Abstract
Virtually all cells in the body have the capacity to recognize and respond to dead cells. Viable cells discriminate apo from nec targets via distinct cell surface receptors. Engagement of these receptors induces “recognition-dependent” signaling events in viable responding cells that differ for apo vs. nec targets. Although “engulfment-dependent” signaling events also contribute to the response by viable cells, these events do not differ for apo vs. nec targets. While many signaling events are conserved across diverse cell lineages, other signaling events, especially those involving Akt, demonstrate lineage-specific variation. Whereas apo targets activate Akt in MΦ, they inhibit Akt in kidney epithelial cells. Differences in the responses to dead targets by viable migratory cells, such as MΦ, and viable fixed cells, such as kidney epithelial cells, permit cell-specific adaptations to local environmental change or stress. We propose that dead cells (apo and nec) act as sentinels to alert nearby viable cells to local environmental change or stress.
Keywords: apo, nec, inflammation, signal transduction, phagocytosis
Distinguishing apo from nec cell death
Until recently, apo cells were thought of as inert debris, rapidly removed by resident or infiltrating phagocytes without any discernible effect on surrounding tissue. A growing literature now suggests that apo cells play a major role in immune homeostasis [1]. One of the defining features of apo cells is their ability to act as potent anti-inflammatory agents [2,3]. This is in stark contrast to nec cells, which are typically associated with inflammation. These opposing effects on inflammation represent one of the major functional differences between apo and nec cell death.
One would predict that such distinct effects on the inflammatory response should be linked to differences in the signaling events elicited in phagocytes upon interaction with apo vs. nec target cells. This is in fact the case. Two well-characterized examples of signaling events differentially affected by apo and nec targets are NF-κB-dependent transcriptional activity [4,5] and activation of the ERK1/2 signaling module [6]. While apo targets potently inhibit both of these activities, nec cells stimulate them. Modulation of these activities by apo and nec targets occurs early (within minutes for ERK1/2), and is a direct consequence of phagocytic interaction with cellular corpses, independent of autocrine/paracrine factors such as TGF-β [5].
Under most circumstances, apo cells are cleared very rapidly before they lose membrane integrity. It is a widespread opinion that apo cells, if not cleared in a timely manner, behave like nec cells, because of release of intracellular contents [4,5,7]. To the contrary, apo cells appear to be functionally equivalent throughout all the stages of their existence, regardless of membrane integrity [5,7]. Rather than mimicking nec cells, late apo cells behave under certain circumstances identically to early apo cells. This is true with respect not only to their anti-inflammatory properties, but also to the inhibition of NF-κB-dependent transcription and ERK1/2 activity [5,7]. These results imply that apo targets deliver a consistent “message” to nearby cells for the duration of their presence.
The uniform effect of apo cells, despite leakage of intracellular contents, suggests caution in attributing the inflammatory effects of nec cells to their swelling and rupture. The only leaked component from nec cells clearly shown to be inflammatory is the nuclear protein HMGB1 [8]. As expected, apo cells do not release HMGB1, even upon loss of membrane integrity [9]. It remains an unresolved issue which effects of nec cells are attributable to HMGB1 and which to the nec cell corpse. At least with respect to ERK1/2 activation, as suggested by differential centrifugation, both HMGB1 and nec corpses possess stimulatory activity [7].
Of note, several observations suggest that the relative potency of nec cells as a stimulus may be less than that of apo cells. For example, in “head-to-head” competition, and with respect to ERK1/2 activity, apo targets were able to negate fully the effect of nec targets, even when present at one-eighth the number of nec cells [7].
Distinguishing between recognition and engulfment of dead target cells
The contrasting inflammatory effects and signaling events induced by apo vs. nec cells indicate that phagocytes can discriminate these two distinct forms of target cell death. MΦ recognition of each type of dead cell occurs via a saturable, receptor-mediated process [4]. Notably, the receptors that mediate binding of apo targets do not compete with those for nec targets, and vice versa. The existence of separate receptors is consistent with the discrete downstream signaling events elicited by apo vs. nec cells, and implies that these two forms of cell death provide independent information to responding phagocytes.
Identification and characterization of these receptors remain an area of active investigation. Although several putative receptors for apo corpses have been described, it is critical to note that their analysis has, in many cases, been incomplete [10–12]. Most functional tests have evaluated apo target “uptake”, without discriminating between actual recognition and engulfment. This is especially important with respect to downstream signaling events, since apo and nec targets share a common pathway for engulfment. Thus, signals and/or outcomes that are a consequence of engulfment must be the same for apo and nec targets. Only those events dependent on receptor-mediated discrimination can differ for apo vs. nec targets.
We and others have begun to distinguish engulfment-dependent from recognition-dependent signaling events. A clear example of the former is activation of the PI3K/Akt axis, occurring upon exposure of MΦ to apo targets, nec targets, or even latex beads [6,7]. Importantly, pharmacologic inhibition of phagocytosis prevents PI3K/Akt activation in response to these targets, demonstrating the dependence on engulfment and the lack of a recognition-dependent Akt response in MΦ [6,7].
Signaling activities, such as NF-κB-dependent transcription and ERK1/2 activity, which differ for apo vs. nec targets are, therefore, attributable to receptor-mediated recognition. These activities, while potently inhibited by apo targets, tend to be activated by nec targets [5–7]. Consistent with independence of engulfment, pharmacologic inhibition of phagocytosis had no effect on the changes in NF-κB-dependent transcriptional activity [4].
Hypothesis: Dead cells are sentinels of local environmental change
Uptake of apo corpses is not limited to professional phagocytes. Similarly, the ability to recognize and signal differentially in response to apo and nec targets extends to non-professional phagocytes. For example, mouse fibroblasts and human epithelial cells demonstrate inhibition of NF-κB-dependent transcription upon specific recognition of apo targets [13]. In fact, we have observed no exceptions to the ubiquity of this inhibitory response. Even non-phagocytic B- and T-lymphocytes exhibit the same conserved response to apo cell recognition [13]. These data indicate that the network of signaling responses to apo and nec targets, as observed in viable cells of multiple lineages, represents an under-appreciated and ubiquitous source of information regarding the environment.
We hypothesize that cell death plays an important role in normal tissue homeostasis. Alterations in the extent and type of cell death, as triggered by such diverse events as ischemia, infection, aging, and acute injury, have the potential to alert neighboring viable cells to environmental changes or stress [14,15]. By this scheme, dead cells transmit a “message” to nearby viable cells about the nature of their immediate environment. In most cases, the signaling events induced in responding cells by apo vs. nec targets are directionally opposite. Such a differential provides neighboring viable cells with a means or gauge by which to assess the overall severity of any environmental change. A predominance of nec cell death would suggest a sudden and severe environmental event. Apo cell death, on the other hand, would represent a more gradual and potentially adaptable change.
This hypothesis has important implications. First, since the signaling responses of viable neighboring cells require physical interaction with cellular corpses, the effects will be local and limited to the close environment of the dead cell. Second, the universal nature of the response to dead targets implies that every cell, tissue, or organ has the capacity to sense and react to environmental change. Finally, the response to environmental change is likely complex, and viable cells will need to integrate information from multiple extracellular cues in addition to those relating to cell death.
The response of macrophages to dead targets
MΦ are the major cell type responsible for the elimination of dead cells [16,17]. MΦ circulate in the bloodstream as non-professional phagocytes and are actively recruited to sites of tissue inflammation or injury by chemokines and other soluble mediators. There, they differentiate into MΦ and begin the task of clearing dead cells and debris. After resolution of the stimulus that attracted them, MΦ do not return to the circulation. The fact that MΦ are migratory means there is no one environment to which they must adapt.
In their capacity as professional phagocytes, MΦ will in general be recruited to tissues having an increased number of dead cells. MΦ interaction with both apo and nec targets leads to activation of the survival kinase Akt through an engulfment-dependent mechanism [6,7], thereby conferring a selective advantage in survival. The presence of dead cells, or other phagocytic targets requiring elimination, thus promotes MΦ longevity.
This raises the interesting question: why should the interaction of MΦ with dead targets, whether apo or nec, lead to Akt activation and enhanced survival? As professional phagocytes, MΦ can clear dead cells and debris more rapidly and efficiently than fixed resident cells. Also, MΦ may need to perform their job under adverse conditions. Two extreme scenarios can be envisioned for which a survival advantage is beneficial. First is severe infection or inflammation. Infiltrating MΦ must survive until full resolution of the inciting process. Resolution entails the clearing of not only resident cells that are damaged or dead but also other infiltrating innate immune cells, such as short-lived neutrophils. On the other hand, local ischemia may lead to an increased rate of cell death because of a relative deficiency of nutrients and growth factors. Here, enhanced MΦ survival permits clearance without further depletion of an already compromised supply of growth factors. Thus, dead cells can be seen as a MΦ survival factor.
Beyond the macrophage to other cell types
Most cells in the body are fixed within organs and lack the capacity to migrate. Any change to their environment requires their adaptation. An increase in the number of dead cells nearby may signal the occurrence of important changes or stresses. The mode of death can provide a clue to the nature of that change and help to guide the form of adaptation of neighboring viable cells. Thus, the response of viable organ-specific, non-migratory cells to cellular corpses in their vicinity should differ in critical aspects from that by MΦ.
We have examined the signaling response by kidney epithelial cells to apo and nec targets [18]. The Akt response of kidney epithelial cells differs in several ways from that of MΦ. Most strikingly, while apo targets activate Akt in MΦ, they inhibit Akt in kidney epithelial cells. In contrast to MΦ, inhibition of Akt in kidney epithelial cells exposed to apo targets is independent of phagocytosis, and triggered exclusively by receptor-mediated recognition. Finally, kidney epithelial cells respond to nec targets by the activation of Akt.
To understand the divergent responses to apo vs. nec targets by kidney epithelial cells, one must consider the distinct implications of an increase in the number of cells undergoing these two forms of cell death. We consider first the case of increased apo cell death. In the setting of progressive atherosclerosis, for example, chronic ischemia may lead to a diminished delivery of oxygen and nutrients. The resulting increase of apo death will partially inhibit Akt in neighboring cells and lead to a reduction in population size. When supply and demand are once again matched, apo death will return to baseline levels, and a new steady state will be achieved.
An increase of nec cell death, as may occur in the setting of vascular occlusion or infarction, carries very different implications. The environmental change is so acute and drastic that compensatory long-term adaptation is not possible, and any cell survival may be of benefit. Increased Akt activity constitutes the cellular equivalent of a “flight-or-fright” response and works to ensure the long-term survival of the organ.
Concluding remarks
Here, we have put forth the hypothesis that dead cells play an important role in the overall homeostasis of tissues by acting as sentinels of environmental change. In evaluating the “message” that dead cells deliver to nearby viable cells, three dichotomies are important to keep in mind (Figure 1). The first is the form of death. Apo death may indicate a gradual and potentially adaptable change, while nec death suggests a more catastrophic change. The second is the distinction between recognition- and engulfment-dependent signals. The third is the nature of the responding cell, migratory and able to evacuate, or fixed and forced to adapt. There is the potential for additional complexity in this response, in that certain viable responder cells may discriminate between apo death caused by different stimuli [19,20].
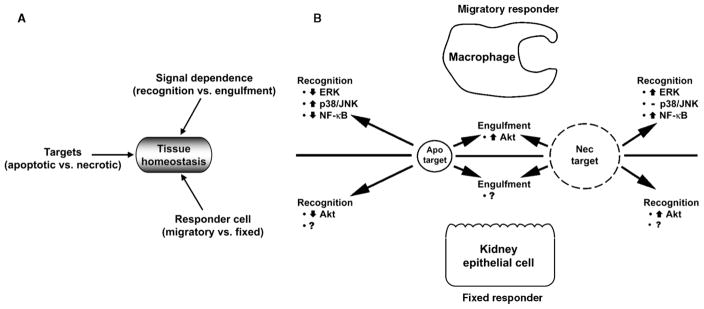
Figure 1.
Dead cells act as sentinels of local environmental change. (A) As depicted, the nature of the response of nearby viable cells to dead target cells is determined by three dichotomies: (1) the form of target cell death (apo vs. nec); (2) the dependence of viable cell signals on receptor-mediated recognition vs. engulfment of the dead targets; and (3) the nature of the responding cell (migratory vs. fixed). (B) The responses of MΦ, a migratory cell, are contrasted with those of fixed and organ-specific kidney epithelial cells.
Acknowledgments
This work was supported by a GRIP Renal Innovations Program Award from Genzyme, Inc. (JSL), NIH grants K01DK071678 (VAP) and AG024234 (DSU), a Veterans Administration Merit Award (WL), and Canadian Institutes of Health Research operating grant MOP-67101 (JR).
References
- 1.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: Clearance of apo cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 2.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apo cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apo cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 4.Cocco RE, Ucker DS. Distinct modes of macrophage recognition for apo and nec cells are not specified exclusively by phosphatidylserine exposure. Mol Biol Cell. 2001;12:919–930. doi: 10.1091/mbc.12.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cvetanovic M, Ucker DS. Innate immune discrimination of apo cells: Repression of pro-inflammatory macrophage transcription is coupled directly to specific recognition. J Immunol. 2004;172:880–889. doi: 10.4049/jimmunol.172.2.880. [DOI] [PubMed] [Google Scholar]
- 6.Reddy SM, Hsiao KH, Abernethy VE, Fan H, Longacre A, Lieberthal W, Rauch J, Koh JS, Levine JS. Phagocytosis of apo cells by macrophages induces novel signaling events leading to cytokine-independent survival and inhibition of proliferation: Activation of Akt and inhibition of extracellular signal-regulated kinases 1 and 2. J Immunol. 2002;169:702–713. doi: 10.4049/jimmunol.169.2.702. [DOI] [PubMed] [Google Scholar]
- 7.Patel VA, Longacre A, Hsiao K, Fan H, Meng F, Mitchell JE, Rauch J, Ucker DS, Levine JS. Apo cells, at all stages of the death process, trigger characteristic signaling events that are divergent from and dominant over those triggered by nec cells: Implications for the delayed clearance model of autoimmunity. J Biol Chem. 2006;281:4663–4670. doi: 10.1074/jbc.M508342200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: Endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by nec cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 10.Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apo thymocytes in vitro. Proc Natl Acad Sci USA. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marguet D, Luciani MF, Moynault A, Williamson P, Chimini G. Engulfment of apo cells involves the redistribution of membrane phosphatidylserine on phagocyte and prey. Nat Cell Biol. 1999;1:454–456. doi: 10.1038/15690. [DOI] [PubMed] [Google Scholar]
- 12.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apo cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 13.Cvetanovic M, Mitchell JE, Patel V, Avner BS, Su Y, van der Saag PT, Witte PL, Fiore S, Levine JS, Ucker DS. Specific recognition of apo cells reveals a ubiquitous and unconventional innate immunity. J Biol Chem. 2006;281:20055–20067. doi: 10.1074/jbc.M603920200. [DOI] [PubMed] [Google Scholar]
- 14.Sanz AB, Santamaría B, Ruiz-Ortega M, Egido J, Ortiz A. Mechanisms of renal apo in health and disease. J Am Soc Nephrol. 2008;19:1634–1642. doi: 10.1681/ASN.2007121336. [DOI] [PubMed] [Google Scholar]
- 15.Bonegio R, Lieberthal W. Role of apo in the pathogenesis of acute renal failure. Curr Opin Nephrol Hypertens. 2002;11:301–308. doi: 10.1097/00041552-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Naito M. Macrophage differentiation and function in health and disease. Pathol Int. 2008;58:143–155. doi: 10.1111/j.1440-1827.2007.02203.x. [DOI] [PubMed] [Google Scholar]
- 17.Geske FJ, Monks J, Lehman L, Fadok VA. The role of the macrophage in apo: Hunter, gatherer, and regulator. Int J Hematol. 2002;76:16–26. doi: 10.1007/BF02982714. [DOI] [PubMed] [Google Scholar]
- 18.Patel VA, Lee DJ, Feng L, Lieberthal W, Ucker DS, Levine JS. Apo cells as sentinels of environmental and local tissue stress: Response pathways activated in viable renal epithelial cells. J Am Soc Nephrol. 2007;18:29A. [Google Scholar]
- 19.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 20.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]