Allergy. 1995;25:7–9. doi: 10.1111/j.1365-2222.1995.tb01124.x. [DOI] [PubMed] [Google Scholar]
- Bernhisel-Broadbenet J., Sampson H. A. Cross-allergenicity in the legume botanical family in children with food hypersensitivity. J Allergy Clin Immunol. 1989;83:435–440. doi: 10.1016/0091-6749(89)90130-9. [DOI] [PubMed] [Google Scholar]
- Bindslev-Jensen C., Briggs D., Osterballe M. Can we determine a threshold level for allergenic foods by statistical analysis of published data in the literature? Allergy. 2002;57:741–746. doi: 10.1034/j.1398-9995.2002.23797.x. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Hill D. J., Hosking C. S. Natural history of cow milk allergy: clinical outcome. J. Pediatr. 1990;116:862–867. doi: 10.1016/s0022-3476(05)80641-9. [DOI] [PubMed] [Google Scholar]
- Bock S. A. The natural history of adverse reactions to food. N Engl Reg Allergy Proc. 1986;7:504–510. doi: 10.2500/108854186779045458. [DOI] [PubMed] [Google Scholar]
- Celik G., Pichler W. J., Adkinson N. F., Jr. Drug allergy. In: Adkinson N. F. Jr., Holgate S. T., Bochner B. S., Lemanske R. F. Jr, Busse W. W., Simons F. E. R., editors. Middleton's Allergy Principles and Practices. 7th ed. Vol. 2. New York: Elsevier Health Sciences; 2009. pp. 1205–1226. [Google Scholar]
- Crevel R. W. R., Briggs D., Hefle S. L., Knulst A. C., Taylor S. L. Hazard characterisation in food allergen risk assessment: the application of statistical approaches and the use of clinical data. Food Chem Toxicol. 2007;45:691–701. doi: 10.1016/j.fct.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Flicker S., Valenta R. Renaissance of the blocking antibody concept in type I allergy. Int. Arch Allergy Immunol. 2003;132:13–24. doi: 10.1159/000073260. [DOI] [PubMed] [Google Scholar]
- Gandhi C., Wasserman S. I., Grammer L., Greenberger P. Patterson's Allergic Diseases. 7th Edition. Philadelphia: Lippincott, Williams and Wilkins; 2009. Biochemical Mediators of Allergic Reactions. [Google Scholar]
- Goodman R. E., Vieths S., Sampson H. A., Hill D., Ebisawa M., Taylor S. L., van Ree R. Allergenicity assessment of genetically modified crops -what makes sense? Nature Biotechnol. 2008;26:73–81. doi: 10.1038/nbt1343. [DOI] [PubMed] [Google Scholar]
- Health Council of the Netherlands. Food Allergy. The Hague: HealthCouncil of the Netherlands; 2007. Publication No. 2007/07. [Google Scholar]
- Hefle S., Nordlee J., Taylor S. L. Allergenic foods. Crit. Rev. FoodSci. Nutr. 1996;36:S91–S118. doi: 10.1080/10408399609527760. [DOI] [PubMed] [Google Scholar]
- Hefle S. L., Furlong T. J., Niemann L., Lemon-Mule H., Sicherer S., Taylor S. L. Consumer attitudes and risks associated with packaged foods having advisory labeling regarding the presence of peanuts. J. Allergy Clin Immunol. 2007;120:171–176. doi: 10.1016/j.jaci.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Hui J. Y., Taylor S. L. Inhibition of in vivo histamine metabolism in rats by foodborne and pharmacologic inhibitors of diamine oxidase, histamine N-methyltransferase, and monoamine oxidase. Toxicol. Appl Pharmacol. 1985;81:241–249. doi: 10.1016/0041-008x(85)90160-7. [DOI] [PubMed] [Google Scholar]
- Jones S. M., Scurlock A. M., Pons L., Kulis M., Perry T. T., Steele P., Kamilaris J., Henry K. A., Burks A. W. Double-blind, placebo-controlled (DBPC) trial of oral immunotherapy (OIT) in peanut allergic children. J. Allergy Clin Immunol. 2009;123:S211. [Google Scholar]
- Julien E., Boobis A., Olin S. S. the ILSI Research Foundation Threshold Working Group. The Key Events Dose-Response framework: A cross-disciplinary, mode-of-action based approach to examining dose-response and thresholds. Crit. Rev. Food Sci Nutr. 2009;49:682–689. doi: 10.1080/10408390903110692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A. K., Blazquez A. B., Zhang S., Mayer L., Sampson H. A., Berin M. C. CD4 T cells activated in the mesenteric lymph node mediate gastrointestingal food allergy in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G1234–G1243. doi: 10.1152/ajpgi.00323.2007. [DOI] [PubMed] [Google Scholar]
- Knippels LM, van Wijk F., Penninks A. H. Food allergy: what do we learn from animal models? Curr Opinion Allergy Clin. Immunol. 2004;4:205–209. doi: 10.1097/00130832-200406000-00012. [DOI] [PubMed] [Google Scholar]
- Kruizinga A. G., Briggs D., Crevel R., Knulst A., van de Bosch L., Houben G. Probabilistic risk assessment model for allergens in food: sensitivity analysis of the minimum eliciting dose and food consumption. Food Chem Toxicol. 2008;46:1437–1443. doi: 10.1016/j.fct.2007.09.109. [DOI] [PubMed] [Google Scholar]
- Lack G. Epidemiologic risks for food allergy. J. Allergy Clin Immunol. 2008;121:1331–1336. doi: 10.1016/j.jaci.2008.04.032. [DOI] [PubMed] [Google Scholar]
- Lehmann K., Schweimer K., Reese G., Randow S., Suhr M., Becker W. M., Vieths S., Rosch P. Structure and stability of 2S albumintype peanut allergens: implications for severity of peanut allergic reactions. Biochem J. 2006;394:463–475. doi: 10.1042/BJ20051728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S. B., Ayuso R., Reese G. Seafood allergy and allergens: a review. Marine Biotechnl. 2003;5:339–348. doi: 10.1007/s10126-002-0082-1. [DOI] [PubMed] [Google Scholar]
- Luccioli S., Brody D. T., Hasan S., Keane-Myers A., Prussin C., Metcalfe D. D. IgE(+), Kit(-), I-A/I-E(-) myeloid cells are the initial source of Il-4 after antigen challenge in a mouse model of allergic pulmonary inflammation. J Allergy Clin Immunol. 2002;110((1)):117–124. doi: 10.1067/mai.2002.125828. [DOI] [PubMed] [Google Scholar]
- Mayer L. Mucosal Immunity. Pediatr. 2003;111((Suppl)):1595–1600. [PubMed] [Google Scholar]
- Moneret-Vautrin D. A., Morisset M. Adult food allergy. Curr Allergy Asthma Rpt. 2005;5:80–85. doi: 10.1007/s11882-005-0060-6. [DOI] [PubMed] [Google Scholar]
- Nelde A., Teufel M., Hahn C., Duschl A., Sebald W., Brocker E. B., Grunewald S. M. The impact of the route and frequency of antigen exposure on the IgE response in allergy. Int. Arch Allergy Immunol. 2001;124((4)):461–469. doi: 10.1159/000053781. [DOI] [PubMed] [Google Scholar]
- Nowak-Wegrzyn A., Sampson H. A. Adverse reactions to foods. Med Clin No Am. 2006;90:97–127. doi: 10.1016/j.mcna.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Ortolani C., Ispano M., Pastorello E., Bigi A., Ansaloni R. The oral allergy syndrome. Ann Allergy. 1988;61:47–52. [PubMed] [Google Scholar]
- Sabroe R. A., Black A. K. Angiotensin-converting enzyme (ACE) inhibitors and angio-edema. Br. J. Dermatol. 1997;136:153–158. [PubMed] [Google Scholar]
- Sampson H. A. Anaphylaxis and emergency treatment. Pediatrics. 2003;111((Suppl)):1601–1608. [PubMed] [Google Scholar]
- Saxon A., Kepley C., Zhang K. Accentuate the negative, eliminate the positive: engineering allergy therapeutics to block allergic reactivity through negative signaling. J. Allergy Clin Immunol. 2008;121((2)):320–325. doi: 10.1016/j.jaci.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Sicherer S. H., Morrow E. H., Sampson H. A. Dose-response in double-blind, placebo-controlled oral food challenges in children with atopic dermatitis. J. Allergy Clin Immunol. 2000;105:582–586. doi: 10.1067/mai.2000.104941. [DOI] [PubMed] [Google Scholar]
- Skripak J. M., Matsui E. C., Mudd K., Wood R. A. The natural history of IgE-mediated cow's milk allergy. J. Allergy Clin Immunol. 2007;120:1172–1177. doi: 10.1016/j.jaci.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Spanjersberg M. Q. I., Kruizinga A. G., Rennen M. A. J., Houben G. F. Risk assessment and food allergy: the probabilistic model applied to allergens. Food Chem Toxicol. 2007;45:49–54. doi: 10.1016/j.fct.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Strobel S., Mowat A. M. Oral tolerance and allergic responses to food proteins. Curr. Opinion Allergy Clin Immunol. 2006;6:207–213. doi: 10.1097/01.all.0000225162.98391.81. [DOI] [PubMed] [Google Scholar]
- Taylor S. L., Hefle S. L. Food allergies and other food sensitivities. Food Technol. 2001;55((9)):68–83. [Google Scholar]
- Taylor S. L., Hourihane J.O'B., Metcalfe D. D., Sampson H. A., Simon R. A. Food Allergy -Adverse Reactions to Foods and Food Additives. 4th ed. Malden MA: Blackwell Publishing; 2008. Food allergen thresholds of reactivity; pp. 82–89. [Google Scholar]
- Taylor S. L., Lehrer S. B. Principles and characteristics of food allergens. Crit. Rev. Food Sci. Nutr. 1996;36:S69–S89. doi: 10.1080/10408399609527761. [DOI] [PubMed] [Google Scholar]
- Taylor S. L., Hefle S. L., Bindslev-Jensen C., Atkins F. M., Andre C., Bruijnzeel-Koomen C., Burks A. W., Bush R. K., Ebisawa M., Eigenmann P. A., Host A., Hourihane J.O'B., Isolauri E., Hill D. J., Knulst A., Lack G., Sampson H. A., Moneret-Vautrin D. A., Rance F., Vadas P. A., Yunginger J. W., Zeiger R. S., Salminen J. W., Madsen C., Abbott P. A consensus protocol for the determination of the threshold doses for allergenic foods: how much is too much? Clin. Exp Allergy. 2004;34:689–695. doi: 10.1111/j.1365-2222.2004.1886.x. [DOI] [PubMed] [Google Scholar]
- Taylor S. L., Crevel R. W. R., Sheffield D., Kabourek J., Baumert J. Threshold dose for peanut: a risk assessment based upon results from challenges of peanut-allergic individuals. Food Chem Toxicol. 2009;47:1198–1204. doi: 10.1016/j.fct.2009.02.011. [DOI] [PubMed] [Google Scholar]
- US FDA/CFSAN Threshold Working Group. Approaches to establish thresholds for major food allergens and for gluten in food. J. Food Prot. 2008;71:1043–1088. doi: 10.4315/0362-028x-71.5.1043. [DOI] [PubMed] [Google Scholar]
- Untersmayr E., Scholl I., Swoboda I., Beil W., Forster-Waldl E., Walter F., Riemer A., Kraml G., Kinaciyan T., Spitzauer S., Boltz-Nitulescu G., Scheiner O., Jensen-Jarolim E. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in Balb/c mice. J Allergy Clin Immunol. 2003;112:616–623. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- Vadas P., Gold M., Perelman B., Liss G. M., Lack G., Blyth T., Simons F. E. R., Simons K. J., Cass D., Yeung J. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N. Engl. J. Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- Van Ree R. Clinical importance of cross-reactivity in food allergy. Curr. Opinion Allergy Clin Immunol. 2004;4:235–240. doi: 10.1097/00130832-200406000-00017. [DOI] [PubMed] [Google Scholar]
- Venter C., Pereira B., Grundy J., Clayton C. B., Arshad S. H., Dean T. Prevalence of sensitization reported and objectively assessed food hypersensitivity amongst six-year-old children: a population-based study. Pediatr Allergy Immunol. 2006;17:356–363. doi: 10.1111/j.1399-3038.2006.00428.x. [DOI] [PubMed] [Google Scholar]
- Wasserman S. I. The mast cell: its diversity of chemical mediators. Int. J. Dermatol. 1980;19:7–17. doi: 10.1111/j.1365-4362.1980.tb01984.x. [DOI] [PubMed] [Google Scholar]
- Weiss S., Robb G. P., Ellis L. B. The systemic effects of histamine in man. Arch. Int Med. 1932;49:360. [Google Scholar]
- Zeiger R. S., Heller S. The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomize study of combined maternal and infant food allergen avoidance. J. Allergy Clin Immunol. 1995;95:1179–1190. doi: 10.1016/s0091-6749(95)70074-9. [DOI] [PubMed] [Google Scholar]


