Abstract
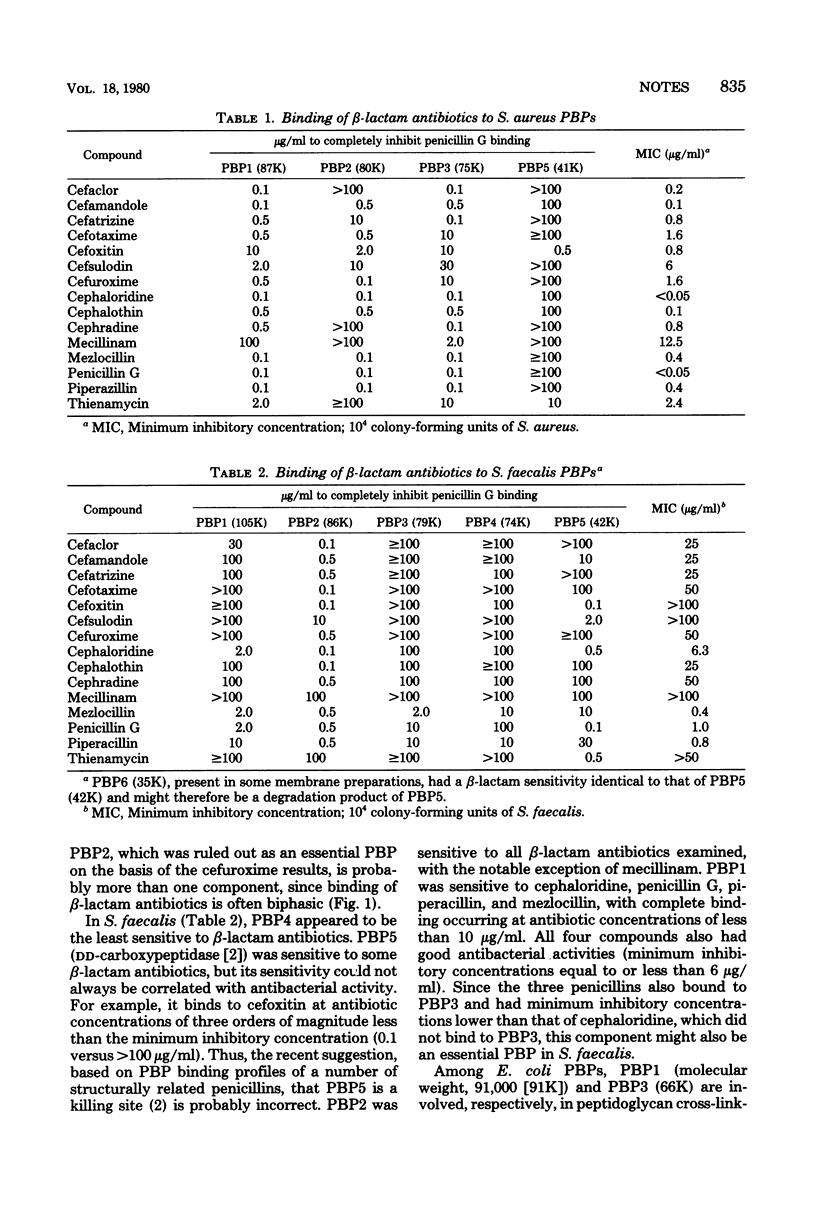
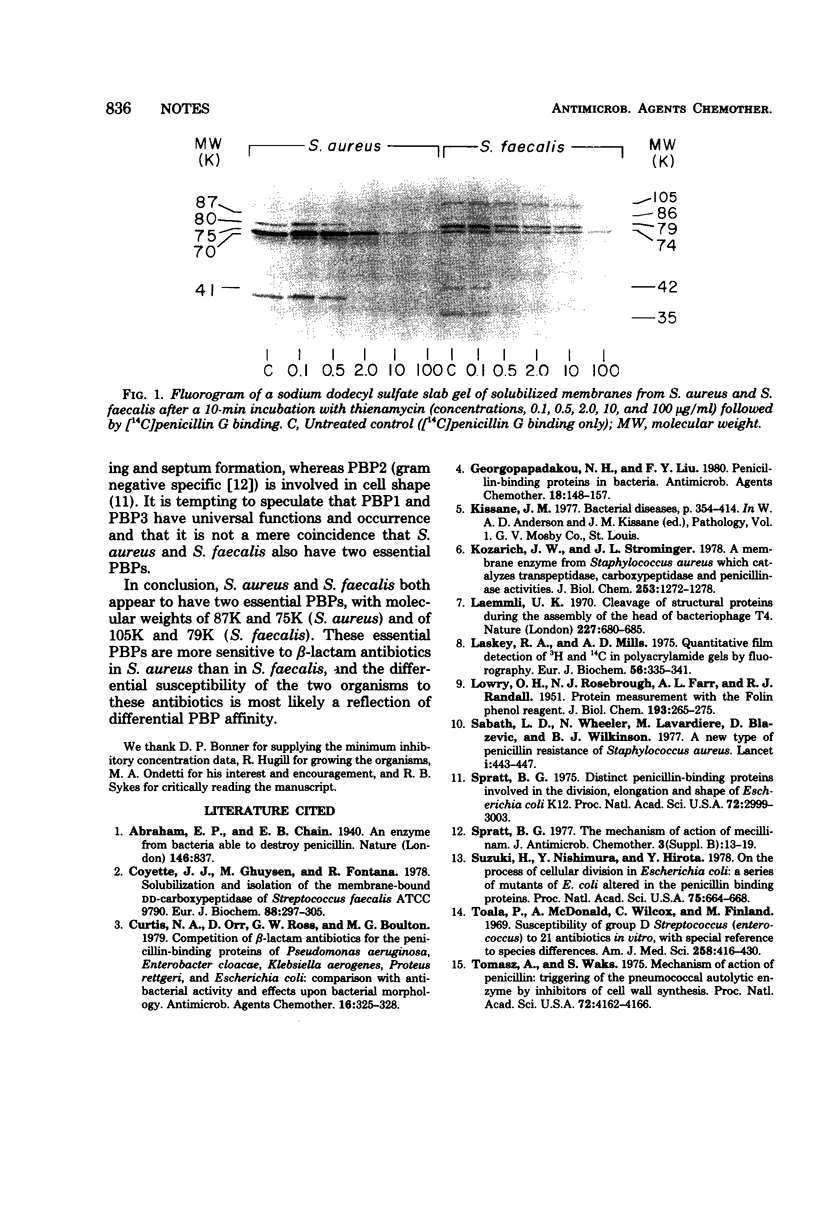
The binding of 14 structurally diverse beta-lactam antibiotics to penicillin-binding proteins of Staphylococcus aureus and Streptococcus faecalis was studied, and the results were examined in the context of the antibacterial activity of the compounds. Penicillin-binding proteins 1 (molecular weight, 87,000) and 3 (molecular weight, 75,000) of S. aureus and penicillin-binding proteins 1 (molecular weight, 105,000) and 3 (molecular weight, 79,000) of S. faecalis bound beta-lactam antibiotics at concentrations comparable to minimum inhibitory concentrations and might therefore be essential. The low affinity of S. faecalis penicillin-binding proteins, relative to that of S. aureus penicillin-binding proteins, toward most beta-lactam antibiotics is probably responsible for the resistance of the former organism to most of these compounds.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coyette J., Ghuysen J. M., Fontana R. Solubilization and isolation of the membrane-bound DD-carboxypeptidase of Streptococcus faecalis ATCC9790. Properties of the purified enzyme. Eur J Biochem. 1978 Jul 17;88(1):297–305. doi: 10.1111/j.1432-1033.1978.tb12450.x. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Competition of beta-lactam antibiotics for the penicillin-binding proteins of Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella aerogenes, Proteus rettgeri, and Escherichia coli: comparison with antibacterial activity and effects upon bacterial morphology. Antimicrob Agents Chemother. 1979 Sep;16(3):325–328. doi: 10.1128/aac.16.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarich J. W., Strominger J. L. A membrane enzyme from Staphylococcus aureus which catalyzes transpeptidase, carboxypeptidase, and penicillinase activities. J Biol Chem. 1978 Feb 25;253(4):1272–1278. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Wheeler N., Laverdiere M., Blazevic D., Wilkinson B. J. A new type of penicillin resistance of Staphylococcus aureus. Lancet. 1977 Feb 26;1(8009):443–447. doi: 10.1016/s0140-6736(77)91941-9. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. The mechanism of action of mecillinam. J Antimicrob Chemother. 1977 Jul;3 (Suppl B):13–19. doi: 10.1093/jac/3.suppl_b.13. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toala P., McDonald A., Wilcox C., Finland M. Susceptibility of group D streptococcus (enterococcus) to 21 antibiotics in vitro, with special reference to species differences. Am J Med Sci. 1969 Dec;258(6):416–430. doi: 10.1097/00000441-196912000-00006. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]




