Abstract
Recent clinic-based and population-based studies have shown evidence of association between ADHD and autistic symptoms in children and adolescents as well as evidence for genetic overlap between these disorders. The objective of the current study was to confirm the association between autistic and ADHD symptoms in a young adult twin sample assessed by self-report, and investigate whether shared genetic and/or environmental factors can explain the association. We performed twin-based structural equation modeling using self-report data from 11 Social Responsiveness Scale (SRS) items and 12 DSM-IV ADHD inattentive and impulsive symptom items obtained from 674 young adult Australian twins. Phenotypic correlation between autistic and ADHD symptoms was moderate. The most parsimonious univariate models for SRS and ADHD included additive genetic effects and unique environmental effects, without sex differences. ADHD and autistic traits were both moderately heritable. In a bivariate model, genetic correlation (rg) between SRS and ADHD was 0.72. Our results suggest that in young adults, a substantial proportion of the genetic influences on self-reported autistic and ADHD symptoms may be shared between the two disorders.
Keywords: ADHD, Autism, comorbidity, heritability, twin study
Clinic-based (Clark, Feehan, Tinline, & Vostanis, 1999; Goldstein & Schwebach, 2004; Santosh & Mijovic, 2004; Sturm, Fernell, & Gillberg, 2004) and population-based (Constantino, Hudziak, & Todd, 2003; Reiersen, Constantino, Volk, & Todd, 2007) studies suggest many children have symptoms consistent with comorbid Attention-Deficit/Hyperactivity Disorder (ADHD) and Autism Spectrum Disorder (ASD). Overlap in linkage peaks between ADHD and autism suggests some genes may influence both of these disorders (Smalley, Loo, Yang, & Cantor, 2005), and a recent twin study also found evidence for genetic overlap between autistic and ADHD symptoms in children (Ronald, Simonoff, Kuntsi, Asherson, & Plomin, 2008).
Constantino, Hudziak, and Todd reported on the first twin study investigating the relationship between ADHD symptoms and autistic traits in children and adolescents (Constantino et al., 2003). In this study of 219 male twin pairs (438 individuals), linear regression was used to investigate the relationship between Child Behavior Checklist (CBCL) syndrome scales and Social Responsiveness Scale (SRS) score. As expected, the CBCL social problems scale showed a statistically significant association with SRS score. Unexpectedly, the CBCL attention problems subscale was also a significant predictor of SRS score. Twin modeling was then used to further investigate the relationship between the CBCL attention problems scale and SRS score. In the preferred bivariate twin model, the genetic factors affecting SRS and ADHD symptoms were separate, and reciprocal causation within individuals accounted for the correlation between SRS and ADHD symptoms.
Ronald and colleagues also recently published a twin study investigating the relationship between ADHD and autistic symptoms in children(Ronald et al., 2008). In their study, the preferred model was a bivariate Cholesky (correlated factors) AE model including sex differences (and a sibling interaction parameter when parent-report data was used for the analysis). They found high heritability for each trait. They estimated genetic correlations (rg) in the range of 0.54–0.57 and nonshared environment correlations (re) in the range of 0.15–0.35., depending on sex and rater (parent vs. teacher). The authors concluded that there was evidence of genetic overlap between autistic traits and ADHD behaviors.
In a population-based phenotypic study using male and female twins, Reiersen and colleagues reported elevated SRS scores in children with ADHD. The level of autistic traits varied depending on ADHD subtype, with the highest levels of autistic traits in children who had DSM-IV combined type ADHD or a population-defined severe combined ADHD subtype defined using latent class analysis (Reiersen et al., 2007).
We are unaware of any published population-based phenotypic studies of the overlap between ADHD and autism spectrum traits or disorders in adults. However, a recent study of 240 adults referred for neuropsychiatric investigation found that 32% of 147 adults with current or remitted ADHD had an autism spectrum diagnosis. Also, 42% of 113 adults with autism spectrum disorder had symptoms consistent with current or remitted ADHD (Anckarsater et al., 2006).
We are unaware of any published twin studies investigating the relationship between ADHD symptoms and autistic traits in adults. The current study evaluates the association between self-reported ADHD symptoms and autistic traits in a sample of young adult Australian twins, and uses twin modeling to examine the evidence for shared genetic and environmental influences affecting both disorders.
Materials & Methods
Subjects included an unselected population-based sample of 699 Australian twins (284 complete pairs) participating in a personality study (Distel et al., 2008). Mean age was 23 (range 18–33) years, and 57% were female. Targeted participants included Australian twins born between 1972 and 1987 who were either voluntarily registered on the Australian Twin Register (ATR) founded in 1978 (Jardine, Martin, & Henderson, 1984), or had previously taken part in twin studies at Queensland Institute of Medical Research (QIMR). Twins consented for the study electronically, by telephone, or on paper, and the study was approved by the QIMR Human Research Ethics Committee.
Of 310 ATR subjects approached for participation, 268 twins (86.4%) completed the survey. Of 808 twins approached directly by QIMR, 431 (53.3%) completed the survey, resulting in a total of 699 completed surveys (493 online, 206 paper). The zygosity of 674 twins (275 complete pairs) was known either from self report answers to standard questions (N = 299), because the twins were of opposite sex (N = 91), or from DNA testing (N = 284). The standard questions used to determine zygosity have been described elsewhere (Martin & Martin, 1975). The zygosity groups include 136 monozygotic males (50 complete pairs), 76 dizygotic males (29 complete pairs), 193 monozygotic females (85 complete pairs), 108 dizygotic females (48 complete pairs), and 161 opposite sex (63 complete pairs). Since full diagnostic criteria for ADHD and autism spectrum disorders were not assessed and we did not ask about diagnoses previously given, we cannot determine the number of subjects with these diagnoses.
The surveys included 11 autistic and 12 DSM-IV ADHD symptom items, which were modified for self-report. Due to the length of the total questionnaire and the need to minimize subject burden, the autism assessment was limited to items 6, 15, 16, 18, 24, 29, 35, 37, 39, 42, and 58 of the Social Responsiveness Scale (SRS), a 65-item quantitative measure of autistic traits (Constantino & Gruber, 2005; Constantino, Przybeck, Friesen, & Todd, 2000). The selected items encompassed the three DSM-IV autism domains, and had strong loadings on the first unrotated factor of a principal components analysis of the SRS in a pediatric sample. Most of the SRS items were related to deficits in reciprocal social behavior, but there were also three items related to stereotyped/repetitive behaviors (SRS items 24, 29, and 39) and an item related to communication impairment (SRS item 35). ADHD items included only those that seemed most applicable to this age group (9 inattentive and 3 impulsive symptoms from DSM-IV). Ideally it would have been useful to include hyperactive symptoms also, but there was a need to limit the total number of items for the survey. Each item was scored on a 4-point scale (0=false, not at all true; 1=slightly true; 2=mainly true; 3=very true). To create the SRS and ADHD scores, the relevant items were summed. Higher scores indicated more severe symptoms. Scores were natural log transformed prior to statistical modeling to approximate a normal distribution.
Structural equation modeling was implemented using Mx software (M.C. Neale, Boker, Xie, & Maes, 2006), including all twins with known zygosity (n=674 twins). Raw data were analyzed so that twin pairs with missing data on one twin could be used in the analysis. The models used information regarding similarity between twins from various zygosity groups to estimate the proportion of variance in scores attributable to hypothesized latent variables representing additive genetic (A), dominance genetic (D), common environmental (C), and unique environmental (E) factors. All models included sex effects on mean SRS and ADHD scores. After components (such as C or D) of a full model were dropped to obtain submodels, we compared the nested models using a difference in log likelihood chi-square test. If p<0.05, this indicated the submodel fit significantly worse than the full model and the full model was accepted. Otherwise the submodel was accepted as the most parsimonious model.
We began with univariate sex-limitation ACE and ADE models, including the 5 different zygosity groups. Sex-limitation models allow testing for sex differences in terms of genetic influences on the measured trait and the relative importance of A, C, and E (or A, D, and E) in producing variance in the measured trait. The full ACE sex limitation model allows estimates of A, C, and E to be different between males and females, and allows genetic covariance for opposite sex pairs to be estimated rather than fixed. Nested models can then be used to evaluate the evidence for sex differences by testing for significant deterioration of fit in models that constrain A, C, and E to be equal between sexes and/or fix the genetic covariance for opposite sex pairs at 0.5. It was important to evaluate for sex limitation effects in univariate models prior to running bivariate models partly because there are certain biases that occur in bivariate Cholesky decomposition sex limitation models that do not apply to univariate sex limitation models (M. C. Neale, Roysamb, & Jacobson, 2006). We used the best fitting univariate models in designing a bivariate Cholesky decomposition model. The bivariate model allows us to estimate the degree of overlap between genetic and environmental factors that affect SRS and those that affect the ADHD score.
Results
Cronbach’s alpha indicated high inter-item reliability for each scale (SRS alpha=0.81, ADHD alpha=0.82). In the entire sample of 699 subjects, the log-transformed SRS and ADHD scores were significantly correlated within individuals (Pearson’s r=0.48, p<0.0001). Correlation and covariance matrices for various zygosity groups are shown in Table 1.
Table 1.
Variance-covariance and correlation statistics for natural log transformed SRS and ADHD scores. Each matrix shows covariances (below diagonal), variances (diagonal), and Pearson’s r (above diagonal, bold). For opposite-sex twins, twin one is female. For other twins, twin one is the first born twin.
| MZ Males, n=50 complete pairs. | ||||
|---|---|---|---|---|
| SRS Twin 1 | ADHD Twin 1 | SRS Twin 2 | ADHD Twin 2 | |
| SRS Twin 1 | 0.66 | 0.48 | 0.44 | 0.36 |
| ADHD Twin 1 | 0.28 | 0.52 | 0.22 | 0.45 |
| SRS Twin 2 | 0.28 | 0.12 | 0.62 | 0.37 |
| ADHD Twin 2 | 0.21 | 0.23 | 0.21 | 0.51 |
| DZ Males, n=29 complete pairs. | ||||
| SRS Twin 1 | ADHD Twin 1 | SRS Twin 2 | ADHD Twin 2 | |
| SRS Twin 1 | 0.47 | 0.24 | 0.04 | −0.12 |
| ADHD Twin 1 | 0.10 | 0.39 | −0.18 | −0.37 |
| SRS Twin 2 | 0.02 | −0.09 | 0.61 | 0.63 |
| ADHD Twin 2 | −0.04 | −0.12 | 0.26 | 0.28 |
| MZ Females, n=85 complete pairs | ||||
| SRS Twin 1 | ADHD Twin 1 | SRS Twin 2 | ADHD Twin 2 | |
| SRS Twin 1 | 0.61 | 0.53 | 0.60 | 0.46 |
| ADHD Twin 1 | 0.30 | 0.53 | 0.35 | 0.50 |
| SRS Twin 2 | 0.39 | 0.21 | 0.69 | 0.52 |
| ADHD Twin 2 | 0.24 | 0.25 | 0.29 | 0.46 |
| DZ Females, n=48 complete pairs. | ||||
| SRS Twin 1 | ADHD Twin 1 | SRS Twin 2 | ADHD Twin 2 | |
| SRS Twin 1 | 0.52 | 0.56 | 0.44 | 0.30 |
| ADHD Twin 1 | 0.30 | 0.54 | 0.21 | 0.38 |
| SRS Twin 2 | 0.23 | 0.11 | 0.51 | 0.48 |
| ADHD Twin 2 | 0.15 | 0.20 | 0.25 | 0.52 |
| DZ Opposite Sex, n=63 complete pairs. | ||||
| SRS Twin 1 | ADHD Twin 1 | SRS Twin 2 | ADHD Twin 2 | |
| SRS Twin 1 | 0.54 | 0.39 | 0.31 | 0.17 |
| ADHD Twin 1 | 0.20 | 0.48 | 0.23 | 0.10 |
| SRS Twin 2 | 0.17 | 0.12 | 0.58 | 0.45 |
| ADHD Twin 2 | 0.08 | 0.04 | 0.23 | 0.45 |
| All MZ Pairs, n=135 complete pairs. | ||||
| SRS Twin 1 | ADHD Twin 1 | SRS Twin 2 | ADHD Twin 2 | |
| SRS Twin 1 | 0.63 | 0.51 | 0.54 | 0.42 |
| ADHD Twin 1 | 0.29 | 0.53 | 0.30 | 0.48 |
| SRS Twin 2 | 0.35 | 0.18 | 0.66 | 0.46 |
| ADHD Twin 2 | 0.23 | 0.24 | 0.26 | 0.47 |
| All DZ Pairs, n=140 complete pairs. | ||||
| SRS Twin 1 | ADHD Twin 1 | SRS Twin 2 | ADHD Twin 2 | |
| SRS Twin 1 | 0.52 | 0.43 | 0.30 | 0.18 |
| ADHD Twin 1 | 0.21 | 0.48 | 0.15 | 0.15 |
| SRS Twin 2 | 0.16 | 0.08 | 0.57 | 0.50 |
| ADHD Twin 2 | 0.09 | 0.07 | 0.26 | 0.45 |
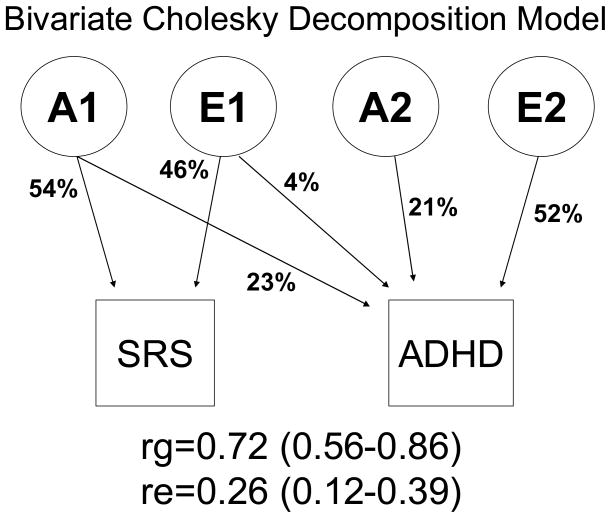
For both SRS and ADHD scores, the most parsimonious univariate model was an AE model without sex limitation effects (Table 2). The bivariate Cholesky decomposition model is shown in figure 1. Because we did not find evidence of significant common environmental or genetic dominance effects in our univariate analyses, we included only A and E in the bivariate analysis. Since we found no significant evidence for sex limitation, we used only two zygosity groups (MZ and DZ) for the bivariate models. There was a significant fit deterioration if the shared component of A (χ12=42.995, p<0.001) or E (χ12=12.664, p<0.001) was dropped. For SRS score, 54% (95% C.I.: 43%–63%) of variance was due to A and 46% (95% C.I.: 37%–57%) due to E. For ADHD score, 44% (95% C.I.: 31%–55%) of variance was due to A and 56% (95% C.I.: 45%–69%) was due to E. The correlation between latent genetic factors affecting SRS and ADHD (rg) suggests about half the genetic variance affecting ADHD symptoms results from genetic influences that also affect autistic traits. In contrast, the environmental correlation (re) suggests only about 7% of the unique environmental variance affecting ADHD is shared with the unique environmental influences on autistic traits.
Table 2.
Univariate models for SRS and ADHD.
| SRS MODELS | −2LL | df | Δχ2 | Δdf | p | Versus: | AIC | A | C | E | OS Cov A |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Full ACE sex limitation model | 1507.614 | 665 | ----- | ----- | ----- | ----- | ---- | 0.32(M) 0.22(F) |
0.08(M) 0.37(F) |
0.60(M) 0.41(F) |
0.50 |
| 2. Equate ACE between sexes | 1512.025 | 668 | 4.411 | 3 | 0.220 | Model 1 | 1.589 | 0.38 (0.01–0.62) | 0.14 (0.00–0.47) | 0.47 (0.38–0.60) | 0.50 |
| 3. Equate DZ additive genetic covariance=0.5 for opposite sex pairs | 1512.025 | 669 | 0.000 | 1 | 1.000 | Model 2 | 2.000 | 0.38 (0.02–0.62) | 0.14 (0.00–0.44) | 0.47 (0.38–0.60) | [0.50] |
| 4. AE model without sex limitation | 1512.729 | 670 | 0.704 | 1 | 0.401 | Model 3 | 1.297 | 0.54 (0.43–0.63) | [0.00] | 0.46 (0.37–0.57) | [0.50] |
| ADHD MODELS | −2LL | df | Δχ2 | Δdf | p | Versus: | AIC | A | C | E | OS Cov A |
| 1. Full ACE sex limitation model | 1375.336 | 665 | ----- | ----- | ----- | ----- | ---- | 0.33(M) 0.29(F) |
0.00(M) 0.23(F) |
0.67(M) 0.49(F) |
−0.36 |
| 2. Equate ACE between sexes | 1379.412 | 668 | 4.076 | 3 | 0.253 | Model 1 | 1.924 | 0.46 (0.11–0.57) | 0.00 (0.00–0.30) | 0.54 (0.43–0.67) | 0.24 |
| 3. Equate DZ additive genetic covariance=0.5 for opposite sex pairs | 1380.270 | 669 | 0.858 | 1 | 0.354 | Model 2 | 1.143 | 0.44 (0.17–0.56) | 0.00 (0.00–0.21) | 0.56 (0.44–0.68) | [0.5] |
| 4. AE model without sex limitation | 1380.270 | 670 | 0.000 | 1 | 1.000 | Model 3 | 2.000 | 0.44 (0.32–0.56) | [0.00] | 0.56 (0.44–0.68) | [0.5] |
Most parsimonious models are shown in bold. OS Cov A = additive genetic covariance for opposite sex twin pairs. Numbers in brackets were fixed rather than estimated. In model 1 for each phenotype, separate estimates for A, C, and E are given for males (M) and females (F). For all other models, A, C, and E are equated between sexes. Proportion of variance accounted for by additive genetics (A), common environment (C), and unique environment (E) are given for each model, and 95% confidence intervals for these parameters are given in parentheses for models 2, 3, and 4.
Figure 1.
Path diagram illustrating the bivariate model. Percent of variance in SRS or ADHD score accounted for by each latent factor is shown on the diagram. A= additive genetic influences. E= environmental influences that lead to differences between twins. Genetic correlation (rg) and environmental correlation (re) are shown with 95% confidence intervals.
Discussion
We cannot be certain whether our young adult self-report SRS and ADHD items truly measure the same problems as ratings by outside observers; however, the genetic structure of our self-report SRS and ADHD scores is basically consistent with studies of SRS (Constantino & Todd, 2003) and ADHD (Faraone et al., 2005) symptoms reported by parents of minors. Our heritability estimates for SRS and ADHD in adults are somewhat lower than those reported for youth, but our study confirms at least moderate heritability for each trait.
In the preferred model from a prior study by Constantino, Hudziak, and Todd, the genetic factors affecting SRS and ADHD symptoms were separate, and reciprocal causation within individuals accounted for the correlation between SRS and ADHD symptoms (Constantino et al., 2003). We did not test a reciprocal causation model using the current dataset because the small sample size and similar genetic structure for the two phenotypes make it impossible to reliably distinguish between our bivariate model and a reciprocal causation model. The expected covariance structure for these two types of models is identical in cases where the A and E variance components for the two phenotypes are the same (Duffy & Martin, 1994). Our findings indicate that pleiotropy (shared genes affecting both SRS and ADHD symptoms) could explain the association between SRS and ADHD symptoms. However, it is still possible that the association between SRS and ADHD symptoms is partly due to measurement overlap or interaction of SRS and ADHD symptoms within individuals (reciprocal causation).
Although different measures of ADHD symptoms and autistic traits were used in our study, our results are remarkably similar to those of Ronald and colleagues(Ronald et al., 2008). Their study of child twins reported somewhat higher heritability for Autistic and ADHD symptoms. They also reported slightly lower rg than our study, but their confidence intervals for rg overlap with ours. Their reported estimates for re were also very similar to ours. Considering both studies, it appears that there is evidence of substantial genetic overlap between ADHD and autistic symptoms in both children and young adults.
This study has some limitations. We did not include DSM-IV hyperactive symptoms in our measure of self-report ADHD symptoms due to limitations on the size of the total survey. However, hyperactive symptoms are less stable than inattentive symptoms from childhood to adulthood (Todd et al., 2008), so inclusion of hyperactive symptoms may not have been very helpful in this age group. Also, our previous study of Missouri twins found that unlike those with inattentive or combined type ADHD, children with the hyperactive-impulsive subtype did not have statistically significant SRS score elevations (Reiersen et al., 2007). Although we did not find significant evidence for common environment effects, genetic dominance, or sex limitation effects, it is possible that our sample size was not large enough to detect these. Also, there are additional types of bivariate models that we did not perform because our sample size provides insufficient power to justify more complex analysis at this stage. We will consider evaluating more complex twin models once a larger sample is available for analysis. Of note, the DZ covariance for ADHD was negative for males in our study. This suggests the possibility of negative sibling interaction effects in males. However, given the small size of the male DZ group, this negative covariation could be due to random factors, and we did not have a large enough sample size to reliably assess for sibling interaction.
Recently we have demonstrated marked gene-environment interaction between prenatal exposure to cigarette smoke and genotype at the DRD4, DAT, and CHRNA4 loci for combined type ADHD (Neuman et al., 2007; Todd & Neuman, 2007). This is the ADHD subtype that is most strongly associated with elevated SRS scores in children (Reiersen et al., 2007). Such gene-environment interaction could result in overestimation of A and underestimation of C in our models. Also, our high estimates for E could be partially due to non-response bias, variable decrease in ADHD symptoms with age, inaccurate self-report, or other forms of error variance. Despite these limitations, we found strong evidence for shared genetic influences on autistic and ADHD symptoms which supports continued study of the association between ADHD and autism.
In future studies, it may be fruitful to search for specific genes that influence both ADHD and autism. Given that the genetic correlation for SRS and ADHD was higher than the environmental correlation, it may be that some genes predispose to both types of symptoms but that environmental factors determine whether individuals have autistic traits, ADHD or both. In further support of this possibility, we have found an association between clinically elevated SRS score and the DRD4 7-repeat allele in Missouri twin subjects with latent-class derived severe combined type ADHD (OR 3.27, p=0.016), but there was no main or interaction effect of prenatal maternal smoking in predicting high SRS score in these children (Reiersen et al., 2008). So, although prenatal maternal smoking appears to interact with genotype to produce severe combined type ADHD, it does not appear that this environmental factor influences SRS score in these children.
In conclusion, our findings indicate that self-reported inattentive/impulsive ADHD symptoms and autistic traits tend to co-occur in adults. This co-occurrence of ADHD and autistic symptoms may be due to substantial overlap of the genetic influences leading to these two types of symptoms. It may be important to assess for autistic features in adult patients with ADHD and measure both autistic traits and ADHD symptoms in studies of either disorder.
Acknowledgments
Acknowledgments & Disclosures: The authors thank the Australian twin participants. This work was supported by the Borderline Personality Disorder Research Foundation and by NIH grant HD 042541 (JNC). One of the authors (JNC) receives royalties on the Social Responsiveness Scale, which is published and distributed by Western Psychological Services.
References
- Anckarsater H, Stahlberg O, Larson T, Hakansson C, Jutblad SB, Niklasson L, et al. The impact of ADHD and autism spectrum disorders on temperament, character, and personality development. Am J Psychiatry. 2006;163(7):1239–1244. doi: 10.1176/ajp.2006.163.7.1239. [DOI] [PubMed] [Google Scholar]
- Clark T, Feehan C, Tinline C, Vostanis P. Autistic symptoms in children with attention deficit-hyperactivity disorder. Eur Child Adolesc Psychiatry. 1999;8(1):50–55. doi: 10.1007/s007870050083. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale (SRS) Manual. Los Angeles, California: Western Psychological Services; 2005. [Google Scholar]
- Constantino JN, Hudziak JJ, Todd RD. Deficits in reciprocal social behavior in male twins: evidence for a genetically independent domain of psychopathology. J Am Acad Child Adolesc Psychiatry. 2003;42(4):458–467. doi: 10.1097/01.CHI.0000046811.95464.21. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Przybeck T, Friesen D, Todd RD. Reciprocal social behavior in children with and without pervasive developmental disorders. J Dev Behav Pediatr. 2000;21(1):2–11. doi: 10.1097/00004703-200002000-00002. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Distel MA, Trull TJ, Derom CA, Thiery EW, Grimmer MA, Martin NG, et al. Heritability of borderline personality disorder features is similar across three countries. Psychol Med. 2008;38(9):1219–1229. doi: 10.1017/S0033291707002024. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Martin NG. Inferring the direction of causation in cross-sectional twin data: theoretical and empirical considerations. Genet Epidemiol. 1994;11(6):483–502. doi: 10.1002/gepi.1370110606. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Goldstein S, Schwebach AJ. The comorbidity of Pervasive Developmental Disorder and Attention Deficit Hyperactivity Disorder: results of a retrospective chart review. J Autism Dev Disord. 2004;34(3):329–339. doi: 10.1023/b:jadd.0000029554.46570.68. [DOI] [PubMed] [Google Scholar]
- Jardine R, Martin NG, Henderson AS. Genetic covariation between neuroticism and the symptoms of anxiety and depression. Genet Epidemiol. 1984;1(2):89–107. doi: 10.1002/gepi.1370010202. [DOI] [PubMed] [Google Scholar]
- Martin NG, Martin PG. The inheritance of scholastic abilities in a sample of twins. I. Ascertainment of the sample and diagnosis of zygosity. Ann Hum Genet. 1975;39:213–218. doi: 10.1111/j.1469-1809.1975.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 7. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry; 2006. [Google Scholar]
- Neale MC, Roysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and G × E interaction. Twin Res Hum Genet. 2006;9(4):481–489. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman RJ, Lobos E, Reich W, Henderson CA, Sun LW, Todd RD. Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biol Psychiatry. 2007;61(12):1320–1328. doi: 10.1016/j.biopsych.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry. 2007;48(5):464–472. doi: 10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- Reiersen AM, Neuman RJ, Reich W, Constantino JN, Volk HE, Todd RD. Intersection of autism and ADHD: Evidence for a distinct syndrome influenced by genes and by gene-environment interactions. In: Hudziak JJ, editor. Developmental Psychopathology and Wellness: Genetic and Environmental Influences. Arlington, VA: American Psychiatric Publishing, Inc; 2008. pp. 191–208. [Google Scholar]
- Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J Child Psychol Psychiatry. 2008;49(5):535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- Santosh PJ, Mijovic A. Social impairment in Hyperkinetic Disorder -relationship to psychopathology and environmental stressors. Eur Child Adolesc Psychiatry. 2004;13(3):141–150. doi: 10.1007/s00787-004-0372-4. [DOI] [PubMed] [Google Scholar]
- Smalley SL, Loo SK, Yang MH, Cantor RM. Toward localizing genes underlying cerebral asymmetry and mental health. Am J Med Genet B Neuropsychiatr Genet. 2005;135(1):79–84. doi: 10.1002/ajmg.b.30141. [DOI] [PubMed] [Google Scholar]
- Sturm H, Fernell E, Gillberg C. Autism spectrum disorders in children with normal intellectual levels: associated impairments and subgroups. Dev Med Child Neurol. 2004;46(7):444–447. doi: 10.1017/s0012162204000738. [DOI] [PubMed] [Google Scholar]
- Todd RD, Huang H, Todorov AA, Neuman RJ, Reiersen AM, Henderson CA, et al. Predictors of stability of attention-deficit/hyperactivity disorder subtypes from childhood to young adulthood. J Am Acad Child Adolesc Psychiatry. 2008;47(1):76–85. doi: 10.1097/chi.0b013e31815a6aca. [DOI] [PubMed] [Google Scholar]
- Todd RD, Neuman RJ. Gene-environment interactions in the development of combined type ADHD: evidence for a synapse-based model. Am J Med Genet B Neuropsychiatr Genet. 2007;144(8):971–975. doi: 10.1002/ajmg.b.30640. [DOI] [PubMed] [Google Scholar]