Abstract
When non-food-deprived rats are given intermittent access to certain substances, consumption of those substances is greater than when more frequent access is provided. The present study examined the effects of three different shortening access conditions on subsequent shortening intake in rats. Each of the three different shortening conditions lasted five weeks and was followed by a five-week period in which shortening access was limited by time (1 hr of availability) on either an Intermittent (Monday, Wednesday, Friday) or Daily schedule of access. In Part 1, limiting the quantity of shortening provided during the 1-hour period of availability attenuated subsequent 1-hr shortening intake in the Intermittent access group, but had no statistically significant effect in the Daily access group. In Part 2, unrestricted availability of shortening (24-hr/day-7days/week) attenuated subsequent 1-hr shortening intake in all groups. In Part 3, shortening non-availability for five weeks enhanced subsequent 1-hr shortening intake in all groups. It was also shown that rats under an Intermittent, but not a Daily, schedule of access consumed as much shortening during a 1 hr period of availability, as was consumed in 24-hr when shortening availability was unrestricted. These results demonstrate that while intermittent access is necessary and sufficient to stimulate binge-type eating in rats, the behavioral history can modulate binge size.
Keywords: animal models, behavioral models, binge eating, dietary fat, eating disorders, high-fat diet, intermittent access, limited access
Introduction
A considerable body of research has shown that behavior directed toward consuming various foods is increased when the time during which those foods are available is decreased. For example, non-food deprived rats drink more of a 1.5% w/v saccharin solution or 20% w/v alcohol during a 24-hr period when provided every other day than when provided on a continuous basis [1,2]. The frequency of dipper presentations and the duration of drinking bouts for 10% alcohol increase when the number of access periods each day is reduced [3]. Other research has also shown that non-food deprived rats consume more of an optional fat (binge) during a 1-2 hr period of access when it is provided intermittently on Mondays Wednesdays and Fridays (MWF) [4-8] or every third day [9] than when it is provided daily for 1-2 hr. “Bingeing” is operationally defined as Intermittent 1-hr shortening intakes > Daily 1-hr shortening intakes in these studies, based upon the DSM-IV criterion of consuming more in a brief period of time than is normally consumed during a similar period of time and under similar circumstances [10]. Only access to the fat is limited; chow and water are always freely available, i.e. the rats are never food-deprived [4-8]. Furthermore, body weight and body fat do not differ between binge rats and chow-fed controls [4]. Similar results have also been obtained for various concentrations of liquid sucrose [11]. The use of intermittent limited access has been proposed as a behavioral model of binge-type eating with good construct and face validity [12]. Recent research suggests the predictive validity of this approach, as well [13].
All of the above studies increase consumption by restricting the time during which the ingestant is available. Time-limited procedures do not promote cumulative overconsumption; rather, they promote brief bouts of excessive intake, i.e. bingeing. This is different from protocols in which extended access (long session duration) promotes cumulative overconsumption, such as that reported for certain drugs of abuse [14], or for fat, in which extended access induces hyperphagia and obesity [15]. Extended access (12 hr vs. 30 min) also has been reported to increase operant responding for sugar [16]. In short, consumption of a variety of items can be modulated by maintaining a constant session duration while altering session frequency (3 times a day, daily, every other day, etc.), or by altering session duration and keeping session frequency constant. Both of these approaches involve alterations in the time during which some commodity is provided. While time-related access has been extensively studied, the effect of limiting the amount provided has not.
Limitation of the quantity available has relevance to the incorporation of “forbidden foods” into the diet [17]. “Forbidden foods” are typically high in fat and those who are trying to lose weight often restrict access to these foods. However, these foods are consumed in large quantities during a binge. The forbidden foods hypothesis of human bingeing suggests that these two conditions are related, i.e. the foods upon which people binge are those to which they have limited their own access [17]. Websites available to the public suggest that incorporation of forbidden foods into the diet will produce “normal” eating patterns, but such ideas have not been subjected to controlled experiments to our knowledge. Would occasional consumption of small quantities of forbidden foods protect against the subsequent development of binge eating? Conversely, would “nibbling” on forbidden foods throughout the day protect against subsequent bingeing? The present study addressed these questions using an established rat model of binge-type eating. The effects of both time-limited and quantity-limited access to fat on subsequent binge-type consumption of fat are reported.
General Methods
Overall design
There were three 10-week parts each consisting of two 5-week sub-parts (Table 1). In the “A” sub-parts, access to shortening was manipulated in different ways; in the “B” sub-parts, our standard limited access protocols (Intermittent or Daily 1-hr access to shortening) were in place. The goal was to determine the effects of the various access manipulations (parts 1A, 2A, 3A) on subsequent binge-type intake (parts 1B, 2B, 3B).
Table 1.
Shortening availability in each part
| PART | ||||||
|---|---|---|---|---|---|---|
| 1A | 1B | 2A | 2B | 3A | 3B | |
| Intermittent (I) | TL1 | TL | Continuous | TL | None | TL |
| Intermittent (I-QL) | QL2 | TL | Continuous | TL | None | TL |
| Daily (D) | TL | TL | Continuous | TL | None | TL |
| Daily (D-QL) | QL | TL | Continuous | TL | None | TL |
| No Shortening (NS) | None | None | None | TL Intermittent | None | TL Intermittent |
Time-limited (1-hr access to full bowl)
Quantity-limited (1-hr access to 2g);
Animals
Fifty male Sprague Dawley rats (Harlan, Indianapolis, IN), 60 days of age and weighing 268-307g (280.7 ± 5.6) at the start of the study, were individually housed in hanging stainless steel wire cages in a temperature- and humidity-controlled environment placed on a 12:12 light:dark cycle. All rats were maintained on a nutritionally complete commercial laboratory rodent chow (Laboratory Rodent Diet 5001, PMI Feeds, Richmond IN; percent of energy as protein: 28.05%, fat: 12.14%, carbohydrate: 59.81%; 3.3 kcal/g). Chow and tap water were available ad libitum throughout all parts of the study. The Pennsylvania State University Institutional Animal Care and Use Committee approved all procedures.
During the first two weeks of adaptation to the vivarium, chow intake was measured on a daily basis, body weights were determined three times per week, and unrestricted access to solid vegetable shortening (Crisco® All-Vegetable Shortening, J.M Smucker Co., Orrville, OH) was provided during a single overnight period. Prior to the start of the experiment, five groups of 10 rats each were matched by body weight, average amount of chow consumed during three consecutive 24-hr periods, and the amount of shortening consumed during the overnight access period [F(4,45)<1, NS for all]. The experimental manipulations for each group are described in detail for each study, below.
Statistics
Body mass-adjusted shortening intake (kcal/body mass0.67; [18]) was used throughout. For Part 1A, 1B, and 2A shortening intake was analyzed using 3-way repeated measures ANOVA: [access schedule (Daily, Intermittent) X amount of shortening available (full bowl, 2 grams) X time (week)]. Differences among groups for each week were assessed using pre-planned LS means comparisons, with a Bonferroni correction applied (p = 0.05/3 comparisons per group = 0.0167). For analyses of shortening intake involving the NS group (Parts 2B, 3A, 3B), as well as all analyses of total energy intake (shortening plus chow) and body weight, 2-way ANOVA was used [group X time (week)] followed by Tukey's HSD post-hoc test. Differences among groups in absolute body weights at the end of week 5 of each sub-part, body weight change during each sub-part, total cumulative energy intake, shortening intake for week 5 of each sub-part, percent protein by weight, percent carbohydrate by weight, and percent fat (chow fat and shortening) of each sub-part were determined using 1-way ANOVA followed by Tukey's HSD.
Part 1
The goal of Part 1 was to determine the effects of quantity-limited shortening consumption on subsequent quantity-unlimited shortening consumption, i.e. on binge-type consumption of shortening. The quantity-limitation provided a way to limit access in the Intermittent group to an even greater extent than the time-limited procedure affords. In addition, it provided a way to control for amount consumed during the access period independent of the schedule of availability, since the amount provided was that which Daily rats normally consume. Chow and water were available ad libitum throughout the study, i.e., the rats were never food-deprived. Only access to the shortening was manipulated.
Methods-Part 1
Part 1A: Quantity-limited access to shortening for some groups, time-limited for all
For the first five weeks, two groups of rats (I, I-QL) were placed on an intermittent schedule of access (Mondays, Wednesdays, and Fridays) to shortening while two other groups were placed on a daily schedule of access (D, D-QL). For all four groups, shortening availability was time-limited to 1 hr. For one of the Intermittent groups (I) and one of the Daily groups (D), the quantity of shortening provided during the 1-hr period of availability was unlimited. That is, the I and D groups received a full bowl during the 1-hr period. For the other Intermittent and Daily groups (I-QL and D-QL, respectively) the quantity of shortening provided during the 1-hr period was limited to ∼2 g (2.0-2.2 g) in that previous work indicated that this was the average amount typically consumed by groups with 1-2 hr of Daily time-limited access to a full bowl of shortening [4-8]. The goal was to “clamp” intake to that normally consumed by Daily rats. In this way, rats in the I-QL, D-QL, and D groups would consume the same amount of shortening within the 1-hr period, regardless of the schedule of availability. This would allow for any effects of the Intermittent or Daily schedules to be determined independent of the amount consumed.
A fifth group of rats (NS) had no shortening access.
Part 1B: Quantity-unlimited and time-limited shortening access
During the next five-week period, access to shortening was limited by time (1 hr), but not by amount. That is, all four groups (I, I-QL, D, D-QL) were given a full bowl of shortening (quantity-unlimited) during the 1-hr period of shortening availability under their respective schedules of access. The NS group again had no access to shortening.
Results – Part 1
Part 1A
Shortening
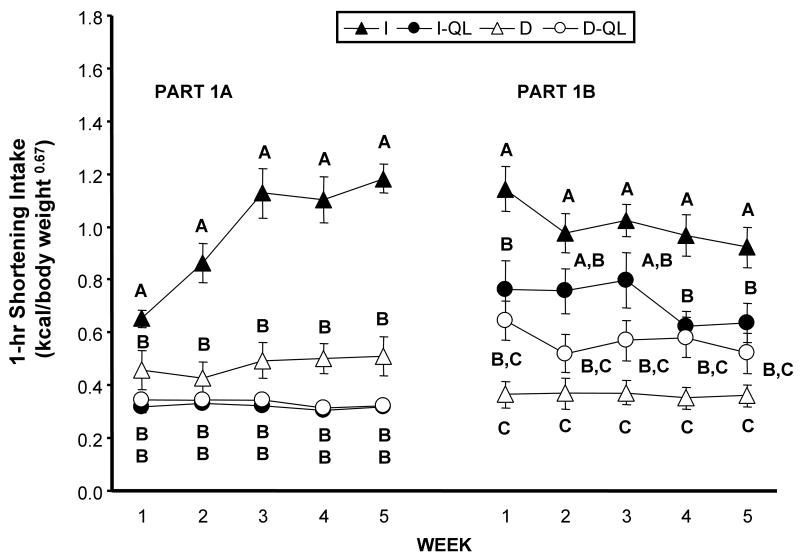
During each week of the initial 5-weeks, the I group consumed significantly more shortening (LS Means, p 0.0167) during the 1 hr period of availability than did any other group, and there were no differences among the I-QL, D, and D-QL groups (Fig. 1, left panel). There were main effects of access schedule (F(1,36) = 32.05, p 0.0001), amount of shortening available (F(1,36) = 86.08, p 0.0001), and an interaction between access schedule and amount (F(1,36) = 35.78, p 0.0001). There was also a main effect of week (F(4,144) = 18.22, p 0.0001), and interactions between week and access schedule (F(4,144) = 13.37, p 0.0001), between week and amount (F(4,144) = 22.25, p 0.0001), and among week, access schedule, and amount (F(4,144) = 11.61, p 0.0001). These results are consistent with previous research [4-8] showing that an intermittent access schedule stimulates fat intake relative to a daily access schedule across a period of several weeks, i.e. rats with intermittent access gradually develop binge-type behavior.
Figure 1.
Average shortening intake (kcal/body weight 0.067) during the 1-hr period that it was available for each week of Part 1. Part 1A: Shortening access was quantity-unlimited (rats received a full bowl) in the I and D groups, but was quantity-limited (rats received 2 g) for the I-QL and D-QL groups. Part 1B: Shortening access was time-limited and quantity-unlimited for all groups. Group designations: D = Daily, I = Intermittent, QL = limited quantity of shortening available during Part 1A. Different letters indicate significant differences among the groups within each week.
The absolute (not body mass-adjusted) average 1-hr shortening intakes during week 5 of Part 1A for all of the groups are shown in Table 2.
Table 2.
| PART | GROUP | Body Weight Week 5 (gm) (SE +/-) |
Body Weight Change (gm) (SE +/-) |
Total 5-Week Energy (kcal) (SE +/-) |
1-hr Shortening Week 5 (kcal) (SE +/-) |
Percent 5-Week Protein by Weight (SE +/-) |
Percent 5-Week Carbohydrate by weight (SE +/-) |
Percent 5-Week Fat by Weight (SE +/-) |
|---|---|---|---|---|---|---|---|---|
| 1A | ||||||||
| I | 416.5 (7.1) |
70.3 A,B (3.0) |
2984.5 (60.5) |
67.3 A (3.3) |
20.7% B,C (0.19%) |
43.2% B,C (0.39%) |
15.7% A,B (0.76%) |
|
| I-QL | 424.0 (6.3) |
82.1 A (2.3) |
3039.4 (36.0) |
18.5 B (0.5) |
22.6% A (0.03%) |
47.4% A (0.06%) |
7.6% C (0.12%) |
|
| D | 415.2 (7.0) |
68.7 B (1.8) |
2947.7 (55.6) |
25.6 B (3.4) |
20.2% C (0.47%) |
42.3% C (0.97%) |
17.6% A (1.90%) |
|
| D-QL | 421.0 (9.7) |
80.1 A,B (5.0) |
2947.1 (68.7) |
18.0 B (0.4) |
21.4% C (0.07%) |
44.8% B (0.15%) |
12.8% B (0.29%) |
|
| NS | 418.4 (7.9) |
70.3 A,B (3.0) |
2970.3 (59.2) |
23.4% B | 49.0% A | 4.5% C | ||
| 1B | ||||||||
| I | 458.2 (8.2) |
46.7 A,B (2.6) |
2865.6 (51.4) |
56.2 A (4.4) |
20.0% B,C (0.26%) |
42.0% B,C (0.54%) |
18.2% A,B (1.05%) |
|
| I-QL | 469.2 (7.7) |
49.5 A,B (2.3) |
2985.9 (31.4) |
39.4 B (4.6) |
21.2% B (0.29%) |
44.4% B (0.61%) |
13.5% B (1.19%) |
|
| D | 455.9 (9.3) |
43.0 A,B (3.6) |
2826.8 (50.0) |
22.4 C (2.6) | 20.5% B (0.40%) |
43.0% B (0.83%) |
16.3% B (1.62%) |
|
| D-QL | 468.7 (10.9) |
52.7 A (2.4) |
2970.7 (74.9) |
32.7 B,C (4.6) |
18.8% C (0.70%) |
39.3% C (1.46%) |
23.47% A (2.85%) |
|
| NS | 455.8 (8.6) |
40.5 B (2.1) |
2876.7 (50.7) |
23.4% A | 49.0% A | 4.5% C |
Total energy intake, body weight
In spite of the differences in shortening intake among the groups with access to shortening, the total energy intake (chow + shortening or chow alone) and body weights did not differ at any week among the I, I-QL, D, D-QL and NS groups (Tukey's HSD, NS). This was due to reductions in chow intake among the groups with shortening access (data not shown). Weight gain of all of the groups was not statistically different from that of the NS group. The macronutrient composition of the diets, although different among the groups, was sufficient to support growth (at least 150 g pro/kg diet, i.e. 15% protein by weight) in all groups as defined by the National Research Council. (Table 2) [19]
Part 1B
Shortening
During the second 5-week period, access to shortening was time-limited only (full bowl provided for 1 hr) for the I, I-QL, D, and D-QL groups (Fig. 1, right panel). The NS group did not receive any shortening during this part of the study. There was a main effect of access schedule [F(1,36) = 35.79, p 0.0001], and an interaction between access schedule and amount [F(1,36) = 13.97, p 0.0006]. There was also a main effect of week [F(4,144) = 7.37, p 0.0001], an interaction between week and access schedule [F(4,144) = 2.77, p 0.0296], and interactions among week, access schedule, and amount [F(4,144) = 2.99, p 0.0208]. The main effect of access schedule, and the interaction between access schedule and amount are accounted for by the fact that the I group consumed significantly more shortening than did any other group during this five-week period (LS Means, p 0.0167), except for weeks 2 and 3, in which there was no difference between the I and I-QL groups (Fig 1, right panel). In addition, the shortening intakes of both the I and I-QL groups were always significantly greater than was that of the D group (LS Means, p 0.0167). The shortening intake of the I-QL and D groups did not differ statistically from that of the D-QL group during any of the five weeks.
Average absolute shortening intakes during the 1 hr period of availability during week 5 of Part 1B are shown in Table 2. The range of total cumulative dietary fat as a percentage of total cumulative energy intake during Part 1B for each of the groups was as follows: I: 38.4 - 41.3%; I-QL: 30.6 - 31.8%; D: 34.5 -37.7%; and D-QL: 43.8-47.8%. The generally higher percentages of the D and D-QL groups were because these groups had access to shortening every day, while the I and I-QL groups only had access three days a week.
Total energy intake, body weight
Total energy intake was affected by access schedule [F(4,45) = 2.83, p 0.0355] and week [F(4,180) = 133.79, p 0.0001]. In addition, access and week interacted [F(16,80) = 2.07, p 0.014]. These effects were due to the significantly greater intakes of the I-QL and D-QL groups during the first week of Part 1B, relative to the D group (p 0.05, Tukey's HSD). Intakes did not differ among the groups (I, I-QL, D, D-QL, NS) at any other time, nor did total cumulative energy intake differ among the groups (Table 2). Body weights also did not differ among the groups at any time (week 5 weights shown in Table 2). However, the D-QL group gained more weight than did the NS group during Part 1B (p 0.05, Tukey's HSD) (Table 2). The macronutrient composition of the diets expressed as percent weight, although different among the groups, was sufficient to support growth (at least 150 g pro/kg diet) in all groups. (Table 2) [19]
Discusson – Part 1
The effects of limiting the quantity of shortening provided during the 1 hr period of availability (I-QL and D-QL groups) in Part 1A were not surprising, in that limiting the quantity artificially restricted shortening consumption. Furthermore, the manipulation of limiting the quantity was successful; the rats in the I-QL group consumed as much as did the D and D-QL groups, but were maintained on the same schedule of availability (Monday, Wednesday, Friday) as was the I group.
Limiting the quantity of shortening provided during the 1 hr period demonstrated that quantity-limited shortening intake reduces subsequent quantity-unlimited (binge) intake under an intermittent schedule of access in rats when shortening is available for 1 hr. That is, the quantity-limited history (I-QL group) reduced subsequent binge size relative to the quantity-unlimited history (I group). Since the I-QL shortening intakes were less than those of the I group during most weeks of Part 2B, it can be said that the quantity-limited history attenuated subsequent binge size. However, since bingeing is operationally defined as an intake greater than what normally would be consumed in the same period, it can be said that the I-QL group still “binged” relative to the D group. This result indicates that the intermittent access schedule is necessary as well as sufficient to promote bingeing, but that binge size can be altered by different behavioral histories (quantity- limited vs. unlimited). In short, not all types of restriction promote bingeing to the same degree in this non-food-deprived binge protocol.
The reasons for this effect are not known. Others have shown in rats that access to sucrose during long periods (12 hr) increases operant responding for sucrose, whereas access to sucrose for short periods (30 min) does not, an effect speculated to involve opioid and/or dopaminergic actions [16]. In humans, eating small amounts of chocolate is considered pleasant, and activates regions of the brain that are different from those activated by eating large amounts of chocolate, which is considered unpleasant [20]. Thus, different neuromodulators and different brain regions may be involved when intakes are quantity-limited relative to when intakes are quantity-unlimited. The fact that the subsequent effects of limiting the quantity were sustained for such a long period of time indicates that the alterations established by the quantity limiting procedure were quite strong.
In contrast to the results obtained with the intermittent access schedule, the amount of shortening consumed under the daily access schedule was not significantly affected by the quantity-limited history. Although the shortening intake of the D-QL group was slightly greater than that of the D group during Part 1B (quantity-unlimited), intakes of the two groups were never statistically different. This is probably not due to insufficiently limiting the quantity during Part 1A, as intakes of the D-QL group were always slightly less than were the intakes of the D group during the first five weeks when the quantity-limited condition was in effect. This suggests that the intakes of the D-QL rats were, indeed, suppressed, i.e. the rats would have eaten slightly more if given the opportunity to do so during Part 1A. In summary, the quantity-limited history had a slight stimulatory effect on subsequent shortening consumption in rats under the daily access schedule and an inhibitory effect in rats under the intermittent access schedule, relative to quantity-unlimited groups under their respective schedules of access. However, relative to each other, the shortening intakes of the I-QL and D-QL groups were not significantly different (see Fig 1, right panel).
These results have potential relevance to clinical recommendations in which the incorporation of preferred fatty foods into the diet is advised to reduce craving for and bingeing on those foods. The present results in rats suggest that occasionally eating small quantities of highly preferred fatty foods may protect against subsequent bingeing to some degree. Whether this would serve as an intervention once bingeing is established cannot be determined from the present results, since the quantity-limited procedure was used in naïve rats, not in rats with previously established binge behavior. In addition, since neither of the Daily groups was subsequently placed onto the Intermittent (binge) protocol, it remains to be seen whether or not daily exposure to preferred foods would have the same protective effect. The present results indicate that the D-QL procedure had slight stimulatory effects on subsequent Crisco intake, when Daily time-limited access was subsequently provided. Whether extended daily exposure would reduce or stimulate intake under binge-inducing conditions (in this case, intermittent access) remains to be determined. Previous research from our group has shown that 2 weeks of exposure to a time-limited D procedure in rats did not reduce subsequent binge behavior when an Intermittent schedule of availability was subsequently introduced (4, and unpublished results). Daily access for longer than 2 weeks, which is then followed by Intermittent access, has not been examined. Finally, whether the present results apply to humans, and whether the effects of quantity-limited access to one food would generalize to other foods is not known.
Part 2
Previous reports indicate that high-fat feeding regimens reduce satiety signaling [21-29] and increase intake of and preference for fatty foods [30-32]. On the other hand, restricting access to palatable foods is also thought to promote their subsequent overconsumption [33]. The second part of the present study addressed the question of whether a history of unrestricted access to shortening (24-hr/day-7 days/week) would alter subsequent shortening consumption when a time-limitation to shortening availability was again imposed. In short, this part of the study sought to determine if a history of unrestricted shortening access (time-unlimited and quantity-unlimited) would alter subsequent binge size (time-limited and quantity-unlimited).
Methods
Part 2A: Quantity-unlimited and time-unlimited (unrestricted) shortening access
For the first five weeks of Part 2A, the same four groups of rats (I, I-QL, D, D-QL) were provided unrestricted access to shortening (24-hr/day, 7 days/week). The NS group again had no shortening access.
Part 2B: Quantity-unlimited and time-limited shortening access
During the second five-week period, access to shortening was limited by time, but not by amount. That is, all groups (I, I-QL, D, D-QL) were given a full bowl of shortening (quantity-unlimited) during the 1-hr period of shortening availability (time-limited) under their respective schedules of access. The NS group that had previously been maintained on continuously available chow for the first 15 weeks of the study was also provided time-limited access to shortening (quantity-unlimited, 1 hr) on the intermittent schedule. This manipulation allowed for comparison of the groups that had a history of shortening exposure (I, I-QL, D, D-QL) to the NS group that had no history of shortening exposure.
Results – Part 2
Part 2A
Shortening
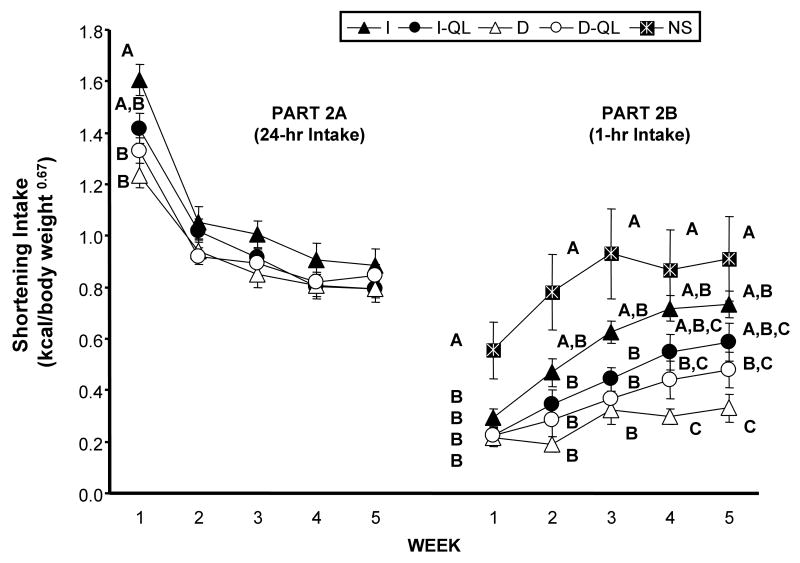
In general, shortening intake for all groups during the five weeks of unrestricted (“24/7”) access was greatest during the first week and then decreased and stabilized over the subsequent four weeks (Fig. 2, left panel). There were main effects of access schedule [F(1,36) = 5.01, p 0.0314] and week [F(4,144) = 329.34, p 0.0001]. There also were interactions between week and access schedule [F(4,144) = 9.81, p 0.0001], as well as among week, access schedule, and amount [F(4,144) = 3.60, p 0.0079]. These results are accounted for by the reductions in intake after week 1, as well as the significant differences among the groups during the first week, but lack thereof in subsequent weeks.
Figure 2.
Average shortening intake (kcal/body weight 0.067) during the 24-hr period (2A) or 1-hr period (2B) that it was available for each week of Part 2. Part 2A: Shortening access was unrestricted (available 24-hr/day - 7-days/week) for the I, I-QL, D, and D-QL groups. Part 2B: Shortening access was time-limited and quantity-unlimited for all groups; the NS (no shortening) group was maintained on the intermittent schedule. Group designations and indications of significance are the same as Figure 1.
During the first week of unrestricted shortening access the I group consumed significantly more shortening than did the D and D-QL groups (LS Means p 0.0167), while the intake of the I-QL group did not differ from any of the other groups (Fig. 2, left panel). During the next 4 weeks there were no significant differences in the weekly average amount of shortening consumed among any of the groups (Fig. 2, left panel).
The absolute average 24-hr shortening intakes during week 5 of Part 2A for all of the groups are shown in Table 3. During Part 2A the average weekly total fat (shortening plus chow fat) intake for the I, I-QL, D, and D-QL groups expressed as a percentage of total energy intake ranged from 64.9% to 78.8%. The highest fat intake as a percentage of total energy intake was the greatest in the first week of Part 2A (73.0-78.8%), and lowest in week five (64.9 - 67.1%). Thus, the diets in Part 2A were “high-fat” relative to what the rats previously had consumed.
Table 3.
| PART | GROUP | Body Weight Week 5 (gm) (SE +/-) |
Body Weight Change (gm) (SE +/-) |
Total 5-Week Energy (kcal) (SE +/-) |
Shortening Week 5 (kcal) (SE +/-) |
Percent 5-Week Protein by Weight (SE +/-) |
Percent 5-Week Carbohydrate by weight (SE +/-) |
Percent 5-Week Fat by Weight (SE +/-) |
|---|---|---|---|---|---|---|---|---|
| 2A | 24-hr | |||||||
| I | 518.2 A,B (11.1) |
55.0 A (6.5) |
3525.7 A (102.5) |
58.2 (4.0) |
12.8% B (0.58%) |
26.9% B (1.21%) |
47.7% A (2.35%) |
|
| I-QL | 528.9 A (6.6) |
55.4 A (4.3) |
3370.8 A,B (71.2) |
53.1 (2.4) |
13.6% B (0.36%) |
28.5% B (0.76%) |
44.5% A (1.48%) |
|
| D | 500.2 A,B (10.8) |
42.0 A (4.6) |
3193.0 B,C (83.4) |
51.1 (3.4) |
14.3% B (0.51%) |
29.9% B (1.06%) |
41.8% A (2.07%) |
|
| D-QL | 519.2 A,B (12.5) |
45.2 A (2.9) |
3223.2 B (43.9) |
55.7 (2.7) |
13.4% B (0.56%) |
28.0% B (1.16%) |
45.4% A (2.27%) |
|
| NS | 482.6 B (8.5) |
23.7 B (1.1) |
2905.7 C (47.7) |
23.4% A | 49.0% A | 4.5% B | ||
| 2B | 1-hr | |||||||
| I | 514.9 (11.5) |
-3.3 C (4.8) |
2435.9 C (94.6) |
48.1 B (3.6) |
21.1% A,B (0.17%) |
44.2% A,B (0.37%) |
13.9% A,B (0.71%) |
|
| I-QL | 530.5 (9.0) |
1.6 B,C (5.8) |
2609.0 B,C (48.5) |
39.2 B,C (4.9) |
21.8% A (0.21%) |
45.7% A (0.43%) |
11.0% B (0.84%) |
|
| D | 509.8 (11.9) |
9.6 B,C (3.5) |
2622.6 B,C (52.0) |
20.7 C (2.9) |
21.0% A,B (0.37%) |
44.1% A,B (0.77%) |
14.1% A,B (1.50%) |
|
| D-QL | 536.0 (14.8) |
17.1 A,B (3.5) |
2734.2 B (83.4) |
31.0 B,C (4.7) |
19.8% B (0.67%) |
41.5% B (1.40%) |
19.0% A (2.72%) |
|
| NS | 514.4 (9.8) |
31.8 A (3.1) |
3071.7 A (61.3) |
70.4 A (8.0) |
20.2% A,B (0.43%) |
42.3% A,B (0.89%) |
17.5% A,B (1.73%) |
Total energy (shortening plus chow)
Differences in total energy intake (shortening plus chow) during the first week of the “24/7” condition reflected the shortening intake (data not shown). Total energy intake was influenced by the access history [main effect of schedule F(4,45) = 9.99, p 0. 0001], as well as week [F(4, 180) = 335.62, p 0.0001]. In addition, access and week interacted [F(16, 180) = 26.93, p 0.0001]. These effects were due to the reductions in intake across time, as well as significant differences among the five groups (I, I-QL, D, D-QL and NS) during the first week of Part 2A. Specifically, during week 1 of Part 2A, the I group consumed significantly more, and the NS group significantly less daily energy than was consumed by any other group (Tukey's HSD, p 0.05). During week 2 of Part 2A, the I group continued to consume more total energy than did D-QL and NS, with NS consuming less than I, I-QL and D (Tukey's HSD, p 0.05). During weeks 3 through 5 there were no differences in total energy intake among the groups.
The macronutrient composition of the diets did not vary among the groups that had access to shortening, but did differ from the chow-maintained (NS) group. The macronutrient composition of the diets is shown in Table 3. The percent dietary protein ranged from ∼13-14% (by weight) in the groups with access to shortening, a level well above that required for weight maintenance in adult male rats (at least 50 g pro/kg diet, i.e. 5% protein by weight) and close to that required for growth (at least 150 g pro/kg diet, i.e. 15% protein by weight) (Table 3) [19].
Body weight
While all of the groups with access to shortening gained significantly more weight than did the NS group during Part 2A (Tukey's HSD, p 0.05) (Table 3), the absolute body weights did not differ among the I, I-QL, D and D-QL groups nor among the I, D, D-QL and NS groups during any week of the five-week period. The body weight of I-QL was significantly greater than that of NS during weeks 2 through 5 (Tukey's HSD, p 0.05) for each week. Body weights during week five are shown in Table 3.
Part 2B
Shortening
Following the five weeks of unrestricted access to shortening, access to shortening was again time-limited and quantity-unlimited (1 hr, full bowl) for all groups under their respective schedules of access for an additional five weeks (Fig 2, right panel). During this time, the NS group also had time-limited, quantity-unlimited (1 hr, full bowl) shortening availability under an intermittent schedule of access (Monday, Wednesday, Friday). During each week of this entire five-week period, the average 1-hr shortening intake of the NS group was greater than that of the other groups; NS intake was statistically greater than that of all four other groups in week 1 of Part 2B.
Since the NS group now had access to shortening, the shortening intake data were analyzed using a 2-way ANOVA (group X time). There were main effects of group [F(4, 45) = 7. 61, p<0. 0001] and week [F(4,180) p < 0. 0001], as well as an interaction between group and week [F(16, 180) = 2.95, p 0.0002]. These main effects and interactions are in part the result of initial low shortening consumption during the first week by the I, I-QL, D and D-QL groups, followed by increased shortening intakes over the five-week period (Fig. 2, right panel).
The average 1-hr absolute shortening intakes during week 5 of Part 2B for all of the groups are shown in Table 3. The percentage of energy consumed as fat ranged from 21.4% to 44.2%. Total fat-derived energy intake was lowest during week 1 (21.4-32%) and highest during week five (31-44.2 %) for all groups including the NS group.
Total Energy (shortening plus chow)
Total energy intake (chow and shortening) reflected the shortening intake. That is, the NS group consumed more energy than did any of the other groups throughout the 5 weeks of Part 2B (Table 3). Intakes of NS were significantly greater than that of all other groups during weeks 1-3, significantly greater than that of I, I-QL and D during week 4, and significantly greater than that of I and I-QL during week 5 (Tukey's HSD, p < 0.05). The I group consumed the least during Part 2B, with total energy intakes being significantly less than those of NS and D during weeks 1-3, and significantly less than D-QL during week 2 (Tukey's HSD, p < 0.05). Overall, total energy intake was significantly influenced by the access history [main effect of group F(4, 45) = 26.20, p < 0.0001] and week [F(4, 180) = 84.85, p < 0.0001]. There also was a group X week interaction [F(16, 180) = 13.73, p<0.0001].
The macronutrient composition of the diets is shown in Table 3. Although different among the groups, dietary protein was sufficient for growth and well above that required for weight maintenance in adult male rats [19].
Body weight
Body weights did not differ among the groups at any time during Part 2B. Body weight change, however, was significantly affected by access schedule [F(4, 45) = 10.59, p < 0.0001], due to a loss of 3.3 g in the I group, and weight gains in the other groups (Table 3). The NS group gained the most weight (31.8 g), a change that was significantly greater than that of the I, I-QL and D groups (-3.3, +1.6, +9.6 respectively) (Tukey's HSD, p < 0.05). The D-QL group gained 17.1 g, a change that was only significantly different from that of the I group (Tukey's HSD, p < 0.05).
Discussion-Part 2
Two new findings are reported in this part of the study. First, the I group consumed more shortening during the first week of unrestricted access in Part 2A than did any of the other groups. Second, unrestricted access (“24/7”) to shortening (a high-fat diet) for five weeks significantly attenuated subsequent shortening intake in all groups relative to control rats with no history of shortening availability.
The first new finding is that the I group consumed more fat under unrestricted access conditions during the first week of Part 2A than did the other groups. Increased consumption under conditions of extended access is considered one measure of “loss of control” in rat models of addiction-like behavior for drug [34]. The present results suggest a history of intermittent bingeing can promote a similar type of phenomenon for fat. Whether the elevated intakes of the I rats were due to their behavioral history (e.g. ‘gorging’) or to the protocol history (e.g. not having one's intake restricted during the period of availability coupled with intermittent access) cannot be determined from the present findings. Body weights did not differ among the groups at the start of Part 2A, suggesting that effects of differential fat cell-mediated satiety signals such as leptin probably do not account for the food intake differences during the first week. Although differences in body fat were not assessed, previous work showed no difference in body fat in male rats maintained on similar protocols and chow-fed controls [4]. The differential intakes in Part 2A were temporary; by the second week of unrestricted access intakes for all groups decreased and significant differences among the groups were no longer seen. Regardless, it is clear that the history of the I group had potent effects for the first week when extended access was provided.
The second new finding reported here is that a significant reduction in shortening intake occurred in Part 2B in the groups that previously had five weeks of unrestricted shortening access (time- and quantity-unlimited) in Part 2A where fat constituted 65-79% of the daily energy intake in all of the groups. The reductions in intake were large enough that weight gain was reduced in three of the groups, and the I group actually lost weight. The results obtained in Part 2B stand in contrast to the results of other studies that report an increase in fat consumption (reduced satiety signaling) after exposure to a high-fat diet (65-70% of the daily energy intake). Several other investigators have reported that high-fat diets result in a greater acceptance of and preference for fatty foods and for flavors associated with intestinal fat infusions, as well as an inhibition of satiety signaling. For instance, it was found that rats maintained on high-fat diets consumed more fats, fat-like substances, and high-fat food (corn oil, safflower oil, olive oil, lard, Vaseline, coconut oil, and chocolate) than did rats maintained on a high carbohydrate diet [30]. Another study [31] reported increased consumption of a high-fat ‘snack’ in rats maintained on a high-fat diet, relative to rats maintained on an isocaloric low-fat diet. Others [32] have reported an increased acceptance of and preference for a high-fat liquid food in rats maintained on a high-fat diet, relative to rats maintained on a low-fat diet. In addition, an enhanced preference for a flavor paired with intestinal fat infusions in rats maintained on a high-fat diet, relative to rats maintained on a low-fat diet has also been reported [35]. Various mechanisms have been proposed to account for these effects, including an enhanced capacity to digest and oxidize fat [30], reduced satiety signaling [21-29], and increased orexigenic signaling [36]. Taken together, these studies all indicate that consumption of a high-fat maintenance diet can have important effects on physiological and neurological mechanisms involved in fat intake, and can promote the consumption of fatty foods.
Given all of the above reports, the present findings were particularly surprising. One difference between this and previous studies is the manner in which the fat was presented. In the present study, the fat was presented as an option to the low-fat maintenance diet (chow), whereas in other studies the fat was mixed into the diet or combined with other non-nutritive substances to obtain iso-caloric diets. It recently has been shown that rats provided time-limited (2-hr) access to shortening every third day (Intermittent) consumed significantly more shortening during the 2-hr access period than did rats with time-limited daily access. However, when shortening was mixed in with chow, 2-hr intakes did not differ significantly between the Intermittent and Daily groups [9]. Apparently the manner in which shortening is presented (separate or mixed with other food substances) has significant effects on intake. Furthermore, the optional “test” fat and the optional unrestricted available fat were the same in the present study. In previous work, on the other hand, the “test” food was different from the continuously available high-fat diet. In addition, in the present study, rats were shifted from a condition in which the optional fat was available all of the time, to a condition in which the optional fat was available only during brief occasional periods. In previous reports, the maintenance diet was still in place when the response to the test foods was assessed. The conditions in the present study, therefore, were different from those previously reported. Such parameters can be important to mechanistic interpretations, an idea supported by previous reports in which the effects of galanin and enterostatin were assessed [39-40].
Why these conditions resulted in reductions in fat intake rather than a stimulation of fat intake remains to be determined. The deprivation condition of the rats cannot explain the different results as non-food-deprived rats were used in the present study as well as in some of the previous reports [30,31]. It is possible that the length of time the rats were maintained on the maintenance conditions may have contributed to the different results. In the present study, rats were maintained in the unrestricted access condition for 35 days whereas in previous studies the length of time on the maintenance high-fat diets ranged from 2 to 26 days. Whether different adaptive changes with extended exposure to high-fat diets can account for the different behavioral results is not known. The amount of fat consumed is probably not the reason. The percentage of total energy intake consumed as fat under the unrestricted access condition in the present study (∼65-79% of energy) is comparable to or greater than that reported in previous studies, e.g., ∼63% [30], ∼70% [31], ∼48% [32, 35]. Rats often will consume less energy when the energy density of the diet is reduced after a period of high-fat diet-induced hyperphagia and body weight gain [41]. Since weight gain was greater among the groups with shortening access relative to the NS group in Part 2A, physiological alterations related to body fat accretion may have contributed to the intake reductions in Part 2B.
In addition, there is evidence that contrast between diets of high and low energy density can contribute to differential intakes [42]. Successive negative contrast refers to reductions in consumatory behavior (e.g. lick rate, intake) that occur when rats are shifted from a highly rewarding nutrient stimulus to one of less reward value, for instance from higher to lower sucrose concentrations or from cafeteria-feeding to chow [42-45]. The behavioral reductions can persist for up to a week, and, when body mass is elevated, some behavioral changes persist for several weeks [42]. Contrast effects on intake of different foods, therefore, have been demonstrated. In addition, others have shown that negative contrast between schedules of reinforcer availability can influence operant responding [46]. One possibility, then, is that contrast between the different conditions of shortening availability in Parts 2A and 2B may have contributed to the reduced intakes in Part 2B.
Part 3
The third part of the present study addressed the question of whether a period of no access to shortening (Part 3A) would affect subsequent shortening intake (Part 3B). The conditions used in Part 3B were the same as those of Parts 1B and 2B, i.e. time-limited access to a quantity-unlimited amount of shortening was provided to all groups including the NS. Based upon other reports in which periods of item-specific deprivation enhanced subsequent intake of alcohol [47], saccharin [48] and nicotine [49], as well as the idea that “perceived deprivation” may stimulate food intake [50], it was inferred that a period of no shortening access would enhance subsequent intake during the 1-hr period of availability.
Method
Part 3A: No Shortening Access
For the first five weeks of this part of the experiment, shortening was not available to any of the rats, and chow and water were continuously available.
Part 3B: Quantity-unlimited and time-limited shortening access
During the next five-weeks, time-limited access to shortening was provided (1 hr, full bowl) to all groups including the NS group.
Results – Part 3
Part 3A
When only chow was available during the first five weeks, the average body mass-adjusted weekly energy intake did not differ among any of the groups during any week except week 4. In week 4, the intake of the NS group was significantly greater than that of the I group (p<0.05; Tukey's HSD). Overall, there was no main effect of access schedule. However, there was an main effect of week [F(4,180)= 75.79, p 0.0001], due to a gradual increase in chow consumption across the 4 weeks. Schedule and week also interacted [F(16,180) = 1.70, p 0.0497], due to slight differences in the rate of increase across time in the different groups. Average absolute daily intake from chow increased by about 10 kcal across the five weeks of Part 3A, ranging from 69-74 kcal in week 1, to 79-84 kcal in week 5. By week 5, energy intake was comparable to that of week 5 of Parts 1B and 2B.
Neither body weight nor body weight change differed among the groups (Table 4). All groups gained weight in each week of Part 3A except NS; NS lost 1.7 g in week 2, but by week 3, weight gain resumed, and body weight was greater than it had been in week 1.
Table 4.
| PART | GROUP | Body Weight Week 5 (gm) (SE +/-) |
Body Weight Change (gm) (SE +/-) |
Total 5-Week Energy (kcal) (SE +/-) |
1-hr Shortening Week 5 (kcal) (SE +/-) |
Percent 5-Week Protein by Weight (SE +/-) |
Percent 5-Week Carbohydrate by weight (SE +/-) |
Percent 5-Week Fat by Weight (SE +/-) |
|---|---|---|---|---|---|---|---|---|
| 3A | ||||||||
| I | 524.3 (11.2) |
9.4 (3.1) |
2656.3 (70.6) |
23.4% | 49.% | 4.5% | ||
| I-QL | 544.2 (9.5) |
13.7 (3.7) |
2760.8 (50.8) |
23.4% | 49.0% | 4.5% | ||
| D | 521.3 (11.9) |
11.5 (3.0) |
2700.8 (75.2) |
23.4% | 49.0% | 4.5% | ||
| D-QL | 545.5 (15.0) |
9.5 (2.4) |
2715.8 (53.3) |
23.4% | 49.0% | 4.5% | ||
| NS | 521.3 (10.4) |
6.9 (2.6) |
2745.7 (55.2) |
23.4% | 49.0% | 4.5% | ||
| 3B | ||||||||
| I | 549.7 (11.4) |
25.4 B (2.3) |
3038.8 (68.9) |
69.6 A (3.4) |
19.7% A (0.17%) |
41.3% A (0.36%) |
19.6% B (0.70%) |
|
| I-QL | 573.1 (10.0) |
28.9 A,B (2.7) |
3064.8 (50.8) |
65.8 A,B (7.6) |
20.5% A (0.28%) |
42.9% A (0.59%) |
16.5% B (1.15%) |
|
| D | 556.3 (10.4) |
35.1 A,B (4.7) |
3080.5 (78.2) |
31.8 B (2.8) |
19.1% A,B (0.50%) |
40.0% A,B (1.05%) |
22.0% A,B (2.05%) |
|
| D-QL | 586.8 (18.4) |
41.3 A (5.7) |
3255 (94.7) |
41.9 B,C (5.1) |
17.7% B (0.83%) |
37.1% B (1.73%) |
27.7% A (3.38%) |
|
| NS | 545.2 (12.9) |
23.9 B (3.3) |
3098.0 (61.4) |
67.7 A (7.8) |
19.7% A,B (0.40%) |
41.2% A,B (0.83%) |
19.7% A,B (1.63%) |
Part 3B
Shortening
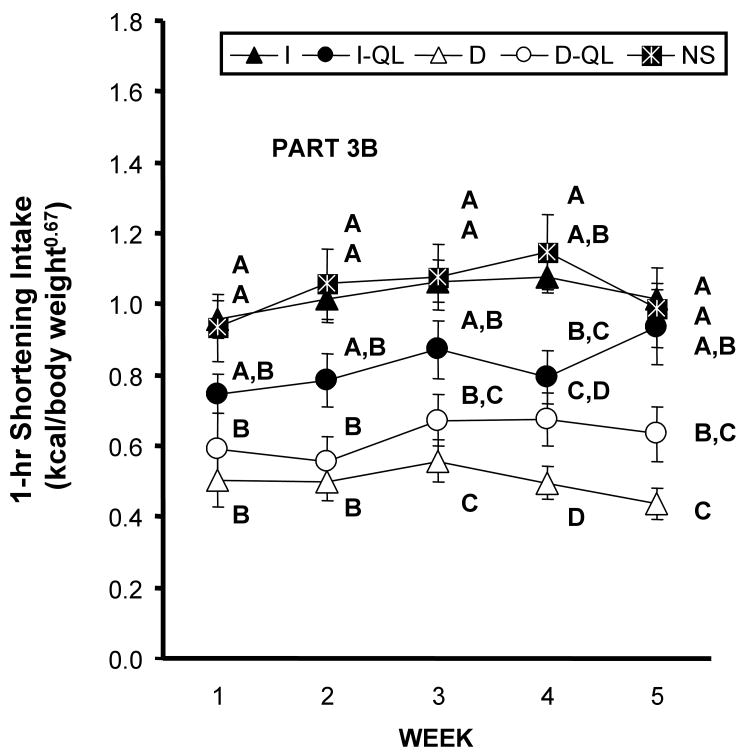
After five weeks of no shortening access, shortening was again provided under time-limited and quantity-unlimited conditions. That is, all rats were provided a unlimited quantity of shortening for 1 hr on their respective access schedules. The NS group had Intermittent access to the shortening. In general, differences in shortening consumption among the groups readily emerged, even during the first week of re-exposure (Fig. 3). There were main effects of group [F(4,45) = 12.42, p 0.0001], week [F(4,180)= 5.74, p 0.0002], and a group by week interaction [F(16, 180) = 1.72 p 0.0456]. The shortening intakes of the NS and I groups were significantly greater than those of the D and D-QL groups during all five weeks (LS Means p 0.0167). The shortening intake of the I-QL group, in contrast, fell between that of the I and NS groups and that of the D and D-QL groups (Fig 3). The D and D-QL intakes did not differ from each other throughout Part 3B. While the I-QL and D-QL shortening intakes during weeks 3 through 5 were not statistically different from one another, the I-QL consumed significantly more shortening than the D group did during these weeks.
Figure 3.
Average shortening intake (kcal/body weight 0.067) during the 1-hr period that it was available for each week of Part 3B. Access was time-limited (1 hr) and quantity-unlimited for all groups. Group designations and indications of significance are the same as Figures 1 and 2.
The average absolute 1-hr shortening intakes during week 5 of Part 3B for all of the groups are shown in Table 4.
Total energy intake, body weight
Total energy intake occasionally differed among the groups (data not shown). Specifically, intakes of the D-QL and NS groups were greater than that of the I-QL group during weeks 1 and 4 of Part 3B, respectively (Tukey's HSD, p 0.05). Intakes did not differ among the groups at any other time. Cumulative intakes, likewise, did not differ (Table 4). Body weights were not statistically different among the groups at any time during Part 3B, though there was a difference in body weight change between the D-QL and NS group (Table 4).
The macronutrient composition of the diets is shown in Table 4. Although different among the groups, dietary protein was sufficient for growth and well above that required for weight maintenance in adult male rats [19].
Comparisons across Parts 1B, 2A, 2B, and 3B
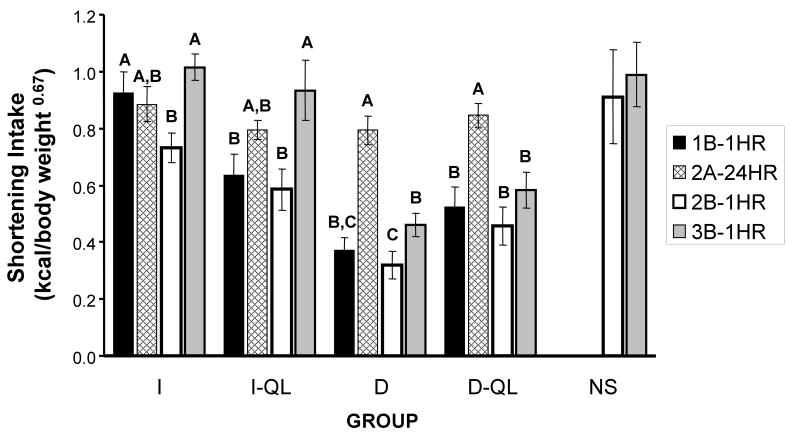
In order to determine if shortening intakes differed within each group in the parts of the study where access to shortening was not limited by quantity, comparisons were made among the average 1-hr shortening intakes for the last week of Parts 1B, 2B, and 3B, and average 24-hr intakes for the last week of Part 2A (Fig. 4). Again, all data were expressed in a body mass-adjusted manner (kcal/body weight0.67) in order to control for changes in body mass across the study.
Figure 4.
Comparison of shortening intake (kcal/body weight 0.067) during the last week of Parts 1B, 2A, 2B and 3B for the I, I-QL, D, and D-QL groups. For the NS group comparison between Part 2B and 3B are shown. Group designations same as previous figures. Different letters indicate significant differences in average shortening intake among the different parts of the study within each group.
Shortening intakes were not statistically different between Parts 1B and 2B in the I-QL, D, and D-QL groups. However, in the I group, intake in Part 2B was less than that of Part 1B (Tukey's HSD, p 0.05). Intakes of I, I-QL, and D were greater in Part 3B than in Part 2B (Tukey's HSD, p 0.05). Intakes in the D-QL and NS groups did not differ between Parts 2B and 3B.
Both the I and I-QL groups consumed as much shortening during 1 hr in Parts 1B, 2B, and 3B (time-limited, quantity-unlimited) as they did in 24-hr in Part 2A (time-unlimited, quantity-unlimited). In contrast, both D and D-QL consumed significantly less shortening in 1 hr Parts 1B, 2B, and 3B than they did in 24 hr in Part 2A.
Discussion-Part 3
In Part 3B, shortening intakes were enhanced after a 5-week period of shortening absence in groups I, I-QL, and D relative to Part 2B. These results were not due to time or growth of the rats as shortening intake was normalized to body weight.
These results bear resemblance to other studies in which removal of some consumable (e.g. alcohol, sucrose, saccharin, nicotine) stimulated subsequent intake of or responding for that item [16, 47, 48, 49, 51, 52, 53]. In the present study, intake of shortening increased in Part 3B relative to Part 2B in three of the groups (I, I-QL, D), suggesting that fat consumption also is sensitive to the intake-stimulating effects of no access. Interestingly, shortening intake in the D-QL group during Part 3B was not significantly affected by shortening removal. The largest change occurred in the I-QL group, with shortening intakes in Part 3B being 58% higher than those of Part 2B. I-QL was also the only group in which the 1-hr shortening intake in Part 3B exceeded that of Part 1B. Why the two groups with the quantity-limited histories differed in their response to shortening removal cannot be determined from these data. However, it is clear that the binge-reducing effect of the quantity-limited history in the I-QL group was attenuated by a period of involuntary shortening removal. Even so, I-QL shortening consumption during the 1-hr period of availability remained intermediate to that of I and D for 4 out of the 5 weeks (Fig 3).
In contrast to the other groups, intake during Part 2B was not suppressed in the NS group. In this case, the removal of shortening did not stimulate intake further in Part 3B. This lack of stimulation after a period of shortening non-availability in the NS group may have been due to an inability to consume any more within the 1 hr period, i.e. a ceiling effect on intake.
The amount of shortening consumed by the I and I-QL groups during the 1-hr period of availability was large. Specifically, the I and I-QL groups consumed as much shortening in 1 hr during Parts 1B, 2B, and 3B, as they did in 24-hr when shortening access was unrestricted. In contrast, the two groups on the daily schedule of access, D and D-QL, consumed significantly less shortening during the 1 hr period of availability in Parts 1B, 2B and 3B than they did during a 24-hr period of unrestricted access in Part 2A (Tukey's HSD, p 0.05). Thus, in addition to consuming more shortening than was consumed by the Daily groups, the large amount of fat consumed by the Intermittent groups supports the interpretation of their intake as binge-like, i.e. eating in a discrete period of time an amount of food that was larger than would normally be consumed in a similar period of time under similar circumstances [10].
Finally, the shortening intakes of the I-QL group again fell between those of the I and D groups, even though the I-QL and I group had access to shortening under exactly the same conditions during all parts of this study except Part 1A. This indicates the potentially powerful attenuating effect that quantity-limited intake of fatty foods can have on subsequent consumption of those foods. Stated otherwise, quantity-limited access, while not eliminating binge-type behavior, was able to reduce binge size across a variety of conditions and over a period of several months.
General Discussion
Several new findings are reported: 1) Limiting the quantity of shortening consumed during a 1-hr period of availability for five weeks produced robust and long-lasting decreases in subsequent binge size when quantity was no longer restricted under Intermittent availability conditions. 2) Five weeks of unrestricted access to shortening also produced robust and long-lasting decreases in subsequent binge size. 3) Five weeks of no access to shortening, on the other hand, produced rapid and long-lasting increases in subsequent binge size. This study demonstrates that intermittent access is necessary and sufficient to promote bingeing on fat in rats, but the size of the binge can be altered by the fat-access history.
The results obtained here have potentially important implications for the translation of findings obtained with rodent models to human ingestive behavior. The consumption of high-fat optional “snack” foods has increased among Americans over the past twenty years [54] and foods such as these are contributing a higher proportion of total dietary fat and energy than in years past [54,55]. Furthermore, those who consume the most snacks also consume the most energy [56,57]. Therefore, findings obtained from behavioral models based upon the consumption of optional sources of dietary fat (“snacks”), such as the one used here, may provide important clues to human food intake patterns that can complement approaches in which the “extra” fat is mixed into the maintenance diet. For instance, a strategy in which small amounts of fatty optional foods are incorporated into the diet might be more effective at preventing subsequent binge eating, than would strategies that involve either complete abstinence from, or periodic ‘gorging’ on, these same foods.
Acknowledgments
Support for this study provided by 1 RO1 MH67943-01 (RLC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pinel JP, Huang E. Effects of periodic withdrawal on ethanol and saccharin selection in rats. Physiol Behav. 1976a;16:693–698. doi: 10.1016/0031-9384(76)90238-9. [DOI] [PubMed] [Google Scholar]
- 2.Pinel JP, Mucha RF, Rovner LI. Temporary effects of periodic alcohol availability. Behav Biol. 1976b;16:227–232. doi: 10.1016/s0091-6773(76)91352-3. [DOI] [PubMed] [Google Scholar]
- 3.Files FJ, Lewis RS, Samson HH. Effects of continuous versus limited access to ethanol on ethanol self-administration. Alcohol. 1994;11(6):523–531. doi: 10.1016/0741-8329(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 4.Corwin RL, Wojnicki FHE, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 5.Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42:139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28:436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MA, Rice HB, Weinstock D, Corwin RL. Effects of aging on food intake and body composition in rats. Physiol Behav. 2002;76:487–500. doi: 10.1016/s0031-9384(02)00800-4. [DOI] [PubMed] [Google Scholar]
- 8.Wojnicki FHW, Roberts DCS, Corwin R. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food-deprived rats. Pharmacol Biochem Behav. 2006;84:197–206. doi: 10.1016/j.pbb.2006.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis JF, Melhorn SJ, Shurdan JD, Heiman JU, Tschop MH, Clegg DJ, Benoit SC. Comparison of hydrogenated vegetable shortening and nutritionally complete high-fat diet on limited access-binge behavior in rats. Physiol Behav. 2007;92(5):924–930. doi: 10.1016/j.physbeh.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DSM-IV™. Diagnostic and Statistical Manual of Mental Disorders. Fourth. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 11.Wojnicki FHW, Stine JG, Corwin RL. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Physiol Behav. 2007;92:566–74. doi: 10.1016/j.physbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Broft AI, Spanos A, Corwin RL, Mayer L, Steinglass J, Devlin MJ, Walsh BT. Baclofen for binge eating: an open-label trial. Int J Eat Disord. 2007;40(8):687–91. doi: 10.1002/eat.20434. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 15.Lucas F, Ackroff K, Sclafani A. Dietary fat-induced hyperphagia in rats as a function of fat type and physical form. Physiol Behav. 1989;45:937–946. doi: 10.1016/0031-9384(89)90218-7. [DOI] [PubMed] [Google Scholar]
- 16.Avena NM, Long KA, Hoebel BG. Sugar dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav. 2005;84:359–62. doi: 10.1016/j.physbeh.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Kales EF. Macronutrient analysis of binge eating in bulimia. Physiol Behav. 1990;48(6):837–40. doi: 10.1016/0031-9384(90)90236-w. [DOI] [PubMed] [Google Scholar]
- 18.Heusner AA. Body size and energy metabolism. Ann Rev Nutr. 1985;5:267–93. doi: 10.1146/annurev.nu.05.070185.001411. [DOI] [PubMed] [Google Scholar]
- 19.National Research Council. Nutrient Requirements of Laboratory Animals. 4th. National Academy Press; Washington, DC: 1995. [Google Scholar]
- 20.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate. Brain. 2001;124:1720–33. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 21.Castiglione KE, Read NW, French SJ. Adaptation to a high-fat diet accelerates emptying of fat but not carbohydrate test meals in humans. Am J Physiol Regulatory Integrative Comp Physiol. 2002;282:R366–R371. doi: 10.1152/ajpregu.00190.2001. [DOI] [PubMed] [Google Scholar]
- 22.Clegg DJ, Benoit SC, Air EL, Jackman A, Tso P, D'Alessio D, Woods SC, Seeley RJ. Increased dietary fat attenuates the anorexic effects of intracerebroventricular injections of MTII. Endocrinology. 2003;144:2941–2946. doi: 10.1210/en.2002-0218. [DOI] [PubMed] [Google Scholar]
- 23.Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides. 1998;19:1407–1415. doi: 10.1016/s0196-9781(98)00096-5. [DOI] [PubMed] [Google Scholar]
- 24.Covasa M, Ritter RC. Reduced sensitivity to the satiation effect of intestinal oleate in rats adapted to high-fat diet. Am J Physiol Regulatory Integrative Comp Physiol. 1999;277:R279–R285. doi: 10.1152/ajpregu.1999.277.1.R279. [DOI] [PubMed] [Google Scholar]
- 25.Covasa M, Ritter RC. Attenuated satiation response to intestinal nutrients in rats that do not express CCK-A receptors. Peptides. 2001;22:1339–48. doi: 10.1016/s0196-9781(01)00461-2. [DOI] [PubMed] [Google Scholar]
- 26.Covasa M, Grahn J, Ritter RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept. 2000a;86:83–88. doi: 10.1016/s0167-0115(99)00084-1. [DOI] [PubMed] [Google Scholar]
- 27.Covasa M, Grahn J, Ritter RC. Reduced hindbrain and enteric neuronal response to intestinal oleate in rats maintained on high-fat diet. Auton Neurosci. 2000b;84:8–18. doi: 10.1016/S1566-0702(00)00176-4. [DOI] [PubMed] [Google Scholar]
- 28.French SJ, Murray B, Rumsey RDE, Fadzlin R, Read NW. Adaptation to high-fat diets: effects on eating behaviour and plasma cholecystokinin. Br J Nutr. 1995;73:179–189. doi: 10.1079/bjn19950022. [DOI] [PubMed] [Google Scholar]
- 29.Samama P, Rumennik L, Grippo JF. The melanocortin receptor MCR4 controls fat consumption. Regul Pept. 2003;113:85–88. doi: 10.1016/s0167-0115(02)00299-9. [DOI] [PubMed] [Google Scholar]
- 30.Reed DR, Friedman MI. Diet composition alters the acceptance of fat by rats. Appetite. 1990;14:219–30. doi: 10.1016/0195-6663(90)90089-q. [DOI] [PubMed] [Google Scholar]
- 31.Savastano DM, Covasa M. Adaptation to a high-fat diet leads to hyperphagia and diminished sensitivity to cholecystokinin in rats. J Nutr. 2005;135:1953–1959. doi: 10.1093/jn/135.8.1953. [DOI] [PubMed] [Google Scholar]
- 32.Warwick ZS, Synowski SJ. Effect of food deprivation and maintenance diet composition on fat preference and acceptance in rats. Physiol Behav. 1999;68:235–239. doi: 10.1016/s0031-9384(99)00192-4. [DOI] [PubMed] [Google Scholar]
- 33.Fisher JO, Birch LL. Restricting access to palatable foods affects children's behavioral response, food selection, and intake. Am J Clin Nutr. 1999;69:1264–1272. doi: 10.1093/ajcn/69.6.1264. [DOI] [PubMed] [Google Scholar]
- 34.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–17. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 35.Lucas F, Sclafani A. The composition of the maintenance diet alters flavor-preference conditioning by intragastric fat infusions in rats. Physiol Behav. 1996;60:1151–1157. doi: 10.1016/0031-9384(96)00136-9. [DOI] [PubMed] [Google Scholar]
- 36.Leibowitz SF, Akabayashi A, Wang J. Obesity on a high-fat diet: role of hypothalamic galanin in neurons of the anterior paraventricular nucleus projecting to the median eminence. J Neurosci. 1998;18(7):2709–19. doi: 10.1523/JNEUROSCI.18-07-02709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corwin RL, Rowe PM, Crawley JN. Galanin and the galanin antagonist M40 do not change fat intake in a fat-chow choice paradigm in rats. Am J Physiol Regulatory Integrative Comp Physiol. 1995;269(38):R511–R518. doi: 10.1152/ajpregu.1995.269.3.R511. [DOI] [PubMed] [Google Scholar]
- 38.Corwin RL, Rice HB. Effects of enterostatin on optional oil or sucrose consumption in non-food deprived rats. Physiol Behav. 1998;65:1–10. doi: 10.1016/s0031-9384(98)00078-x. [DOI] [PubMed] [Google Scholar]
- 39.Rice HB, Corwin RL. Intracerebroventricular enterostatin stimulates food intake in non-food deprived rats. Peptides. 1996;17:885–888. doi: 10.1016/s0196-9781(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 40.Rice HB, Corwin RL. Effects of enterostatin on consumption of optional foods in non-food deprived rats. Obes Res. 1998;6(1):54–61. doi: 10.1002/j.1550-8528.1998.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 41.Sclafani AS. Dietary Obesity. In: Stunkard AJ, editor. Obesity. W.B. Saunders, Co.; Philadelphia: 1980. [Google Scholar]
- 42.Rogers RJ. Returning ‘cafeteria-fed’ rats to a chow diet: Negative contrast and effects of obesity on feeding behaviour. Physiol Behav. 1985;35:493–499. doi: 10.1016/0031-9384(85)90129-5. [DOI] [PubMed] [Google Scholar]
- 43.Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-Dependent Anticipatory Negative Contrast and Binge-Like Eating in Rats with Limited Access to Highly Preferred Food. Neuropsychopharmacology. 2008;33(3):524–53. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- 44.Flaherty C, Geary N. Contrast in consummatory behavior. Appetite. 1993;21:81. doi: 10.1006/appe.1993.1038. [DOI] [PubMed] [Google Scholar]
- 45.Flaherty DF, Grigson PS, Demetrikopoulos MK, Weaver MS, Krauss KL, Rowan GA. Effect of serotonergic drugs on negative contrast in consummatory behavior. Pharmacol Biochem Behav. 1990;36(4):799–806. doi: 10.1016/0091-3057(90)90080-2. [DOI] [PubMed] [Google Scholar]
- 46.Marcucella H, Macdonall JS. A molecular analysis of multiple schedule interactions: negative contrast. J Exp Anal Behav. 1977;28:71–82. doi: 10.1901/jeab.1977.28-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ. Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav. 2004;79(3):439–450. doi: 10.1016/j.pbb.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Neznanova ON, Zvartau EE, Bespalov AY. Behavioral analysis of the saccharin deprivation effect in rats. Behav Neurosci. 2002;116(5):747–56. doi: 10.1037/0735-7044.116.5.747. [DOI] [PubMed] [Google Scholar]
- 49.O'Dell LE, Koob GF. ‘Nicotine deprivation effect’ in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007 Feb;86(2):346–53. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowe MR, Levine AS. Eating motives and the controversy over dieting: Eating less than needed versus less than wanted. Obesity Research. 2005;13(5):797–806. doi: 10.1038/oby.2005.90. [DOI] [PubMed] [Google Scholar]
- 51.Sinclair JD, Li L. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6(6):505–509. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- 52.Sinclair JD, Walker S, Jordan W. Behavioral and physiological changes associated with various durations of alcohol deprivation in rats. Q J Stud Alcohol. 1973;34(3):744–757. [PubMed] [Google Scholar]
- 53.Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, McBride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- 54.Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obes Res. 2002;10(5):370–378. doi: 10.1038/oby.2002.51. [DOI] [PubMed] [Google Scholar]
- 55.Popkin BM, Siega-Riz AM, Haines PS, Jahns L. Where's the fat? Trends in U.S. diets 1965-1996. Preventive Medicine. 2001;32:245–254. doi: 10.1006/pmed.2000.0807. [DOI] [PubMed] [Google Scholar]
- 56.Hampl JS, Heaton CL, Taylor CA. Snacking patterns influence energy and nutrient intakes but not body mass index. J Hum Nutr Diet. 2003;16(1):3–11. doi: 10.1046/j.1365-277x.2003.00417.x. [DOI] [PubMed] [Google Scholar]
- 57.Kerver JM, Yang EJ, Obayashi S, Bianchi L, Song WO. Meal and snack patterns are associated with dietary intake of energy and nutrients in US adults. J Am Diet Assoc. 2006;106(1):46–53. doi: 10.1016/j.jada.2005.09.045. [DOI] [PubMed] [Google Scholar]