Abstract
The authors propose a heuristic model of the social outcomes of childhood brain disorder that draws on models and methods from both the emerging field of social cognitive neuroscience and the study of social competence in developmental psychology/psychopathology. The heuristic model characterizes the relationships between social adjustment, peer interactions and relationships, social problem solving and communication, social-affective and cognitive-executive processes, and their neural substrates. The model is illustrated by research on a specific form of childhood brain disorder, traumatic brain injury. The heuristic model may promote research regarding the neural and cognitive-affective substrates of children’s social development. It also may engender more precise methods of measuring impairments and disabilities in children with brain disorder and suggest ways to promote their social adaptation.
Keywords: social competence, childhood brain disorder, social neuroscience, traumatic brain injury
Surprisingly little is known about the extent, basis, and consequences of the social problems associated with neurological dys-function and brain insults occurring during childhood, despite the significant long-term implications of social development for children’s functioning at home, in school, and in the community (Parker, Rubin, Erath, Wojslawowicz, & Buskirk, 2006; Rubin, Bukowski, & Parker, 2006). Until recently, the lack of measurement tools and articulated models of social functioning has limited our ability to address social outcomes in children with brain disorder. The development of more sensitive measures and explicit models of social functioning would help researchers and clinicians to target children with brain disorders for further study and intervention.
Now is an excellent time to consider social outcomes in children with brain disorder. The emerging field of social cognitive neuroscience provides a critical perspective on the social impact of childhood brain disorder. Social neuroscience not only supplies tools needed to better understand the neural substrates and social-cognitive processes associated with social functioning, but also provides a foundation for a multilevel, integrative analysis of the social difficulties arising from neurological insults (Brothers, 1990; Cacioppo, Berntson, Sheridan, & McClintock, 2000; Moss & Damasio, 2001; Ochsner & Lieberman, 2001; Posner, Rothbart, & Gerardi-Caulton, 2001). Although social neuroscience to date has focused primarily on adults, in part because of the inability to study the developing brain in vivo, this no longer need be the case. With contemporary neuroimaging, various elegant methods are available that can inform researchers about brain development and neuropathology in the study of social behavior in children with brain disorder (Toga & Thompson, 2005).
The methods and models derived from social neuroscience will be particularly powerful when combined with those associated with the study of social competence in developmental psychology and developmental psychopathology (Parker et al., 2006; Rubin, Bukowski, & Parker, 2006). The latter approaches reflect a developmental perspective that can enhance the field of social neuroscience. In short, we now have the tools and models to begin to understand how children’s daily functioning in the social world is associated with their abilities to identify, think about, produce, and regulate emotions; to consider other people’s perspectives, beliefs, and intentions; and to solve interpersonal problems. Furthermore, we can model this association in terms of developmental processes and brain pathology.
In this article, we propose an integrative, heuristic model of the social outcomes of childhood brain disorder, grounded in concepts and methods drawn from both the emerging field of social neuroscience and the study of social competence in developmental psychology/psychopathology. The model attempts to specify the relations between social adjustment, peer interactions and relationships, social problem solving and communication, social-affective and cognitive-executive processes, and their brain substrates. The model also takes into account the distinct but related developmental trajectories that occur within these domains.
We use the broad, generic term childhood brain disorder because we believe the model may be germane to a wide range of central nervous system abnormalities and insults, both developmental and acquired in origin. For instance, the model may be applicable to neurodevelopmental disorders, such as autism (Baron-Cohen & Belmonte, 2005), disorders arising from prenatal exposure to teratogens, such as fetal alcohol syndrome (Schonfeld, Mattson, & Riley, 2005), or to acquired brain injuries, such as childhood stroke (Coelho-Mosch, Max, & Tranel, 2005). This does not mean that we expect all childhood brain disorders to affect social development to the same degree or in the same way. Rather, the likelihood that any specific brain disorder will affect social development will be a function primarily of the nature and timing of the brain insult with which it is associated, rather than of the specific etiology involved. For instance, an ischemic stroke and a traumatic brain injury arising from closed-head trauma can potentially occur at the same age and affect comparable brain regions, and therefore would be likely to give rise to similar social outcomes.
To illustrate the application of the model to a specific form of childhood brain disorder, we draw on research regarding traumatic brain injury (TBI). TBI, also referred to as closed-head injury, is a form of acquired brain injury that arises as a result of blunt trauma to the head (Yeates, 2000). TBI is a leading cause of death and disability in youth under the age of 15, and several lines of research suggest that children with TBI are vulnerable to poor social outcomes; nevertheless, the social outcomes of childhood TBI remain largely uncharacterized and poorly understood. We review the existing research in line with our proposed heuristic model and discuss how the model may help to guide future research on childhood TBI.
The model may also help to further characterize social competence in healthy children and thereby has the potential to contribute to our understanding of both normal and aberrant social development. Indeed, our hope is that the model will provide a heuristic framework for future research regarding the neural and cognitive-affective substrates of children’s social behavior. Practically speaking, the model may further the development of more precise methods of measuring impairments and disabilities in children with brain disorder, help clinicians target children with poor social outcomes for further intervention, and prove valuable in designing interventions to promote better social outcomes following childhood brain disorder.
Definitions and Distinctions in the Study of Social Competence
The study of social outcomes in childhood brain disorder rests in part on a definition of social competence. Researchers studying social development have proffered many definitions (Dodge, Pettit, McClaskey, & Brown, 1986; Rose-Krasnor, 1997; Rubin, Booth, Krasnor, & Mills, 1995). Most have suggested that social competence involves the effectiveness of a person’s functioning as an individual, in dyadic relationships, and in groups (Bukowski, Rubin, & Parker, 2001). Rubin and Rose-Krasnor (1992; Rubin & Krasnor, 1986) have defined social competence as the ability to achieve personal goals in social interaction while simultaneously maintaining positive relationships with others over time and across situations. A significant feature of this definition is its implicit recognition of the importance of both individual and social goals. Bukowski et al. (2001) suggested that this emphasis reflects an essential duality of self and other, placing the individual within a social and personal context. Thus, Rubin and Rose-Krasnor’s definition highlights the complex goals that persons confront as individuals (satisfying personal goals) and as members of groups (while maintaining positive relationships).
On the basis of this definition, social competence may be viewed as a transactional construct. That is, social competence depends on personal characteristics of the child, the interactions between the child and members of his or her social world, and the interpretations of the self and others that the child’s actions are acceptable and successful. Social competence from this perspective also is viewed as a developmental construct that is both time and context dependent (Rubin & Krasnor, 1986; Rubin & Rose-Krasnor, 1992).
Rubin, Bukowski, and Parker (2006) have suggested that the study of social competence can be guided in part by distinguishing among several levels of social complexity: individuals, interactions, and relationships. Children bring certain individual characteristics to bear in their interactions with others (e.g., the ability to regulate emotion; ways of thinking about how to solve social problems; a repertoire of means to achieve social goals; the capacity to predict the consequences of strategies selected to meet social goals, for both the self and others; thoughts and feelings about the self’s ability to be successful in the social world). In many ways, these individual characteristics may be thought to comprise children’s social intelligence. In turn, children’s social interactions also depend on the individual characteristics and behavior of the children and adults with whom they are involved. Social interactions may be characterized as involving actions that bring individuals together (i.e., sociable and prosocial behaviors), actions that move people against each other (i.e., aggression), and actions that isolate individuals from each other (i.e., social withdrawal; Rubin, Bukowski, & Parker, 2006). Finally, interactions are frequently embedded in and give rise to longer term relationships. Relationships are defined in part by the members’ individual characteristics and the quality of their interactions but have distinct properties of their own, such as closeness and commitment. Friendship is a prototypical relationship.
A closely related set of distinctions was made by Nassau and Drotar (1997) in their review of social competence in children with chronic health conditions affecting the central nervous system. Drawing on Cavell’s (1990) tri-component model of social competence, they distinguished between social skills, social performance, and social adjustment. Social skills are the individual abilities or characteristics needed to behave competently in social settings. Social performance refers to children’s actual behavior in social interactions and to whether their responses are effective both in achieving their own goals and in maintaining positive relationships. Social adjustment, finally, reflects the extent to which children attain socially desirable and developmentally appropriate goals. Social adjustment encompasses the quality of children’s relationships as perceived by others but also includes self-perceptions of loneliness, social support, or social self-esteem.
Children whose individual social skills and social interactions engender social success are popular among their peers and viewed by teachers and parents as well adjusted (Parker et al., 2006). In contrast, children who are less competent are typically rejected by peers and rated by teachers and parents as maladjusted. For example, children who frequently seek to attain their personal goals by means of aggression (i.e., moving against their social partners) are often viewed by teachers and parents as having adjustment problems of an externalizing nature. Children who retreat when others approach them or who attempt to meet their social goals by requesting that adults act on their behalf are often viewed by teachers and parents as having problems of an internalizing nature (Parker et al., 2006).
Previous studies pertaining to social outcomes in childhood brain disorders have focused largely on social adjustment, which in this population has been assessed primarily via parent ratings. Few researchers of childhood brain disorder have examined children’s social skills and other individual characteristics that affect social behavior, and investigators have yet to directly examine social interactions and relationships among children with brain disorder. Moreover, in only a handful of studies have researchers investigated the relations among different aspects of social competence. We contend that a comprehensive portrayal of social outcomes in childhood brain disorder must encompass the three levels that characterize recent definitions of social competence (i.e., individual characteristics and social skills, social performance and interaction, and social adjustment), as well as the relations between the levels. The three levels provide distinct but interrelated windows on social competence.
An Integrative, Multilevel Approach to the Study of Social Competence
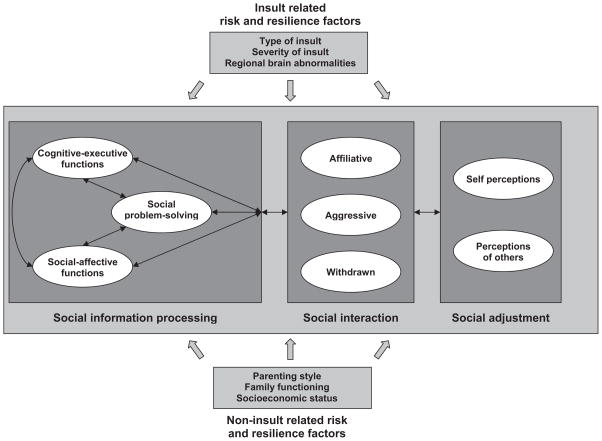
Research in developmental psychology and developmental psychopathology has provided a more detailed characterization of the individual characteristics and social skills, interactions, and various aspects of social adjustment that constitute social competence. Additionally, it has shown how deficits in those areas are linked to social maladaptation. On the basis of that research, we propose a multilevel, integrative, heuristic model of social competence, as illustrated in Figure 1, which details specific components at each level and articulates the relations among levels.
Figure 1.
An integrative, heuristic model of social competence in children with brain disorder.
Model Components
At the level of individual characteristics and social skills, social information processing is frequently seen as a critical determinant of social competence (Crick & Dodge, 1994; Rubin & Krasnor, 1986). Social information processing is conceived as involving a series of distinct problem-solving steps that are implemented when children respond to social situations. Such steps would commonly involve interpreting cues, clarifying goals, generating alternative responses, selecting and implementing a specific response, and evaluating the outcome. Social problem solving is often assessed by asking children to reflect on and answer questions about hypothetical social dilemmas (Dodge, Laird, Lochman, & Zelli, 2002). Children’s reasoning about such dilemmas varies systematically as a function of the specific situations presented, such as ones involving peer provocation versus group entry (Burgess, Wojslawowicz, Rubin, Rose-Krasnor, & Booth-LaForce, 2006; Dodge et al., 2002).
Recent theorists have recognized that social information processing depends on other cognitive and affective factors and have incorporated into their models such constructs as language pragmatics, executive function, and emotion regulation (Dodge et al., 2002; Guralnick, 1999; Lemerise & Arsenio, 2000). The latter variables are typically treated as stable individual characteristics (i.e., “latent knowledge” per Dodge et al., 2002; “foundation processes” per Guralnick, 1999). They are assumed to play a critical role in the implementation of interpersonal problem solving, which is seen as a more situation-specific and “online” social skill. The models assume that the effects of these cognitive and affective factors on social interaction and adjustment are mediated in part through their effects on social problem solving.
Research on children’s social interactions has shown that they vary depending on both the type of social situation and the nature of children’s relationships with the individuals with whom they interact (Parker et al., 2006; Rubin, Bukowski, & Parker, 2006). For instance, children exhibit different behaviors when attempting to enter a peer group activity than when responding to peer provocation, and they use different strategies when attempting to gain access to objects than when attempting to gain the attention of others (e.g., Krasnor & Rubin, 1983). Similarly, children interact differently with friends than with unfamiliar peers (Dunn, Cutting, & Fisher, 2002; Newcomb & Bagwell, 1995). Notably, the range and flexibility of children’s social behaviors across different contexts and relationships are often considered hallmarks of social competence (Dodge et al., 1986; Rose-Krasnor, 1997; Rubin et al., 1995).
A detailed understanding of children’s social interactions cannot be attained using conventional rating scales or questionnaires but instead requires direct observation in a variety of contexts. Many observational protocols and coding schemes have been developed to study children’s social interactions (Bierman, 2004; Rubin, Bukowski, & Parker, 2006). Regardless of the context in which children are observed or with whom they interact, coding schemes frequently focus on the three broad behavioral tendencies noted earlier: (a) moving toward others (i.e., prosocial, affiliative behavior), (b) moving against others (i.e., aggressive or agonistic behavior), and (c) moving away from others (i.e., socially withdrawn behavior).
Research on social adjustment has shown that it too varies along several important dimensions. One critical distinction, consistent with the incorporation of both individual and social goals in our definition of social competence, is whether social adjustment is evaluated based on self-perceptions versus the perceptions of others, such as peers, parents, or teachers (Parker et al., 2006; Rubin, Bukowski, & Parker, 2006). This distinction may be especially important for children with brain disorder, who may lack awareness of their own deficits (Prigatano, 1991; Prigatano, Altman, & O’Brien, 1990) and might therefore tend to evaluate their social adjustment more positively than do others.
Social adjustment from the perspective of others can be assessed via classroom peer nominations and ratings of peer acceptance and behavioral reputation. These indices are not independent of one another but are conceptually and empirically distinct and have different implications for long-term adjustment (Asher, Parker, & Walker, 1996; Gest, Graham-Bermann, & Hartup, 2001; Nangle, Erdley, Newman, Mason, & Carpenter, 2003). For instance, in early adolescence, some forms of aggression are linked to perceived popularity among peers; however, they also result in significant constraints on reciprocal friendships (Cillessen & Rose, 2005), which increase in importance as children grow older (Rubin, Wojslawowicz, Rose-Krasnor, Booth-La Force, & Burgess, 2006).
Social adjustment also can be measured from the perspective of the self. In early and middle childhood, aggressive children tend to believe that they are well accepted by peers and that they are socially skilled, but their peers think otherwise (Boivin, Vitaro, & Poulin, 2005). Indeed, the friendships of aggressive children are marked by instability and mistrust (Hektner, August, & Realmuto, 2000). In contrast, children who withdraw from social interaction tend to view themselves as lacking in social competence (Rubin, Chen, & Hymel, 1993). They are also inclined to indicate feelings of loneliness and depression (Rubin, Burgess, & Coplan, 2002). These socially wary and withdrawn children are like their aggressive counterparts, however, in that they are often unpopular in the peer group and have close relationships with others much like themselves (Boivin, Hymel, & Bukowski, 1995; Rubin, Wojslawowicz, et al., 2006).
A substantial literature suggests that social information processing, social interactions, and social adjustment are closely interrelated (Parker et al., 2006; Rubin, Bukowski, & Parker, 2006). Children who display deficits in social information processing are more often aggressive or socially anxious and withdrawn in their interactions with other children. Those interactions typically result in peer rejection and being considered less desirable as friends. As noted above, anxious and withdrawn children tend to view themselves and their social skills relatively negatively, whereas aggressive children often have an exaggerated opinion of their social competence. In contrast, children whose social information processing skills are intact tend to be more skilled in initiating and maintaining positive relationships, and rely on behaviors that are more prosocial. They are more likely to be socially accepted by peers and to have satisfactory friendships. Thus, Figure 1 incorporates pathways between the three levels of social competence. The pathways are designated as bidirectional; thus, social information processing can affect social interactions, which in turn affect social adjustment. Conversely, the perceptions of self and other can affect social interactions and help to shape social information processing.
Recent models of social competence also have acknowledged that there are a variety of risk and resilience factors that can hamper or promote social development (Guralnick, 1999; Masten et al., 1999). Some of those factors are intrinsic to the child (e.g., intellectual functioning), whereas others involve environmental influences (e.g., socioeconomic status, parenting behaviors, and parent–child relationships). For instance, neurological dysfunction or acquired brain injury can be conceptualized as risk factors that increase the likelihood of deficits in social information processing, atypical social interaction, and poor social adjustment (Janusz, Kirkwood, Yeates, & Taylor, 2002; Warschausky, Cohen, Parker, Levendosky, & Okun, 1997).
On the environmental side of the ledger, research suggests that parenting beliefs and behaviors and the quality of the parent–child relationship can influence children’s social interactions and social adjustment (Rubin & Burgess, 2002). More general aspects of the family environment, including poverty and parental unemployment, parental conflict, and parent mental health, also may affect social competence (Du Rocher Schudlich, Shamir, & Cummings, 2004; Zahn-Waxler, Duggal, & Gruber, 2002). Even broader sociocultural influences, such as the stigmatization that can result from perceived disability, may have an effect on psychosocial adjustment (Kendall & Terry, 1996).
Risk and resilience factors, whether endogenous or exogenous to the child, can act both as independent predictors of social competence and as moderators of the relations among its various components. For instance, parental warmth and authoritative control tend to predict more appropriate social behavior, which in turn predicts better social adjustment (Ladd & Pettit, 2002; Rubin & Burgess, 2002). Effective parenting also may moderate the relation between children’s social information processing and their social adjustment by promoting more appropriate social interactions in children whose social information processing skills are deficient. Insult-related and noninsult-related risk and resilience factors do not necessarily operate independently of one another. Indeed, they may even interact to predict the social outcomes of childhood brain disorder. For example, children from lower socioeconomic status homes may be more likely to suffer a TBI (Parslow, Morris, Tasker, Forsyth, & Hawley, 2005). Similarly, the social outcomes of childhood TBI have been found to be moderated by the quality of the family, with better outcomes in children from more advantaged backgrounds (Yeates et al., 2004).
Figure 1 acknowledges the possibility that both insult-related and noninsult-related variables may act as risk and resilience factors in determining the social outcomes of childhood brain disorder. Although the model represented in Figure 1 focuses on the influence of the brain and other individual factors on social competence because of our emphasis on children with brain disorder, the model also acknowledges the important role of noninsult-related risk and resilience factors (i.e., environmental influences) as potential contributors to or moderators of outcome.
The Emerging Discipline of Social Cognitive Neuroscience
Until recently, the study of social competence in children has not been strongly informed by neuroscience. The emerging field of social cognitive neuroscience, however, now provides a basis for integrating knowledge about brain structure and function into the study of children’s social development. Social cognitive neuroscience uses methods such as neuroimaging, neuropsychological assessment, and the study of brain disorders to understand the neural substrates of social functioning. The field promotes integrative, multilevel studies of the links between brain, emotion and cognition, and social behavior (Brothers, 1990; Cacioppo et al., 2000; Moss & Damasio, 2001; Ochsner & Lieberman, 2001; Posner et al., 2001).
A growing literature in social cognitive neuroscience indicates that a distributed network of interdependent brain regions subserve a variety of social-cognitive and affective processes that gradually become integrated during the course of social development (Adolphs, 2001; Grady & Keightley, 2002; Johnson et al., 2005). Because the network involves multidirectional and recursive connections, the relationship between structure and process is not strictly 1:1. Any single process typically depends on a variety of structures, and a single structure can be involved in several processes (Adolphs, 2003). Thus, regional specialization likely reflects different patterns of activation across structures rather than activity in a single structure.
Nevertheless, many brain regions have been found to play especially important roles in specific processes. For example, the fusiform gyrus and superior temporal sulcus have been implicated in the perception of faces and the movement of living things (Adolphs, 2003), and the amygdala plays an especially important role in emotion, particularly fear, and the response to danger or threat (Adolphs, 2002; Adolphs, Baron-Cohen, & Tranel, 2002). The anterior cingulate and ventromedial, orbital, and dorsolateral prefrontal cortex are other brain structures that appear to play an important role in other aspects of social cognition, such as the understanding of other’s mental statues (i.e., theory of mind) and emotional regulation (Allman, Hakeem, Erwin, Nimchinsky, & Hof, 2001; Amodio & Frith, 2006; S. Anderson, Bechara, Damasio, Tranel, & Damasio, 1999; Bechara, Damasio, & Damasio, 2000; Frith & Frith, 2001; Gallagher & Frith, 2003; Goel, Grafman, Sadato, & Hallett, 1995; Grattan & Eslinger, 1989; Mah, Arnold, & Grafman, 2004; Siegal & Varley, 2002).
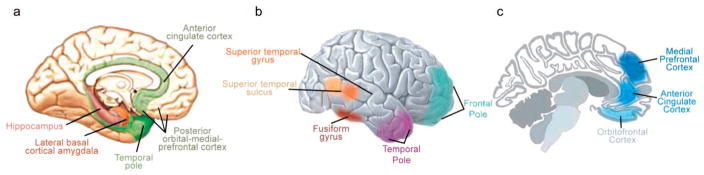
Figure 2 portrays the various brain regions that have been implicated in social cognition and behavior, and Table 1 summarizes some of the links between those regions and specific social-cognitive and affective processes that have been the focus of research to date. As Table 1 indicates, most brain regions are involved in multiple functions, and most specific functions draw on multiple brain regions, although some regional specialization is also apparent. Although most of the previous research has been based on adults, the brain regions illustrated in Figure 2 and listed in Table 1 follow predictable developmental sequences that relate to social development and that can be disrupted or impaired by childhood brain disorders (Johnson et al., 2005).
Figure 2.
Brain regions implicated in social cognition and executive function. Panel a shows medial surface of the right hemisphere depicting the limbic lobe, laterobasal-cortical amygdala, orbital-medial frontal cortex, and hippocampus. The laterobasal-cortical amygdala and hippocampus are projected on the surface of the parahippocampal gyrus. Panel b shows lateral surface of right hemisphere depicting the superior temporal sulcus, superior temporal gyrus, fusiform gyrus, and temporal and frontal poles. Panel c shows midsaggital section depicting orbitofrontal cortex, medial prefrontal cortex, and anterior cingulate cortex. Adapted, with permission, from “Autism: A Window Onto the Development of the Social and the Analytic Brain,” by S. Baron-Cohen and M. K. Belmonte, 2005, Annual Review of Neuroscience, 28, p. 113. Copyright 2005 by Annual Reviews (www.annualreviews.org). Also adapted, with permission, from “The Limbic Lobe and Its Output Channels: Implications for Emotional Functions and Adaptive Behavior,” by L. Heimer and G. W. Van Hoesen, 2006, Neuroscience and Biobehavioral Reviews, 30, p. 135. Copyright 2006 by Elsevier.
Table 1.
Links Between Brain Structures and Social-Affective and Cognitive-Executive Processes
| Brain structure | Social-affective and cognitive-executive functions |
|---|---|
| Somatosensory cortices | Representation of emotional response |
| Viewing others’ actions | |
| Fusiform gyrus | Face perception |
| Superior temporal gyrus | Representation of perceived action |
| Face perception | |
| Perception of gaze direction | |
| Perception of biological motion | |
| Amygdala | Motivational evaluation |
| Self regulation | |
| Emotional processing | |
| Gaze discrimination | |
| Linking internal somatic states and external stimuli | |
| Ventral striatum | Motivational evaluation |
| Self regulation | |
| Linking internal somatic states and external stimuli | |
| Hippocampus and temporal poles | Modulation of cognition |
| Memory for personal experiences | |
| Emotional memory retrieval | |
| Basal forebrain | Modulation of cognition |
| Cingulate cortex | Modulation of cognition |
| Error monitoring | |
| Emotion processing | |
| Theory of mind | |
| Orbitofrontal cortex | Motivational evaluation |
| Self-regulation | |
| Theory of mind | |
| Medial frontal cortex | Theory of mind |
| Action monitoring | |
| Emotional regulation | |
| Emotional responses to socially relevant stimuli | |
| Monitoring of outcomes associated with punishment and reward | |
| Dorsolateral frontal cortex | Cognitive executive functions |
| Working memory |
Notably, the brain regions known to regulate cognitive-executive function overlap substantially with those implicated in social-cognitive and emotional functioning. Indeed, many of these regions play a dual role not only in social cognition but in various aspects of memory and executive function. Thus, diffuse injury to frontotemporal and limbic regions is likely to affect both the cognitive and emotional aspects of social behavior in children (Levin & Hanten, 2005). On the other hand, early focal lesions to particular regions of the social brain network may have more specific effects. For instance, dorsolateral frontal lesions may lead to cognitive deficits in executive functions without significant emotional or social impairment, whereas damage to the orbital and ventromedial prefrontal cortex often results in profound deficits in self-regulation, emotion, and social behavior (Cummings, 1993; Eslinger, Flaherty-Craig, & Benton, 2004; Eslinger, Grattan, Damasio, & Damasio, 1992).
The role of anterior brain regions in social behavior may vary as a function of hemispheric specialization. Developmental researchers have interpreted asymmetries in frontal EEG activation in terms of motivational systems of approach and withdrawal (Davidson, 1992; Fox, 1994). The left frontal region appears to facilitate the approach to appetitive stimuli, whereas the right frontal region is thought to evoke withdrawal from aversive stimuli. Fox and colleagues have demonstrated that right-frontal EEG asymmetry is associated with high levels of behavioral inhibition and social reticence during infancy and early childhood (Fox et al., 1995; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001; Henderson, Marshall, Fox, & Rubin, 2004). Left-frontal asymmetry, on the other hand, has been associated with social approach and positive peer interaction (Fox et al., 1995; Henderson et al., 2004). These findings are consistent with related research on older children and adults (Gray, 1990; Muris, Meesters, de Kanter, & Timmerman, 2005). Studies of emotional expression in individuals with prefrontal lesions also provide evidence of hemispheric asymmetries in social-affective behavior; right frontal lesions are associated with excessive emotionality and disinhibited behavior, whereas left frontal lesions are associated with negative emotions, such as depression and fearfulness, as well as withdrawal (Powell & Voeller, 2004). Qualitative differences of this sort parallel the broad behavioral tendencies that have been identified in studies of children’ social interactions, as described earlier.
In summary, social cognitive neuroscience provides a more detailed picture of the cognitive and affective constructs that also are incorporated in recent models of social information processing and also points to potential neural substrates for specific types of social interactions. More broadly, we believe that the cognitive and emotional processes that are the focus of social cognitive neuroscience provide a critical bridge between knowledge regarding the brain substrates of social behavior and models of social competence from developmental psychology and developmental psychopathology. Specifically, the cognitive-executive and social-affective functions in Figure 1 reflect aspects of social information processing that are linked to a network of specific brain regions (Adolphs, 2001; Grady & Keightley, 2002). At the same time, they also represent the stable individual characteristics (i.e., latent knowledge or foundation processes) described in recent models of social competence (Dodge et al., 2002; Guralnick, 1999; Lemerise & Arsenio, 2000).
Social cognitive neuroscience also links research on children’s social development to the study of childhood brain disorder. Many childhood brain disorders involve insults to the largely anterior brain regions implicated in social information processing. Deficits in social information processing, in turn, are known to be associated with atypical social interactions and poor social adjustment, across a variety of normal and atypical populations (Parker et al., 2006; Rubin, Bukowski, & Parker, 2006; Yeates et al., 2004). The insults associated with many childhood brain disorders, therefore, are likely to have negative consequences for children’s social competence at multiple levels. By linking a network of specific brain regions to deficits in social-cognitive and emotional processes, social cognitive neuroscience provides a foundation for a multilevel analysis of the social problems arising from childhood brain disorder—an analysis that bridges brain, cognition and emotion, and action (Brothers, 1990; Cacioppo et al., 2000; Moss & Damasio, 2001).
Developmental Considerations
The brain regions implicated in social behavior are subject to changes with age, just as social behavior is itself. The changes are likely related, moreover, such that brain maturation correlates with increases in children’s capacities for social information processing, which in turn are related to changes in the complexity of their social behavior (Dennis, 2006; Paus, 2005; Stuss & Anderson, 2004). Understanding the distinct but linked developmental trajectories within these domains, and how they may be altered by childhood brain disorders, will be important for any model of social adaptation and maladaptation.
Brain development
The anterior regions of the brain that are linked to social behavior undergo gradual development, and the prefrontal cortex is particularly slow to mature. Morphological development of the frontal cortex is not complete until around puberty, with further changes continuing into adulthood (Klingberg, Vaidya, Gabrieli, Moseley, & Hedehus, 1999; Orzhek-hovskaya, 1981; Yakovlev, 1962). Similarly, the prefrontal cortex is not fully myelinated until mid-to-late adolescence (Giedd et al., 1999; Klingberg et al., 1999; Sowell et al., 1999; Yakovlev, 1962; Yakovlev & Lecours, 1967). Synaptogenesis occurs at the same rate in most cortical regions (Rakic, Bourgeois, Eckenhoff, Zecevic, & Goldman-Rakic, 1986), although the prefrontal cortex may lag behind the rest of the brain (Chugani, Phelps, & Mazziotta, 1987; Huttenlocher, 1979). White matter may also undergo protracted development within anterior brain regions (Klingberg et al., 1999; Sowell et al., 1999).
Magnetic resonance imaging (MRI) studies have shown rapid growth spurts in the frontal lobes relative to the temporal lobes in the first 2 years after birth (Matsuzawa et al., 2001). After age 5, brain volumes remain relatively stable (Reiss, Abrams, Singer, Ross, & Denckla, 1996), but the ratio of gray to white matter lessens with increasing age (Pfefferbaum et al., 1994; Sowell & Jernigan, 1998) because of decreases in gray matter volumes between childhood and early adulthood (Gogtay et al., 2004; O’Donnell, Noseworthy, Levine, Brandt, & Dennis, 2005). Gray matter loss progresses evenly across the brain at an early age; by adolescence, though, the decreases are localized to the frontal and parietal lobes (Sowell et al., 1999; Sowell, Trauner, Gamst, & Jernigan, 2002). Recent longitudinal studies of cortical gray matter development have shown that higher order association cortices mature only after lower order somatosensory and visual cortices (Gogtay et al., 2004). Within the frontal lobes, maturation proceeds in a back-to-front direction, beginning in the primary motor cortex (precentral gyrus) and spreading anteriorly over the superior and inferior frontal gyri, with the prefrontal cortex developing last. Within the prefrontal cortex, the frontal pole and precentral cortex mature early and the dorsolateral cortex matures last, coinciding with its later myelination.
Development of social information processing
Social information processing also shows developmental changes, in a manner that likely relates to brain development (V. Anderson, Levin, & Jacobs, 2002; Diamond, 2002). The executive functions involved in social behavior, particularly inhibitory control and working memory, undergo gradual development. For instance, during the preschool years, children become more able to delay responses, to suppress responses in a go–no go paradigm, and to respond correctly in the presence of a conflicting response option (Diamond & Taylor, 1996; Gerstadt, Hong, & Diamond, 1994; Kochanska, Murray, Jacques, Koenig, & Vandegeest, 1996; Livesey & Morgan, 1991). The development of working memory and inhibitory control occurs in tandem (Cowan, 1997; Hulme & Roodenrys, 1995), with a close relationship between working memory and inhibitory control beginning to emerge during the preschool years (Dowsett & Livesey, 2000).
Theory of mind is a more specific form of social information processing that also demonstrates ongoing development. Theory of mind involves the ability to think about mental states and to use them to understand and predict what other people know and how they will act (Bibby & McDonald, 2005). In adults, frontal lesions impair performance on theory of mind tasks (Stuss, Gallup, & Alexander, 2001). Theory of mind begins to become apparent early in childhood; infants display expectations about the actions of others and by 18 months are able to understand intentions (Kain & Perner, 2003; Meltzoff, 1995; Meltzoff, Gopnik, & Repacholi, 1999). Children first become able to understand desires and intentions (Bartsch & Wellman, 1989) and later begin to understand false beliefs (Sodian, Taylor, Harris, & Perner, 1991). The emergence of theory of mind appears to be closely related to executive functions, such as working memory and inhibitory control (Moses, 2001). Indeed, the emergence of theory of mind correlates closely with the development of executive skills, although they become less closely coupled at later ages (Carlson & Moses, 2001; Gordon & Olson, 1998; Hughes, 2002; Hughes & Ensor, 2005; Rowe, Bullock, Polkey, & Morris, 2001).
The ability to use and understand forms of nonliteral language, such as irony and deceptive praise, in which a speaker’s affective message does not correspond to the words spoken, also follows a protracted developmental course (Dennis, Purvis, et al., 2001). Early in development, children do not understand the concept of saying one thing while meaning another (Demorest, Meyer, Phelps, Gardner, & Winner, 1984). Later in development, children are able to recognize deliberate falsehoods and take into consideration both the facts of the situation and what they believe the speaker believes (Demorest et al., 1984). By middle childhood, children begin to correctly interpret white lies (Demorest et al., 1984). They also begin to understand ironic criticism and to distinguish it from deceptive intent (Demorest et al., 1984). The ability to understand ironic criticism becomes well established by early adolescence (Winner, 1988).
As they mature, children also are increasingly able to think reflectively about more complex social dilemmas, and their growing social problem-solving skills contribute to more successful social function (Crick & Dodge, 1994; Dodge et al., 2002). Young children have knowledge about prosocial problem solving that is not reflected in their spontaneous behavior (Rudolph & Heller, 1997). Children become more skilled at several different aspects of social problem solving, ranging from the retrieval or construction of possible solutions to the evaluation, selection, and enactment of behavioral responses (Mize & Ladd, 1988; Yeates, Schultz, & Selman, 1991). These changes may reflect an increasingly sophisticated ability to coordinate social perspectives (Yeates et al., 1991).
Development of social behavior
With increasing age and brain maturation, children’s social information-processing abilities grow, and their social behavior becomes more diverse, complex, and integrated (Rubin, Bukowski, & Parker, 2006). Changes are apparent both in children’s specific interactions and in their relationships (e.g., friendships). For instance, as their motor and language skills grow, toddlers begin to engage in increasingly lengthy interactions with peers and their play becomes more organized (Eckerman & Stein, 1990). They also display the beginnings of meaningful relationships, preferring to play and engage in complex interactions with familiar as opposed to unfamiliar playmates (Howes, 1988; Howes & Phillipsen, 1998).
Pretend play is a particularly important form of social interaction during the preschool years (Goncu, Patt, & Kouba, 2002; Rubin, Fein, & Vandenberg, 1983). By the third year of life, children are able to share symbolic meanings through social pretense (Howes, 1988). Goncu (1993) has reported quantitative differences in the extent to which the social interchanges of 3-versus 4.5-year-olds reflect shared meaning. For example, the social interactions of older preschoolers involve longer sequences or turns. With increasing age, play partners become better able to agree with each other about the roles, rules, and themes of their pretense. They are also better able to maintain their play interactions by adding new dimensions to their expressed ideas. These developments reflect preschoolers’ growing capacity to take the perspective of the play partner and the increasing sophistication of their nascent theory of mind (Watson, Nixon, Wilson, & Capage, 1999).
By middle childhood, children are spending significantly more time interacting with peers than they did when younger, and their peer interactions are less supervised. Pretend and rough-and-tumble play becomes less common and is replaced by games and activities structured by adults (Pellegrini, 2002). Children become increasingly concerned with acceptance by peers during middle childhood (Kuttler, Parker, & La Greca, 2002). Verbal and relational aggression (i.e., insults, derogation, threats, gossip) gradually replace direct physical aggression when conflict occurs. Children’s conceptualizations of friendship begin to shift from being more instrumental to more empathic, perhaps contingent on their growing ability to coordinate social perspectives (Selman & Schultz, 1990). Their friendships become more stable and are more likely to be reciprocated (Berndt & Hoyle, 1985).
Many of these trends continue during adolescence. Adolescents spend almost one third of their waking hours with peers, nearly double what they spend with parents and other adults (Csikszentmihalyi & Larson, 1984). Their interactions are more likely to occur outside adult guidance and control than they were at earlier ages as well as to involve members of the opposite sex (Brown & Klute, 2003). Friends become increasingly important as sources of support and advice, and friendship begins to involve much more intimacy and self-disclosure (Buhrmester & Furman, 1986). Adolescents develop clear conceptions of the properties that distinguish romantic relationships from friendships, and the two kinds of relationships have distinct implications for adolescent adjustment (Collins, 2003; Connolly, Craig, Goldberg, & Pepler, 1999).
Developmental linkages among brain and social behavior
Relatively little is currently known about the association between brain development and social development. The field of developmental neuroscience holds substantial promise for linking developmental changes in social information processing and social behavior with those that occur in brain structure and function (Munakata, Casey, & Diamond, 2004). Generally speaking, studies of structural and functional brain development suggest that infants and children demonstrate more widely distributed patterns of brain function than adults (Casey, Giedd, & Thomas, 2000), suggesting that regional specialization evolves gradually over the course of development.
Consistent with this general finding, Johnson et al. (2005) recently reviewed the development of the social brain network, emphasizing the concept of interactive specialization. In contrast to a maturational perspective, which suggests that brain functions emerge once a brain region reaches a certain state of maturity, interactive specialization suggests that functional brain development occurs gradually, as a result of the activation and interaction of multiple brain regions. Over time, organizational changes occur in the neural network and certain brain regions ascend in their control or primacy over processing and responding to certain stimuli. Thus, regional specialization occurs, but “the response properties of a specific region are partly determined by its patterns of connectivity to other regions, and their patterns of activity” (Johnson et al., 2005, p. 600). Johnson et al. (2005) presented data from studies of face processing in infants indicating that the entire social brain network is partially active from at least 3 months of age but shows less specialized functionality than in adults, so that children display more widespread brain activation to faces than do adults (Passarotti et al., 2003).
One corollary of the interactive specialization perspective is that an essential ingredient for normal brain development is connectivity. Connectivity in the brain involves white matter pathways, and hence the development of white matter becomes essential for the emergence of the social brain network. In a post-mortem study, Kinney, Brody, Kloman, and Gilles (1988) outlined the staging of myelin development during infancy and used that information to make projections about myelination throughout childhood (see also Haynes et al., 2005; Kinney, 2005). Herbert et al. (2004) used the myelination indices developed by Kinney et al. (1998) in the study of autism, a disorder intimately linked to aberrations in the development of the social brain and associated deficits in gaze cuing and joint attention (Johnson et al., 2005). They found that white matter volume increases primarily in later or longer myelinating brain regions.
Another corollary of the interactive specialization perspective is that the social brain network may be especially vulnerable to early insults. If brains gradually undergo more regional specialization through interactive processes that depend on connectivity, then early insults may disrupt connectivity in such a way that they have a widespread impact on brain development that may be quite remote from the specific location of the insult itself. Notably, the frontal pole, temporal pole, and corpus callosum are the three brain regions with the most protracted white matter development (Haynes et al., 2005; Kinney, 2005; Kinney et al., 1988). The frontal and temporal lobes include key components of the social brain network, and numerous studies have demonstrated the vulnerability of white matter in those regions to certain childhood brain disorders, such as TBI (Gorrie, Duflou, Brown, Gibson, & Waite, 2001; Gorrie, Oakes, Duflou, Blumbergs, & Waite, 2002; Tasker et al., 2005; Wilde, Chu, et al., 2006; Wilde et al., 2005). If early insults disrupt the development of the social brain network more than later insults, then they also are likely to result in more profound consequences for social behavior. Indeed, studies of early focal lesions to the prefrontal cortex suggest that they have more profound effects on social outcomes than similar lesions occurring in adulthood (Eslinger et al., 2004).
Developmental Dimensions in Childhood Brain Disorders
The outcomes associated with brain disorders in childhood are themselves dependent on developmental factors, especially in the case of acquired brain injuries or brain disorders that have their onset after birth. Specifically, outcomes vary along three distinct but interrelated dimensions: the age of the child at the onset of the disorder or time of insult, the amount of time that has passed since the disorder began or insult occurred, and the child’s age at the time of outcome assessment (Taylor & Alden, 1997).
Most studies of school-age children and adolescents have not found a strong relationship between age at onset or insult and outcomes. However, recent studies of preschool children with traumatic brain injuries indicate that injuries sustained during infancy or early childhood are associated with more persistent deficits than are brain insults occurring during later childhood and adolescence (V. Anderson, Catroppa, Morse, Haritou, & Rosenfeld, 2005;V. A. Anderson et al., 1997; Ewing-Cobbs et al., 1997). Similar findings have been obtained in children with other brain insults, including congenital hemiplegia (Banich, Levine, Kim, & Huttenlocher, 1990), brain tumors (Reimers et al., 2003), and diabetes (Rovet, Ehrlich, & Czuchta, 1990).
With regard to time since insult, longitudinal studies have indicated that children generally display a gradual recovery over the first few years after acquired brain injuries, with the most rapid improvement occurring soon after the insult. The initial rate of recovery is often more rapid among children with severe injuries than among those with milder injuries, but severe injuries also are associated with persistent deficits after the rate of recovery slows (Taylor et al., 2002; Yeates et al., 2002). Because very few long-term follow-up studies lasting 5 or more years have been completed, we do not know whether children with acquired brain injuries show any progressive deterioration in functioning relative to healthy peers after their initial recovery. However, younger children appear to demonstrate a slower rate of change over time and more significant residual deficits after their recovery plateaus than do older children with injuries of equivalent severity (V. Anderson et al., 1997, 2005; Ewing-Cobbs et al., 1997). In addition, other neurologically at-risk groups, such as children with bacterial meningitis or with extremely low birth weight, acquire some skills more slowly with age compared with unaffected children (Taylor, Minich, Klein, & Hack, 2004; Taylor, Schatschneider, & Minich, 2000).
The influence of age at testing has been the focus of the least research. The effects of age at testing would be reflected in demonstrations of latent or delayed sequelae resulting from children’s failure to meet new developmental demands as a result of a brain disorder. For instance, because adolescence is associated with substantial maturational changes in the frontal lobes, the effects of frontal lesions might not become fully apparent until then, even if they occurred much earlier in life. The phenomenon of “growing into a lesion” or time-lagged effects has been reported in case studies showing the delayed onset of social problems in children with early frontal lobe lesions (Eslinger et al., 1992), but group studies illustrating this phenomenon are difficult to locate. Latent effects are especially difficult to detect, because they require evidence that differences in the consequences of acquired injuries are due specifically to age at testing, as opposed to age at insult or time since insult. Disentangling these dimensions is difficult, even in the context of longitudinal research (Taylor & Alden, 1997).
Summary: An Integrative, Multilevel Model of Social Outcomes
Figure 1 represents an integrative, multilevel model of the social outcomes of childhood brain disorder grounded in concepts and methods drawn from both the emerging field of social cognitive neuroscience and the study of social competence in developmental psychology/psychopathology. The model specifies general relationships among social information processing, social interaction, and social adjustment, and reflects the possibility of bidirectional relations among those different levels of social competence (e.g., self-perceptions of adjustment may affect social interactions and vice versa). The model acknowledges the brain substrates for social cognition and affect regulation, and it indicates that factors related directly to the neurological insult, as well as those independent of it, can influence social competence at all levels and the relations among them. The model as portrayed in Figure 1 is largely heuristic in nature in that it portrays the relationships among the levels of social competence and their association with insult-related and noninsult-related risk and resilience factors in a general fashion that does not necessarily lead to specific predictions. However, when the existing research literature about the individual components of the model is taken into account, the model can give rise to more specific hypotheses.
For instance, when applied to childhood brain disorders, the model implies that some disorders—but not all—will be associated with insults predominantly to the temporal cortices, amygdala, anterior cingulate, basal forebrain, and prefrontal cortex (Wilde, Bigler, et al., in press; Wilde et al., 2005). In the presence of such selective damage, children are likely to have difficulties understanding the emotional expressions and mental states of others as well as regulating their own emotions (Dennis, Barnes, Wilkinson, & Humphreys, 1998; Dennis, Purvis, Barnes, Wilkinson, & Winner, 2001). Additionally, the children will have difficulty thinking about multiple social perspectives while deciding how to respond to social stimuli (Janusz et al., 2002). Possibly because of associated deficits in executive functions, they will have difficulty thinking flexibly about how to respond (Janusz et al., 2002) and instead may act impulsively because of their poor inhibitory control (Dennis, Guger, Roncadin, Barnes, & Schachar, 2001). The combination of deficits in these cognitive-executive and social-affective functions may influence children’s reflective social problem solving (Yeates et al., 2004). They would be predicted to (a) choose instrumental over prosocial goals, (b) misinterpret the intent of others, and (c) generate fewer and less effective responses to social dilemmas.
In their actual interactions, children with brain disorders affecting social information processing would be hypothesized to behave in ways that do not promote social affiliation but rather involve aggression, social withdrawal, or other inappropriate social behaviors. As a result, they would be expected to be poorly accepted by peers, as are other children who behave this way (Parker et al., 2006; Rubin, Wojslawowicz, et al., 2006). They also would be anticipated to have fewer reciprocal friendships, which would be characterized by more avoidance or discord. Peers, teachers, and parents will describe them as less socially competent and as displaying more social problems than other children (Dennis, Guger, et al., 2001; Yeates et al., 2004). Additionally, the children are expected to report low levels of social self-esteem and high levels of emotional distress as well as negative social relationships (Andrews, Rose, & Johnson, 1998; Bohnert, Parker, & Warschausky, 1997). However, we might predict that some children with brain disorder would be relatively unaware of their social problems and might actually overestimate their social functioning, as aggressive children have been shown to do (Boivin et al., 2005).
The social outcomes of brain disorder are likely to be moderated by the developmental factors outlined earlier, particularly age at insult and time since insult. An earlier age at insult appears to be a risk factor for a number of negative social outcomes, such as persistent deficits in the understanding of social emotions (Dennis et al., 1998). Social information processing could be predicted to be particularly vulnerable at early ages because executive functions and theory of mind are more tightly linked during childhood than adulthood (Hughes, 2002). Children with early brain insults may tend to show little or no improvement in their social adjustment across time, despite recovery of other cognitive abilities. Indeed, focal frontal lobe lesions in young children appear to result in more persistent social deficits than are sometimes apparent in older children and adults (Eslinger, Biddle, & Grattan, 1997).
The poor social outcomes that occur in association with some childhood brain disorders may tend to persist even when they occur later in childhood (Yeates et al., 2004). Deficits in social information processing may potentially limit social experiences and hinder peer interactions, so that social functioning may become more divergent from that of peers with increasing time since insult. Indeed, given the transactional nature of social competence, negative social outcomes might persist even in the face of partial or complete recovery of social information processing. A cascade of negative changes in peer interactions and relationships, and consequently in broader aspects of social adjustment, including the perceptions of peers and adults, could engender a negative developmental spiral leading to chronic social problems that become very difficult to reverse even if children’s social information processing improves following a brain insult (Coie, 1990).
Applying the Model to Pediatric TBI
TBI provides an excellent example of how childhood brain disorders can give rise to significant social problems as well as an apt illustration of the model portrayed in Figure 1. In the United States, TBI is a leading cause of death and disability in youth under the age of 15 and therefore represents a major public health problem (Centers for Disease Control and Prevention, 1999). More than 1 million children and adolescents sustain TBI annually, resulting in approximately 150,000 hospitalizations and 5,000 deaths (Kraus, 1995; Langlois, Rutland-Brown, & Thomas, 2005). Injury severity is typically related to the outcomes associated with TBI, so that moderate and severe injuries account for much of the mortality and morbidity, despite representing only about 15% of all cases (Kraus, 1995). As improved medical treatment has led to more frequent survival, concern has increasingly focused on the subsequent cognitive, emotional, and behavioral morbidity, especially among children with more severe injuries (Yeates, 2000). Estimates suggest that over 17,000 children are left with permanent disabilities as a result of TBI each year (Kraus, 1995).
Notwithstanding the growing interest in postacute sequelae, the social outcomes of childhood TBI remain largely uncharacterized and poorly understood. Despite the importance of social competence as a predictor of numerous other outcomes, including psychological adjustment, academic performance, and health status (Cacioppo et al., 2002; Rubin, Bukowski, & Parker, 2006), we know little about the nature, basis, and consequences of social problems among children with TBI. Nevertheless, because of its critical developmental implications, poor social functioning is likely to play a major role in the reductions in quality of life reported following childhood TBI (Stancin et al., 2002).
Several different lines of research suggest that children with TBI are vulnerable to poor social outcomes. First, children with developmental disabilities and chronic health conditions affecting the central nervous system, such as epilepsy and cerebral palsy, are rated as less socially accepted and less socially competent than peers (Nassau & Drotar, 1997). Second, neuroimaging research has revealed an anterior-posterior gradient in the focal lesions associated with TBI. Larger and more numerous lesions are found in frontal and anterior temporal regions (Levin et al., 1989; Mendelsohn et al., 1992; Wilde et al., 2005), which are the same regions that have been implicated as the neural substrates of social information processing and the regulation of social behavior (Adolphs, 2001; Grady & Keightley, 2002). Third, the few previous studies of social outcomes in childhood TBI have shown that children with severe TBI are less skilled at social problem solving and are rated as less socially competent and lonelier than healthy children or children with injuries not involving the brain and that their poor social outcomes persist over time (Andrews et al., 1998; Bohnert et al., 1997; Dennis, Guger, et al., 2001; Janusz et al., 2002; Max et al., 1998; McGuire & Rothenberg, 1986; Papero, Prigatano, Snyder, & Johnson, 1993; Yeates et al., 2004).
Nevertheless, previous research on the social outcomes of childhood TBI is limited in quantity and has not made use of state-of-the-art measures and models of social function, thereby precluding a comprehensive portrayal of social outcomes following childhood TBI. The model in Figure 1 could serve as an organizing framework for future research examining between-group differences (i.e., TBI vs. no TBI) at each level of the model and within-group associations across levels. In the following sections, we describe several recent studies that illustrate elements of the model. The review is by no means exhaustive but instead highlights findings that help set the stage for multilevel, integrative studies of social outcomes in childhood TBI. The studies also illustrate the kinds of research that the model could give rise to with regard to childhood brain disorders more generally.
Regional Brain Damage in Childhood TBI
Although clinical neuroimaging studies indicate that childhood TBI often results in focal frontal lesions, we do not know whether these insults result in structural changes to the broader regions implicated in social information processing. Wilde et al. (2005) recently used in vivo MRI volumetric analysis to evaluate the extent of tissue loss following childhood TBI. The study is the first of which we are aware to examine specific subregions of the prefrontal and temporal lobes, which are regions implicated by social neuroscience as critical for social information processing and social interaction. Volumetric MRI was used to evaluate brain volume differences in the whole brain, as well as prefrontal, temporal, and posterior regions. Participants included 16 children with moderate to severe TBI ranging from 9 to 16 years of age and 16 uninjured children of similar age and demographic characteristics. The children had been injured between 3 and 13 years of age and were between 1 and 10 years postinjury at the time of the study.
The TBI group had significantly reduced whole brain, prefrontal, and temporal regional tissue volumes as well as increased cerebrospinal fluid (CSF). Specific regional differences were found for gray matter and white matter in the superior medial and ventromedial prefrontal regions; white matter in the lateral frontal region; and gray matter, white matter, and CSF in the temporal region. In the TBI group, whole brain volume and total brain gray matter were reduced, and total ventricular volume, total CSF volume, and ventricle-to-brain ratio were increased. Comparisons of volumetric data from typically developing children and subgroups of children who sustained TBI with and without regional focal lesions suggested that gray matter loss in frontal regions was primarily attributable to focal injury (i.e., gray matter loss occurred only in the TBI subgroup with focal frontal lesions). In contrast, white matter loss in the frontal and temporal lobes was related to both diffuse and focal injury (i.e., white matter loss occurred in TBI subgroups both with and without focal lesions).
Notably, volumetric measures of preserved frontotemporal tissue were related to functional recovery as measured by the Glasgow Outcome Scale (Jennett & Bond, 1975), with greater tissue preservation predicting better recovery. Tissue preservation in the posterior cortex was not related to recovery. These results indicate that tissue loss may occur in many of the regions implicated in social information processing. However, the study did not include specific measurement of all the structures that have been implicated in research on the neural substrates of social behavior, such as the cingulate gyrus and the amygdala.
Figure 3, adapted from Bigler (2005), provides an additional illustration of the vulnerability of frontotemporal and limbic regions to TBI. The figure was generated with statistical parametric mapping (SPM) techniques, which permit a voxel-by-voxel comparison, also referred to as voxel-based morphometry, to compare a group of individuals with TBI with age-matched controls (see Salmond, Ashburner, Vargha-Khadem, Gadian, & Friston, 2000, for details on these methods). In the lower portion of Figure 3, the areas in red represent regions where significant differences were found between the density of gray matter pixels in the two groups. At the cortical level, the differences are located predominantly in frontal and temporal regions of the brain. In the gray-scale SPM plots at the top of Figure 3, the darker regions represent the most significant differences between the TBI group and controls. The red arrow points to where the most significant changes were observed in patients with TBI. We found it interesting that the most significant changes occur in the basal forebrain, diencephalon, and ventral striatum. According to Adolphs (2001, 2003), the ventral striatum plays a critical role in self-regulation. Likewise, this region has been a focus of many theories of dysregulation for a variety of neuropsychiatric disorders (Heimer, 2003; Heimer & Van Hoesen, 2006).
Figure 3.
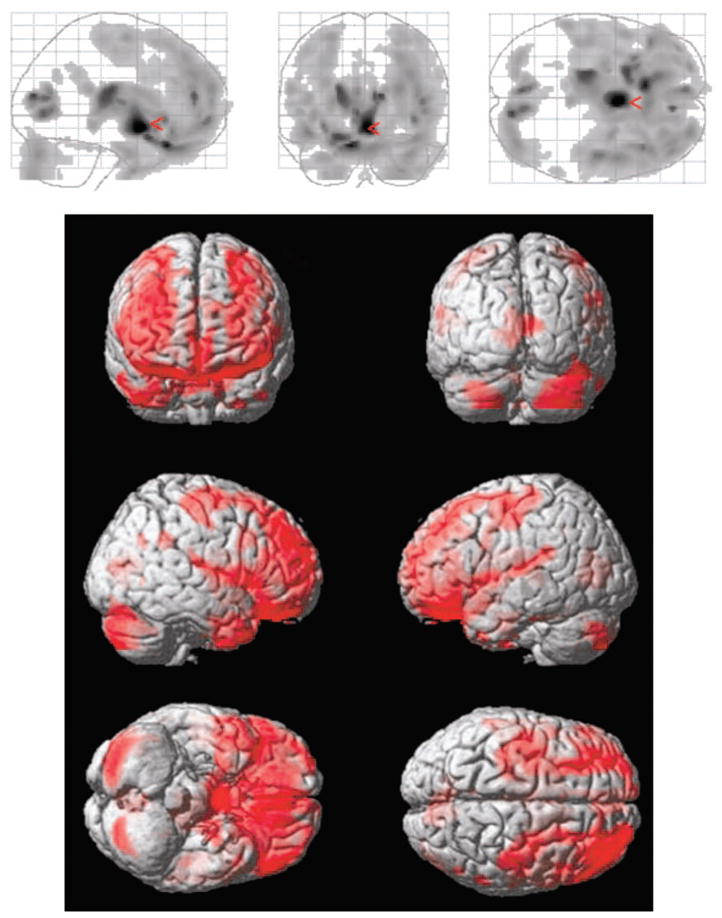
Regional brain abnormalities following traumatic brain injury in children. Results are based on voxel-based morphometry analyses through use of statistical parametric mapping (SPM) techniques. Participants were 6 adolescents (mean age = 16 years, SD = 5.1), all with severe TBI (i.e., Glasgow Coma Scale scores of 8 or below) and associated frontal and temporal contusions, compared with 18 control subjects of similar age (3 control subjects per TBI patient within 2 years of age). The gray scale SPM plots at the top of the figure illustrate significant differences (p < .001) between TBI patients and controls. Darker shading represents areas with less density of gray matter pixels (i.e., greater atrophy) within a given voxel among TBI subjects as compared with controls. The red arrow points to where the most significant changes were observed. In the colored three-dimensional portrayal in the bottom portion of the figure, the areas in red represent the regions where significant differences were found in the density of gray matter pixels within the comparison voxels. Adapted, with permission, from “Structural Imaging” by E. D. Bigler, in Textbook of Traumatic Brain Injury (2nd ed.), 2005, Washington, DC: American Psychiatric Publishing. Copyright 2005 by American Psychiatric Publishing.
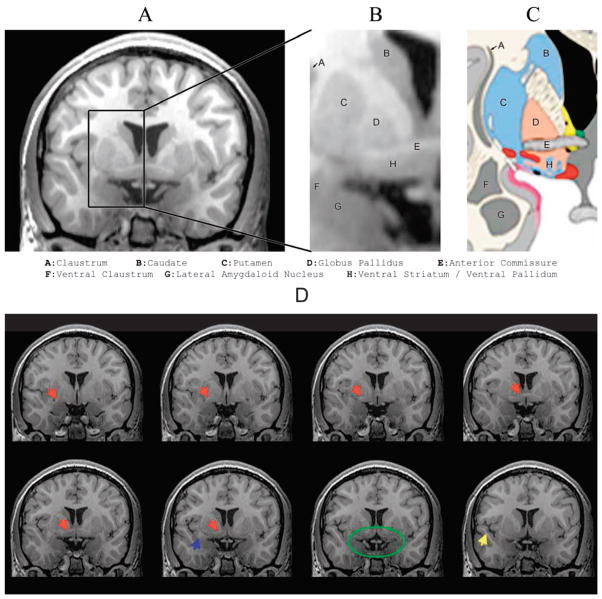
The region of the ventral striatum may be especially important because damage in that region likely relates to deficits in social cognition as well as memory and executive function (Salmond, Chatfield, Menon, Pickard, & Sahakian, 2005). The reason for this can be seen in Figure 4, which is taken from a 3 Tesla MRI of a 14-year-old healthy male, highlighting the basal ganglia, ventral striatum, and amygdala. The close proximity of the gray matter involving these structures emphasizes their interrelatedness (Heimer, 2003). Coursing through this region is also a band of white matter fibers constituting the anterior commissure, another structure sensitive to TBI in children (Wilde, Bigler, et al., 2006). The anterior commissure not only links the two amygdaloid nuclei but also has projections to the hippocampi and the superior temporal gyrus. The superior temporal gyrus is one of the regions that may be critical for social cognition (Adolphs, 2001, 2003; see Table 1). The superior temporal gyrus also has been shown to be one of the temporal lobe structures that atrophies in response to TBI (Bigler et al., 1997).
Figure 4.
Proximity of basal ganglia, ventral striatum, and amygdala. Panel A depicts a coronal thin section (1 mm) 3 Tesla MRI (T1 inversion recovery sequence) at the level of the basal ganglia and amygdaloid complex also showing location of the superior temporal gyrus (STG; Panel D, bottom row, blue arrow). The highlighted region shown in the box is enlarged in Panel B and labeled according to Heimer’s (2003) classification system, as shown in Panel C. The coronal sections in the bottom two rows of Panel D are 1 mm apart, with the section in Panel A shown in the outline in the bottom row of Panel D (green oval). The blue arrow identifies the STG, which can be viewed in each subsequent coronal section, and the yellow arrow identifies the temporal stem. Red arrows show the position of the anterior commissure, and the green oval outlines the region of the ventral striatum, parahippocampal gyrus, and amygdala. Adapted, with permission, from “The Limbic Lobe and Its Output Channels: Implications for Emotional Functions and Adaptive Behavior,” by L. Heimer and G. W. Van Hoesen, 2006, Neuroscience and Biobehavioral Reviews, 30, p. 128. Copyright 2006 by Elsevier.
Thus, damage to the basal forebrain and ventral-striatal area as a result of TBI involves structures that play critical roles in emotional regulation, social cognition, memory, and executive function. Of course, moderate to severe TBI typically involves not only focal lesions but also diffuse abnormalities (Yeates, 2000). The brain damage associated with TBI is not usually restricted to a single region, such as the ventral striatum, but instead involves a broad range of anterior structures (Bigler, in press). Indeed, Wilde, Bigler, et al. (in press) have shown that the ventromedial frontal region, temporal lobe, and amygdala show particularly marked volumetric reductions in association with childhood TBI. Thus, the entire social brain network is potentially vulnerable to TBI. Damage anywhere in the social brain network will disrupt the normal functioning of the system, placing the child with TBI at greater risk for deficits in social information processing, although the exact nature of those deficits may vary according to the specific combination of focal and diffuse damage that occurs in any given case.
Social-Affective Functions in Childhood TBI
A variety of studies have shown that children with TBI display impairments in social-affective functions, including pragmatic language, understanding of emotions, and appreciation of mental states. For instance, Dennis et al. (1998) examined the effect of TBI on children’s appreciation of emotional states and ability to differentiate between internally experienced versus socially expressed emotion. The sample consisted of 59 children with TBI ranging from 6 to 15 years of age and 87 normally developing, age-matched controls. The children with TBI sustained their injuries between 1 and 15 years of age and were from 6 months to 14 years postinjury at the time of the study. They completed a task that assesses the ability to understand real and deceptive emotions in brief narratives. Children with TBI were able to identify felt emotions but had difficulty identifying expressed emotions when they were incongruent with the actual emotion. Children who sustained their TBI before age 7, or who had associated frontal lobe injury, were the most impaired.
In a separate study, Dennis, Purvis, Barnes, Wilkinson, and Winner (2001) examined the appreciation of mental states and affective communication in children with TBI. Participants included 42 school-age children, 13 with severe TBI, 13 with mild TBI, and 16 age-matched healthy controls. On average, the children with TBI were injured at 7 years of age and were 4 years postinjury. The participants were administered a task that involves the presentation of a pictured scenario and a tape-recorded speech act made by one participant to another. The speech acts take the form of literal truth, ironic criticism, or empathic praise. Children were asked a series of questions to determine their understanding of the protagonist’s intentions (as reflected in literal truth statements) and emotive communication (as reflected in ironic criticism and empathic praise). Overall, children with TBI did not differ from controls in understanding literally true statements but performed more poorly than controls in understanding statements involving ironic criticism or empathic praise. Although children with severe TBI were most impaired, even children with mild TBI were less able than controls to differentiate ironic from empathic statements.
Collectively, the two studies indicate that children with TBI display deficits in the social-affective functions represented in Figure 1. These functions have been linked to specific neural substrates by research in social neuroscience and also have been incorporated in recent models of social problem solving drawn from developmental psychology and psychopathology. However, we cannot tell from the results whether deficits in social-affective functions are directly related to social behavior or adjustment.
Social Problem Solving in Childhood TBI
Research has also shown that social problem solving is impaired in children with TBI. Janusz, Kirkwood, Yeates, and Taylor (2002) examined social problem solving in a sample of 6- to 12-year-old children recruited prospectively from several hospitals. Participants included 53 children with severe TBI, 56 with moderate TBI, and 80 with orthopedic injuries but without TBI who were part of a larger study of the outcomes of childhood TBI (Taylor et al., 2002; Yeates et al., 2002). The groups did not differ in age, gender, race, or socioeconomic status. The children and their families were assessed on three occasions: at baseline and at 6 and 12 months postinjury. Three additional assessments occurred at yearly intervals, beginning on average 4 years postinjury.
The long-term effects of childhood TBI on social problem solving were examined with data collected on average 4 years postinjury, when the participants were between 9 and 18 years of age. Data were available for 35 children with severe TBI, 40 children with moderate TBI, and 46 children with orthopedic injuries. The children were administered a semistructured interview to assess the developmental level of their responses to hypothetical social dilemmas (Yeates et al., 1991; Yeates, Selman, & Schultz, 1990). They responded to a series of questions about two dilemmas involving social conflict, one between peers and one between a child and a parent. Children in the severe TBI group defined social dilemmas and generated alternative strategies to solve dilemmas at the same developmental level as did children in the orthopedic injury group. However, they articulated lower level strategies as the best way to solve dilemmas and used lower level reasoning to evaluate the effectiveness of their chosen strategies. In regression analyses controlling for group membership, race, socioeconomic status, IQ, and age, the level of children’s strategies for resolving conflicts predicted parents’ ratings of social competence, aggressive behavior, and academic performance.
The findings suggest that children with severe TBI demonstrate long-term deficits in their social problem-solving skills that help to account for their poor social outcomes. However, the assessment of social problem solving was limited to dilemmas involving social conflict. Previous research (Warschausky et al., 1997) has suggested that children with TBI display greater deficits in social problem solving when situations involve peer-group entry as opposed to social conflict. Thus, the scenarios used by Yeates et al. (2004) may not have been as sensitive to impairments in social information processing. Future studies are needed to determine the extent to which deficits in social problem solving extend across multiple types of situations. Additionally, outcomes were limited to parent report; future research is needed that examines social interaction and adjustment using methods such as peer assessments and behavioral observations.
Social Information Processing and Social Outcomes in Childhood TBI
Yeates et al. (2004) subsequently conducted a more detailed examination of social outcomes of pediatric TBI and their relationships to social information processing using data from the same project. They conducted growth curve analyses of social outcomes across the first four assessments, from baseline to the 4-year follow-up. Additionally, they performed path analyses focusing on the prediction of social outcomes at the 4-year follow-up using contemporaneous measures of executive functions, language pragmatics, and social problem solving. Outcome measures included the Socialization scale from the Vineland Adaptive Behavior Scales (Sparrow, Balla, & Cicchetti, 1984) and the Social Competence and Social Problems scales from the Child Behavior Checklist (Achenbach, 1991).
Growth curve analyses revealed that childhood TBI was associated with adverse long-term social outcomes, which were exacerbated by fewer family resources and poorer family functioning. The path analyses indicated that social outcomes were accounted for, in part, by specific social-affective and cognitive-executive skills and by social problem solving, even after controlling for group membership, age, race, socioeconomic status, and IQ. However, the findings did not provide much evidence that social problem solving mediates the influence of cognitive-executive and social-affective abilities on social outcomes. Pragmatic language was predictive of social problem solving, but executive function was not. Pragmatic language and executive function predicted a different set of social outcomes than did social problem solving, and the strength of their association with social outcomes was not significantly weaker after taking social problem solving into account.
The findings indicate that the social outcomes of childhood TBI are moderated by environmental risks, and they also illustrate how cognitive-executive and social-affective functions and social problem solving may be related to social adjustment. The findings regarding the role of social problem solving as a mediator of social-affective and cognitive-executive functions were largely negative. However, the assessment of cognitive-executive and social-affective functions did not incorporate measures of developmentally significant abilities, such as the appreciation of intentionality or the recognition of emotional states, that may have a more direct bearing on social problem solving and social adjustment. More generally, the measurement of social outcomes relied exclusively on parent report and did not involve peer ratings or observational assessments of social interactions.
Social Outcomes and Frontal Lobe Injury in Childhood TBI
Few studies have examined the link between focal brain injury and social outcomes in childhood TBI. Levin et al. (2004) recently published a study that focused on psychosocial outcomes in children with TBI with and without focal frontal lesions. They compared 22 school-age children who sustained TBI with unilateral frontal lesions to a matched sample of 22 children with TBI and nonfrontal focal lesions. They also compared 18 children who sustained a TBI with nonfrontal focal lesions to 18 children with TBI but without focal lesions. Participants were drawn from both a prospective cohort who sustained their injuries between 5 and 15 years of age and completed MRI at 3 months postinjury and from a cross-sectional cohort who were between 5 and 18 years of age at the time of the study and at least 2 years postinjury. The primary outcome measure was the Vineland Adaptive Behavior Scales (VABS; Sparrow et al., 1984). Children with frontal lesions displayed deficits on the VABS Socialization scale and Maladaptive Behavior scale when compared to children with nonfrontal lesions, despite the absence of differences on the VABS Communication scale or on cognitive tests of memory, expressive language, and processing speed. The volume of frontal lesions was a significant predictor of the VABS Socialization scale, with larger lesions associated with poorer outcomes. Nonfrontal lesions were not associated with poorer psychosocial outcomes. These findings confirm the importance of the frontal lobes in social functioning, but they are limited by the omission of measures of lesions in other brain regions implicated in social information processing and by the restricted assessment of social outcomes. For example, the authors did not distinguish between aggression and social withdrawal as potential outcomes, although those behaviors are qualitatively different and may reflect the lateralization of frontal lobe injury (Fox, Calkins, & Bell, 1994; Fox et al., 1995, 2001). Furthermore, the quality of children’s relationships in the peer group at large, and more specifically with friends, was not assessed.
In another recent study, changes in social behavior were linked to lesions in the dorsal prefrontal cortex. Max and colleagues (Max et al., 2005, 2006) studied 177 children between 5 and 14 years of age who were recruited prospectively following TBI and followed to 24 months postinjury. They completed the Neuropsychiatric Rating Scale (Max et al., 1998), a semistructured psychiatric interview designed to identify symptoms of personality change due to TBI. Persistent personality change occurred in 18% of the children with TBI during the first 6 months postinjury, 13% between 6 and 12 months after injury, and 12% in the 2nd year after injury. The most common changes involved affective lability, aggressive behavior, and poor social judgment (e.g., tactless comments about the listener, inappropriate sharing of personal information). Persistent personality change was more common among children with more severe injuries and was associated with lesions to several distinct regions as seen on MRI, but only lesions in the superior frontal gyrus and frontal lobe white matter accounted for unique variance. These regions connect to the ventral system discussed previously and likely play a key role in the effortful regulation of affective states produced by that system. The findings again point to the role of the frontal lobes in social functioning, although the Neuropsychiatric Rating Scale is clearly limited as a measure of social outcomes.
Research Lacunae
Previous research on the social outcomes of childhood TBI has not been guided by methods or models derived from social cognitive neuroscience and has only rarely capitalized on the extensive literature on social competence in developmental psychology and developmental psychopathology. Only a handful of researchers have examined social adjustment in children with TBI. The few existing studies have been characterized by a limited range of outcome measures, usually relying on parent or teacher ratings, which are subject to bias and provide only an indirect index of social functioning. Only two studies have examined self-perceptions of social competence among children with TBI (Andrews et al., 1998; Bohnert et al., 1997). These studies indicate that children report concern about losing friends and describe themselves as less socially competent and lonelier than children without brain injuries, but they do not report fewer or qualitatively different friendships. We are unaware of any previous studies in which classroom peer nominations or ratings were used to examine peer acceptance, behavioral reputation, or reciprocal friendships in children with TBI.
Although the discourse skills of children with TBI were examined in one study (Chapman et al., 2004) and found to be impaired, broader aspects of peer interactions and social communication have not been observed directly in previous research. Moreover, only a few studies have examined social information processing in children with TBI. Although they have consistently documented deficits in abilities such as the understanding of mental states and the generation of effective solutions to social dilemmas, those deficits may not necessarily cut across all situations (Janusz et al., 2002; Warschausky et al., 1997; Yeates et al., 2004). Moreover, the two recent studies described earlier apparently are the only ones that have investigated the relation between social information processing in children with TBI and their social adjustment (Janusz et al., 2002; Yeates et al., 2004).
As noted earlier, neuroimaging studies of childhood TBI have suggested that focal lesions are larger and more numerous in the brain’s anterior regions, which have been broadly implicated in social behavior (Levin et al., 1989; Mendelsohn et al., 1992). However, with the exception of the recent series of studies by Wilde and colleagues (Wilde, Bigler, et al., in press, 2006; Wilde, Chu, et al., 2006; Wilde et al., 2005), the existing neuroimaging studies were not designed to examine the discrete brain regions specifically implicated in social cognition. Moreover, no previous studies of childhood TBI have attempted to link the results of neuroimaging analyses directly to variations in social information processing, although preliminary links between frontal lobe lesions and poor social adjustment have been demonstrated (Levin et al., 2004; Max et al., 2005, 2006). The studies of frontal asymmetries and social development described previously are also relevant here and indicate a need to study the contribution of specific brain subregions to social behavior.
Previous research also has largely not attended to developmental considerations. Most studies of TBI in preschool children have focused on cognitive outcomes, so that the impact of early injuries on social outcomes is largely unknown. Additionally, social outcomes after TBI have been examined longitudinally in only a few studies. Although the existing research provides evidence of persisting deficits in social adjustment despite considerable cognitive recovery (Yeates et al., 2004), the neural and social cognitive substrates of the persisting deficits are unclear.
Future Directions and Significance
An integrated, multilevel model, such as one presented herein, is critical to understanding social outcomes (Cacioppo et al., 2000). More restricted models do not promote an examination of the links between brain, cognition and emotion, and action, as portrayed in Figure 1. Future research is likely to be especially informative if it entails a contemporaneous examination of each level in the model (regional brain abnormalities, social information processing, social interaction, and social adjustment) and their interrelationships. In that sense, the model encourages research that reflects an integrative, biopsychosocial approach to health problems (Zerhouni, 2003).
The selection of constructs included in the model was made in a principled fashion, so as to avoid its being overly inclusive. For instance, the model does not encompass all possible neuropsychological outcomes but instead focuses on specific cognitive-executive functions that have been shown empirically to relate to social cognition, particularly those implicated in studies of theory of mind (Grattan & Eslinger, 1989; Hughes, 1998). Because of its comprehensive approach to the study of social competence, however, the model may nevertheless encourage a major expansion of knowledge about the outcomes of childhood brain disorders. Additionally, the model is potentially germane not only to children with brain disorders but also to those with neurodevelopmental disorders and to healthy children, and it may therefore inform research regarding the neural and cognitive-affective substrates of social behavior more generally.
Currently, we know relatively little about outcomes at each level of the model and even less about the connections among them. Children with brain disorders are likely to exhibit deficits at each level, but the magnitude of deficits may vary across the different levels as a function of the specific neuropathology involved. We also are unsure which levels will prove to have stronger relations and whether the relations will be different for children with brain disorder than for healthy children. Future research is needed to examine whether childhood brain disorders alter the connections between levels of social competence.
For instance, the association between social problem solving and social interaction may be weaker among children with brain disorder, who may be able to articulate more appropriate responses in a reflective interview than they can actually implement during ongoing interactions. In other words, they may know more than they can do (Dennis et al., 1998). This is certainly true for children identified as socially withdrawn, who also are typically found to demonstrate left-frontal hypoactivity (Fox et al., 1995, 2001). That is, extremely withdrawn school-age children do not demonstrate deficits in hypothetical-reflective social thinking, but they do display social ineptitude in actual interactions (Rubin et al., 2002). One explanation of this mismatch between thought and behavior is that these children know what to do in a wide variety of social circumstances, but they are unable to regulate emotions adequately; as a consequence, their emotions override competent thinking, thereby resulting in incompetent social behavior. The multilevel, integrated nature of the model proposed here will allow future research to address this sort of possibility in children with brain disorder.
Practically speaking, the clinical application of more sensitive measures, such as those used to assess the understanding of emotion or the comprehension of mental states, may help clinicians target children with poor social outcomes for further intervention. In turn, the refinement of a multilevel, integrated causal model should prove valuable in designing interventions to promote better social outcomes following childhood TBI (Bierman, 2004; Cooley, Glang, & Voss, 1997; Glang, Todis, Cooley, Wells, & Voss, 1997; Guralnick, 1989, 1990). In this sense, the model will afford an opportunity to improve the long-term quality of life of children and families affected by brain disorders.
Acknowledgments
The preparation of this article was supported in part by Grant K02 HD44099 from the National Institute of Child Health and Human Development to Keith Owen Yeates and Grant U19 HD35476 from the National Institute of Child Health and Human Development to Erin D. Bigler. Aside from Keith Owen Yeates, the order of authorship is alphabetical.
Contributor Information
Keith Owen Yeates, The Ohio State University and Columbus Children’s Research Institute.
Erin D. Bigler, Brigham Young University and University of Utah
Maureen Dennis, University of Toronto and Hospital for Sick Children.
Cynthia A. Gerhardt, The Ohio State University and Columbus Children’s Research Institute
Kenneth H. Rubin, University of Maryland
Terry Stancin, Case Western Reserve University and MetroHealth Medical Center.
H. Gerry Taylor, Case Western Reserve University and Rainbow Babies and Children’s Hospital.
Kathryn Vannatta, The Ohio State University and Columbus Children’s Research Institute.
References
- Achenbach TM. Manual for the Child Behavior Checklis/4–18 and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R. Cognitive neuroscience of human social behaviour. Nature Reviews: Neuroscience. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. Journal of Cognitive Neuroscience. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- Allman J, Hakeem A, Erwin J, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Annals of the New York Academy of Sciences. 2001;935:107–117. [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson S, Bechara A, Damasio H, Tranel D, Damasio H. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116:1374–1382. doi: 10.1542/peds.2004-1728. [DOI] [PubMed] [Google Scholar]
- Anderson V, Levin HS, Jacobs R. Executive functions after frontal lobe injury: A developmental perspective. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 504–527. [Google Scholar]
- Anderson VA, Morse SA, Klug G, Catroppa C, Haritou F, Rosenfeld J, et al. Predicting recovery from head injury in young children: A prospective analysis. Journal of the International Neuropsychological Society. 1997;3:568–580. [PubMed] [Google Scholar]
- Andrews TK, Rose FD, Johnson DA. Social and behavioural effects of traumatic brain injury in children. Brain Injury. 1998;12:133–138. doi: 10.1080/026990598122755. [DOI] [PubMed] [Google Scholar]
- Asher SR, Parker JG, Walker DL. Distinguishing friendship from acceptance: Implications for intervention and assessment. In: Bukowski WM, Newcomb AF, Hartup WW, editors. The company they keep: Friendship in childhood and adolescence. New York: Cambridge University Press; 1996. pp. 366–405. [Google Scholar]
- Banich M, Levine S, Kim H, Huttenlocher P. The effects of developmental factors on IQ in hemiplegic children. Neuropsychologia. 1990;28:35–47. doi: 10.1016/0028-3932(90)90084-2. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Belmonte MK. Autism: A window onto the development of the social and the analytic brain. Annual Review of Neuroscience. 2005;28:109–126. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- Bartsch K, Wellman HM. Young children’s attribution of action to beliefs and desires. Child Development. 1989;60:946–964. [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Berndt TJ, Hoyle SG. Stability and change in childhood and adolescent friendships. Developmental Psychology. 1985;21:1007–1015. [Google Scholar]
- Bibby H, McDonald S. Theory of mind after traumatic brain injury. Neuropsychologia. 2005;43:99–114. doi: 10.1016/j.neuropsychologia.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Bierman KL. Peer rejection: Developmental processes and intervention. New York: Guilford; 2004. [Google Scholar]
- Bigler ED. Structural imaging. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. 2. Washington, DC: American Psychiatric Publishing; 2005. pp. 79–105. [Google Scholar]
- Bigler ED. Anterior and middle cranial fossa in traumatic brain injury (TBI): Relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology. doi: 10.1037/0894-4105.21.5.515. in press. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Blatter DD, Anderson CV, Johnson SC, Gale SD, Hopkins RO, et al. Hippocampal volume in normal aging and traumatic brain injury. AJNR American Journal of Neuroradiology. 1997;18:11–23. [PMC free article] [PubMed] [Google Scholar]
- Bohnert AM, Parker JG, Warschausky SA. Friendship and social adjustment of children following a traumatic brain injury: An exploratory investigation. Developmental Neuropsychology. 1997;13:477–486. [Google Scholar]
- Boivin M, Hymel S, Bukowski WM. The roles of social withdrawal, peer rejection, and victimization by peers in predicting loneliness and depressed mood in childhood. Development & Psycho-pathology. 1995;7:765–785. [Google Scholar]
- Boivin M, Vitaro F, Poulin F. Peer relationships and the development of aggressive behavior in early childhood. In: Tremblay R, Hartup WW, Archer J, editors. Developmental origins of aggression. New York: Guilford Press; 2005. pp. 376–397. [Google Scholar]
- Brothers L. The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- Brown BB, Klute C. Friends, cliques, and crowds. In: Adams GR, Berzonsky MD, editors. Blackwell handbook of adolescence. Malden, MA: Blackwell; 2003. pp. 330–348. [Google Scholar]
- Buhrmester D, Furman W. The changing functions of friends in childhood. A neo-Sullivan perspective. In: Derlega VJ, Winstead BA, editors. Friendship and social interaction. New York: Springer-Verlag; 1986. pp. 41–62. [Google Scholar]
- Bukowski W, Rubin KH, Parker J. Social competence. In: Smelser NJ, Baltes PB, editors. International encyclopedia of social and behavioral sciences. Oxford, England: Elsevier; 2001. pp. 14258–14264. [Google Scholar]
- Burgess KB, Wojslawowicz JC, Rubin KH, Rose-Krasnor L, Booth-LaForce C. Social information processing and coping styles of shy/withdrawn and aggressive children: Does friendship matter? Child Development. 2006;77:371–383. doi: 10.1111/j.1467-8624.2006.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Sheridan JF, McClintock MK. Multilevel integrative analyses of human behavior: Social neuroscience and the complementing nature of social and biological approaches. Psychological Bulletin. 2000;126:829–843. doi: 10.1037/0033-2909.126.6.829. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, et al. Loneliness and health: Potential mechanisms. Psychosomatic Medicine. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Moses LJ. Individual differences in inhibitory control and children’s theory of mind. Child Development. 2001;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KN. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cavell TA. Social adjustment, social performance, and social skills: A tri-component model of social competence. Journal of Clinical Child Psychology. 1990;19:111–122. [Google Scholar]
- Centers for Disease Control and Prevention. Traumatic brain injury in the United States: A report to Congress. Atlanta, GA: U.S. Department of Health and Human Services; 1999. [Google Scholar]
- Chapman SB, Sparks G, Levin HS, Dennis M, Roncadin C, Zhang L, et al. Discourse macrolevel processing after severe pediatric traumatic brain injury. Developmental Neuropsychology. 2004;25:37–60. doi: 10.1080/87565641.2004.9651921. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of Neurology. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Cillessen AHN, Rose AJ. Understanding popularity in the peer system. Current Directions in Psychological Science. 2005;14:102–105. [Google Scholar]
- Coelho-Mosch S, Max JE, Tranel D. A matched lesion analysis of childhood versus adult-onset brain injury due to unilateral stroke: Another perspective on neural plasticity and recovery of social functioning. Cognitive and Behavioral Neurology. 2005;18:5–17. doi: 10.1097/01.wnn.0000152207.80819.3c. [DOI] [PubMed] [Google Scholar]
- Coie JD. Toward a theory of peer rejection. In: Asher SR, Coie JD, editors. Peer rejection in childhood. Cambridge, England: Cambridge University Press; 1990. pp. 365–401. [Google Scholar]
- Collins WA. More than a myth: The developmental significance of romantic relationships during adolescence. Journal of Research on Adolescence. 2003;13:1–24. [Google Scholar]
- Connolly J, Craig W, Goldberg A, Pepler D. Conceptions of cross-sex friendships and romantic relationships in early adolescence. Journal of Youth & Adolescence. 1999;28:481–494. [Google Scholar]
- Cooley EA, Glang A, Voss J. Making connections: Helping children with ABI build friendships. In: Glang A, Singer GHS, Todis B, editors. Students with acquired brain injuries: The school’s response. Baltimore: P. H. Brookes; 1997. pp. 255–275. [Google Scholar]
- Cowan N. The development of working memory. In: Cowan N, Hulme C, editors. The development of memory in childhood. Hove, England: Psychology Press; 1997. pp. 163–200. [Google Scholar]
- Crick NR, Dodge KA. A review and reformulation of social information-processing mechanisms in children’s social adjustment. Psychological Bulletin. 1994;115:74–101. [Google Scholar]
- Csikszentmihalyi M, Larson R. Being adolescent. New York: Basic Books; 1984. [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain & Cognition. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- Demorest A, Meyer C, Phelps E, Gardner H, Winner E. Words speak louder than actions: Understanding deliberately false remarks. Child Development. 1984;55:1527–1534. [Google Scholar]
- Dennis M. Prefrontal cortex: Typical and atypical development. In: Risberg J, Grafman J, editors. The frontal lobes: Development, function and pathology. New York: Cambridge University Press; 2006. pp. 128–162. [Google Scholar]
- Dennis M, Barnes MA, Wilkinson M, Humphreys RP. How children with head injury represent real and deceptive emotion in short narratives. Brain and Language. 1998;61:450–483. doi: 10.1006/brln.1997.1886. [DOI] [PubMed] [Google Scholar]
- Dennis M, Guger S, Roncadin C, Barnes M, Schachar R. Attentional-inhibitory control and social-behavioral regulation after childhood closed head injury: Do biological, developmental, and recovery variables predict outcome? Journal of the International Neuropsychological Society. 2001;7:683–692. doi: 10.1017/s1355617701766040. [DOI] [PubMed] [Google Scholar]
- Dennis M, Purvis K, Barnes MA, Wilkinson M, Winner E. Understanding of literal truth, ironic criticism, and deceptive praise after childhood head injury. Brain and Language. 2001;78:1–16. doi: 10.1006/brln.2000.2431. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 466–503. [Google Scholar]
- Diamond A, Taylor C. Development of an aspect of executive control: Development of the abilities to remember what I said and to “Do as I say, not as I do. Developmental Psychobiology. 1996;29:315–334. doi: 10.1002/(SICI)1098-2302(199605)29:4<315::AID-DEV2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Dodge KA, Laird R, Lochman JE, Zelli A. Multidimensional latent-construct analysis of children’s social information processing patterns: Correlations with aggressive behavior problems. Psychological Assessment. 2002;14:60–73. doi: 10.1037//1040-3590.14.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA, Pettit GS, McClaskey CL, Brown MM. Social competence in children. Monographs of the Society for Research in Child Development. 1986;51(2) Serial No. 213. [Google Scholar]
- Dowsett SM, Livesey DJ. The development of inhibitory control in preschool children: Effects of “executive skills” training. Developmental Psychobiology. 2000;36:161–174. doi: 10.1002/(sici)1098-2302(200003)36:2<161::aid-dev7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Dunn J, Cutting A, Fisher N. Old friends, new friends: Predictors of children’s perspective on their friends at school. Child Development. 2002;73:621–635. doi: 10.1111/1467-8624.00427. [DOI] [PubMed] [Google Scholar]
- Du Rocher Schudlich TD, Shamir H, Cummings EM. Marital conflict, children’s representations of family relationships, and children’s dispositions towards peer conflict strategies. Social Development. 2004;13:171–192. [Google Scholar]
- Eckerman CO, Stein MR. How imitation begets imitation and toddler’s generation of games. Developmental Psychology. 1990;26:370–378. [Google Scholar]
- Eslinger PJ, Biddle KR, Grattan LM. Cognitive and social development in children with prefrontal cortex lesions. In: Krasnegor NA, Lyon GR, Goldman-Rakic PS, editors. Development of the prefrontal cortex: Evolution, neurobiology, and behavior. Baltimore: H. Brookes; 1997. pp. 295–336. [Google Scholar]
- Eslinger PJ, Flaherty-Craig CV, Benton AL. Developmental outcomes after early prefrontal cortex damage. Brain and Cognition. 2004;55:84–103. doi: 10.1016/S0278-2626(03)00281-1. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ, Grattan LM, Damasio H, Damasio AR. Developmental consequences of childhood frontal lobe damage. Archives of Neurology. 1992;49:764–769. doi: 10.1001/archneur.1992.00530310112021. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Fletcher JM, Levin HS, Francis DJ, Davidson K, Miner ME. Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. Journal of the International Neuropsychological Society. 1997;3:581–591. [PubMed] [Google Scholar]
- Fox NA. Dynamic cerebral processes underlying emotion regulation. Monographs of the Society for Research in Child Development. 1994;59(2–3) Serial No. 240. [PubMed] [Google Scholar]
- Fox NA, Calkins SD, Bell MA. Neural plasticity and development in the first two years of life: Evidence from cognitive and socioemotional domains of research. Development and Psychopathology. 1994;6:677–696. [Google Scholar]
- Fox NA, Henderson HA, Rubin K, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first 4 years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, et al. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1770–1784. [PubMed] [Google Scholar]
- Frith U, Frith C. The biological basis of social interaction. Current Directions in Psychological Science. 2001;10:151–155. [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of “theory of mind. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gerstadt CL, Hong YJ, Diamond A. The relationship between cognition and action: Performance of children 3.5–7 years old on a Stroop-like day–night test. Cognition. 1994;53:129–153. doi: 10.1016/0010-0277(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Gest S, Graham-Bermann S, Hartup W. Peer experience: Common and unique features of number of friendships, social network centrality, and sociometric status. Social Development. 2001;10:23–40. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Lui J, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glang A, Todis B, Cooley E, Wells J, Voss J. Building social networks for children and adolescents with traumatic brain injury: A school-based intervention. Journal of Head Trauma Rehabilitation. 1997;12:32–47. [Google Scholar]
- Goel V, Grafman J, Sadato N, Hallett M. Modeling other minds. NeuroReport. 1995;6:1741–1746. doi: 10.1097/00001756-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy ofSciences, USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncu A. Development of intersubjectivity in the dyadic play of preschoolers. Early Childhood Research Quarterly. 1993;8:99–116. [Google Scholar]
- Goncu A, Patt MB, Kouba E. Understanding young children’s pretend play in context. In: Smith PK, Hart CH, editors. Blackwell handbook of childhood social development. Malden, MA: Blackwell; 2002. pp. 418–437. [Google Scholar]
- Gordon AC, Olson DR. The relation between acquisition of a theory of mind and the capacity to hold in mind. Journal of Experimental Child Psychology. 1998;68:70–83. doi: 10.1006/jecp.1997.2423. [DOI] [PubMed] [Google Scholar]
- Gorrie C, Duflou J, Brown J, Gibson T, Waite PM. Extent and distribution of vascular brain injury in pediatric road fatalities. Journal of Neurotrauma. 2001;18:849–860. doi: 10.1089/089771501750451776. [DOI] [PubMed] [Google Scholar]
- Gorrie C, Oakes S, Duflou J, Blumbergs P, Waite PME. Axonal injury in children after motor vehicle crashes: Extent, distribution, and size of axonal swellings using beta-APP immunohistochemistry. Journal of Neurotrauma. 2002;19:1171–1182. doi: 10.1089/08977150260337976. [DOI] [PubMed] [Google Scholar]
- Grady CL, Keightley ML. Studies of altered social cognition in neuropsychiatric disorders using functional neuroimaging. Canadian Journal of Psychiatry. 2002;47:327–336. doi: 10.1177/070674370204700403. [DOI] [PubMed] [Google Scholar]
- Grattan L, Eslinger P. Higher cognition and social behavior: Changes in cognitive flexibility and empathy after cerebral lesions. Neuropsychology. 1989;3:175–185. [Google Scholar]
- Gray JA. Brain systems that mediate both emotion and cognition. Cognition and Emotion. 1990;4:269–288. [Google Scholar]
- Guralnick MJ. Social competence as a future direction for early intervention programs. Journal of Mental Deficiency Research. 1989;33:275–281. doi: 10.1111/j.1365-2788.1989.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Guralnick MJ. Social competence and early intervention. Journal of Early Intervention. 1990;14:3–14. [Google Scholar]
- Guralnick MJ. Family and child influences on the peer-related social competence of young children with developmental delays. Mental Retardation and Developmental Disabilities Research Reviews. 1999;5:21–29. [Google Scholar]
- Haynes RL, Borenstein NS, Desilva TM, Folkerth RD, Liu LG, Volpe JJ, et al. Axonal development in the cerebral white matter of the human fetus and infant. Journal of Comparative Neurology. 2005;484:156–167. doi: 10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. American Journal of Psychiatry. 2003;160:1726–1739. doi: 10.1176/appi.ajp.160.10.1726. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neuroscience and Biobehavioral Reviews. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hektner JM, August GJ, Realmuto GM. Patterns and temporal changes in peer affiliation among aggressive and nonaggressive children participating in a summer school program. Journal of Clinical Child Psychology. 2000;29:603–614. doi: 10.1207/S15374424JCCP2904_12. [DOI] [PubMed] [Google Scholar]
- Henderson H, Marshall P, Fox NA, Rubin KH. Psycho-physiological and behavioral evidence for varying forms of nonsocial behavior in preschoolers. Child Development. 2004;75:251–263. doi: 10.1111/j.1467-8624.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, et al. Localization of white matter volume increase in autism and developmental language disorder. Annals of Neurology. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- Howes C. Peer interaction of young children. Monographs of the Society for Research in Child Development. 1988;53(1 No 217) [Google Scholar]
- Howes C, Phillipsen L. Continuity in children’s relationships with peers. Social Development. 1998;7:340–349. [Google Scholar]
- Hughes C. Executive function in preschoolers: Links with theory of mind and verbal ability. British Journal of Developmental Psychology. 1998;16:233–253. [Google Scholar]
- Hughes C. Executive functions and development: Emerging themes. Infant and Child Development. 2002;11:201–209. [Google Scholar]
- Hughes C, Ensor R. Executive function and theory of mind in 2 year olds: A family affair? Developmental Neuropsychology. 2005;28:645–668. doi: 10.1207/s15326942dn2802_5. [DOI] [PubMed] [Google Scholar]
- Hulme C, Roodenrys S. Practitioner review: Verbal working memory development and its disorders. Journal of Child Psychology and Psychiatry. 1995;36:373–398. doi: 10.1111/j.1469-7610.1995.tb01297.x. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Janusz JA, Kirkwood MW, Yeates KO, Taylor HG. Social problem-solving skills in children with traumatic brain injury: Long-term outcomes and prediction of social competence. Child Neuropsychology. 2002;8:179–194. doi: 10.1076/chin.8.3.179.13499. [DOI] [PubMed] [Google Scholar]
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Griffin R, Csibra G, Halit H, Farroni T, De Haan M, et al. The emergence of the social brain network: Evidence from typical and atypical development. Development and Psychopathology. 2005;17:599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain W, Perner J. Do children with ADHD not need their frontal lobes for theory of mind? A review of brain imaging and neuropsychological studies. In: Brune M, Ribbert H, Schiefenhovel W, editors. The social brain: Evolution and pathology. Chichester, England: John Wiley; 2003. pp. 197–230. [Google Scholar]
- Kendall E, Terry DJ. Psychosocial adjustment following closed head injury: A model for understanding individual differences and predicting outcome. Neuropsychological Rehabilitation. 1996;6:101–132. [Google Scholar]
- Kinney HC. Human myelination and perinatal white matter disorders. Journal of Neurological Sciences. 2005;228:190–192. doi: 10.1016/j.jns.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy: II. Patterns of myelination in autopsied infants. Journal of Neuropathology and Experimental Neurology. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JDE, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. NeuroReport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray K, Jacques TY, Koenig AL, Vandegeest KA. Inhibitory control in young children and its role in emerging internalization. Child Development. 1996;67:490–507. [PubMed] [Google Scholar]
- Krasnor L, Rubin KH. Preschool social problem solving: Attempts and outcomes in naturalistic interaction. Child Development. 1983;54:1545–1558. [Google Scholar]
- Kraus JF. Epidemiological features of brain injury in children: Occurrence, children at risk, causes and manner of injury, severity, and outcomes. In: Broman SH, Michel ME, editors. Traumatic head injury in children. New York: Oxford University Press; 1995. pp. 22–39. [Google Scholar]
- Kuttler AF, Parker JG, La Greca AM. Developmental and gender differences in preadolescents’ judgments of the veracity of gossip. Merrill-Palmer Quarterly. 2002;48:105–132. [Google Scholar]
- Ladd GW, Pettit GS. Parenting and the development of children’s peer relationships. In: Bornstein MH, editor. Handbook of parenting: Vol. 5. Practical issues in parenting. 2. Mahwah, NJ: Erlbaum; 2002. pp. 269–309. [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: Differences by race. Journal of Head Trauma Rehabilitation. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Lemerise EA, Arsenio WF. An integrated model of emotion processes and cognition in social information processing. Child Development. 2000;71:107–118. doi: 10.1111/1467-8624.00124. [DOI] [PubMed] [Google Scholar]
- Levin H, Amparo E, Eisenberg H, Miner M, High W, Jr, Ewing-Cobbs L, et al. MRI after closed head injury in children. Neurosurgery. 1989;24:223–227. doi: 10.1227/00006123-198902000-00011. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G. Executive functions after traumatic brain injury in children. Pediatric Neurology. 2005;33:79–93. doi: 10.1016/j.pediatrneurol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Levin HS, Zhang L, Dennis M, Ewing-Cobbs L, Schachar R, Max J, et al. Psychosocial outcome of TBI in children with unilateral frontal lesions. Journal of the International Neuropsychological Society. 2004;10:305–316. doi: 10.1017/S1355617704102129. [DOI] [PubMed] [Google Scholar]
- Livesey DJ, Morgan GA. The development of response inhibition in 4- and 5-year-old children. Australian Journal of Psychology. 1991;43:133–137. [Google Scholar]
- Mah L, Arnold MC, Grafman J. Impairment of social perception associated with lesions of the prefrontal cortex. American Journal of Psychiatry. 2004;161:1247–1255. doi: 10.1176/appi.ajp.161.7.1247. [DOI] [PubMed] [Google Scholar]
- Masten AS, Hubbard JJ, Gest SD, Tellegen A, Garmezy N, Ramirez M. Competence in the context of adversity: Pathways to resilience and maladaptation from childhood to late adolescence. Development and Psychopathology. 1999;11:143–169. doi: 10.1017/s0954579499001996. [DOI] [PubMed] [Google Scholar]
- Matsuzawa J, Matsui M, Konishi T, Noguchi K, Gur RC, Bilker W, Miyawaki T. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cerebral Cortex. 2001;11:335–342. doi: 10.1093/cercor/11.4.335. [DOI] [PubMed] [Google Scholar]
- Max JE, Koele SL, Lindgren SD, Robin DA, Smith WL, Jr, Sato Y, Arndt S. Adaptive functioning following traumatic brain injury and orthopedic injury: A controlled study. Archives of Physical Medicine and Rehabilitation. 1998;79:893–899. doi: 10.1016/s0003-9993(98)90084-3. [DOI] [PubMed] [Google Scholar]
- Max JE, Levin HS, Landis J, Schachar RJ, Saunders AE, Ewing-Cobbs L, et al. Predictors of personality change due to traumatic brain injury in children and adolescents in the first six months after injury. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44:434–442. doi: 10.1097/01.chi.0000156280.66240.61. [DOI] [PubMed] [Google Scholar]
- Max JE, Levin HS, Schachar RJ, Landis J, Saunders AE, Ewing-Cobbs L, et al. Predictors of personality change due to traumatic brain injury in children and adolescents six to twenty-four months after injury. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:21–32. doi: 10.1176/jnp.18.1.21. [DOI] [PubMed] [Google Scholar]
- McGuire TL, Rothenberg MB. Behavioral and psychosocial sequelae of pediatric head injury. Journal of Head Trauma Rehabilitation. 1986;1:1–6. [Google Scholar]
- Meltzoff AN. Understanding of the intentions of others: Reenactment of intended acts by 18-month-old children. Developmental Psychology. 1995;31:838–850. doi: 10.1037/0012-1649.31.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN, Gopnik A, Repacholi BM. Toddlers’ understanding of intentions, desires and emotions: Explorations of the dark ages. In: Zelazo PD, Astington JW, Olson DR, editors. Developing theories of intention: Social understanding and self-control. Mahwah, NJ: Erlbaum; 1999. pp. 17–41. [Google Scholar]
- Mendelsohn D, Levin HS, Bruce D, Lilly M, Harward H, Culhane KA, Eisenberg HM. Late MRI after head injury in children: Relationship to clinical features and outcome. Child’s Nervous System. 1992;8:445–452. doi: 10.1007/BF00274405. [DOI] [PubMed] [Google Scholar]
- Mize J, Ladd GW. Predicting preschoolers’ peer behavior and status from their interpersonal strategies: A comparison of verbal and enactive responses to hypothetical social dilemmas. Developmental Psychology. 1988;24:782–788. [Google Scholar]
- Moses LJ. Executive accounts of theory of mind development. Child Development. 2001;72:688–690. doi: 10.1111/1467-8624.00306. [DOI] [PubMed] [Google Scholar]
- Moss H, Damasio A. Emotion and the human brain. Annals of the New York Academy of Sciences. 2001;935:101–106. [PubMed] [Google Scholar]
- Munakata Y, Casey BJ, Diamond A. Developmental cognitive neuroscience: Progress and potential. Trends in Cognitive Sciences. 2004;8:122–128. doi: 10.1016/j.tics.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Muris P, Meesters C, de Kanter E, Timmerman PE. Behavioural inhibition and behavioural activation system scales for children: Relationships with Eysenck’s personality traits and psychopathological symptoms. Personality and Individual Differences. 2005;38:831–841. [Google Scholar]
- Nangle DW, Erdley CA, Newman JE, Mason CA, Carpenter E. Popularity, friendship quantity, and friendship quality: Interactive influences on children’s loneliness and depression. Journal of Clinical Child and Adolescent Psychology. 2003;32:546–555. doi: 10.1207/S15374424JCCP3204_7. [DOI] [PubMed] [Google Scholar]
- Nassau JH, Drotar D. Social competence among children with central nervous system-related chronic health conditions: A review. Journal of Pediatric Psychology. 1997;22:771–793. doi: 10.1093/jpepsy/22.6.771. [DOI] [PubMed] [Google Scholar]
- Newcomb A, Bagwell C. Children’s friendship relations: A meta-analytic review. Psychological Bulletin. 1995;117:306–347. [Google Scholar]
- Ochsner KN, Lieberman MD. The emergence of social cognitive neuroscience. American Psychologist. 2001;56:717–734. [PubMed] [Google Scholar]
- O’Donnell S, Noseworthy M, Levine B, Brandt M, Dennis M. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage. 2005;24:948–954. doi: 10.1016/j.neuroimage.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Orzhekhovskaya NS. Fronto-striatal relationships in primate ontogeny. Neuroscience and Behavioural Physiology. 1981;11:379–385. doi: 10.1007/BF01184205. [DOI] [PubMed] [Google Scholar]
- Papero PH, Prigatano GP, Snyder HM, Johnson DL. Children’s adaptive behavioural competence after head injury. Neuropsychological Rehabilitation. 1993;3:321–340. [Google Scholar]
- Parker J, Rubin KH, Erath S, Wojslawowicz JC, Buskirk AA. Peer relationships and developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental psychopathology: Risk, disorder, and adaptation. 2. Vol. 2. New York: Wiley; 2006. pp. 419–493. [Google Scholar]
- Parslow RC, Morris KP, Tasker RC, Forsyth RJ, Hawley CA. Epidemiology of traumatic brain injury in children receiving intensive care in the UK. Archives of Disease in Childhood. 2005;90:1182–1187. doi: 10.1136/adc.2005.072405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Paul BM, Bussiere JR, Buxton RB, Wong EC, Stiles J. The development of face and location processing: An fMRI study. Developmental Science. 2003;6:100–117. [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pellegrini AD. Rough-and-tumble play from childhood through adolescence: Development and possible functions. In: Smith PK, Hart CH, editors. Blackwell handbook of childhood social development. London: Blackwell; 2002. pp. 438–453. [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Posner M, Rothbart M, Gerardi-Caulton G. Exploring the biology of socialization. Annals of the New York Academy of Sciences. 2001;935:208–216. doi: 10.1111/j.1749-6632.2001.tb03482.x. [DOI] [PubMed] [Google Scholar]
- Powell KB, Voeller KS. Prefrontal executive function syndromes in children. Journal of Child Neurology. 2004;19:785–797. doi: 10.1177/08830738040190100801. [DOI] [PubMed] [Google Scholar]
- Prigatano GP. Disturbances of self-awareness of deficit after traumatic brain injury. In: Prigatano GP, Schacter DL, editors. Awareness of deficit after brain injury: Clinical and theoretical issues. New York: Springer; 1991. pp. 111–126. [Google Scholar]
- Prigatano GP, Altman I, O’Brien K. Behavioral limitations that traumatic brain injured patients tend to underestimate. The Clinical Neuropsychologist. 1990;4:1–14. [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986 April 11;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Reimers TS, Ehrenfels S, Mortensen EL, Schmiegelow M, Sonderkaer S, Carstensen H, et al. Cognitive deficits in long-term survivors of childhood brain tumors: Identification of predictive factors. Medical and Pediatric Oncology. 2003;40:26–34. doi: 10.1002/mpo.10211. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rose-Krasnor L. The nature of social competence: A theoretical review. Social Development. 1997;6:111–135. [Google Scholar]
- Rovet JF, Ehrlich RM, Czuchta D. Intellectual characteristics of diabetic children at diagnosis and one year later. Journal of Pediatric Psychology. 1990;15:775–788. doi: 10.1093/jpepsy/15.6.775. [DOI] [PubMed] [Google Scholar]
- Rowe AD, Bullock PR, Polkey CE, Morris RG. “Theory of mind” impairments and their relationship to executive functioning following frontal lobe excisions. Brain. 2001;124:600–616. doi: 10.1093/brain/124.3.600. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Booth C, Krasnor LR, Mills RSL. Social relationships and social skills: A conceptual and empirical analysis. In: Shulman S, editor. Close relationships and socioemotional development. Norwood, NJ: Ablex Publishing; 1995. pp. 63–95. [Google Scholar]
- Rubin KH, Bukowski W, Parker J. Peer interactions, relationships, and groups. In: Eisenberg N, editor. Handbook of child psychology: Social, emotional, and personality development. 6. New York: Wiley; 2006. pp. 571–645. [Google Scholar]
- Rubin KH, Burgess K. Parents of aggressive and withdrawn children. In: Bornstein M, editor. Handbook of parenting. 2. Vol. 1. Hillsdale, NJ: Erlbaum; 2002. pp. 383–418. [Google Scholar]
- Rubin KH, Burgess K, Coplan R. Social withdrawal and shyness. In: Smith PK, Hart CH, editors. Blackwell handbook of childhood social development. Malden, MA: Blackwell; 2002. pp. 330–352. [Google Scholar]
- Rubin KH, Chen X, Hymel S. Socioemotional characteristics of withdrawn and aggressive children. Merrill-Palmer Quarterly. 1993;39:518–534. [Google Scholar]
- Rubin KH, Fein G, Vandenberg B. Play. In: Mussen PH, Hetherington EM, editors. Handbook of child psychology: Vol. 4. Socialization, personality and social development. New York: Wiley; 1983. pp. 693–774. [Google Scholar]
- Rubin KH, Krasnor LR. Social-cognitive and social behavioral perspectives on problem solving. In: Perlmutter M, editor. Cognitive perspectives on children’s social and behavioral development: The Minnesota Symposia on Child Psychology. Vol. 18. Hills-dale, NJ: Erlbaum; 1986. pp. 1–68. [Google Scholar]
- Rubin KH, Rose-Krasnor L. Interpersonal problem-solving. In: Van Hassett VB, Hersen M, editors. Handbook of social development. New York: Plenum; 1992. pp. 283–323. [Google Scholar]
- Rubin KH, Wojslawowicz JC, Rose-Krasnor L, Booth-LaForce CL, Burgess KB. The friendships of socially shy/withdrawn children: Prevalence, stability, and relationship quality. Journal of Abnormal Child Psychology. 2006;34:139–153. doi: 10.1007/s10802-005-9017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph K, Heller T. Interpersonal problem solving, externalizing behavior, and social competence in preschoolers: A knowledge-performance discrepancy? Journal of Applied Developmental Psychology. 1997;18:107–117. [Google Scholar]
- Salmond CH, Ashburner J, Vargha-Khadem F, Gadian DG, Friston KJ. Detecting bilateral abnormalities with voxel-based morphometry. Human Brain Mapping. 2000;11:223–232. doi: 10.1002/1097-0193(200011)11:3<223::AID-HBM80>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond CH, Chatfield DA, Menon DK, Pickard JD, Sahakian BJ. Cognitive sequelae of head injury: Involvement of basal forebrain and associated structures. Brain. 2005;128:189–200. doi: 10.1093/brain/awh352. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN, Riley EP. Moral maturity and delinquency after prenatal alcohol exposure. Journal of Studies on Alcohol. 2005;66:545–554. doi: 10.15288/jsa.2005.66.545. [DOI] [PubMed] [Google Scholar]
- Selman RL, Schultz LH. Making a friend in youth: Developmental theory and pair therapy. Chicago: University of Chicago Press; 1990. [Google Scholar]
- Siegal M, Varley R. Neural systems involved in “theory of mind. Nature Reviews Neuroscience. 2002;3:463–471. doi: 10.1038/nrn844. [DOI] [PubMed] [Google Scholar]
- Sodian B, Taylor C, Harris PL, Perner J. Early deception and the child’s theory of mind: False trails and genuine markers. Child Development. 1991;62:468–483. [Google Scholar]
- Sowell ER, Jernigan TL. Further MRI evidence of late brain maturation: Limbic volume increases and changing asymmetries during childhood and adolescence. Developmental Neuropsychology. 1998;14:599–617. [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. NeuroImage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. Development of cortical and subcortical brain structures in childhood and adolescence: A structural MRI study. Developmental Medicine and Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales: Interview edition. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Stancin T, Drotar D, Taylor HG, Yeates KO, Wade SL, Minich NM. Health-related quality of life of children and adolescents following traumatic brain injury. Pediatrics. 2002;109:e34. doi: 10.1542/peds.109.2.e34. Retrieved March 7, 2007, from http://pediatrics.aappublications.org/cgi/content/full/109/2/e34. [DOI] [PubMed]
- Stuss DT, Anderson V. The frontal lobes and theory of mind: Developmental concepts from adult focal lesion research. Brain and Cognition. 2004;55:69–83. doi: 10.1016/S0278-2626(03)00271-9. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Gallup GG, Alexander MP. The frontal lobes are necessary for “theory of mind. Brain. 2001;124:279–286. doi: 10.1093/brain/124.2.279. [DOI] [PubMed] [Google Scholar]
- Tasker RC, Salmond CH, Westland AG, Pena A, Gillard JH, Sahakian BJ, et al. Head circumference and brain and hippocampal volume after severe traumatic brain injury in childhood. Pediatric Research. 2005;58:302–308. doi: 10.1203/01.PDR.0000169965.08854.25. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Alden J. Age-related differences in outcome following childhood brain injury: An introduction and overview. Journal of the International Neuropsychological Society. 1997;3:555–567. [PubMed] [Google Scholar]
- Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight. Journal of the International Neuropsychological Society. 2004;10:1–15. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Schatschneider C, Minich NM. Longitudinal outcomes of Haemophilus influenzae meningitis in school-age children. Neuropsychology. 2000;14:509–518. doi: 10.1037//0894-4105.14.4.509. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcomes after traumatic brain injury in children: Behavior and achievement. Neuropsychology. 2002;16:15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Genetics of brain structure and intelligence. Annual Review of Neuroscience. 2005;28:1–23. doi: 10.1146/annurev.neuro.28.061604.135655. [DOI] [PubMed] [Google Scholar]
- Warschausky S, Cohen EH, Parker JG, Levendosky AA, Okun A. Social problem-solving skills of children with traumatic brain injury. Pediatric Rehabilitation. 1997;1:77–81. doi: 10.3109/17518429709025850. [DOI] [PubMed] [Google Scholar]
- Watson AC, Nixon CL, Wilson A, Capage L. Social interaction skills and theory of mind in young children. Developmental Psychology. 1999;35:386–391. doi: 10.1037//0012-1649.35.2.386. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Bigler ED, Haider JM, Chu Z, Levin HS, Li X, Hunter JV. Vulnerability of the anterior commissure in moderate to severe pediatric traumatic brain injury. Journal of Child Neurology. 2006;21:769–776. doi: 10.1177/08830738060210090201. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Bigler ED, Hunter JV, Fearing MA, Scheibel RS, Newsome MR, et al. Morphometric findings in the hippocampus, amygdala, and basal ganglia in children after moderate to severe traumatic brain injury. Developmental Medicine & Child Neurology. doi: 10.1111/j.1469-8749.2007.00294.x. in press. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, et al. Diffusion tensor imaging fiber tracking in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2006;23:1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, et al. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2005;22:333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Winner E. The point of words: Children’s understanding of metaphor and irony. Cambridge, MA: Harvard University Press; 1988. [Google Scholar]
- Yakovlev PI. Morphological criteria of growth and maturation of the nervous system in man. Research Publications Association for Research in Nervous and Mental Disease. 1962;39:3–46. [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional development of the brain in early life. Oxford, England: Blackwell; 1967. pp. 3–70. [Google Scholar]
- Yeates KO. Closed-head injury. In: Yeates KO, Ris MD, Taylor HG, editors. Pediatric neuropsychology: Research, theory, and practice. New York: Guilford; 2000. pp. 92–116. [Google Scholar]
- Yeates KO, Schultz LH, Selman KO. The development of interpersonal negotiation strategies in thought and action: A socialcognitive link to behavioral adjustment and social status. Merrill-Palmer Quarterly. 1991;37:369–406. [Google Scholar]
- Yeates KO, Selman RL, Schultz LH. Bridging the gaps in child-clinical assessment: Toward the application of social-cognitive developmental theory. Clinical Psychology Review. 1990;10:567–588. [Google Scholar]
- Yeates KO, Swift E, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. Short- and long-term social outcomes following pediatric traumatic brain injury. Journal of the International Neuropsychological Society. 2004;10:412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term neuropsychological outcomes after pediatric traumatic brain injury. Neuropsychology. 2002;16:514–523. doi: 10.1037//0894-4105.16.4.514. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Duggal S, Gruber R. Parental psychopathology. In: Bornstein MH, editor. Handbook of parenting: Vol. 4. Social conditions and applied parenting. 2. Mahwah, NJ: Erlbaum; 2002. pp. 295–327. [Google Scholar]
- Zerhouni E. The NIH roadmap. Science. 2003 October 3;302:63–64. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]