Abstract
Binge eating is more common in females than in males. This study investigated the effects of ovarian hormones on binge-eating behavior in a diet-related rat model. Six groups of ovariectomized Sprague-Dawley rats were used (n=13/group). All rats had continuous access to chow and water throughout the study. One half of the rats were injected every fourth day with estradiol benzoate (2μg/100μl sesame oil) and progesterone (500μg/100μl sesame oil); the other half received only the sesame oil vehicle. Three feeding protocols were tested in each hormone injection condition: (1) chow only: no additional dietary fat access; (2) low-restriction: 1-h fat access every day; (3) high-restriction: 1-h fat access on Monday, Wednesday, and Friday. As previously reported in intact male and female rats, the high-restriction groups exhibited binge-like increases in 1-h energy intake during fat access. The major new finding of this study is that 1-h energy intake was tonically, but not cyclically, reduced in the hormone-treated high-restriction (binge) rats. Specifically, both low- and high-restriction hormone-treated rats consumed significantly less energy than did the oil-treated rats during the 1-h fat period (p<0.0001) and overall (p<0.0001), indicating a tonic inhibition of eating. However, food intake during the 1-h fat access period was also cyclically reduced in the hormone-treated low-restriction rats, but not in the hormone-treated high-restriction rats. These results indicate that the normal cyclic inhibitory influence of ovarian hormones on eating, but not their normal tonic inhibitory influence, is disrupted by conditions leading to binge-type eating.
Keywords: binge eating, bulimia, estradiol, progesterone, fat intake, female
Introduction
Bingeing-related eating disorders including binge eating disorder (BED) and bulimia nervosa (BN) have become important health issues in western countries (1-4). Like other eating disorders, BED and BN are more common in females than in males. American women are 1.5 times more likely than men to develop BED and 3 times more likely to develop BN (4-6). In Norway, the female-male ratio is 1.7:1 for lifetime prevalence of BED and 3:1 for lifetime prevalence of BN in adolescents (7). Furthermore, people who do not meet the criteria for bingeing-related eating disorders (bulimia nervosa, binge eating disorder, binge/purge subtype of anorexia nervosa) also binge eat. For instance, one study reported a binge eating prevalence of 24% in a randomly sampled population of women, whereas the prevalence of bulimia nervosa was only 1.5% (8).
Although biological sex differences ultimately arise from the different genotypes of males (XY) and females (XX), after early development most sex differences are mediated through hypothalamic-pituitary-gonadal (HPG) axis function, especially the actions of gonadal steroid hormones – androgens, estrogens and progesterones (9, 10). Several effects of gonadal steroid hormones on food intake have been well documented in both humans and animals (11-13). Food intake in women varies with the phase of the menstrual cycle, with a decrease in the peri-ovulatory phase, when plasma estradiol concentration peaks; conversely, food intake generally increases in the luteal phase, when plasma progesterone levels are high (14-16). Adult female rats and mice also eat different amounts of food across the estrous cycle, which is usually 4 d in length. Rats and mice eat least near the time of ovulation, during what is called the estrus phase, which occurs just after estradiol peaks, and eat most during diestrus, when estradiol levels are lower (11, 12). This cyclic food intake pattern is thought to be due to inhibitory effects of estradiol on eating (17). In rats, pharmacological progesterone treatment can reduce the intake-reducing effects of estradiol, but so far no physiological action of progesterone on eating has been shown (11-13). The decrease in eating during the peri-ovulatory phase of the ovarian cycle is referred to as the cyclic inhibitory effect of estradiol on eating (18). In addition, ovariectomy (OVX) dramatically increases food intake and body weight in rats and mice, and administration of estradiol brings food intake and body weight back to a normal physiological level (11-13). There is some evidence for a similar effect in women (14). Thus, in addition to its cyclic effects, estradiol also has tonic inhibitory effects on eating (11, 12, 18).
The frequency of binge–type eating has been reported to change during the menstrual cycle. In women with bulimia nervosa, binge frequency increased during the luteal phase and menses (19-21). In one study of women with BN, a significant negative association between estradiol and binge frequency as well as a significant positive association between progesterone and binge frequency were reported (22). In a community sample of women, changes in a modified Emotional Eating subscale of the Dutch Eating Behavior Questionnaire (DEBQ) also were associated with cyclic hormone fluctuations. Specifically, higher scores (consistent with binge eating) were obtained when estradiol was low and progesterone was high (23). In none of these studies, however, have alterations in binge size with the menstrual cycle been reported. Finally, bingeing may disrupt menstrual cyclicity and ovarian hormone function (24-26); in three studies, 37-64% of women with BN experienced oligomenorrhea (27-29). How binge behavior and HPG function might interact has not been established, and the necessary mechanistic studies are difficult in human subjects. Animal models, therefore, are needed.
Several animal models have been developed to study binge eating (30). In the present study a limited-access binge-eating model is used. In this model, rats are given access to a source of dietary fat for one or two hours per day three times a week, with nutritionally complete rat chow and water always freely available. Fat intakes during the fat-access period are much higher under this 3-day limited-access condition than when rats are offered fat for similar periods every day (31-35). The model has face validity in that it reproduces a key criterion for human binge eating: the consumption of more food during a brief period than is normally consumed under similar circumstances. In addition, body weight typically does not differ between binge rats and chow controls. This is also similar to the maintenance of normal body weight by most patients with bulimia nervosa (1) as well as recent data showing that most people who binge are not obese (4). Therefore, rats with 3-day limited access are referred to as bingeing rats, and the three weekly fat access days are called binge days. Although the rats consume large amounts of energy on binge days relative to controls, they eat less chow on the non-binge days. Due to this “overeat/undereat” or “sawtooth” intake pattern, body weight typically does not differ between binge rats and chow controls. Thus, factors involved in binge behavior can be studied without obesity-related confounds that might influence food intake.
Although both intact female and intact male rats exhibit binge behavior with this protocol, the energy consumed during the limited-access period by females is much smaller than that consumed by males (32, 33, 35). In addition, the day-to-day intake patterns are different; that is, the overeat/undereat pattern is not as regular in females (33). A possible reason for the smaller binge size in intact females compared to males may be the tonic inhibitory effect of estradiol on eating. The irregular day-to-day intake pattern, on the other hand, may be due to estradiol's cyclic inhibitory effect during the estrus phase, which falls randomly on binge and non-binge days.
The rationale for the present study rests on reports, first, that higher levels of estradiol have been associated with decreased binge frequency in women with BN (22) and, second, that estradiol can elicit both tonic and cyclic inhibitory effects on eating under non-binge conditions in women and in female animals (17, 36). Tonic and cyclic effects of ovarian hormones on binge behavior, however, have not been investigated. Therefore, we sought to investigate the tonic and cyclic effects of ovarian hormones on binge eating behavior in the limited fat access animal model. Specifically, we hypothesized that in this model: 1) binge size and daily food intake would be reduced in hormone-treated binge OVX rats compared to vehicle-treated binge OVX rats due to tonic inhibitory effects of estradiol on eating, 2) binge size and daily food intake would be reduced on the day of the hormone treatment cycle modeling estrus (day 4) due to cyclic inhibitory effects of estradiol, and 3) day-to-day intake patterns of daily food intake would be different in hormone-treated and vehicle-treated binge rats due to the cyclic influence of ovarian hormones.
Materials and methods
1. Subjects
Seventy-eight female Sprague-Dawley rats (Harlan, Indianapolis, IN; 60 days of age and initially weighing 184-218 g) were individually housed in stainless-steel cages (Length : Width : Height = 48.5 cm : 30 cm : 20.5 cm) with continuous access to water and pelleted chow (Laboratory Rodent Diet 5001, PMI Feeds, Richmond, IN; macronutrient content (g/kg diet, kcal/kg diet, percent of calories): protein (234, 936, 28%), fat (45, 405, 12%), carbohydrate (490, 1960, 60%); total, 3.3 kcal/g). The vivarium was maintained at 22±2°C with a 12/12h light-dark cycle (lights off at 1900 h). All procedures were approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
2. OVX and cyclic hormone treatment
After a one-week period of adaptation to the vivarium, rats were given overnight access to a bowl of solid fat [Crisco® All-Vegetable Shortening (partially hydrogenated vegetable oil), J.M. Smucker Co., Orrville, OH; 9.17 kcal/g] clipped to the front of cage, in addition to their continuously available chow and water. This was done to prevent neophobia during the rest of the study. Three days later, the rats were anaesthetized (1 ml/kg body weight, intraperitoneally: IP) with a mixture of 70 mg/kg Ketamine (Phoenix Science Inc., St. Joseph, MO) and 2 mg/kg Xylazine (Phoenix Science Inc., St. Joseph, MO), with 0.2 ml/kg supplements given as needed, and a bilateral OVX was performed using a dorsal approach.
After 4-5 days of postoperative recovery, when body weights had returned to their pre-surgical levels, rats were matched for body weight and overnight fat intake and divided into two groups. One group (OVX+EP, n = 39) was subcutaneously (SC) injected with 17-β-estradiol-benzoate (Sigma, 2 μg/100μl sesame oil) in the middle of the light phase every fourth day and with progesterone (Sigma, 500 μg/100μl sesame oil) 1 day later; the other group (OVX+OIL, n = 39) was injected with the sesame oil vehicle on the same days. Injection days were followed by 2 non-injection days. The hormone treatment regimen is shown in Table 1. These hormone injection regimens produce near-physiological levels of estradiol (17) and progesterone (37), and maintain normal body weight, food intake, spontaneous meal patterns, and sexual receptivity (lordosis) in OVX rats (17). Note that the day of estradiol injection is labeled day 2 of the treatment cycle and the progesterone injection day is labeled day 3. This is done so that the last day of the cycle models the last day of the typical 4-d estrus cycle of intact rats; that is, Day 1: Diestrus 1, Day 2: Diestrus 2, Day 3: Proestrus, Day 4: Estrus.
Table 1.
Cyclic ovarian hormone treatment regimen
| Day of treatment cycle | ||||
|---|---|---|---|---|
| Group | Day 1 | Day 2 | Day 3 | Day 4 |
| EP | — | E | P | — |
| OIL | — | Oil | Oil | — |
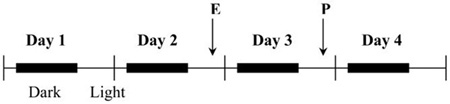
Cyclic ovarian hormone treatment regimen used throughout the study. All rats were ovariectomized. The 24-h test days begin and end at the tick marks, i.e. at 1600 h, 3 h prior to lights off. Hormones were injected at 1300 h (the middle of the light phase) on Day 2 and Day 3 of the cycle, so that Day 4 of the treatment cycle models the estrus phase of the ovarian cycle in intact rats. E = 2 μg β-estradiol 3-benzoate/100μl sesame oil/rat; P = 500 μg progesterone/100μl sesame oil/rat.
3. Feeding protocols
After two cycles of hormone treatment or one cycle of vehicle treatment, rats within each group were matched for current body weight and assigned to one of three subgroups (n = 13/group), which were then maintained on one of the three fat-access schedules shown in Table 2: Chow only (C), which had no additional fat; Low-restriction access (L), which had access to a bowl of additional fat for 1 h/day every day, 2 h prior to lights off; and High-restriction access (H), which had access to a bowl of additional fat for 1 h/day on Monday, Wednesday and Friday, 2 h prior to lights off. All rats had continuous access to chow and water throughout the study. These schedules were based on previous work from our laboratory (32, 33, 35). The H group is also referred to as the bingeing group. Chow intake was measured every 24-h prior to the fat access period. One-h fat and chow intakes were measured at the end of the 1-h fat access period. Food intake and weekly body weight were monitored as an indicator of the efficacy of the OVX and hormone-treatment protocols.
Table 2.
Experimental Groups
| Group | Hormone Treatment | Fat Access |
|---|---|---|
| EP-C | Estradiol + Progesterone | None |
| EP-L | Estradiol + Progesterone | Low-restriction |
| EP-H | Estradiol + Progesterone | High-restriction |
| OIL-C | OIL | None |
| OIL-L | OIL | Low-restriction |
| OIL-H | OIL | High-restriction |
4. Body composition
Body composition analysis was undertaken to investigate the source of the body weight differences observed at the end of the study. After 12 weeks on their respective diet protocols, rats were sacrificed via CO2 asphyxiation. First, the gonadal, abdominal (mesenteric + non-gonadal), and retroperitoneal fat pads were removed, weighed and returned to the carcass for analysis. Then, body water, fat, ash, and protein were analyzed using methods previously described (32).
5. Data analysis
Data were analyzed using SAS 9.1 for Windows (SAS Institute, Cary, NC). The outcomes analyzed were 1-h energy intake (kcal), 24-h energy intake (kcal), weekly energy intake (kcal), body weight (g), fat pad weight (g), and body composition (dry mass in g, and water, mineral, fat, and protein in g and percent). All data are presented as means ± SEM. One-h energy intake and 24-h energy intake on binge days and non-binge days were analyzed by 3-way ANOVA (fat access schedule × hormone treatment × cycle day), with cycle day as a repeated factor. Cycle day 1 data was calculated by averaging all of the day 1 food intake data for each 4-day hormone treatment cycle on binge days (Mon, Wed, Fri) or non-binge days (Tues, Thurs, Sat, Sun) across the 6-week study. Similarly, food intake data on days 2, 3, or 4 of the cycle were averaged across all 6 weeks. Within each treatment group and under each fat access schedule, 1-h energy intake and 24-h energy intake on each cycle day were analyzed by 1-way ANOVA, with day as a repeated factor. Following the 1-way ANOVA, Tukey's HSD post hoc tests were used to determine significant differences among cycle days. Within each hormone treatment group, average weekly 24-h energy intake was analyzed by 2-way ANOVA (fat access schedule × week), with week as a repeated factor. Body weights (before OVX, 5 d postovariectomy and 45 d postovariectomy), total and individual fat pad weights (gonadal, abdominal and retroperitoneal), and body composition data were analyzed by 2-way ANOVA (fat access schedule × hormone treatment). Planned comparisons among groups were examined using a Least Square differences (LS means) table, with the Bonferroni correction applied to insure an experiment-wide α < 0.05. Tukey tests (for repeated measures) and ANOVA outcomes were considered significant when α <0.05.
Results
1. 1-h energy intake
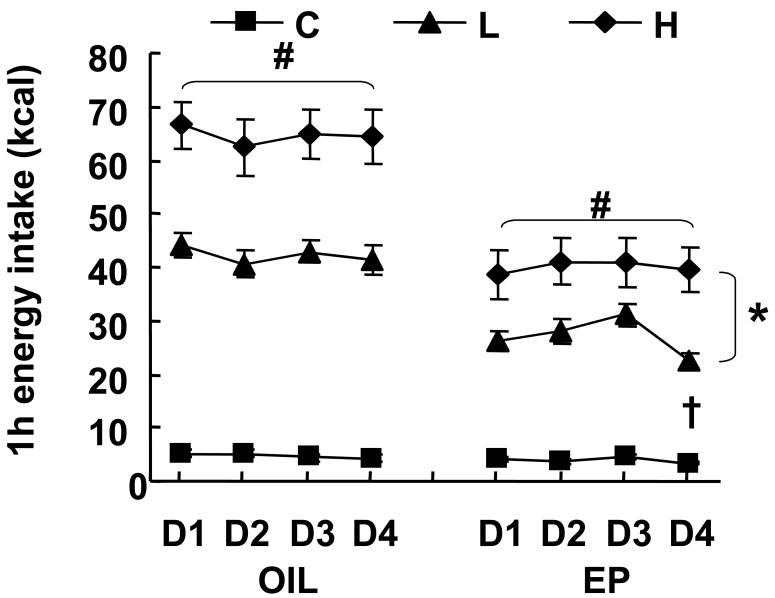
Energy intake in the 1-h fat access period was significantly affected by fat access schedule, ovarian hormone treatment, and day of treatment cycle (Fig 1). There were main effects of the fat access schedule [main effect of fat access F(2,72)=148.22, p<0.0001]. In both the OIL and EP groups, the 1-h energy intake of the H rats was significantly greater than that of the L rats, which in turn was significantly greater than that of the C rats (p<0.0001; Fig 1). In other words, bingeing, as operationally defined by H intake greater than L intake, occurred in both OIL and EP groups.
Fig 1.
Effect of cyclic hormone treatment and fat access schedule on 1-h energy intake. D=Day. # indicates significant differences among the C, L, and H groups within the OIL and EP rats (P<0.0001). * indicates significant differences between OIL and EP groups on the H and L feeding schedules (P<0.0001). † indicates intake on day 4 significantly different from intake on day 2 in EP-L group (P<0.05).
There were also main effects of ovarian hormone treatment due to greater energy intake overall by the OIL rats relative to EP rats [main effect of hormone treatment F(1,72)=34.62, p<0.0001]. In other words, the EP treatment tonically reduced 1-h energy intake (EP < OIL) in both H and L rats (p<0.0001; Fig 1).
Hormone treatment interacted with fat access schedule [F(2,72)=8.99, p<0.0005], apparently because intake was reduced only in the groups with access to dietary fat (H and L), but not in the group with access to only chow (C). This was likely due to a floor effect in the chow groups, as 1-h chow intake was quite low. Hormone treatment also interacted with cycle day [F(3,216)=5.25, p<0.05] due to lower 1-h energy intake on Day 4 relative to Day 2 in the EP-L rats only [main effect of day F(3,216)=4.38, p<0.01]. That is, the cyclic effect of EP treatment [1-h food intake in EP-treated rats was less on cycle day 4 than on cycle day 2, (17)] occurred only in the L rats (1-way repeated ANOVA F (3,36)= 16.92, p<0.0001), not in the H rats (1-way repeated ANOVA F (3,36)=1.07, NS). There was no 1-h energy intake difference between day 2 and day 4 in any OIL group (Fig 1).
2. Daily energy intake
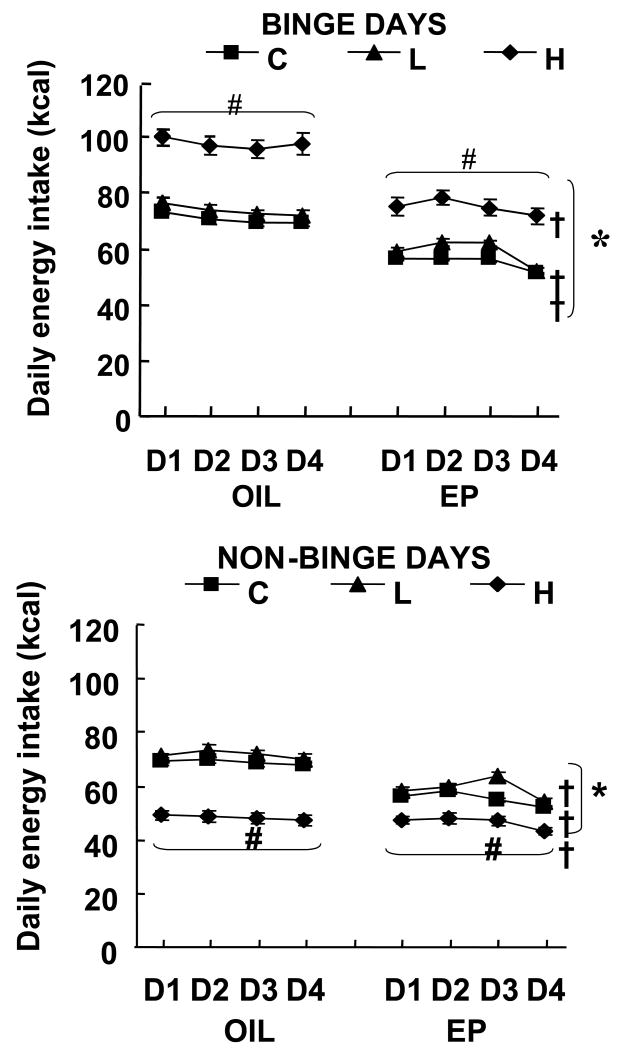
Twenty-four hour energy intake was significantly affected by fat access schedule, hormone treatment, and day of cycle on binge days as well as on non-binge days (Fig 2). There were main effects of the fat access schedule [binge days: main effect of fat access F(2,72)=85.04, p<0.0001; non-binge days: main effect of fat access F(2,72)=11.43, p<0.0001]. These were due to the fact that in both OIL and EP groups, H rats ate significantly more than did L rats and C rats on binge days and significantly less on non-binge days (p<0.0001), i.e. the H rats exhibited an overeat/undereat, sawtooth pattern of consumption.
Fig 2.
Effect of cyclic hormone treatment and fat access schedule on 24-h energy intake on binge days (Mon, Weds, Fri) and non-binge days (Tues, Thurs, Sat, Sun). D=Day. # indicates H rats ate significantly more than C and L rats on binge days (P<0.0001) and significantly less on non-binge days (P<0.0001). * indicates significant differences between OIL and EP groups on the C, L and H feeding schedules on binge days, and in C and L groups on non-binge days (P<0.0001). † indicates intake on day 4 significantly different from intake on day 2 within the EP-C, EP-L and EP-H groups (P<0.05).
The main effect of ovarian hormone treatment revealed tonic inhibitory effects of EP on daily energy intake in all EP-treated groups on binge days (main effect of hormone F(1,72)=124.24, p<0.0001) and EP-L and EP-C groups on non-binge days (main effect of hormone F(1,72)=73.85, p<0.0001; Fig 2). In other words, the 24-h energy intakes were significantly lower in EP rats than OIL rats in the L and C groups on both binge and non-binge days, and in the H group on binge days (EP < OIL; p<0.0001). The lack of tonic inhibitory effect of EP in H groups on non-binge days resulted in an interaction between hormone treatment and fat access on non-binge days [F(2,72)=11.43, p<0.0001].
There also were cyclic effects of EP on 24-h energy intakes (Day 4 < Day 2). On binge days there were interactions between cycle day and fat access [F(6,216)=2.5, p<0.05] as well as between cycle day and hormone treatment [F(3,216)=15.95, p<0.0001]. On non-binge days, a similar profile emerged, i.e. there were interactions between cycle day and fat access [F(6,216)=4.62, p<0.005], as well as cycle day and hormone treatment [F(3,216)=10.74, p<0.0001]. These results were due to significant daily energy intake differences between day 2 and day 4 (day 4 < day 2) in all EP-treated groups on both binge and non-binge days, while there was no significant daily energy intake difference between day 2 and day 4 in any of the OIL groups. On binge days 1-way ANOVA F (3,36) and p values for the EP-L, EP-H and EP-C groups, respectively, were 23.32, P<0.0001, 6.58, p<0.005, and 19.97, p<0.0001, and on non-binge days, were 14.26, P<0.0001, 9.88, p<0.0001, and 31.93, p<0.0001.
3. Weekly energy intake
Weekly average energy intakes were compared between chow control and fat-restricted rats in order to assess the compensatory abilities of the different groups (Table 3). In the OIL groups, the L rats consumed more energy than was consumed by the C rats in the first three weeks and then ate statistically the same amount of energy as did C controls thereafter. The H rats, on the other hand, consumed statistically the same amount as did the chow rats during all six weeks (interaction of week × fat access F(10,180)=6.71, p<0.0001; main effect of fat access F(2,36)=3.27, p<0.05).
Table 3.
Weekly cumulative energy intake across the study
| Wk 1 | Wk 2 | Wk 3 | Wk 4 | Wk 5 | Wk 6 | |
|---|---|---|---|---|---|---|
| OIL GROUPS | ||||||
| C | 521.3±6.8a | 510.2±8.9a | 497.6±8.0a | 481.7±9.5 | 470.3±9.3 | 452.5±7.8 |
| L | 590.1±10.8b | 562.9±14.5b | 535.8±12.9b | 490.4±17.0 | 451.9±10.8 | 453.6±10.2 |
| H | 536.5±6.2a | 517.4±7.5a | 493.1±9.4a | 475.7±9.6 | 454.2±9.0 | 449.9±10.3 |
| EP GROUPS | ||||||
| C | 403.8±4.7a | 391.6±4.2a | 394.7±5.6a | 379.3±5.5 | 384.4±5.7 | 372.1±5.7a |
| L | 454.8±8.2b | 433.6±6.5b | 422.7±9.4b | 393.2±9.8 | 386.5±5.1 | 402.7±8.2b |
| H | 437.7±6.8b | 423.5±4.7b | 411.9±10.1a | 400.7±7.1 | 396.6±6.7 | 401.6±6.3b |
Different lower case letters indicate different weekly energy intake within OIL or EP groups in each week (p<0.05).
In the EP-L rats, results were similar to those described above for the OIL-L rats. That is, the EP-L rats consumed more energy than did the EP-C rats in the first three weeks and then ate statistically the same amount of energy as did C controls for the next two weeks. By week 6, however, L intakes again exceeded C intakes. Results differed in the EP-H rats, in that average weekly intakes of the EP-H group exceeded those of the C group in the first two weeks and in week six, but were statistically similar to the chow group during weeks 3-5 [interaction of week × fat access F(10,180)=4.66, p<0.0001; main effect of fat access F(2,36)=7.15, p<0.01].
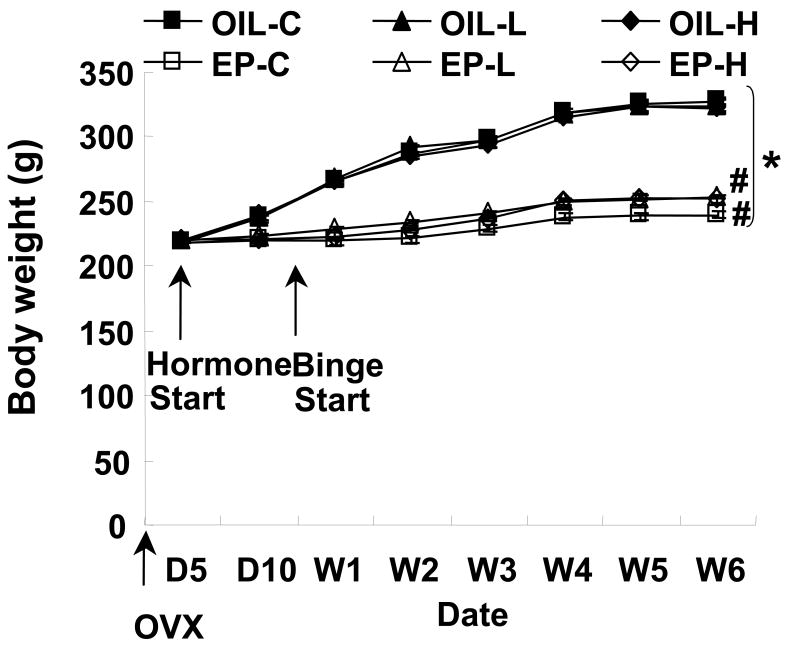
4. Body weight
Body weight before OVX and at day 5 post-OVX did not differ significantly among groups. Body weight at the 6th week of the study, however, was significantly affected by hormone treatment, with the OIL rats (324 ± 2 g) weighing significantly more than the EP rats (248 ± 2 g) [Fig 3; interaction effect of (hormone treatment × fat access) F(2,72)=3.68, p<0.05; main effect of hormone treatment: F(1,72)=818.66, p<0.0001]. The interaction effect was due to significantly greater body weights in the EP-L and EP-H relative to EP-C (p<0.0167). Such differences did not emerge in the OIL rats. OIL rats gained 106 ± 4 g between day 5 and day 45 post-OVX, whereas EP rats gained only 29 ± 2 g during the same period. There was no significant difference in final body weight among the OIL groups.
Fig 3.
Body weight at day 5 of postovariectomy and weekly across the 6-week study. # indicates final body weight of L and H rats greater than C rats in EP groups (P<0.05). * indicates significant difference between OIL and EP groups that had the same feeding schedule (P<0.001).
Due to an intervening drug study, during which the EP and OIL treatments were maintained, there was an ∼40-day interval between the end of the 6-wk feeding study and the day of sacrifice for the carcass analysis. Rats were sacrificed about 10 days after the end of the drug study, which allowed sufficient time for the drug to clear. The overall pattern of group differences was not affected by the drug study. Specifically, there was no significant difference in sacrifice body weight among the OIL groups. However, among the EP groups, only the sacrificed body weight of EP-L was statistically greater than that of EP-C. EP-H was ∼10 g greater than EP-C; however, this was no longer statistically significant.
5. Body composition
As analyses of the masses of the retroperitoneal, abdominal, and ovarian fat pads yielded similar results as anlysis of the sum of the three pads' weights, only the latter are presented (Table 4). The summed masses of the OIL groups' fat pads were significantly greater than those of the EP groups (Table 4). In addition, fat pad mass was greater in OIL-L rats than in OIL-H rats, which in turn were greater than OIL-C rats (Table 4; p<0.0167). Fat pad mass was not significantly affected by fat access in the EP rats. Carcass analysis was done to determine if total body fat changed in proportion to the retroperitoneal, abdominal, and ovarian fat pads and to determine if there were also differences in fat deposition in regions other than the fat pads measured (e.g. subcutaneous fat). This analysis revealed that total body fat content, body protein, body water, body mineral, and wet carcass weight were all increased in OIL rats compared to EP rats (Table 4; P<0.0167), with the relative changes quite similar to those observed in the three fat pads measured. Body mineral content, body water content and wet carcass were not different among the different diet protocols in the OIL rats, although in EP rats, body water and wet carcass were greatest in the EP-L group (p<0.0167). Protein mass was not affected by fat access in either OIL or EP rats.
Table 4.
Body composition data
| C | H (Binge) | L | |
|---|---|---|---|
| Sacrifice body weight | |||
| OIL | 331.2±4.11 | 326.4±4.21 | 326.9±5.51 |
| EP | 250.7±2.8b, 2 | 260.2±2.9ab, 2 | 263.5±3.0a, 2 |
| Fat pads† | |||
| OIL | 10.8±0.7b, 1 | 12.8±1.3ab, 1 | 15.1±0.6a, 1 |
| EP | 7.1±0.42 | 8.5±0.52 | 8.8±0.62 |
| Wet mass | |||
| OIL | 240.2±3.51 | 241.1±3.81 | 248.9±4.21 |
| EP | 175.9±2.6b, 2 | 181.2±2.5ab, 2 | 190.5±1.6a, 2 |
| Water | |||
| OIL | 144.1±1.61 | 140.0±1.91 | 140.1±2.81 |
| EP | 107.9±1.8b, 2 | 109.5±1.9ab, 2 | 115.2±1.0a, 2 |
| Dry mass | |||
| OIL | 96.2±2.4b, 1 | 101.7±2.7ab, 1 | 108.8±4.9a, 1 |
| EP | 68.0±1.12 | 71.7±1.1 2 | 75.3±1.2 2 |
| Mineral | |||
| OIL | 8.3±0.11 | 8.3±0.21 | 7.7±0.2 |
| EP | 7.1±0.1a, 2 | 6.2±0.1b, 2 | 7.3±0.2a |
| Fat mass | |||
| OIL | 33.5±2.2b, 1 | 39.2±2.3ab, 1 | 47.3±4.3a, 1 |
| EP | 22.5±0.8 2 | 25.3±1.2 2 | 26.9±1.2 2 |
| Protein | |||
| OIL | 54.4±0.75 1 | 53.6±1.05 1 | 53.7±1.08 1 |
| EP | 38.4±0.56 2 | 40.1±0.77 2 | 41.2±0.59 2 |
Different lower case letters indicate significant differences within OIL or EP groups. Different numbers indicate significant differences between OIL and EP groups which had the same fat access schedule (p<0.0167).
Fat pads are the combined mass of gonadal, abdominal and retroperitoneal fat pads.
Discussion
The major finding of this study is that binge size was tonically, but not cyclically, reduced in the EP-H rats, i.e., rats in which highly restricted scheduled access to fat led to binge-like, excessive intake in 1-h tests. Specifically, both EP-H and EP-L rats consumed significantly less energy than did the OIL rats during the 1-h fat access period, indicating a tonic reduction of binge size in the EP rats. However, 1-h energy intake did not vary cyclically across the 4-day hormone treatment cycle in the EP-H rats, but did vary cyclically in the EP-L rats. One-h energy intake also did not vary in the EP-C rats, but we attribute this to the very low 1-h intake in this group. Finally, 24-h energy intakes varied cyclically in all three groups. Together these data indicate that, whereas the normal tonic inhibitory effect of ovarian hormones on eating persists in this binge eating model, the normal cyclic inhibitory effect on eating is disrupted during binge-type eating episodes.
The tonic and cyclic decreases in eating produced by cyclic hormone treatment in OVX EP-L rats extends reports of such effects under several other conditions (11, 12, 17, 18, 38) and indicates that these effects occur even with limited access to an optional source of dietary fat. Cyclic estradiol, however, failed to produce cyclic inhibition of eating during the 1-h fat access period in EP-H rats. The fact that cyclic effects on 1-h fat intake were evident in the L rats indicates that the lack of effect in the binge rats is not simply due to the availability of fat in addition to chow. Rather, it appears to be related to the consumption of large amounts in brief periods of time relative to controls, e.g. bingeing. Estradiol's cyclic inhibitory effect on eating under non-binge conditions is expressed as reduced meal size, with no reduction in meal frequency (17, 38). Thus, the present results suggest that the cyclic inhibitory effects of estradial are compromised by binge-type consumption of large fatty meals.
We know of no comparable human data. Although binge frequency and subjective correlates of eating have been reported to change across the menstrual cycle in women with bulimia nervosa (22, 23), alterations in binge size across the menstrual cycle have not. However, there are some reports indicating that the ability to limit meal size is compromised in women with bulimia nervosa. For instance, patients with bulimia nervosa eat significantly more of both single and multiple-item meals than do control women when instructed to binge eat (39); patients with bulimia nervosa also need to eat more than do controls to produce equivalent self-reported fullness during a meal (40). Such results suggest that the normal physiological inhibitory controls of eating, including the effects of estradiol, may be weakened in women who binge frequently. These effects may be related to a reduced satiation effect of CCK, because both blunted postprandial cholecystokinin release and delayed gastric emptying were seen in women with bulimia nervosa (11, 12, 41, 42). In contrast, in one report using a 24-h naturalistic laboratory feeding situation, the majority of meals were of normal size in bulimic women; binges represented the minority of meals but were calorically rich, primarily due to increased consumption of fatty foods (43).
Because pharmacological doses of progesterone (1 mg or more) can reverse estradiol's inhibitory effect on eating in rats (44), it is possible that the loss of estradiol's cyclic effects during the binge was due to an enhanced effect of progesterone at this time. This does not seem likely, however, because there was still a cyclic decrease in 24-h intake in the same rats and because our progesterone dose (0.5 mg) was smaller than those reported to antagonize estradiol's eating-inhibitory effect.
Alternatively, the lack of cyclic hormonal effects during the binge may have been due to other influences of estradiol that might affect binge behavior. The inhibitory action of estradiol on eating is generally thought to relate to “homeostatic” rather than “hedonic” controls of food intake (45-47). In addition, however, estradiol can have stimulatory effects on other behaviors, including drug intake (48, 49) and drug-induced locomotor activity (50), that are considered hedonic rather than homeostatic. Binge eating is also likely to be more related to non-homeostatic, hedonic processes (45, 46). This suggests that it is possible that the hedonic, stimulatory effects of estradiol predominated during consumption of the binge food in the EP-H rats, while the homeostatic, inhibitory effects of estradiol predominated during consumption of the chow. The net result would be an elevated binge size in the estrus phase of the ovarian cycle in the EP-H rats, eliminating the typical cyclic food intake pattern during the bingeing period, while still leaving cyclic effects on chow and overall daily intake intact.
Although the cyclic eating-inhibitory effect of estradiol on binge intake was eliminated in the binge rats, the tonic eating-inhibitory effect was still present, both in 1-h and in 24-h energy intake. In general, the average 24-h energy intake of the EP rats was lower than that of the OIL rats on both binge days and non-binge days. The one exception to this occurred in the EP-H group; 24-h intake of EP-H was not significantly lower than OIL-H on non-binge days (Fig 2). This appeared to be due to the failure of the EP-H groups to undereat on non-binge days during the first few weeks of the study (24-h intake data not shown), thus increasing the 6-week mean data presented. During the latter weeks of the study, the tonic inhibitory effect of EP was indeed more clear.
Previous studies in male rats indicate that H rats overeat on binge days when fat is present and undereat on non-binge days, resulting in a net energy intake comparable to C rats (35). This occurred here in the OIL-H group but not in the EP-H group. In OIL groups, weekly average energy intake did not differ between H and C control rats in any of the 6 weeks. In contrast, weekly average energy intakes were greater in the EP-H rats relative to the EP-C controls in the first two weeks and in week six. However, in a previous study from our laboratory (33) intact bingeing female rats tended to undereat more than they overate until the fifth week. Whether this difference represents an activational effect of ovarian hormones on energy homeostasis warrants further investigation.
The effects we report here are likely to be of physiological relevance because we used near physiological amounts and patterns of estradial and progesterone. For example, in previous work with OVX Long Evans rats, cyclic 2 μg estradiol benzoate administered on day 3 of a 4-day cycle produced estradiol concentrations comparable to those of intact cycling rats, with low and high levels all close to minimum and maximum intact values (10-30 and 180-300 pmol/L, respectively). Furthermore, normalized body weight and food intake patterns have been reported when this estradiol replacement regimen was used (17). Others have shown that in OVX Sprague-Dawley rats, progesterone doses larger than those used here (∼1000 μg/rat) injected 20 h following estradiol, produced peak plasma progesterone levels that were near to peak levels assayed during the proestrus phase in intact females (37, 51-53) Therefore, the 500 μg/rat progesterone dose used in this study was lower than that which would be considered physiological. Although relatively low, this dose of progesterone has been reported to produce normal sexual receptivity in E-treated OVX rats (17). The EP rats in the present study gained 29 ± 2 g during the initial 6-weeks of the study, which is comparable to the 35 g body weight gain reported previously in intact female Sprague-Dawley rats maintained for 6 weeks on the same feeding schedule (33). Thus, the 4-day cycle of estradiol/progesterone administration used herein would not be considered pharmacological.
We included progesterone in the injection protocol in the present study because progesterone seems to be important for ligand binding to GABA-B receptors in the neocortex of female rats (37), and because the GABA-B agonist baclofen has been shown to reduce binge-type eating in rats (54) and to produce promising results in an open-label trial in humans (55). In addition, estradiol was found to negatively regulate GABA-B receptors in the pituitary and hypothalamus of female rats (56) and to rapidly attenuate the potency of baclofen in hypothalamic POMC neurons in female guinea pigs (57). Thus, desensitization of GABA-B receptors by estradiol could theoretically interfere with inhibitory/compensatory controls under binge-type conditions. Whether such alterations can explain the present results remains to be determined. We included progesterone also because high progesterone levels were associated with increased binge frequency independent of estradiol in women with BN (22). More recently, progesterone was independently positively associated with higher emotional eating scores in a community sample (23). Therefore, it would be of interest to determine the individual roles of estradiol and progesterone under binge-type conditions.
The delayed compensatory behavior, however, is not likely due to alterations in the ability of the EP-H rats to learn how to adapt to the feeding protocol, because increases in estradiol or progesterone in serum, cortex, and hippocampus have been reported to enhance cognitive performance in both intact and hormone-primed OVX rats (58).
One aspect of human binge eating that group analyses using this model do not capture is that of individual differences in susceptibility. It may be possible, however, to pursue this issue in the context of this model, as individual variability in the amount of shortening consumed has been seen in this study as well as in previous studies using male rats. Within the EP-H (binge) rats in the current study, for instance, shortening intakes ranged from 13.4 to 73.3 kcal, with the top 24% of the rats (3 rats) consuming 60.5 kcal, and the bottom 24% consuming 16.5 kcal. Thus, as in humans (8), individual vulnerability to the effects of exposure to binge-inducing stimuli appears to exist under the conditions used in the present study.
As previously reported (11, 13, 59), OIL rats weighed significantly more than EP rats. Body weight and wet mass did not differ among the OIL rats; however, dry mass of the OIL-L group was significantly greater than that of the OIL-C controls (Table 4). The elevated dry mass of the OIL-L rats was primarily due to a higher fat mass; body water, mineral, and protein mass did not significantly differ among the OIL rats. This effect in the OIL rats is similar to a previous study in intact females (33), in which fat mass accounted for the somewhat higher carcass mass of the L rats relative to C controls. This similarity suggests that repeatedly consuming large amounts of fat in brief periods of time may have disrupted estrus cycling in the previous study.
In summary, this study reports that administration of estradiol and progesterone no longer exerts cyclic inhibitory effects on the size of brief bouts of fat intake under binge-type eating conditions. Clearly, further work is warranted to determine the mechanisms involved in this effect and to determine if the phenomenon is of relevance to binge size in human binge-type eating.
Acknowledgments
The authors thank Allison Brown for her excellent technical assistance with the carcass composition analysis. We also would like to thank Kim Feeney, Lauren Chuday, Jessica Hedden, and Maggie Sikora for their assistance with data collection. This work was supported by NIH 1-R01-MH6794301, as well as the Women in Science and Engineering Research Program (WISER) funded by the Pennsylvania Space Grant Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Basdevant A, Pouillon M, Lahlou N, Le Barzic M, Brillant M, Guy-Grand B. Prevalence of binge eating disorder in different populations of French women. Int J Eat Disord. 1995;18:309–315. doi: 10.1002/1098-108x(199512)18:4<309::aid-eat2260180403>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Gotestam KG, Agras WS. General population-based epidemiological study of eating disorders in Norway. Int J Eat Disord. 1995;18:119–126. doi: 10.1002/1098-108x(199509)18:2<119::aid-eat2260180203>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spitzer RL, Yanovski S, Wadden T, Wing R, Marcus MD, Stunkard A, Devlin M, Mitchell J, Hasin D, Horne RL. Binge eating disorder: its further validation in a multisite study. Int J Eat Disord. 1993;13:137–153. [PubMed] [Google Scholar]
- 6.Spitzer RL, Stunkard A, Yanovski S, Marcus MD, Wadden T, Wing R, Mitchell J, Hasin D. Binge eating disorder should be included in DSM-IV: a reply to Fairburn et al.'s “the classification of recurrent overeating: the binge eating disorder proposal”. Int J Eat Disord. 1993;13:161–169. [PubMed] [Google Scholar]
- 7.Kjelsas E, Bjornstrom C, Gotestam KG. Prevalence of eating disorders in female and male adolescents (14-15 years) Eat Behav. 2004;5:13–25. doi: 10.1016/S1471-0153(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 8.Kinzl JF, Traweger C, Trefalt E, Mangweth B, Biebl W. Binge eating disorder in females: a population-based investigation. Int J Eat Disord. 1999;25:287–292. doi: 10.1002/(sici)1098-108x(199904)25:3<287::aid-eat6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Gorski R. Sexual differenation of the nervous system. 4th. New York: MacGraw-Hill; 2000. [Google Scholar]
- 10.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 11.Geary N. The estrogenic inhibition of eating. In: Sticker E, Woods SC, editors. Neurobiology of food and fluid intake. 2nd. New York: Kluwer Academic Publishing; 2004. pp. 305–343. [Google Scholar]
- 12.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- 14.Geary N, Lovejoy J. Sex differences in energy metabolism, obesity, and eating behavior. In: Becker JB, B K, Geary N, Hampson E, Herman JP, Young EA, editors. Sex differences in the brain: from genes to behavior. Oxford, UK: Oxford University Press; 2008. pp. 253–274. [Google Scholar]
- 15.Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav. 1995;58:1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- 16.Dye L, Blundell JE. Menstrual cycle and appetite control: implications for weight regulation. Hum Reprod. 1997;12:1142–1151. doi: 10.1093/humrep/12.6.1142. [DOI] [PubMed] [Google Scholar]
- 17.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 18.Drewett RF. Oestrous and dioestrous components of the ovarian inhibition on hunger in the rat. Anim Behav. 1973;21:772–780. doi: 10.1016/s0003-3472(73)80103-4. [DOI] [PubMed] [Google Scholar]
- 19.Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: relation to menstrual-cycle phase and cortisol levels. Psychol Med. 2003;33:51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- 20.Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. Am J Psychiatry. 1987;144:1592–1595. doi: 10.1176/ajp.144.12.1592. [DOI] [PubMed] [Google Scholar]
- 21.Price WA, Torem MS, DiMarzio LR. Premenstrual exacerbation of bulimia. Psychosomatics. 1987;28:378–379. doi: 10.1016/s0033-3182(87)72511-0. [DOI] [PubMed] [Google Scholar]
- 22.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2006:1–11. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- 23.Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. Psychol Med. 2008:1–9. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leigh AJ, Stock MJ, Lacey JH, Wilson CA. Diet-induced loss of cyclic ovarian function at normal body weight in a rodent model for bulimia nervosa. J Reprod Fertil. 1998;112:217–223. doi: 10.1530/jrf.0.1120217. [DOI] [PubMed] [Google Scholar]
- 25.Gendall KA, Bulik CM, Joyce PR, McIntosh VV, Carter FA. Menstrual cycle irregularity in bulimia nervosa. Associated factors and changes with treatment. J Psychosom Res. 2000;49:409–415. doi: 10.1016/s0022-3999(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 26.Crow SJ, Thuras P, Keel PK, Mitchell JE. Long-term menstrual and reproductive function in patients with bulimia nervosa. Am J Psychiatry. 2002;159:1048–1050. doi: 10.1176/appi.ajp.159.6.1048. [DOI] [PubMed] [Google Scholar]
- 27.Cantopher T, Evans C, Lacey JH, Pearce JM. Menstrual and ovulatory disturbance in bulimia. Bmj. 1988;297:836–837. doi: 10.1136/bmj.297.6652.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairburn CG, Cooper PJ. The clinical features of bulimia nervosa. Br J Psychiatry. 1984;144:238–246. doi: 10.1192/bjp.144.3.238. [DOI] [PubMed] [Google Scholar]
- 29.McCluskey SE, Lacey JH, Pearce JM. Binge-eating and polycystic ovaries. Lancet. 1992;340:723. doi: 10.1016/0140-6736(92)92257-g. [DOI] [PubMed] [Google Scholar]
- 30.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 31.Corwin RL. Bingeing rats: a model of intermittent excessive behavior? Appetite. 2006;46:11–15. doi: 10.1016/j.appet.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas MA, Rice HB, Weinstock D, Corwin RL. Effects of aging on food intake and body composition in rats. Physiol Behav. 2002;76:487–500. doi: 10.1016/s0031-9384(02)00800-4. [DOI] [PubMed] [Google Scholar]
- 33.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28:436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 34.Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42:139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 36.Drewett RF. The meal patterns of the oestrous cycle and their motivational significance. Q J Exp Psychol. 1974;26:489–494. doi: 10.1080/14640747408400438. [DOI] [PubMed] [Google Scholar]
- 37.al-Dahan MI, Thalmann RH. Progesterone regulates gamma-aminobutyric acid B (GABAB) receptors in the neocortex of female rats. Brain Res. 1996;727:40–48. [PubMed] [Google Scholar]
- 38.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- 39.LaChaussee JL, Kissileff HR, Walsh BT, Hadigan CM. The single-item meal as a measure of binge-eating behavior in patients with bulimia nervosa. Physiol Behav. 1992;51:593–600. doi: 10.1016/0031-9384(92)90185-5. [DOI] [PubMed] [Google Scholar]
- 40.Guss JL, Kissileff HR. Microstructural analyses of human ingestive patterns: from description to mechanistic hypotheses. Neurosci Biobehav Rev. 2000;24:261–268. doi: 10.1016/s0149-7634(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 41.Geracioti TD, Jr, Kling MA, Joseph-Vanderpool JR, Kanayama S, Rosenthal NE, Gold PW, Liddle RA. Meal-related cholecystokinin secretion in eating and affective disorders. Psychopharmacol Bull. 1989;25:444–449. [PubMed] [Google Scholar]
- 42.Devlin MJ, Walsh BT, Guss JL, Kissileff HR, Liddle RA, Petkova E. Postprandial cholecystokinin release and gastric emptying in patients with bulimia nervosa. Am J Clin Nutr. 1997;65:114–120. doi: 10.1093/ajcn/65.1.114. [DOI] [PubMed] [Google Scholar]
- 43.Weltzin TE, Hsu LK, Pollice C, Kaye WH. Feeding patterns in bulimia nervosa. Biol Psychiatry. 1991;30:1093–1110. doi: 10.1016/0006-3223(91)90180-t. [DOI] [PubMed] [Google Scholar]
- 44.Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol. 1975;88:183–193. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- 45.Lowe MR, Levine AS. Eating motives and the controversy over dieting: eating less than needed versus less than wanted. Obes Res. 2005;13:797–806. doi: 10.1038/oby.2005.90. [DOI] [PubMed] [Google Scholar]
- 46.Corwin RL, Hajnal A. Too much of a good thing: neurobiology of non-homeostatic eating and drug abuse. Physiol Behav. 2005;86:5–8. doi: 10.1016/j.physbeh.2005.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geary N, Trace D, Smith GP. Estradiol interacts with gastric or postgastric food stimuli to decrease sucrose ingestion in ovariectomized rats. Physiol Behav. 1995;57:155–158. doi: 10.1016/0031-9384(94)00271-6. [DOI] [PubMed] [Google Scholar]
- 48.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 49.Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- 50.Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- 51.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 52.Nequin LG, Alvarez J, Schwartz NB. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod. 1979;20:659–670. doi: 10.1095/biolreprod20.3.659. [DOI] [PubMed] [Google Scholar]
- 53.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 54.Buda-Levin A, Wojnicki FH, Corwin RL. Baclofen reduces fat intake under binge-type conditions. Physiol Behav. 2005;86:176–184. doi: 10.1016/j.physbeh.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Broft AI, Spanos A, Corwin RL, Mayer L, Steinglass J, Devlin MJ, Attia E, Walsh BT. Baclofen for binge eating: An open-label trial. Int J Eat Disord. 2007 doi: 10.1002/eat.20434. [DOI] [PubMed] [Google Scholar]
- 56.Rey-Roldan EB, Bianchi MS, Bettler B, Becu-Villalobos D, Lux-Lantos VA, Libertun C. Adenohypophyseal and hypothalamic GABA B receptor subunits are downregulated by estradiol in adult female rats. Life Sci. 2006;79:342–350. doi: 10.1016/j.lfs.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 57.Malyala A, Kelly MJ, Ronnekleiv OK. Estrogen modulation of hypothalamic neurons: activation of multiple signaling pathways and gene expression changes. Steroids. 2005;70:397–406. doi: 10.1016/j.steroids.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 58.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallen WJ, Belanger MP, Wittnich C. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr. 2001;131:2351–2357. doi: 10.1093/jn/131.9.2351. [DOI] [PubMed] [Google Scholar]