Abstract
Reduced uterine perfusion initiated in late gestation in the rat results in intrauterine growth restriction (IUGR) and development of hypertension by 4 weeks of age. We hypothesize that the renin angiotensin system (RAS), a regulatory system important in the long term control of blood pressure, may be programmed by placental insufficiency and may contribute to the etiology of IUGR hypertension. We previously reported that RAS blockade abolished hypertension in adult IUGR offspring; however, the mechanisms responsible for the early phase of hypertension are unresolved. Therefore, the purpose of this study was to examine RAS involvement in early programmed hypertension and to determine whether temporal changes in RAS expression are observed in IUGR offspring. Renal renin and angiotensinogen mRNA expression were significantly decreased at birth (80 and 60 percent, respectively); plasma and renal RAS did not differ in conjunction with hypertension (mean increase of 14 mmHg) in young IUGR offspring; however, hypertension (mean increase of 22 mmHg) in adult IUGR offspring was associated with marked increases in renal ACE activity (122%) and renal renin and angiotensinogen mRNA (7-fold and 7.4-fold, respectively), but no change in renal angiotensin II or angiotensin type 1 receptor. ACE inhibition (enalapril, 10 mg/kg/day, administered from 2 to 4 weeks of age) abolished hypertension in IUGR at 4 weeks of age (decrease of 15 mmHg, respectively) with no significant depressor effect in control offspring. Therefore, temporal alterations in renal RAS are observed in IUGR offspring and may play a key role in the etiology of IUGR hypertension.
Keywords: intrauterine growth restriction, hypertension, kidney, brain, renin, angiotensin, rat
INTRODUCTION
Epidemiological and experimental studies provide strong evidence to suggest cardiovascular disease and hypertension are programmed by exposure to adverse conditions in utero (1, 6, 11, 12, 13, 17, 30, 51, 55). The inverse relationship observed in epidemiological studies between birth weight and blood pressure suggests that factors involved in prenatal development, which affect fetal growth, are responsible for the in utero programming of arterial blood pressure (6, 7, 25, 31). Mechanisms involved in the pre-natal programming of hypertension are unclear. However, animal studies suggest a role for the kidneys (1, 2, 30, 51, 55, 54, 56) in the pathogenesis of pre-natal programmed hypertension with inclusion of the renin angiotensin system (RAS) (30, 44, 51, 55).
Numerous investigators have examined the role of the RAS in models of prenatal programming induced by protein restriction during gestation in the rat (33, 34, 40, 43, 45, 48, 51, 52, 55) and pre-natal exposure to glucocorticoids (XX). Suppression of the RAS observed at birth (51, 55) may lead to permanent structural changes associated with the pathogenesis of hypertension in offspring from protein restricted dams (55). Up-regulation of the renal angiotensin type 1 receptor (AT1R) following late gestational protein restriction is observed as early as 4 weeks of age by some investigators (30, 33, 51), but not others (35), and characterization of the renal RAS including AT1R has not yet been determined in the adult hypertensive offspring. Marked increases in plasma renin activity (PRA) following late gestational protein restriction are also observed as early as 4 weeks of age by some investigators (30), but not by other investigators who observe suppression of PRA at 4 weeks of age with a gradual increase leading to inappropriate activation at 6 months of age (30). Thus, temporal alterations in the RAS are reported by investigators utilizing similar models of pre-natal programming induced by gestational protein restriction (30, 33, 48, 51, 52, 55). More importantly, the critical role of the RAS in the etiology of hypertension programmed by protocols of in utero protein restriction is indicated by RAS blockade studies (24, 48). Furthermore, alterations in the RAS may also be responsible for marked increases in blood pressure programmed by pre-natal exposure to glucocorticoids indicating fetal insults lead to similar pathways of programmed hypertension (XX).
Our laboratory utilizes a model of prenatal programmed hypertension initiated by reduced uterine perfusion that may better reflect the patho-physiological induction of intrauterine growth restriction (IUGR) in humans in the Western world. IUGR induced by placental insufficiency is associated with development of hypertension in male growth-restricted offspring (1, 40). We previously reported that ACE inhibition abolishes hypertension in adult male growth-restricted offspring (40) suggesting that the RAS plays a critical role in the maintenance of established hypertension in this model of IUGR. Thus, the purpose of this study was to determine whether temporal alterations in the RAS are present in male growth restricted offspring from reduced uterine perfusion dams, and to de termine the quantitative importance of the RAS in the early phase of programmed hypertension in male growth restricted offspring.
MATERIALS AND METHODS
All experimental procedures were done in accordance with National Institute of Health guidelines with approval by the Animal Committee at the University of Mississippi Medical Center. Rats were housed in a temperature-controlled room (23°C) with a 12:12 hour light/dark cycle with food and water available ad libitum. At day 14 of gestation, female timed pregnant Sprague Dawley rats (Harlan Inc., Indianapolis, IN) destined for reduced uterine perfusion were clipped as described below. All dams were allowed to deliver at term with one control litter size-matched to a reduced uterine perfusion litter. Minimum litter size was 8 pups. Birth weight was recorded within 12 hours and offspring from all litters were weaned at 3 weeks of age. Only male offspring were utilized for all studies since only male IUGR offspring develop and remain hypertensive into adulthood. For ACE inhibition studies young male offspring from 12 control and 11 reduced uterine perfusion litters were administered either enalapril, 10 mg/kg/day by gavage, utilizing a dose shown previously by our laboratory or others (47) to result in an effective blockade of the ANG II response as determined by a decrease in arterial pressure and increase in PRA, or vehicle (water and corn syrup, 50:50). Administration of enalapril or vehicle was initiated at 2 weeks of age and continued for 2 weeks of until 4 weeks of age. Mean arterial pressure was measured at 4 weeks of age in one group of animals, and at 5 weeks of age in a second group. For measurement of RAS components at birth, tissues were pooled from offspring of a single litter for collection of adequate tissue for analysis. Six litters of control and 8 litters of reduced uterine perfusion dams were utilized. For studies involving quantitation of RAS components in older animals, kidneys were not pooled as kidneys were sufficient in size for analysis, and therefore, each measurement represents one kidney per animal. For these determinations, tissues and serum were collected from offspring born to a total of 12 control rats and 13 reduced uterine perfusion rats. Kidneys and plasma were collected from animals following decapitation.
Reduced uterine perfusion in the pregnant rat
As previously described (1) reduced uterine perfusion was utilized to induce IUGR. Briefly, all rats undergoing surgical procedures were anesthetized with 2% isoflurane (W.A. Butler Co.) delivered by Vaporizer (Ohio Medical Products, Madison, WI). At day 14 of gestation, a midline abdominal incision was done and the lower abdominal aorta was isolated and a silver clip (0.203 mm ID) was placed around the aorta above the iliac bifurcation. Since compensation of blood flow to the placenta occurs through an adaptive increase in ovarian blood flow, flow to both the right and left ovarian arteries that supply the uterus was reduced using silver clips (0.100 mm ID). Pregnant rats used for the control group were not exposed to surgical procedures. Based on previous observations, no differences have been observed between offspring from pregnant rats undergoing a sham operation and offspring from pregnant rats not exposed to surgical procedures. (Alexander BT, unpublished data, 2003).
Acute arterial pressure measurements in conscious rats
Mean arterial pressure (MAP) was measured acutely as previously described (1). Briefly, rats were anesthetized with isoflurane as described above and surgically instrumented with a carotid arterial catheter (PE 50 tubing). Following 3 days of recovery, rats acclimated to restraint were placed in modified restraining cages with arterial pressure monitored with a pressure transducer connected to a data acquisition kit (DATAQ Instruments, Inc., Akron, OH) and computer for continuous recording. All animals were acclimated to restraint for two hours prior to a one-hour equilibration period followed by 2-twenty minute pressure determinations. All pressure measurements were made during mid morning hours.
Measure of plasma renin activity
Renin activity in plasma was measured by radioimmunoassay (RIA) using a modification of Haber and associates (22) with AI standards, tracer, and antibody from the National Bureau of Standards, Perkin Elmer Life Sciences, and Arnel, respectively.
ACE Activity Assay
Isolated kidney cortices were homogenized in an assay buffer consisting of (in mmol/L) 50 HEPES, pH 7.4, 150 NaCl, 0.5% Triton X-100, 10 μM ZnCl2, and 1.0 PMSF, and then clarified by centrifugation at 10,000g for 15 minutes. ACE activity against a synthetic substrate (p-hydroxybenzoyl-glycyl-L-hisidyl-L-leucine) was determined using the colorimetric based ACEcolor kit (Fujirebio Inc). For the assay, tissue samples were standardized to 1 μg protein/μl. For serum ACE activity 50 μl of serum was used. Optical density was read at 505 nm with a spectrophotometer. Results were calculated as mIU/mg of protein. All data were reported as mean +/−SE.
Isolation of total cellular RNA and Real-time PCR
Total RNA was utilized for quantitation of the mRNA message by Real-Time PCR. Kidneys were removed, quick frozen in liquid nitrogen, and stored at −80°C. Each kidney was first ground using a liquid nitrogen chilled mortar and pestle and total RNA was isolated using a guanidine thicyanide, acid phenol:chloroform procedure (ToTALLY RNA kit, Ambion, Austin, TX). Total RNA concentration and purity were determined spectrophotometrically using A260 and A260/280 ratio, respectively. Total RNA integrity was checked using 1% agarose gel electrophoresis. All RNA isolates were DNA free by treatment with DNAse (DNA-free kit, Ambion, Austin, TX). 2 μg of total RNA were reverse transcribed using a modified MMLV-derived reverse transcriptase and a unique blend of oligo (dT) and random hexamer primers (iScript™cDNA Synthesis Kit, BIO-RAD). 1μl of the resulting cDNA was amplified by real-time PCR using SYBR Green (iQ™ SYBR Green Supermix, BIO-RAD) as fluorophore in an iCycler real-time thermal cycler (BIO-RAD). Specific pairs of primers was used for each gene amplification. PCR conditions were optimized for each pair of primers. DNA sequencing of the fragment amplified confirmed specificity of the PCR products.
| Renin (37) | Forward: GCTACATGGAGAATGGGACTGAA |
| Reverse: ACCACATCTTGGCTGAGGAAAC | |
| Angiotensinogen (37) | Forward: AGCACGGACAGCACCCTATT |
| Reverse: AGAACTCATGGAGCCCAGTCA | |
| AT2R primers (34): | Forward: AATTACCCGTGACCAAGTCTTGA |
| Reverse: AGAACATGGAAGGGAAGCCA | |
| AT1R primers (34): | Forward: GGCCAGTGTTTTTCTTTTGAATTTAGCAC |
| Reverse: GTTCCCCTTGTTTGGTGTAT |
ACE mRNA expression was assessed using RT2 PCR Primer Set for Rat ACE (SuperArray Bioscience Corporation). Results were calculated using the 2−ΔΔCT method and expressed in folds increase/decrease of the gene of interest in IUGR vs. control rats. All reactions were performed in triplicates and β-actin was used as an internal control (RT-PCR Primer and Control Set, Invitrogen). Level of mRNA expression was calculated using the mathematical formulas for delta/delta CT recommended by Applied Biosystems (Applied Biosystems User Bulletin, No. 2, 1997).
Radioimmunoassay for angiotensin II
Briefly, frozen kidneys were quickly weighed and quick frozen in liquid nitrogen. For analysis they were homogenized, and extracted using Sep-Pak columns. Solvent was evaporated and recovery of radiolabeled angiotensin added to the samples was determined during the extraction. Recovery of radiolabeled peptide was corrected for recovery. Ang II was measured using the Alpco Diagnostics kit (Windham, NH).
Radioligand autoradiography for ANG II receptors and ACE
Quantitative autoradiography was utilized to determine renal AT1R, AT2R, and ACE binding from whole kidneys as previously described (5, 28, 29). Briefly, kidneys were snap-frozen in liquid nitrogen, cut sagitally into sequential 20-μm thick frozen sections within 1 week of harvest, thaw-mounted onto gelatin-coated slides, air-dried, and stored at −20 C. Within one week of sectioning, the sections were thawed and the autoradiography procedure was carried out. For AT1 and AT2 receptor autoradiography, sections were pre-incubated for 30 minutes at room temperature in isotonic buffer (50 mM NaPO4, 150 mM NaCl, 5 mM EDTA, 0.1 mM bacitracin, pH 7.1–7.2). Consecutive sections were incubated for 2 hours in isotonic-buffer containing 0.4 nM 125I-SI ANG II plus a saturation concentration (3μM) of ANG II to block both AT1 and AT2 receptors for nonspecific binding; 0.4 nM 125I-SI ANG II plus 10 mM PD123177 (an AT2R-selective compound) for measurement of AT1R; and 0.4 nM 125I-SI ANG II plus 10 mM losartan (an AT1-selective antagonist) for measurement of AT2R, as previously described (35). For ACE autoradiography, sections were pre-incubated in 50 mM Tris, 100 mM NaCl buffer (pH 7.4 at 22°C) for 30 min at room temperature and then incubated with 125I-351A plus or minus 1μM MK-422 for 1 hour at room temperature for determination of ACE as previously described (4, 24). Following radioligand incubation, sections were rinsed, dried, and exposed to Kodak Biomax MR1 X-ray film, at −20°C for ~36 hours. A standard slide with calibrated concentrations ranging from 1 to 600 nCi/mg 125I (Microscales, GE Healthcare Biosciences, Piscataway, NJ) was included in each cassette. The film was developed using an automated film processor.
Image Analysis
Images were captured by use of an AIS image analysis system (Imaging Research Inc) via an analog video camera. The density of Ang II receptors was referenced to the 125I standards processed with each film.
Statistics
All data are presented as means ± Std error. Statistical differences were evaluated using repeated-measures one-way ANOVA, followed by Student’s t-test. The criterion for statistical significance was set at P<0.05.
RESULTS
Birth and body weights
As reported previously (1), weight at birth was significantly reduced in growth-restricted offspring as compared to control offspring (5.7±0.1 vs. 6.4±0.3 grams, P<0.01, IUGR vs. control, respectively). However, body weight was not significantly decreased at either 4 (80±3 grams) or 5 weeks of age in growth-restricted offspring (126±4 grams) as compared to control (80±3 and 119±4 grams, respectively). However, treatment with enalapril from 2 to 4 weeks of age led to a significant reduction in body weight in growth-restricted offspring relative to their untreated counterparts at 4 weeks of age (66±3 grams, P <0.05) that persisted at 5 weeks of age (103±5 gram, P<0.05); enalapril had no significant effect on body weight in control offspring at either 4 (76±2 grams) or 5 weeks of age (121±2 grams). Body weight remained similar at 16 weeks of age in growth-restricted offspring (413±50 grams) compared to control offspring (411±5 grams).
Effect of renin angiotensin system blockade on mean arterial pressure
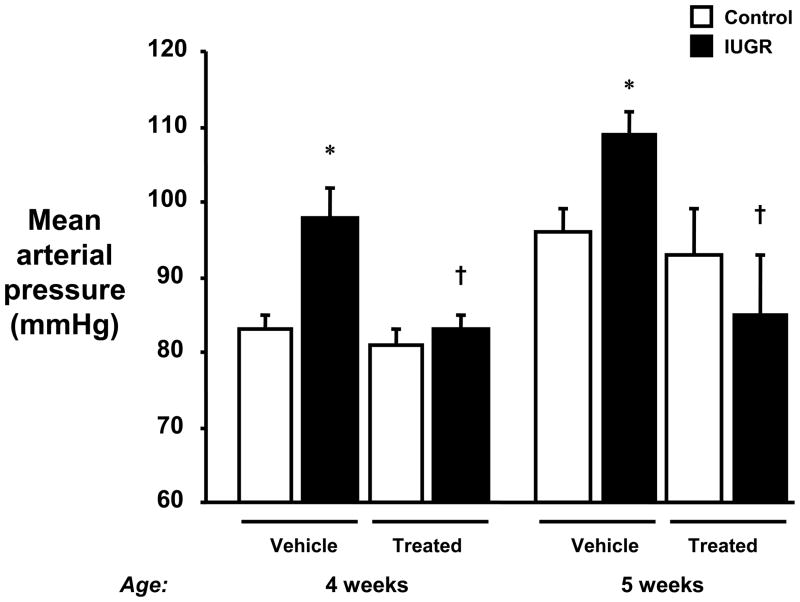
To determine the importance of the RAS in the early phase of hypertension in this model of IUGR, enalapril was administered from 2 to 4 weeks of age. The increase in MAP at both 4 and 5 weeks of age in growth-restricted offspring was abolished by ACE inhibitor treatment. ACE inhibition did not significantly alter blood pressure in control offspring (Figure 1).
Figure 1.
Inhibition of the renin angiotensin system in intrauterine growth restricted (IUGR) offspring. Mean arterial pressure was measured in conscious chronically instrumented offspring at 4 and 5 weeks of age following treatment with either vehicle or enalapril (treated; 10mg/kg/day by gavage from 2 to 4 weeks of age). 4 week group: control vehicle (n=11), IUGR vehicle (n=7), control treated (n=10), and IUGR treated (n=9). 5 week group: control vehicle (n=8), IUGR vehicle (n=5), control treated (n=7), and IUGR treated (n=6). *P<0.05 vs. untreated control. †P<0.05 vs. untreated IUGR. All data are expressed as mean ± SEM.
Measurement of mean arterial pressure in adult growth-restricted offspring
For characterization of RAS components in adult growth-restricted offspring, tissues were collected at 16 weeks of age, a time at which MAP was significantly elevated in growth-restricted offspring (145±2 mmHg, P<0.05) as compared to control (123±2 mmHg). These values, as determined in conscious chronically instrumented animals, are comparable to values of MAP obtained by telemetry (measurement at 16 weeks of age following full recovery from probe implantation at 10 weeks of age) (40).
Intra-renal expression of renin angiotensin system components
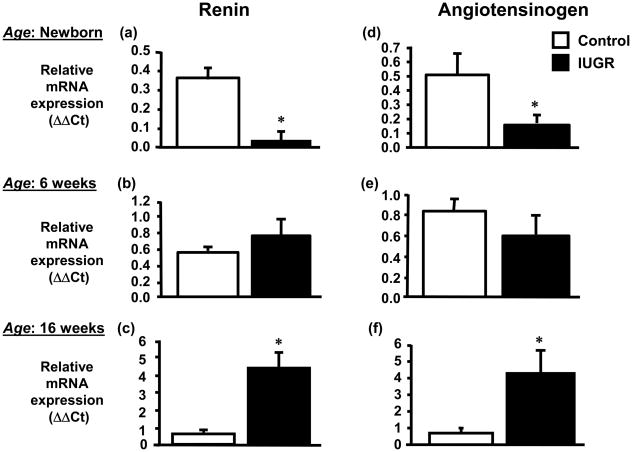
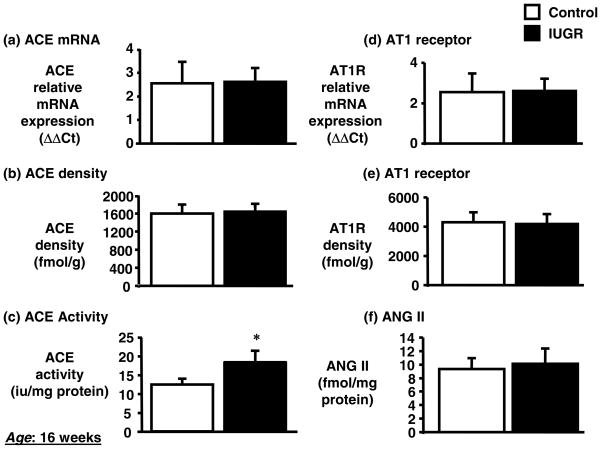
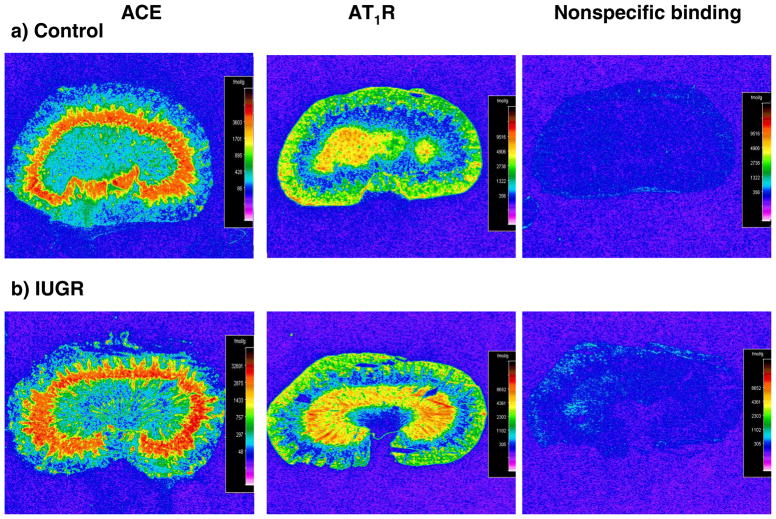
Expression of renin mRNA in the kidney was significantly decreased by 80% (day 1) in growth-restricted offspring relative to control offspring (Figure 2a). At 6 weeks of age no difference in expression of renin in the renal cortex was observed in growth-restricted versus control offspring (Figure 2b). However, cortical renin mRNA expression was significantly increased by 7-fold at 16 weeks of age in growth-restricted offspring (Figure 2c) as compared to control offspring. Renal angiotensinogen mRNA expression followed a similar trend. Renal angiotensinogen mRNA expression was significantly decreased at birth (day 1) (Figure 2d), did not differ at 6 weeks of age (Figure 2e), but was significantly increased at 16 weeks of age (Figure 2f). Renal cortical ACE mRNA expression (Figure 3a) or renal ACE density (Figure 3b) determined by radioligand autoradiography did not differ at 16 weeks of age; however, a significant increase in renal ACE activity (Figure 3c) was observed in growth-restricted offspring at 16 weeks of age relative to control offspring. Renal AT1 receptor mRNA (Figure 3d) or renal AT1 receptor density (Figure 3e) were not altered in growth-restricted offspring as compared to control offspring at 16 weeks of age, and despite a significant increase in renal ACE activity, renal angiotensin II (ANG II) levels were also not increased at 16 weeks of age (Figure 3f). AT1R binding was greater in the medulla relative to the cortex in both control and growth-restricted offspring (Figure 4); an observation previously reported by others (20, 57). AT1 receptor binding as determined by radioligand autoradiography also did not differ at 4 weeks of age. Binding to the angiotensin type 2 (AT2) receptor was not detectable in growth-restricted or control offspring at either 4 or 16 weeks of age; AT2 receptor mRNA expression was also not detectable.
Figure 2.
Temporal changes in renal renin and angiotensinogen in intrauterine growth restricted (IUGR) offspring. Real time PCR was utilized to assess renal renin mRNA expression in newborn (day 1) (a), 6 weeks of age (b), and 16 weeks of age (c) or renal angiotensinogen mRNA expression of in newborn (day 1) (d), 6 weeks of age (e), and 16 weeks of age (f). Quantitation of renal RAS components was normalized relative to renal β-actin mRNA expression levels. For newborn samples mRNA expression was quantitated from whole kidney homogenates representing a pool of tissues collected from a single litter, n = 8 IUGR litters and 6 control litters; individual cortical sections were analyzed from 8 IUGR and 8 control offspring at 6 weeks of age; 7 IUGR and 8 control offspring at 16 weeks of age. Standard error is calculated from at least 3 determinations from at least three independent experiments. *P<0.05 vs. control. All data are expressed as mean ± SEM.
Figure 3.
Renal ACE, AT1 receptor, and angiotensin II in intrauterine growth restricted (IUGR) offspring at 16 weeks of age. Real time PCR was utilized to assess renal ACE (a) and AT1 receptor (d) mRNA expression. Quantitation of renal RAS components was normalized relative to renal mRNA β-actin mRNA expression levels. Individual cortical sections were analyzed from 7 IUGR and 8 control offspring at 16 weeks of age. Standard error was calculated from at least 3 determinations from at least three independent experiments. Quantitative binding of renal ACE (b) and AT1 receptor (e) was determined in 8 IUGR and 8 control offspring. Renal ACE activity (c) and renal ANG II (f) was determined in 6 IUGR and 5 control offspring. All data are expressed as mean ± SEM.
Figure 4.
Quantitative autoradiography of angiotensin type 1 (AT1R) receptors and angiotensin converting enzyme (ACE) in whole kidney from control and IUGR offspring at 16 weeks of age. Representative autoradiographs are shown of ACE (a), AT1R (b), and non-specific binding (c) of 125 I-SI ANG II.
Plasma renin activity
Plasma renin activity (PRA) did not differ between control and growth-restricted offspring at either 4 (5.7±2.6 vs. 6.5±2.2 nmoles AI/L/Hr) or 5 weeks of age (5.2±1.6 vs. 8.1±0.6 nmoles AI/L/Hr), respectively. ACE inhibition from 2 to 4 weeks of age resulted in a significant increase in PRA at 4 weeks of age in control and growth-restricted treated offspring (114±42.2 and 95.6±37.4 nmoles AI/L/Hr). At 5 weeks of age PRA returned to baseline values in control (6.3±1.7nmoles AI/L/Hr) or below in growth-restricted offspring (3.2±1.7 nmoles AI/L/Hr, P<0.05 vs. untreated growth-restricted).
Serum ACE activity
Serum ACE activity was not significantly increased at 16 weeks of age in growth-restricted offspring (13.5±1.2 vs. 15.2±1.1 IUnits ACE/L, control vs. growth-restricted, respectively).
DISCUSSION
Fetuses subjected to stressful influences resulting in IUGR are predisposed to the development of cardiovascular disease and hypertension in later life (1, 30, 51, 55). In the rat reduced uterine perfusion initiated in late gestation results in growth-restricted offspring that develop marked elevations in MAP (1, 40). Significant elevations in MAP observed at 12 weeks of age in growth-restricted offspring are not associated with marked changes in glomerular filtration rate or renal plasma flow (1). Therefore, regulatory systems for control of sodium balance and arterial pressure may be altered by placental insufficiency in utero. We previously reported that RAS blockade by use of the ACE inhibitor enalapril abolishes hypertension in adult growth-restricted offspring from reduced uterine perfusion dams (40). This suggests an important role for RAS involvement in the established phase of IUGR-induced hypertension. In the present study we determined the quantitative importance of the RAS in the early phase of IUGR-induced hypertension. In addition, we also determined whether temporal alterations in the RAS were associated with the etiology of hypertension in this model of IUGR produced in response to reduced uterine perfusion.
An important role for RAS involvement in the development of hypertension in models of pre-natal programming induced by low protein is suggested by RAS blockade studies. Early blockade of the RAS by the ACE inhibitor, enalapril (33), or by AT1R blockade (48) prevents development of hypertension in models of programming induced by gestational protein restriction. Although elevated levels of intrarenal ANG II (30, 45) or systemic (32) RAS may not be present at this time, investigators report offspring of protein restricted dams present increased sensitivity to ANG II (35, 46) that may (46) or may not (35) be associated with increased intra-renal expression of AT1 receptors. In our studies hypertension is present in growth-restricted offspring from reduced uterine perfusion dams between 4 to 6 weeks of age (1). To examine the quantitative importance of the RAS in the early phase of hypertension in this model of IUGR, we administered the ACE inhibitor, enalapril, for two weeks starting at 2 weeks of age. Nephrogenesis is complete by 2 weeks of age so the initiation of an ACE inhibitor at this age in the rat will not interfere with proper renal development (21). Although intra-renal expression of AT1 receptors was not increased at 4 weeks of age in growth restricted offspring, early ACE inhibition abolished hypertension. In addition, the effect of ACE inhibition on arterial pressure was sustained since MAP remained normalized in treated growth-restricted offspring relative to control, treated and untreated, at 5 weeks of age, or one week past treatment with enalapril. Thus, the RAS contributes to early hypertension in this model of IUGR induced by placental insufficiency and increased sensitivity to ANG II may contribute to the etiology of hypertension in growth-restricted offspring.
We next examined whether temporal alterations in the RAS were observed in this model of pre-natal programmed hypertension induced by placental insufficiency. RAS components are highly expressed in the developing kidney and play a role in mediating proper nephrogenesis (20, 57). AT1R blockade during the nephrogenic period in the rat leads to a reduction in nephron number and development of hypertension (57) suggesting that the intra-renal RAS contributes to proper nephrogenesis. Models of programming induced by gestational protein restriction consistently exhibit marked reductions in nephron number and kidney size associated with significant increases in arterial pressure in adulthood (30, 51, 55). In one model of gestational protein restriction utilized by Woods et al., suppression of the RAS is present at birth (55). Thus, in utero exposure to low protein may lead to alterations in the RAS resulting in permanent structural changes and subsequent development of hypertension in adult offspring (55). Similar to observations noted by Woods with the low protein model, we observed a significant decrease in renal mRNA expression of renin and angiotensinogen in newborn growth-restricted offspring. This reduction in renin and angiotensinogen mRNA expression at birth in growth-restricted offspring may also be associated with reductions in nephron number. Kidney weight is reduced in growth-restricted offspring in response to reduced uterine perfusion (1) and reductions in nephron number have been reported by other investigators using similar models of reduced uteroplacental flow in the rat (36, 42) and other species (8, 10).
The RAS is an important regulator of arterial pressure and body fluid balance through the systemic actions of ANG II (23). Inappropriate activation of the peripheral RAS, noted by a marked increase in PRA, is observed in conjunction with established hypertension in a model of gestational protein restriction utilized by Vehaskari and colleagues (32). Vehaskari also notes that RAS blockade abolishes established hypertension in adult offspring of gestational protein restricted dams (32). Furthermore, alterations in the RAS may also contribute to marked increases in blood pressure induced by pre-natal exposure to glucocorticoids (XX). Therefore, a role for RAS involvement is indicated in animal models of hypertension produced in response to an adverse insult during fetal development. However, in growth-restricted offspring from reduced uterine perfusion dams, established marked elevations in MAP were not associated with changes in the peripheral RAS as we previously reported by measure of PRA and plasma renin substrate (40), and in this study, by serum ACE activity. Thus, inappropriate activation of the peripheral RAS is not associated with hypertension in this model of IUGR. However, as suggested by studies in transgenic animals, intrarenal ANG II can contribute to development of hypertension despite the absence of an increase in the peripheral RAS (14). Therefore, the intra-renal RAS, not the peripheral RAS, may contribute to the etiology of hypertension in growth-restricted offspring from reduced uterine perfusion dams.
All components of the RAS are present in the kidney (19). Since RAS blockade abolishes hypertension in adult growth-restricted offspring suggesting RAS involvement (40), we characterized the renal RAS in growth-restricted offspring to determine if alterations were present in this model of IUGR-induced hypertension. Renal renin and angiotensinogen mRNA expression were no longer suppressed at 6 weeks of age as previously observed at birth, and were significantly elevated at 16 weeks of age in the cortex of adult growth-restricted offspring relative to control offspring. A marked increase in renal ACE activity was also observed at 16 weeks of age in adult growth-restricted offspring. However, ACE and AT1R mRNA expression did not differ upon comparison of adult growth-restricted offspring to control. Quantitative autoradiography also showed no difference between ACE and AT1R expression in the kidneys of adult growth-restricted offspring relative to control. Although renal ACE activity was increased in adult growth restricted offspring at 16 weeks of age in conjunction with increased levels of renin substrate, ANG II formation was not increased.
Angiotensinogen of hepatic origin can serve as the substrate for generation of intra-renal ANG II; however, angiotensinogen is also present within the proximal tubule (27) and can be stimulated by testosterone (39). We previously reported that plasma testosterone levels are elevated two-fold in adult male growth restricted offspring (40). Regulation of renal angiotensinogen by testosterone can occur in a dose dependent fashion (16) suggesting that elevated testosterone in adult male growth restricted offspring may serve as the stimulus for enhanced renal angiotensinogen observed in this model of IUGR hypertension. Enhanced renal angiotensinogen levels can lead to activation of the RAS and hypertension (26) and we have demonstrated the importance of the RAS in mediating hypertension in adult male growth restricted offspring (40). However, despite enhanced intra-renal angiotensinogen, increased intra-renal ANG II was not observed in conjunction with established hypertension in adult male IUGR. Discrepancies in intra-renal angiotensinogen and ANG II are also observed in the Dahl salt-sensitive (28) and Zucker Diabetic rat (50), yet the importance of ANG II in these experimental models is demonstrated by the renoprotective effects mediated via AT1 receptor blockade (33, 39, 50). Therefore, the intrarenal RAS, independent of the peripheral RAS, may play a role by contributing to impaired sodium reabsorption and hypertension in adult growth-restricted offspring from reduced uterine perfusion dams.
ANG II blockade studies support a role for RAS involvement in both the development and maintenance of established hypertension in this model of IUGR induced by placental insufficiency. Moreover, temporal alterations in the renal RAS are evident in this model of IUGR. However, it appears that the factors that initiate hypertension in this model of IUGR may differ from the factors that maintain established hypertension. Fetal responses to reduced uterine perfusion indicate suppression of intra-renal RAS at birth, no alteration in the intra-renal RAS in young animals, and marked alterations in the intra-renal RAS in the adult hypertensive animal; in addition, no temporal changes are observed in the peripheral RAS. Since sex differences are observed in this model of IUGR, only male growth-restricted offspring remain hypertensive after puberty, differential expression of the RAS by sex hormones may contribute to established hypertension in adult male growth-restricted offspring.
Animal studies provide evidence suggesting that insults during gestation result in structural and physiological alterations leading to hypertension associated with permanent changes in the regulatory systems involved in the long-term control of arterial pressure. Alterations in intra-renal RAS contribute to the etiology of hypertension in offspring from reduced uterine perfusion dams, an observation similar to that observed by other investigators using models of gestational protein restriction and pre-natal glucocorticoid exposure. The pathogenesis of hypertension is multifactorial; and although insight provided by different animal models highlights the complexities involved in the developmental origins of adult disease, the presence of common mechanistic pathways is suggested.
Acknowledgments
GRANTS
This work was supported by NIH grant HL074927.
Footnotes
DISCLOSURES
The authors have no disclosures.
References
- 1.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41(3):457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R1–R10. doi: 10.1152/ajpregu.00417.2005. [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low birth weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45(4):754–758. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- 4.Allen AM, Zhuo J, Mendelsohn FA. Localization of angiotensin AT1 and AT2 receptors. J Am Soc Nephrol. 1999;10(S11):S23–29. [PubMed] [Google Scholar]
- 5.Bagby SP, LeBard LS, Luo Z, Ogden BE, Corless C, McPherson ED, Speth RC. ANG II AT(1) and AT(2) receptors in developing kidney of normal microswine. Am J Physiol Renal Physiol. 2002;283(4):F755–F764. doi: 10.1152/ajprenal.00313.2001. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ. The fetal and infant origin of disease. Eur J Clin Investig. 1995;25(7):457–463. doi: 10.1111/j.1365-2362.1995.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 8.Bassan H, Trejo LL, Kariv N, Bassan M, Berger E, Fattel A, Gozes I, Harel S. Experimental intrauterine growth retardation alters renal development. Pediatr Nephrol. 2000;15(3–4):192–195. doi: 10.1007/s004670000457. [DOI] [PubMed] [Google Scholar]
- 9.Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11beta-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142(7):2841–2853. doi: 10.1210/endo.142.7.8238. [DOI] [PubMed] [Google Scholar]
- 10.Briscoe TA, Rehn AE, Dieni S, Duncan JR, Wlodeck ME, Owens JA, Rees SM. Cardiovascular and renal disease in the adolescent guinea pig after chronic placental insufficiency. Am J Obstet Gyneco. 2004;191(3):847–855. doi: 10.1016/j.ajog.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 11.Campbell DM, Hall MH, Barker DJ, Cross J, Shiell AW, Godfrey KM. Diet in pregnancy and the offspring blood pressure 40 years later. Br J Obstet Gynaecol. 1996;103(3):273–280. doi: 10.1111/j.1471-0528.1996.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 12.Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94(6):1310–1315. doi: 10.1161/01.cir.94.6.1310. [DOI] [PubMed] [Google Scholar]
- 13.Curhan GC, Willet WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetus mellitus, and obesity in US men. Circulation. 1996;94(12):3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 14.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics. 1999;1(1):3–9. doi: 10.1152/physiolgenomics.1999.1.1.3. [DOI] [PubMed] [Google Scholar]
- 15.DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11(2):197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Ding Y, Sigmund CD. Androgen-dependent regulation of human angiotensinogen expression in KAP-hAGT transgenic mice. Am J Physiol Renal Physiol. 2001;280(1):F54–60. doi: 10.1152/ajprenal.2001.280.1.F54. [DOI] [PubMed] [Google Scholar]
- 17.Forsen T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJ. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ. 1999;319(7222):1403–1407. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005;565(Pt 1):137–147. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez RA, Lynch KR, Chevalier RL, Wilfong N, Everett A, Carey RM, Peach MJ. Renin and angiotensinogen gene expression in maturing rat kidney. Am J Physiol. 1988;254(4 Pt 2):F582–F587. doi: 10.1152/ajprenal.1988.254.4.F582. [DOI] [PubMed] [Google Scholar]
- 20.Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. J Hypertens. 2000;18(2):123–137. doi: 10.1097/00004872-200018020-00001. [DOI] [PubMed] [Google Scholar]
- 21.Guron G, Marcussen N, Nilsson A, Sundelin B, Friberg P. Postnatal time frame for renal vulnerability to enalapril in rats. J Am Soc Nephrol. 1999;10(7):1550–1560. doi: 10.1681/ASN.V1071550. [DOI] [PubMed] [Google Scholar]
- 22.Haber E, Koerner T, Page LB, Kliman B, Purnode A. Application of a radioimmunoassay for angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocr Metab. 1969;29(10):1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- 23.Hadoke PWF, Lindsay RS, Seckl JR, Walker BR, Kenyon CJ. Altered vascular contractility in adult female rats with hypertension programmed by prenatal glucocorticoid exposure. J Endocrinol. 2006;188(3):435–442. doi: 10.1677/joe.1.06506. [DOI] [PubMed] [Google Scholar]
- 24.Hall JE, Guyton AC, Mizelle HL. Role of the renin-angiotensin system in control of sodium excretion and arterial pressure. Acta Physiol Scand Suppl. 1990;591:48–62. [PubMed] [Google Scholar]
- 25.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002;282(1):F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18(7):815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 27.Ichihara A, Kobori H, Nishiyama A, Navar LG. Renal renin-angiotensin system. Contrib Nephrol. 2004;143:117–30. doi: 10.1159/000078716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobori H, Harrison-Bernard LH, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12(3):431–9. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41(3):592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krebs LT, Hanesworth JM, Sardinia MF, Speth RC, Wright JW, Harding JW. A novel angiotensin analog with subnanomolar affinity for angiotensin-converting enzyme. J Pharmacol Exp Ther. 2000;293(1):260–267. [PubMed] [Google Scholar]
- 31.Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 1996;91(5):607–615. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- 32.Law CM, Shiell AW. Is blood pressure inversely related to birth weight? The strength of evidence from a sympathethic review of the literature. J Hypertens. 1996;14(8):935–941. [PubMed] [Google Scholar]
- 33.Manning J, Vehaskari VM. Low birth weight associated adult hypertension in rat. Pediatr Nephrol. 2001;16(5):417–422. doi: 10.1007/s004670000560. [DOI] [PubMed] [Google Scholar]
- 34.Manning J, Vehaskari VM. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am J Physiol regul Integr Comp Physiol. 2005;288(1):R80–R84. doi: 10.1152/ajpregu.00309.2004. [DOI] [PubMed] [Google Scholar]
- 35.McMullen S, Gardner DS, Langley-Evans SE. Prenatal programming of angiotensin II type 2 receptor expression in the rat. Br J Nutr. 2003;91(1):133–140. doi: 10.1079/bjn20031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMullen S, Gardner DS, Langley-Evans SC. Prenatal programming of angiotensin II type 2 receptor expression in the rat. Br J Nutr. 2004;91(1):133–140. doi: 10.1079/bjn20031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merlet-Benichou C, Glibert T, Muffat-Joly M, Lelievre-Pegorier M, Leroy B. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr Nephrol. 1994;8(2):175–180. doi: 10.1007/BF00865473. [DOI] [PubMed] [Google Scholar]
- 38.Naito Y, Tsujino T, Fujioka Y, Ohyanagi M, Iwasaki T. Augmented diurnal variations of the cardiac renin-angiotensin system in hypertensive rats. Hypertension. 2002;40(6):827–833. doi: 10.1161/01.hyp.0000039960.66987.89. [DOI] [PubMed] [Google Scholar]
- 39.Navar LG, Von Thun AM, Zou L, el-Dahr SS, Mitchell KD. Enhancement of intrarenal angiotensin II levels in 2 kidney 1 clip and angiotensin II induced hypertension. Blood Press Suppl. 1995;2:88–92. [PubMed] [Google Scholar]
- 40.Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of AT1 receptor blockade onr enal injury and mitogen-activated protein activity in Dahl salt –sensitive rats. Kidney Int. 2004;65(3):972–81. doi: 10.1111/j.1523-1755.2004.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Reagan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287(5):E863–E870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- 42.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R758–63. doi: 10.1152/ajpregu.00311.2006. [DOI] [PubMed] [Google Scholar]
- 43.Peers A, Campbell DJ, Wintour EM, Dodic M. The peripheral renin-angiotensin system is not involved in the hypertension of sheep exposed to prenatal dexamethasone. Clin Exp Pharmacol Physiol. 2001;28(4):306–311. doi: 10.1046/j.1440-1681.2001.03443.x. [DOI] [PubMed] [Google Scholar]
- 44.Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp Physio. 2003;285(5):R962–R970. doi: 10.1152/ajpregu.00201.2003. [DOI] [PubMed] [Google Scholar]
- 45.Rasch R, Skriver E, Woods LL. The role of the RAS in programming of adult hypertension. Acta Physiol Scand. 2004;181(4):537–542. doi: 10.1111/j.1365-201X.2004.01328.x. [DOI] [PubMed] [Google Scholar]
- 46.Riviere G, Michaud A, Breton C, VanCamp G, Laborie C, Enache M, Lesage J, Deloof S, Corvol P, Vieau D. Angiotensin-converting enzyme 2 (ACE2) and ACE activities display tissue-specific sensitivity to undernutrition-programmed hypertension in the adult rat. Hypertension. 2005;46(5):1169–1174. doi: 10.1161/01.HYP.0000185148.27901.fe. [DOI] [PubMed] [Google Scholar]
- 47.Sahajpal V, Ashton N. Increased glomerular angiotensin II binding in rats exposed to a maternal low protein diet in utero. J Physiol. 2005;563(Pt 1):193–201. doi: 10.1113/jphysiol.2004.078642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahajpal V, Ashton N. Renal function and angiotensin AT1 receptor expression in young rats following intrauterine exposure to maternal low-protein diet. Clin Sci (Lond) 2003;104(6):607–614. doi: 10.1042/CS20020355. [DOI] [PubMed] [Google Scholar]
- 49.Schiffrin EL, Gutkowska J, Thibault G, Genest J. Effect of enalapril (MK-421), an orally active angiotensin I converting enzyme inhibitor, on blood pressure, active and inactive plasma renin, urinary prostaglandin E2, and kallikrein excretion in conscious rats. Can J Physiol Pharmacol. 1984;62(1):116–123. doi: 10.1139/y84-019. [DOI] [PubMed] [Google Scholar]
- 50.Sherman RC, Langley-Evans SC. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 2000;98(3):269–275. [PubMed] [Google Scholar]
- 51.Speth RC, Husain A. Distribution of angiotensin-converting enzyme and angiotensin II-receptor binding sites in the rat ovary. Biol Reprod. 1988;38(3):695–702. doi: 10.1095/biolreprod38.3.695. [DOI] [PubMed] [Google Scholar]
- 52.Suzaki Y, Ozawa Y, Kobori H. Intrarenal oxidative stress and augmented angiotensinogen are precedent to renal injury in Zucker Diaetic Fatty rats. Int J Biol Sci. 2007;3(1):40–6. doi: 10.7150/ijbs.3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol. 2004;287(2):F262–F267. doi: 10.1152/ajprenal.00055.2004. [DOI] [PubMed] [Google Scholar]
- 54.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59(1):238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 55.von Lutterotti N, Camargo MJ, Mueller FB, Timmermans PB, Laragh JH. Angiotensin II receptor antagonist markedly reduces mortality in salt-loaded Dahl S rats. Am J Hypertens. 1991;4(4 Pt 2):346S–349S. doi: 10.1093/ajh/4.4.346s. [DOI] [PubMed] [Google Scholar]
- 56.Wintour EM, Johnson K, Koukoulas I, Moritz K, Tersteeg M, Dodic M. Programming the cardiovascular system, kidney and the brain--a review. Placenta. 2003;24(A):S65–S71. doi: 10.1053/plac.2002.0927. [DOI] [PubMed] [Google Scholar]
- 57.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49(4):460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Woods LL. Fetal origin of adult hypertension: a renal mechanism? Curr Opin Nephrol Hypertens. 2000;9(4):419–425. doi: 10.1097/00041552-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 59.Woods LL, Rasch R. Perinatal Angiotensin II programs adult blood pressure, glomerular number and renal function in rats. Am J Physiol Regul Integr Comp Physiol. 1998;275(5 Pt 2):R1593–R1599. doi: 10.1152/ajpregu.1998.275.5.R1593. [DOI] [PubMed] [Google Scholar]