Abstract
This study assessed the effects of the opioid antagonist naltrexone, the dopamine 2-like (D2) antagonist raclopride, and the GABAB agonist baclofen on consumption of fat/sucrose mixtures (FSM) using a limited access protocol. Sixty male Sprague-Dawley rats were grouped according to two schedules of access (Daily [D] or Intermittent [I]) to an optional FSM. Each FSM was created by whipping 3.2% (L), 10% (M), or 32% (H) powdered sugar into 100% vegetable shortening in a w/w manner (n=10 per group). One-hour intakes of the IL and IM groups were significantly greater than intakes of the respective DL and DM groups, thus fulfilling our operational definition of binge-type eating in these groups. Baclofen reduced intakes of the L and M mixtures regardless of access schedule, but failed to reduce intake of the H mixture. Naltrexone reduced intake in all groups, but potency was greater in IL rats than in DL rats. Furthermore, potency was attenuated in Intermittent rats, but enhanced in Daily rats, at higher sucrose concentrations. Raclopride reduced intake in the DL and stimulated intake in the IL groups, reduced intake in both M groups, and was without effect in both H groups. These results indicate that fat/sucrose mixtures containing relatively low concentrations of sucrose allow distinctions to be made between: 1) intakes stimulated by different access schedules and 2) opioid and dopaminergic modulation of those intakes. These results also suggest that brief bouts of food consumption involving fatty, sugar-rich foods may prove to be particularly resistant to pharmacological intervention.
Keywords: binge eating, bulimia, behavioral models, dopamine, eating disorders, food intake, GABA, ingestive behavior, opioids, reward
INTRODUCTION
Binge eating is characterized by the loss of short-term control of meal size, and is operationally defined as the repeated, intermittent consumption of an amount of food in discrete periods of time that is greater than would normally be consumed under similar circumstances (American Psychiatric Association, 2000). Several animal models have been developed to emulate the intermittent overeating characteristic of binge-type eating (Corwin & Buda-Levin, 2004; Hagan & Moss, 1997; Hagan et al., 2002; Rada et al., 2005). Some of these models use sugar solutions as the binge food (e.g. Rada et al., 2005), and some use foods, such as cookies, that contain both fat and sugar (e.g. Hagan & Moss, 1997; Hagan et al., 2002). The model developed in this laboratory has historically used an optional source of dietary fat as the binge food. Optional fat is offered intermittently (three times a week) or daily for 1–2-hr periods to rats that are never food-deprived. Rats with intermittent access to the optional fat consume significantly more during the 1–2 hr period than do rats with daily 1–2 hr access (Corwin, 2004; Corwin et al, 1998; Dimitriou et al., 2000; Thomas et al, 2002; Wojnicki et al., 2008b). Furthermore, rats with intermittent access to the optional fat consume as much fat in the 1–2 hr period as rats with continuous access (24 hr/day-7 days/week) consume in a 24-hr period (Dimitriou et al., 2000; Wojnicki et al., 2008a). Bingeing during the limited access period is operationally defined in this protocol as Intermittent intakes > Daily intakes. The limited access binge protocol is an isomorphic model with good face validity in that it fulfills the definition of binge eating, i.e. that of repeatedly consuming more in a discrete amount of time than is normally consumed under similar circumstances (Corwin & Buda-Levin, 2004). A recent study suggests that the limited access protocol may also have good predictive validity (Broft et al., 2007).
While each of the different models have provided information relevant to human binge eating, none have systematically examined the effects of manipulating the fat and sugar concentrations of the binge food on the binge behavior. This is important for two reasons: 1) Many of the foods upon which humans binge typically contain both fat and sugar (American Psychiatric Association, 2000). Therefore, it would be useful to understand the contribution of these components to the behavior and neurobiology of bingeing. 2) When working with animal models of binge eating, it is important to be able to distinguish binge-type consumption from that which is driven simply by palatability. It is possible that some combinations of fat and sucrose would be so palatable that both control and binge rats would consume large amounts. This would make it impossible to distinguish binge- from palatability-driven intake. One goal of the present research, therefore, was to use our binge model to determine concentrations of sucrose that would permit the assessment of bingeing when combined with fat.
Another goal of the present research was to examine how the incremental addition of sucrose to a dietary fat option influences the efficacy of GABAB, opioid, and dopaminergic receptor ligands under binge and control feeding conditions using our limited access model. In recent years, attention has turned to the relevance of neural systems associated with food reward to binge eating, with both clinical and preclinical evidence implicating the involvement of GABAB (Broft et al., 2007; Buda-Levin et al. 2005; Rao et al., 2008; Wojnicki et al, 2006), opioid (Boggiano et al., 2005; Drewnowski et al., 1995), and dopamine receptors (Avena et al., 2006; Corwin & Wojnicki, 2006; Jimerson et al., 1992; Kaye et al., 1990; Rada et al., 2005; Rao et al, 2008). In spite of these advances, pharmacological options for the clnical treatment of binge eating are limited (Reas & Grilo, 2008; Zhu & Walsh, 2002). Furthermore, interpretation of results in the preclinical studies has been complicated by the use of different binge foods (sugar solutions, solid fat, cookies), as well as different binge protocols (e.g. Avena et al., 2006; Boggiano et al., 2005; Buda-Levin et al. 2005; Rao et al., 2008). In the present research, therefore, GABAB, opioid, and dopaminergic receptor ligands were tested in our limited access binge protocol, using three different optional fat/sucrose mixtures.
Three receptor ligands were tested: 1) the GABAB agonist baclofen, 2) the opioid antagonist naltrexone, and 3) the D2 receptor antagonist raclopride. Baclofen was included because of evidence in rats that it reduces consumption of high-fat foods (Buda-Levin et al. 2005; Corwin & Wojnicki, 2006; Rao et al., 2008; Sato, et al., 2007), while generally stimulating, or having no effect on, consumption of low-fat foods (Buda-Levin et al. 2005; Corwin & Wojnicki, 2006; Ebenezer, 1995, 1996; Ebenezer & Prabhaker, 2007; Ebenezer & Pringle, 1992; Higgs & Barber, 2004; Janak & Gill, 2003; Patel & Ebenezer, 2008; Rao et al., 2008; but see also Sato, et al., 2007). Although the effects of baclofen appear to be fat-specific, rather than binge-specific (Buda-Levin et al., 2005; Corwin & Wojnicki, 2006), a recent open label trial nevertheless suggests the potential usefulness of baclofen in the treatment of bingeing-related eating disorders (Broft et al., 2007). Since the foods upon which people binge generally contain both fat and sugar, the present investigation included baclofen in order to determine its effect when fatty sweet foods were used in a rat binge model.
In contrast to the effects of baclofen, opioids, particularly mu-opioid ligands, appear to modulate the consumption of sugar and sweet tastants (Beczkowska et al., 1992, 1993; Cooper, 1983; Czirr & Reid, 1986; Fantino et al., 1986; Glass et al., 2001; Gosnell & Majchrzak, 1989; Levine et al., 1995; Rockwood & Reid, 1982), as well as fatty foods (Glass et al., 1999; Hagan et al., 1997; Mizushige et al., 2006; Will et al., 2003; Zhang & Kelley, 2000). Opioid blockade may be particularly effective at reducing intake of optional snack-like fatty foods, even if a sweeter food is preferred (Hagan et al., 1997). In addition, both preclinical and clinical evidence suggests that opioid blockade can reduce binge-type consumption of foods containing both sugar and fat (e.g. cookies, chocolate) (Boggiano et al, 2005; Drewnowski et al., 1995). Taken together, these reports suggest that when fat/sucrose mixtures serve as the binge food, opioid blockade is more effective at reducing binge relative to control intake, but that potency may be reduced when sugar concentrations increase.
Dopamine D2-like receptors also have been associated with food reward and consumption, although their association with binge eating has not yet been clarified. The administration of the D2 antagonist raclopride to sham-fed rats dose-dependently decreased sham intake of corn oil (Weatherford et al., 1988, 1990). However, differential effects of D2 blockade on fatty food consumption also have been reported. For instance, in ad libitum fed rats with daily limited access to a high-fat diet, raclopride reduced consumption of the high-fat diet at high dosages, but increased intake at lower dosages (Baker et al, 2001). In addition, the schedule of access to fatty foods may modulate the effects of D2 blockade. When limited access to pure fat was provided, raclopride was generally less effective at reducing intake in non-deprived rats with intermittent (Mon., Weds., Fri.) 1-hr access to the fat, relative to rats with daily 1-hr access (Corwin & Wojnicki, 2006; Rao et al., 2008).
In contrast to effects on consumption of optional fatty foods, effects of D2 blockade on consumption of optional sweet foods appear to be modulated by sucrose concentration, rather than access schedule or drug dose. D2 antagonists blocked the expression of flavor preferences conditioned by sucrose (Hsiao and Smith, 1995; Yu et al., 2000) and also reduced sucrose intake (Schneider et al., 1986, 1990; Smith, 1995). However, the intake-reducing effects of raclopride were attenuated with increasing sucrose concentration (Muscat & Willner, 1989; Phillips, et al, 1991a,b; Weatherford et al., 1990). When limited access to sucrose was provided, raclopride was equally effective at reducing intake in rats on either an intermittent or a daily access schedule (Corwin & Wojnicki, 2006).
Overall, it appears that the effects of D2 blockade on sucrose intake can be modulated by sucrose concentration whereas the effects of D2 blockade on fat intake can be modulated by the access schedule. Which of these effects would predominate when fat and sucrose are combined is not known. Therefore, the present study examined the effects of raclopride when increasing concentrations of sucrose were added to an optional source of pure fat under two different access schedules.
METHODS
Fat/sucrose Mixtures
Fat/sucrose mixtures (FSM) were created by blending vegetable shortening (Crisco All-Vegetable shortening, J.M. Smucker, Orrville, OH; 9.17 kcal/g) and powdered sugar (Domino Foods, Inc., Yonkers, NY; 3.8 kcal/g) to produce a homogenous, solid, icing-like substance. These mixtures were kept at room temperature and did not separate. A fresh batch was prepared on a weekly basis. Three different sucrose concentrations (3.2%, 10%, 32%) were whipped into 100% shortening on a w/w basis. The sucrose concentrations spanned those that produce inverted U-shaped intake and fixed-ratio functions, linear progressive ratio functions (see Sclafani & Ackroff, 2003), and differential effects of dopamine receptor blockade (Muscat & Willner, 1989; Phillips et al., 1991, a, b; Weatherford et al., 1990) under non-binge conditions. These concentrations also span the range that promotes (3.2%, 10%) and does not promote (32%) binge-type consumption under limited access conditions similar to those used in the present study (Wojnicki et al., 2007). The three different FSM, expressed as a percentage of total weight were: Low sucrose (L) 96.8% fat/3.2% sucrose, 8.99 kcal/g; Medium sucrose (M) 90% fat/10% sucrose, 8.63 kcal/g; and High sucrose (H) 68% fat/32% sucrose, 7.45 kcal/g.
Animals
Sixty male Sprague-Dawley rats (Harlan Sprague Dawley, Inc., Indianapolis, IN), 60 days of age and weighing 267–308 g (284.5 ± 1.1 g) at the start of the study, were individually housed in hanging stainless steel wire cages in a temperature- and humidity-controlled vivarium under a 12-hr light:12-hr dark cycle. Throughout the entire study, all rats had ad libitum access to tap water and a nutritionally complete, commercially available, pelleted laboratory rodent diet [Laboratory Rodent Diet 5001, PMI Feeds, Richmond, IN; 3.3 kcal/g: protein (28.05% of energy; 23.4 % of weight), fat (12.14% of energy; 4.5% of weight), carbohydrate (59.81% of energy; 49% of weight)]. All procedures were approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
Procedures
During the 1-week adaptation period, chow intake was measured on a daily basis, while body weights were measured three times a week. Six groups of 10 animals each were then matched by body weight and the average amount of chow consumed during the last 3 consecutive 24-hr periods (F (5,54<1, NS for all). All rats were then given overnight access to an assigned FSM, two groups per concentration, to prevent neophobia. Three of the groups were placed on a Daily schedule of access (n=10 each, Daily, D) on which they received their assigned FSM during a 1-hr period of availability every day of the week two hours prior to the start of the dark cycle in addition to their continuously available chow and water. The remaining three groups were placed on an Intermittent schedule of access (n=10 each, Intermittent, I) on which they received their assigned FSM during a 1-hr period of availability on Mondays, Wednesdays, and Fridays, two hours prior to the start of the dark cycle, in addition to their continuously available chow and water. Three days following the overnight exposure to the mixtures, all rats began their assigned schedules of access for a pre-drug period of 6 weeks. During week 6, 24-hr chow data in addition to 1-hr chow and FSM data were collected for 7 days. Body weight was measured on Thursdays and Sundays throughout the study.
Drug administration followed the 6-week pre-drug period. All rats received all drug dosages, which were assigned using a uniform Latin square. Drugs (Tocris, Ellisville, MO) were administered on Mondays and Fridays, with Wednesdays serving as non-drug baseline days. Chow and FSM were simultaneously available during the 1-hr test period; 1-hr chow and FSM intakes were recorded. All drugs were dissolved in saline and administered intraperitoneally (i.p.) at a volume of 1 ml/kg.
The first drug administered was the GABAB agonist (R-S)-baclofen; (0.0, 0.6, 1.0, 1.8 mg/kg, 30-min pretreatment). It was initially determined that the highest dosage of baclofen to be injected was 1.8 mg/kg based on previous research (Buda-Levin et al., 2005; Wojnicki et al., 2006). However, due to negative results, an additional dosage (3.2 mg/kg) of baclofen was administered to all rats at the end of the baclofen injection series. After baclofen the following drugs were administered: the predominantly mu-opioid antagonist naltrexone (0.0, 0.03, 0.1, 0.3 mg/kg, 20-min pretreatment), and the dopamine (D2) antagonist raclopride (0.0, 0.03, 0.1, 0.3 mg/kg, 20-min pretreatment). A period of one week with no injections was allowed between drugs. All rats received all drugs in the same order.
Statistics
SAS v. 9.1 (SAS Institute, Cary, NC) was used to analyze all data. One-hr FSM, 1-hr chow, and average weekly 24-hr intakes during the 6th week of the pre-drug period were analyzed using a 2-way analysis of variance (ANOVA) (concentration × access). Significant 1-hr intake differences among groups were assessed using preplanned LS Means comparisons with a Bonferroni correction applied such that α = 0.0167 (0.05/3 comparisons per mean). Significant daily 24-hr intake differences between groups within each concentration (DL vs. IL, DM vs. IM, DH vs. IH) were assessed using t-tests. FSM and chow intake after drug administration were analyzed using 3-way ANOVA (Concentration × Access × Dosage). Dose effect functions were analyzed using 1-way repeated ANOVA with significant effects defined by Tukey’s HSD. To assess baseline stability and to rule out order effects of the different drugs, 1-way ANOVA followed by Tukey’s HSD post-hoc test was used for two different analyses: 1) FSM intake on non-injection days during each drug test (Wednesdays) was compared to FSM intake after saline injections; 2) FSM intakes on non-injection days were compared across all drug testing periods with energy intakes expressed as kcal/body weight0.67 (Heusner, 1985). Alpha was set at 0.05. If F was <1, results were not significant and p-values are not reported.
RESULTS
Pre-drug FSM and chow intake
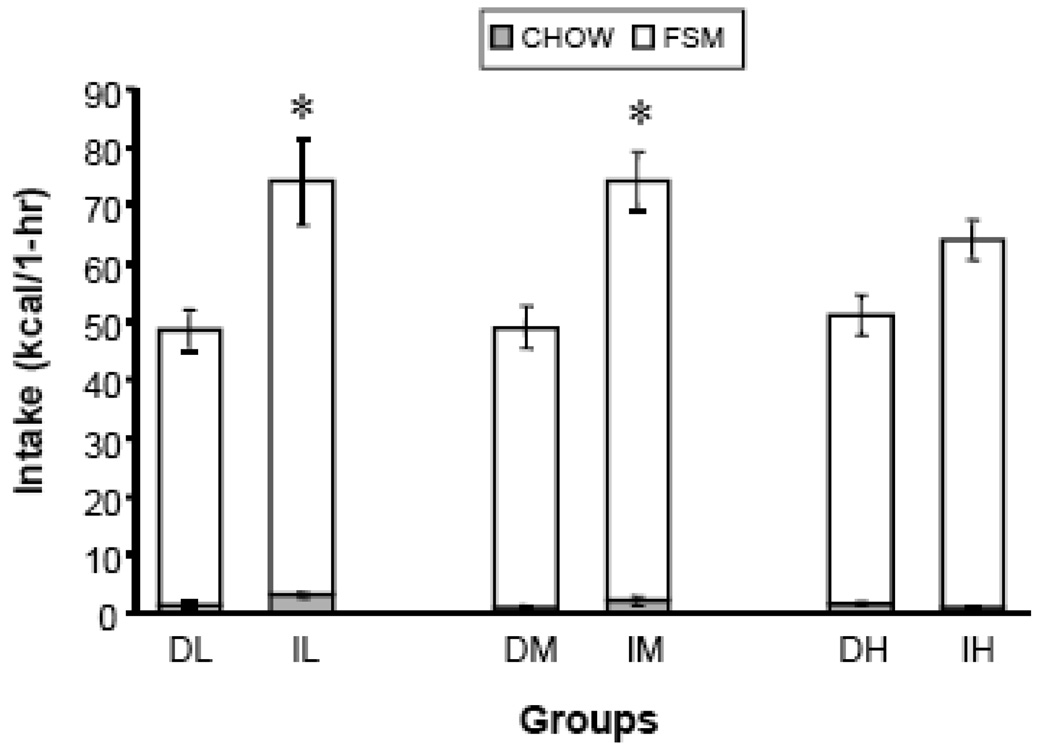
1-hr FSM energy intakes (kcal/1-hr) were significantly affected by schedule [F (1,53)=29.47, p < 0.0001]. The significant effect was due to greater FSM intake in the Intermittent groups than in the Daily groups (Fig. 1, white bars). Specifically, the IL and IM groups consumed significantly more FSM than did the corresponding Daily groups (p < 0.0167, LS Means). DH and IH intakes were not statistically different. There was no significant effect of sucrose concentration and no interaction between access schedule and concentration. Analysis of 1-hr mixture intakes in grams yielded a similar result [main effect of schedule of access: F (1,53)=30.20, p < 0.0001] (data not shown).
Figure 1.
1-hr chow and FSM energy intakes. Asterisks indicate significant differences in total intake (Intermittent > Daily) at a specific concentration. D=Daily, I=Intermittent, L= 3.2% sucrose (by wt) mixed into vegetable shortening, M = 10% sucrose (by wt) mixed into vegetable shortening, H = 32% sucrose (by wt) mixed into vegetable shortening; Vertical lines represent Standard Error of the Mean (SEM).
For 1-hr chow energy intake (kcal/1-hr) there was a schedule × concentration interaction [F (2,53)=3.99, p < 0.024], but no main effects (Fig. 1, shaded bars). The significant interaction was due to greater chow intake in IL relative to IH (p < 0.0167, LS Means) and relatively equal chow consumption across all Daily groups. In addition, IL consumed significantly more chow than did DL (p < 0.0167, LS Means). An analysis of 1-hr chow intakes in grams yielded similar results (interaction of schedule × concentration [F (2,53)=3.84, p < 0.028]) (data not shown).
Total 1-hr combined chow and FSM energy intakes were significantly affected by schedule of access [F(1,53)=32.15, p < 0.0001]. The significant effect was due to generally greater intake in the Intermittent groups than in the Daily groups (Fig. 1, stacked white and shaded bars). Specifically, IL consumed significantly more than did DL (p < 0.0167, LS Means) and IM consumed significantly more than did DM (p < 0.0167, LS Means). However, intakes of the IH and DH groups were not statistically different. There was no significant effect of sucrose concentration and no interaction between access schedule and concentration. The analysis of 1-hr gram intakes of chow and mixture yielded a similar result (main effect of schedule of access [F (1,53)=36.44, p < 0.0001]) (data not shown).
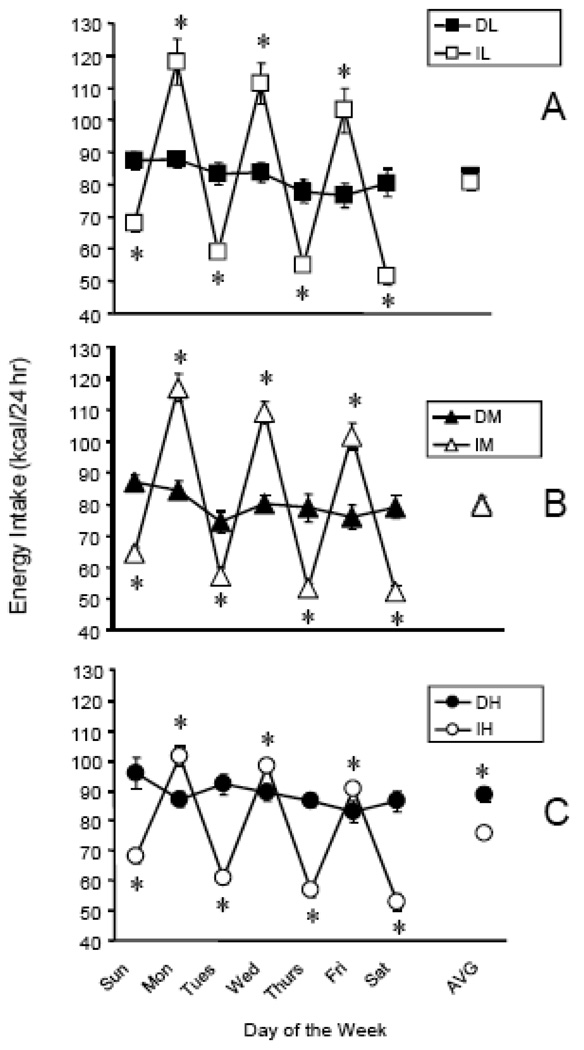
Average weekly total energy intake was significantly affected by access schedule [F (1,53)=8.58, p<0.0050], which was due to significantly greater intake in the DH group relative to the IH group (p < 0.05, t-test) (Fig. 2C, AVG symbol). While there was no main effect of sucrose concentration, access schedule and concentration interacted [F (2,53)=5.12, p < 0.0093]. The interaction was again due to the significant difference between DH and IH, but no differences at the lower sucrose concentrations (Fig. 2A–C, AVG symbols). Although the average weekly total energy intake did not differ between Daily and Intermittent groups consuming the L (3.2%) and M (10%) mixtures, daily intakes did differ. Specifically, the intermittent groups consumed significantly more 24-hr energy on the days that the mixtures were available (Mon, Weds, Fri) and significantly less energy on the days that the mixtures were not available (Tues, Thurs, Sat, Sun), relative to the groups getting mixtures every day (p < 0.05, t-test) (Fig 2A–C). This intake pattern was demonstrated with all three FSM.
Figure 2.
24-hour total daily energy intake (chow + FSM) over the course of week 6. A: DL and IL groups, B: DM and IM groups, C: DH and IH groups. Asterisks indicate a significant difference between the Daily and Intermittent group for that 24 hr period or for the week’s average (AVG). Abbreviations and vertical lines as in Fig. 1.
Analysis of total cumulative energy intake during week 6 revealed a significant effect of schedule [F (1, 53) = 8.56, p < 0.01] and a schedule × concentration interaction [F (2, 53) = 5.11, p < 0.01]. These differences were likely caused by DH consuming significantly more energy than IH or DM consumed (p < 0.0167, LS Means) (data not shown). The intake differences were reflected in slight differences among body weights, with DH weighing about 20g more than IH, and about 10g more than DM. However, none of the body weight differences were statistically reliable (data not shown).
Baclofen
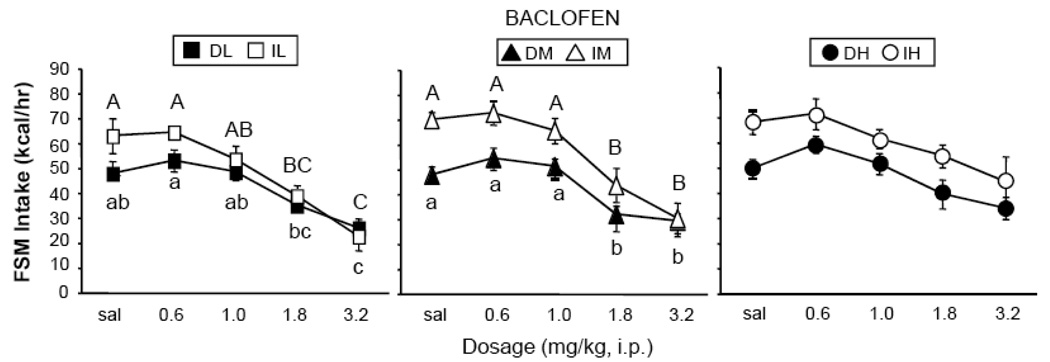
Baclofen significantly reduced 1-hr FSM intake. There was a main effect of dosage [F (4, 216) = 44.15, p < 0.0001], but no interactions with schedule of access or concentration. Post-hoc analysis revealed that baclofen significantly reduced intake in the DL and IL, as well as DM and IM, groups at the 1.8 and 3.2 mg/kg dosages, but had no effect in either the DH or IH group at any dosage (p < 0.05, Tukey’s HSD, Fig. 3). There were also main effects of schedule of access [F (1, 54) = 15.85, p < 0.001] and concentration [F (2, 54) = 13.67; p < 0.0001], but no interactions. The effect of access was due to the higher intakes of the Intermittent groups. The effect of concentration was due to the overall higher intakes of DH and IH, due to a lack of response to the drug in those groups.
Figure 3.
Effects of baclofen on consumption of FSM. Significant effects of baclofen dosage within the Intermittent groups are marked with capital letters (A, AB, B…); Significant differences within the Daily groups are marked with lower-case letters (a, ab, b…). Different letters represent significant differences among dosages. Abbreviations and vertical lines as in Fig. 1.
Baclofen stimulated 1-hr chow intake (data not shown). There was a main effect of dosage [F (4, 216) = 14.48, p <0.0001], but no interaction with schedule of access or concentration. Further analysis showed that baclofen stimulated intake only in the DM and DH groups at 1.8-mg/kg, and in the DL group at 3.2-mg/kg (p < 0.05, Tukey’s HSD). Baclofen had no significant effect on chow intake in the Intermittent groups (ns, Tukey’s HSD). For all groups, there were no significant differences in 1-hr FSM intakes between non-drug baseline days (Wednesdays) and days in which saline was injected.
Naltrexone
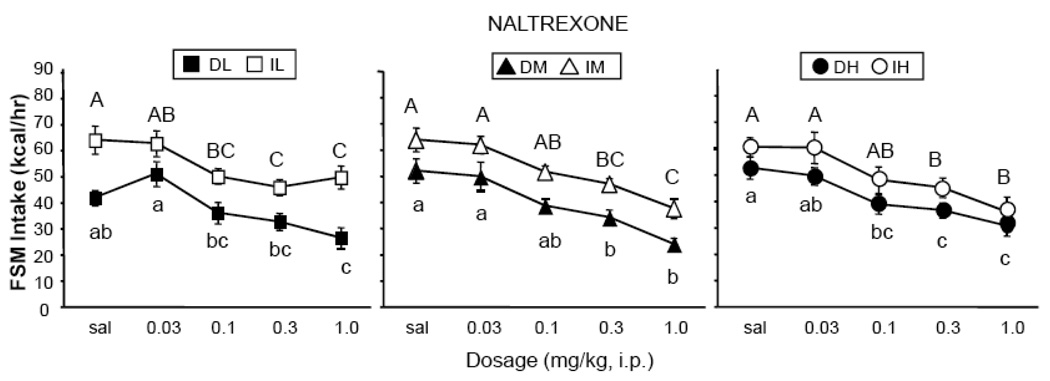
Naltrexone significantly decreased 1-hr FSM intake in all groups (Fig. 4). There was a main effect of dosage [F (4,216) = 52.91, p < 0.0001], but no interaction with schedule of access or sucrose concentration. Although there were no interactions with drug dosage, slight differences in sensitivity to naltrexone emerged in the Daily and Intermittent groups. More specifically, the minimally effective dosage of naltrexone in the Daily groups was 1.0 mg/kg in DL, 0.3 mg/kg in DM, and 0.1 mg/kg in DH (p < 0.05, Tukey’s HSD, Fig. 4). Thus, the potency of naltrexone was somewhat enhanced at higher sucrose concentrations in the Daily groups. On the other hand, the minimally effective dosage in the Intermittent groups was 0.1-mg/kg in IL, and 0.3-mg/kg in IM and IH (p < 0.05, Tukey’s HSD, Fig. 4). That is, the potency of naltrexone was somewhat reduced at higher sucrose concentrations in the Intermittent groups. When comparing results between Intermittent and Daily groups, naltrexone was more potent in IL than in DL by a full log unit, and more potent in DH than in IH by a half log unit. There were main effects of access schedule [F (1,54) = 20.81, p < 0.0001] and concentration [F (2,54) = 3.69, p < 0.05], but no interaction. The effect of access was due to the overall higher intakes in the Intermittent groups. The main effect of concentration was due to the collapsed overall higher intakes of the DH and IH groups.
Figure 4.
Effects of naltrexone on consumption of FSM. Labeling conventions from Figure 3 used.
Naltrexone had no significant effect on chow intake in any of the groups (data not shown). For all groups, there were no significant differences in 1-hr mixture intakes between non-drug baseline days (Wednesdays) and days in which saline was injected.
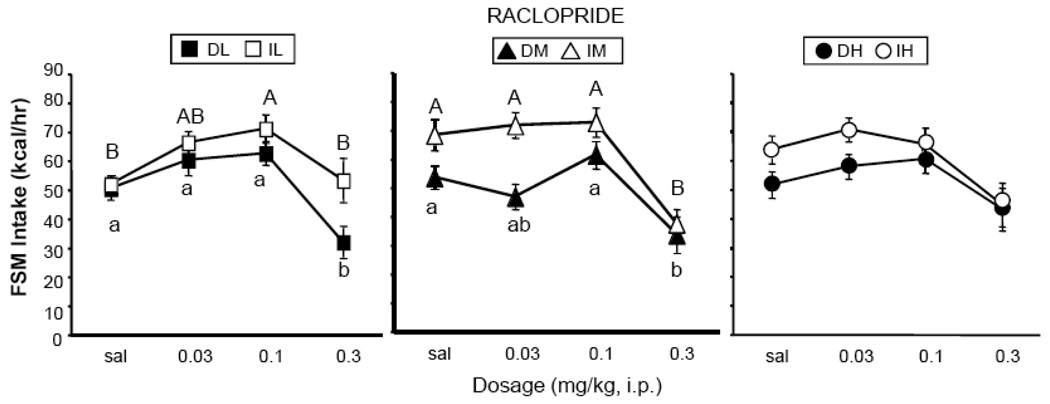
Raclopride
The effects of raclopride varied among the different groups (Fig. 5). There was a main effect of dosage [F (3, 162) = 33.08, p < 0.0001], but no interaction with schedule of access or sucrose concentration. Although the interactions were not significant, post-hoc analyses revealed a decrease at 0.3 mg/kg in the DL, IM and DM groups (p < 0.05, Tukey’s HSD, Fig. 5); however, raclopride had no effect in either the DH or IH group. Furthermore, raclopride (0.1 mg/kg) significantly stimulated intake in the IL group (Fig. 5). There were main effects of access schedule [F (1,54) = 9.32, p < 0.01] and concentration [F (2,54) = 5.06, p < 0.01], but no interaction. The effect of access was due to the higher intakes of the Intermittent groups. The effect of concentration was due to the higher intakes of the DH and IH groups due to a lack of response to the drug.
Figure 5.
Effects of raclopride on consumption of FSM. Labeling conventions from Figure 3 used.
Raclopride had no significant effect on 1-hr chow intake in any of the groups (data not shown). There were, however, main effects of access schedule [F (1,54) = 19.12, p < 0.0001] and a schedule × concentration interaction [F (2,54) = 7.32, p < 0.01] due mainly to the large amount to chow consumed by IL and IM relative to DL and DM, respectively. For all groups, there were no significant differences in 1-hr FSM intakes between non-drug baseline days (Wednesdays) and days in which saline was injected.
Stability of FSM intake across time
For all groups, FSM intake (normalized to body weight0.67) on non-injection days (Wednesdays) did not differ significantly across the baclofen, naltrexone, and raclopride test periods. In other words, there was no evidence of drug effects on baseline intake across the length of the study.
DISCUSSION
Several new findings are reported: (1) Operationally defined binge-type behavior (Intermittent > Daily) occurred in the L and M groups, but not in the H groups; (2) Drug effects differed with sucrose concentration and access schedule. These results indicate that the presence of sucrose in an optional dessert-type food can influence operational definitions of bingeing, as well as the response to pharmacological manipulations.
Pre-drug Intakes
Binge behavior is defined as greater intakes in a brief period of time than would normally be consumed under similar circumstances. This criterion for bingeing has been met operationally in previous studies in which Intermittent rats consumed more 100% vegetable shortening than Daily rats consumed (Corwin et al., 1998; Dimitriou et al., 2000; Thomas et al., 2002; Wojnicki et al., 2006, 2008a, b); the criterion was also met in a report in which Intermittent rats consumed more 3.2% and 10% sucrose than Daily rats consumed (Wojnicki et al., 2007). The criterion was not met, however, when limited access to an optional 32% sucrose solution was provided (Wojnicki et al., 2007). In the present study, the operational criterion for bingeing (Intermittent > Daily) was met in the IL and IM groups, but not in the IH group. The slightly elevated energy intakes of the DH rats relative to the other two daily groups, and the slightly lower energy intakes of the IH rats relative to the other two intermittent groups diminished the binge effect in the H rats. Thus, bingeing in this study occurred at the same sucrose concentrations as was reported previously for liquid sucrose solutions (Wojnicki et al., 2007).
Gram intakes of the FSM were higher than those previously reported for male rats consuming 100% shortening (Corwin et al., 1998; Wojnicki et al., 2008b). Furthermore, although energy intake decreased in the Intermittent groups as sucrose concentration increased, gram intakes actually increased slightly across sucrose concentrations in the Intermittent (IL [7.9 g]; IM [8.3 g]; IH [8.5 g]) as well as the Daily groups (DL [5.3 g]; DM [5.7 g]; DH [6.6 g]). The slight decrease in energy intake that occurred in the IH group can be explained by the reduced energy density of the 32% FSM. For the FSM energy intake in the IH group to have equaled that of the IL group, the IH rats would have had to consume, on average, 9.5 g of the 32% FSM, an amount that approaches the maximal capacity of the rat stomach (Bull & Pitts, 1971). The failure to distinguish binge-type intake from control intake in the 32% FSM groups may have been due to ceiling effects in the IH group.
In another report in which 2-hr access to a dietary option containing 45% fat and 17% sucrose was provided, intake of the option also did not differ between Intermittent and Daily access groups (Berner et al., 2008), a finding similar to that reported here in the IH and DH groups. Taken together, these reports indicate that when high fat sugary optional foods are provided, sucrose concentrations greater than 10% produce robust intakes in rats with Daily as well as Intermittent limited access to the options. Thus, it becomes difficult to operationally distinguish binge-type eating from eating induced simply by palatability.
These results, as well as those reported previously (Wojnicki et al., 2007), indicate that the choice of sucrose concentration is critical to one’s operational definition of binge eating when working with animal models. Results from this study, our previous report (Wojnicki et al, 2007), as well as reports using a sucrose binge model (e.g. Rada et al., 2005), indicate that 3.2 and 10% sucrose concentrations allow for the distinction between binge groups and controls. Distinctions between binge and control intakes may not be possible with higher sucrose concentrations, however, because they promote robust consumption when mixed with fats (e.g. Lucas & Sclafani, 1990; Naleid et al., 2008; Reed & Friedman, 1990), regardless of access conditions, as was the case in the DH group in the present report. If bingeing were simply defined as “large” intakes, without comparison to a control group with access under similar circumstances (American Psychiatric Association, 2000), then it would appear that the high sucrose mixture promoted “bingeing” in both the Daily, as well as the Intermittent, groups.
The well-documented over-eat/under-eat consumption pattern of Intermittent rats (Corwin et al., 1998; Dimitriou et al., 2000, Rao, et al., 2008; Wojnicki et al., 2007) was maintained in the IL and IM groups, i.e. Intermittent rats ate considerably less energy on days in which FSM was not provided. Although the daily intake patterns of the Intermittent and Daily rats differed, average overall intakes of the Intermittent and Daily rats consuming either the L or the M mixture did not differ. However, the overall intake of DH was significantly greater than that of IH, due to daily relatively large intake of the 32% FSM and failure to reduce chow intake sufficiently to compensate for the energy provided by the H option. The Intermittent rats, in contrast, only had access to the 32% FSM 3 times per week and consumed sufficiently less chow to compensate. This may have been related to maintenance of nutritional status. The National Research Council estimates that the protein requirement for growth in laboratory rats is 150 g of protein per kg of diet consumed (1995). The requirement was exceeded in the IH rats at ~187 g protein/kg total diet consumed (chow + FSM) across a 1-week period, but was barely achieved in the DH rats at ~149 g protein/kg total diet consumed. The DH rats could not reduce chow consumption any further without compromising nutrient intake.
The possibility that rats will not reduce chow intake below that required for protein nutriture when low-protein dietary options are provided is supported by a recent report in which a fat/sucrose option was used (Mathes et al., 2008). The fat/sucrose option in that study (33.3% sugar by weight whipped into 100% vegetable shortening) was similar to the H mixture used in the present report (32% sugar by weight whipped into 100% vegetable shortening), but was provided continuously, rather than for only 1 hour each day. Rats with continuous access to the fat/sucrose option consumed more energy and gained more weight than did rats with continuous access to a 6% sugar gel option or with continuous access to only chow. Based upon the data presented in that report, a rough calculation of protein intake in the rats receiving the fat/sucrose option indicates that protein comprised about 140 g/kg diet, a quantity close to that recommended for growth in rats, and similar to that consumed by the DH rats in the present report. In other work, when 100% shortening was provided as an option for only 2 hours per day, protein intake was well above the National Research Council recommendations for growth (Corwin et al., 1998; Dimitriou et al., 2000), and energy intakes did not differ from those of chow-only controls. However, when 100% shortening was provided continuously, rats consumed more total energy than did chow-only controls, and protein intake was maintained at about 140–150 g/kg diet (Dimitriou et al., 2000; Lucas et al., 1989; Wojnicki et al., 2008b).
In contrast, Berner et al. (2008) recently reported that when a nutritionally complete high fat/high sucrose optional food was provided to rats on either an Intermittent or Daily schedule of access, body weight did not differ between these two groups. That is, when the option provided nutritionally adequate levels of protein, body weight did not differ between Intermittent and Daily groups. Thus, the lack of protein in the FSM of the present report, and the need to defend protein nutriture with higher consumption of chow, may have contributed to the elevated energy intake of the DH rats of the present report. It is important to note, however, that body weight of the Daily limited access group in the Berner et al. (2008) report was significantly greater than that of chow-only controls. Furthermore, in a previous report using female rats provided Daily limited access to 100% vegetable shortening, body weight did not differ statistically from chow-only controls, but body fat accretion was significantly greater than that of the chow controls (Dimitriou et al., 2000). These reports suggest that, in contrast to Intermittent limited access, daily limited access to fatty dietary options may promote weight gain or body fat accretion in rats in some cases.
Baclofen: GABAB Agonist
The intake reducing effects of the GABAB agonist baclofen were comparable in the Intermittent and Daily groups. In addition, effects were attenuated with increasing sucrose concentrations. Baclofen significantly reduced FSM intake in the Daily and the Intermittent L (3.2%) and M (10%) groups. However, baclofen had no effect on consumption of the 32% FSM in either the Intermittent or the Daily group. All decreases in FSM intake were accompanied by either an increase or no change in chow intake at the same dosages, demonstrating that motor function was maintained after drug administration.
Baclofen reduced intake of the L and M mixtures, rather than stimulating intake as reported by others for chow and sucrose pellets (see introduction for citations). This result is likely not due to ceiling effects, since the Daily rats consumed significantly less of the L and M mixtures than did the Intermittent rats, but baclofen still did not stimulate intake in the DL and DM groups. The results with the L and M mixtures are, however, consistent with previous reports using solid fats of different concentrations. Baclofen decreased intake of 100% and 56% vegetable shortening in rats regardless of access schedule, while either stimulating or having no effect on chow intake (Buda-Levin et al., 2005; Rao et al., 2008). Similarly, others have reported that baclofen reduced intake of a 60% fat diet in mice (Sato et al., 2007). These reports indicate that baclofen may selectively reduce intake of high-fat foods. Why, then, did baclofen not reduce consumption of the H (32%) FSM, which contained 68% fat? In contrast to the effects on fat intake, previous research has shown that GABAB activation had no effect on intake of 3.2, 10, and 32% sucrose solutions in rats maintained on limited access protocols (Corwin & Wojnicki, 2006), but stimulated intake of sucrose pellets in 24-hr food-deprived mice (Higgs & Barber, 2004). Thus, the lack of effect in the H (32%) groups of the present study can be explained by the relatively high sucrose concentration in the H mixture. That is, the higher sugar concentration may have attenuated the ability of baclofen to reduce intake, while the near maximal intakes precluded the assessment of any stimulatory effects.
The mechanisms accounting for this sucrose effect are not known, but may relate to the ability of sucrose to stimulate dopamine release in the nucleus accumbens. In rats, accumbens dopamine is repeatedly released in rats bingeing on sucrose but not in controls (Avena et al., 2006; Rada et al., 2005), and the stimulatory effect of sucrose on dopamine release has been shown to be concentration, not volume, dependent (Hajnal et al., 2004). It is possible, then, that the inhibitory effects of peripherally administered baclofen on dopamine cell bodies (Erhardt et al., 2002) were not strong enough to overcome the stimulatory effect of the high sucrose concentration on dopamine release and associated signaling involved in palatable food consumption.
Whether a similar attenuation of efficacy would occur in human binge eaters is not known. The sugar content of “forbidden foods” upon which people binge ranges from ~15% (pudding) to ~50% (chocolate candy), and within a small patient population, the sugar content of binge episodes was reported to be somewhat greater among bulimics than among subjects with binge eating disorder (Gayle et al., 2004). Baclofen recently was tested in an open label clinical trial and was shown to reduce binge frequency in women with binge eating disorder or bulimia nervosa (Broft et al., 2007). However, binge content was not assessed in that study and reductions in binge frequency ranged from 22% to 100%. Whether the sucrose content of the binge foods would influence the efficacy of baclofen as a treatment strategy for binge eating bears investigation.
Naltrexone: Non-Specific mu-Opioid Antagonist
The ability of naltrexone to reduce FSM intake varied according to sucrose concentration in the different groups. The IL rats were more sensitive to the intake-reducing effects of naltrexone than were the DL rats. Furthermore, effects in the Intermittent rats were somewhat attenuated as sucrose concentration increased. However, effects in the Daily rats were enhanced as sucrose concentration increased.
Others have shown that mu-receptor binding is enhanced in rats that consume large amounts of sugar in a protocol providing 12 hours of daily access to a sugar solution (Colantuoni et al., 2001). Since our DH rats exhibited more sensitivity to naltrexone, their daily sucrose exposure and greater overall sucrose intake may have enhanced mu-receptor binding. Sugar-bingeing rats in Colantuoni et al. are estimated to have consumed between 26–77 g sucrose/week throughout the duration of the study. The amount of sucrose consumed by the DH rats of the present report was ~15 g sucrose/week, while their Intermittent counterparts consumed only ~8 g sucrose/week. Both Daily and Intermittent rats of the remaining two FSM groups consumed markedly less sucrose in a week. Differences in weekly sucrose intake can be explained by two constraints: 1) Colantuoni et al. allowed daily 12-hr access to sucrose, whereas our rats were given only 1-hr; 2) Colantuoni et al. provided liquid sucrose and chow, whereas our rats consumed solid fat/sucrose mixtures and chow, i.e. the time constraint of our protocol and energy density of our mixtures may have prevented the excessive sucrose intake reported by Colantuoni, et al. Regardless, the apparent enhanced sensitivity to opioid blockade in the DH rats may represent enhanced mu-receptor binding due to the greater overall sucrose intake and/or the daily exposure to a high sucrose concentration in this group.
In contrast to the effects obtained with the H option, IL rats were more sensitive to the intake reducing effects of naltrexone than were the DL rats, an effect similar to that reported in a model involving binge intake of cookies (Boggiano et al., 2005). This is in contrast to a report in which naltrexone was equally potent (0.1 mg/kg, ip) in Intermittent and Daily rats consuming 100% vegetable shortening (Corwin & Wojnicki, 2006). Why the addition of 3.2% sucrose would induce a differential response of Intermittent and Daily rats to opioid blockade is not clear. The differential baselines are not likely responsible, as baseline intakes differed in the report mentioned above, yet naltrexone was equally effective in both groups (Corwin & Wojnicki, 2006). One possibility is that opioid receptor involvement in incubation may have been greater in the Intermittent rats than in the Daily rats due to the 48-hr period of forced abstinence between FSM access opportunities. Others have shown that the potency of naloxone to reduce responding for a sucrose-paired cue is greater in rats with prolonged forced abstinence from a sucrose solution (Grimm et al., 2007). Furthermore, the ability of naloxone to reduce sham intake of sucrose solutions is reduced at higher sucrose concentrations (Kirkham & Cooper, 1988). Thus, the differences in abstinence periods may have mediated the Intermittent/Daily differences at low sucrose concentrations, but this effect may have been attenuated by the higher sucrose concentrations.
Whether the present results would translate to human binge eating is not known. Opioid blockade has shown promise in the treatment of bulimia nervosa in a double-blind placebo-controlled trial (Marrazzi et al., 1995), and significantly reduced consumption of high-fat high-sugar foods (cookies ~38% sugar, ~21% fat; chocolate candy ~ 50% sugar, ~30% fat) in binge eaters, but not in non-bingeing subjects (Drewnowski et al., 1995). For preclinical drug assessment of opioid involvement in binge eating using rat models, therefore, the low sucrose FSM of the present report, or foods that are lower in fat, such as cookies (Boggiano et al., 2005), may be the best choices for distinguishing binge from non-binge sensitivity to the intake reducing effects of opioid blockade.
Raclopride: Dopamine D2 Receptor Antagonist
Raclopride had differential effects in the Intermittent and Daily groups consuming the L (3.2%) mixture. However, differential effects were not seen with the 10% and 32% mixtures. Furthermore, any effects that raclorpide had were completely eliminated at the highest sucrose concentration.
Raclopride stimulated intake in the IL group at a lower dosage (0.1 mg/kg) than was required to reduce intake in other groups. Furthermore, raclopride had no significant effect on intake in the IL group at the highest dosage tested (0.3 mg/kg). In contrast, the highest dosage of raclopride significantly reduced intake in the DL group. Others have reported stimulatory effects of raclopride on consumption of a 33% fat diet or of pure vegetable shortening at lower dosages than were required to reduce intake (Baker et al., 2001; Corwin & Wojnicki, 2006). This intake stimulatory effect at low dosages has been proposed to be attributable to a disinhibition of dopamine release via preferential blockade of presynaptic D2 receptors (Baker et al., 2001).
If raclopride-induced disinhibition of dopamine release accounts for results obtained with the L sucrose concentration, then the differential response of the IL and DL rats to raclopride would be consistent with differential processing of dopamine signaling in these groups. While this is intuitively appealing, similar differential effects were not seen at the higher sucrose concentrations in the present study.
The effects obtained with the 10% and 32% FSM are consistent with results reported by other groups. Specifically, the efficacy of raclopride for reducing consumption of sucrose has been reported to be inversely related to the sucrose concentration (Muscat & Willner, 1989; Phillips, et al, 1991a,b; Weatherford et al., 1990). Since sucrose consumption stimulates dopamine release in the nucleus accumbens in a concentration-related manner (Hajnal et al., 2004), the present results could be due to the enhanced release of dopamine in the terminal fields at the higher sucrose concentrations. In addition, rats maintained on daily 12-h access to 10% sucrose showed decreased D2 receptor binding in the nucleus accumbens, a result that also would be consistent with reduced efficacy of raclopride (Colantuoni et al., 2001). The enhanced dopamine release coupled with possible reduced D2 receptor binding would attenuate the efficacy of raclopride and obscure possible schedule effects on dopamine signaling. While higher dosages would overcome this effect, they can have non-specific effects on behavior that interfere with feeding; higher dosages, therefore, were not tested in this study.
While this scenario can account for the different profiles obtained with the D groups, and with the IM and IH groups, the stimulation of intake with the IL group cannot be explained by the sucrose concentration. Since raclopride also stimulated intake in rats with Intermittent access to 100% shortening (Wojnicki & Corwin, 2006), the present results suggest that dopamine signaling, at least that involving D2 receptors, can be modulated not only by the sucrose (and possibly fat) content of the food, but also by the food availability and/or the manner in which it is consumed.
Conclusions
In conclusion, involvement of GABAB, opioid, and dopamine receptors in the consumption of fat/sucrose mixtures is influenced by the sucrose concentration of the food, and to some extent, by the manner is which the food is presented and consumed. Differential pharmacological effects in the Intermittent (binge) and Daily (non-binge) rats were most evident when the fatty option containing 3.2% sucrose was provided. The IL rats were more sensitive to the intake reducing effects of opioid blockade than were the DL rats; in addition, intake was stimulated in the IL rats, but not in the DL rats, when D2 receptors were blocked. This suggests that alterations in opioid and dopamine signaling may result in this rat model of binge eating when mildly sweet, but predominantly fatty, foods are consumed. However, the response profiles changed at higher sucrose concentrations. D rats became more sensitive, but I rats less sensitive, to the intake reducing effects of opioid blockade when options with higher sucrose concentrations were consumed. In addition, both baclofen and raclopride were ineffective at the highest sucrose concentration in Intermittent and Daily groups. These results highlight the importance of the fat and sucrose concentrations of the binge foods when assessing preclinical efficacy of potential pharmacological interventions. In addition, the present results suggest that brief bouts of food consumption involving sugar-rich, fatty foods may prove to be particularly resistant to pharmacological intervention.
ACKNOWLEDGEMENTS
Funding for this research was provided by the National Institutes of Health 1-R01-MH67943 (RLC). We thank one of the reviewers for suggestions regarding interpretation of the dietary protein issue and regarding studies that could be conducted to address this issue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of the mental disorders. 4th ed, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neurosci. 2006;139(3):813–820. doi: 10.1016/j.neuroscience.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Baker RW, Osman J, Bodnar RJ. Differential actions of dopamine receptor antagonism in rats upon food intake elicited by either mercaptoacetate or exposure to a palatable high-fat diet. Pharmacol Biochem Behav. 2001;69:201–208. doi: 10.1016/s0091-3057(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Beczkowska IW, Bowen WD, Bodnar RJ. Central opioid receptor subtype antagonists differentially alter sucrose and deprivation-induced water intake in rats. Brain Res. 1992;589:291–301. doi: 10.1016/0006-8993(92)91289-q. [DOI] [PubMed] [Google Scholar]
- Beczkowska IW, Koch JE, Bostock ME, Leibowitz SF, Bodnar RJ. Central opioid receptor subtype antagonists differentially reduce intake of saccharin and maltose dextrin solutions in rats. Brain Res. 1993;618:261–270. doi: 10.1016/0006-8993(93)91274-v. [DOI] [PubMed] [Google Scholar]
- Berner LA, Avena NM, Hoebel BG. Bingeing, Self-restriction, and Increased Body Weight in Rats With Limited Access to a Sweet-fat Diet. Obesity (Silver Spring) 2008 Jun 26; doi: 10.1038/oby.2008.328. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CD, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119:1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- Broft AI, Spanos A, Corwin RL, Mayer L, Steinglass J, Devlin MJ, Attia E, Walsh BT. Baclofen for binge eating: an open-label trial. Int J Eat Disord. 2007;40(8):687–691. doi: 10.1002/eat.20434. [DOI] [PubMed] [Google Scholar]
- Buda-Levin A, Wojnicki FHE, Corwin RL. Baclofen reduces fat intake under binge-type conditions. Physiol Behav. 2005;86:176–184. doi: 10.1016/j.physbeh.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull LS, Pitts GC. Gastric capacity and energy absorption in the force-fed rat. J Nutr. 1971;101 doi: 10.1093/jn/101.5.593. 593-5996. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy K, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Cooper SJ. Effects of opiate agonists and antagonists on fluid intake and saccharin choice in the rat. Neuropharmacology. 1983;22:232–238. doi: 10.1016/0028-3908(83)90247-2. [DOI] [PubMed] [Google Scholar]
- Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42:139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Buda-Levin A. Behavioral models of binge-type eating. Physiol Behav. 2004;82:123–130. doi: 10.1016/j.physbeh.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FHE, Fisher KO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FHE. Program # 457.8. 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. Differential effects of D1, D2, opioid, and GABAB receptor ligands in rats with intermittent access to fat and sucrose. Online. [Google Scholar]
- Czirr SA, Reid LD. Demonstrating morphine’s potentiating effects on sucrose-intake. Brain Res Bull. 1986;17(5):639–642. doi: 10.1016/0361-9230(86)90195-4. [DOI] [PubMed] [Google Scholar]
- Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28:436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Krahn DD, Demitrack MA, Nairn K, Gosnell BA. Naloxone, an opiate blocker, reduces the consumption of sweet high-fat foods in obese and lean female binge eaters. Am J Clin Nutr. 1995;61:1206–1212. doi: 10.1093/ajcn/61.6.1206. [DOI] [PubMed] [Google Scholar]
- Ebenezer IS. Intraperitoneal administration of baclofen increases consumption of both solid and liquid diets in rats. Eur J Pharmacol. 1995;273:183–185. doi: 10.1016/0014-2999(94)00707-e. [DOI] [PubMed] [Google Scholar]
- Ebenezer IS. Baclofen pretreatment attenuates the suppressant effect of intraperitoneal administration of cholecystokinin (CCK) on food intake in rats. Brain Res Bull. 1996;41:269–271. doi: 10.1016/s0361-9230(96)00188-8. [DOI] [PubMed] [Google Scholar]
- Ebenezer IS, Prabhaker M. The effects of intraperitoneal administration of the GABA(B) receptor agonist baclofen on food intake in CFLP and C57BL/6 mice. Eur J Pharmacol. 2007;569:90–93. doi: 10.1016/j.ejphar.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Ebenezer IS, Pringle AK. The effect of systemic administration of baclofen on food intake in rats. Neuropharmacology. 1992;31:39–42. doi: 10.1016/0028-3908(92)90158-l. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Mathé JM, Chergui K, Engberg G, Svensson TH. GABA(B) receptor-mediated modulation of the firing pattern of ventral tegmental area dopamine neurons in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2002;365(3):173–180. doi: 10.1007/s00210-001-0519-5. [DOI] [PubMed] [Google Scholar]
- Fantino M, Hosotte J, Apfelbaum M. An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Physiol. 1986;251:R91–R96. doi: 10.1152/ajpregu.1986.251.1.R91. [DOI] [PubMed] [Google Scholar]
- Gayle JL, Fitzgibbon ML, Martinovich Z. A preliminary analysis of binge episodes: comparison of a treatment-seeking sample of Black and White women. Eat Behav. 2004;5:303–313. doi: 10.1016/j.eatbeh.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Glass MJ, O’Hare E, Cleary JP, Billington CJ, Levine AS. The effect of naloxone on food-motivated behavior in the obese Zucker rat. Psychopharmocology (Berl) 1999;131:378–384. doi: 10.1007/s002130050847. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Grace MK, Cleary JP, Billington CJ, Levine AS. Naloxone's effect on meal microstructure of sucrose and cornstarch diets. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1605–R1612. doi: 10.1152/ajpregu.2001.281.5.R1605. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Majchrzak MJ. Centrally administered opioid peptides stimulate saccharin intake in nondeprived rats. Pharmacol Biochem Behav. 1989;33(4):805–810. doi: 10.1016/0091-3057(89)90474-7. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Manaois M, Osincup D, Wells B, Buse C. Naloxone attenuates incubated sucrose craving in rats. Psychopharmacology (Berl) 2007;194:537–544. doi: 10.1007/s00213-007-0868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MM, Moss DE. Persistence of binge-eating pattern after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disord. 1997;22:411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286(1):R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- Heusner AA. Body size and energy metabolism. Ann Rev Nutr. 1985;5:267–293. doi: 10.1146/annurev.nu.05.070185.001411. [DOI] [PubMed] [Google Scholar]
- Higgs S, Barber DJ. Effects of baclofen on feeding behavior examined in the runway. Progress in neuropsychopharmacology and biological psychiatry. 2004;28:405–408. doi: 10.1016/j.pnpbp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hsiao S, Smith GP. Raclopride reduces sucrose preference in the rat. Pharmacol Biochem Behav. 1995;50(1):121–125. doi: 10.1016/0091-3057(95)00315-n. [DOI] [PubMed] [Google Scholar]
- Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30(1):1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Jimerson DC, Lesem MD, Kaye WH, Brewerton TD. Low serotonin and dopamine metabolite concentrations in cerebrospinal fluid from bulimic patients with frequent binge episodes. Arch Gen Psychiatry. 1992;49:132–138. doi: 10.1001/archpsyc.1992.01820020052007. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Ballenger JC, Lydiard RB, Stuart GW, Laraia MT, O'Neil P, Fossey MD, Stevens V, Lesser S, Hsu G. CSF monoamine levels in normal-weight bulimia: evidence for abnormal noradrenergic activity. Am J Psychiatry. 1990;147:225–229. doi: 10.1176/ajp.147.2.225. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Cooper SJ. Naloxone attenuation of sham feeding is modified by manipulation of sucrose concentration. Physiol Behav. 1988;44(4–5):491–494. doi: 10.1016/0031-9384(88)90310-1. [DOI] [PubMed] [Google Scholar]
- Levine AS, Weldon DT, Grace M, Cleary JP, Billington CJ. Naloxone blocks that portion of feeding driven by sweet taste in food-restricted rats. Am J Physiol. 1995;268:R248–R252. doi: 10.1152/ajpregu.1995.268.1.R248. [DOI] [PubMed] [Google Scholar]
- Lucas F, Sclafani A. Hyperphagia in rats produced by a mixture of fat and sugar. Physiol Behav. 1990;47:51–55. doi: 10.1016/0031-9384(90)90041-2. [DOI] [PubMed] [Google Scholar]
- Lucas F, Ackroff K, Sclafani A. Dietary fat-induced hyperphagia in rats as a function of fat type and physical form. Physiol Behav. 1989;45(5):937–946. doi: 10.1016/0031-9384(89)90218-7. [DOI] [PubMed] [Google Scholar]
- Marrazzi MA, Bacon JP, Kinzie J, Luby ED. Naltrexone use in the treatment of anorexia nervosa and bulimia nervosa. Int Clin Psychopharmacol. 1995;10:163–172. doi: 10.1097/00004850-199510030-00005. [DOI] [PubMed] [Google Scholar]
- Mathes CM, Ferrara M, Rowland NE. Cannabinoid-1 receptor antagonists reduce caloric intake by decreasing palatable diet selection in a novel dessert protocol in female rats. Am J Physiol Regul Integr Comp Physiol. 2008 Jul;295(1):R67–R75. doi: 10.1152/ajpregu.00150.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushige T, Matsumura S, Yoneda T, Tsuzuki S, Inoue K, Fushiki T. Daily increase of fat ingestion mediated via mu-opioid receptor signaling pathway. Biomed Res. 2006;27:259–263. doi: 10.2220/biomedres.27.259. [DOI] [PubMed] [Google Scholar]
- Muscat R, Willner P. Effects of dopamine receptor antagonists on sucrose consumption and preference. Psychopharmacology (Berl) 1989;99:98–102. doi: 10.1007/BF00634461. [DOI] [PubMed] [Google Scholar]
- Naleid AM, Grimm JW, Kessler DA, Sipols AJ, Aliakbari S, Bennett JL, Wells J, Figlewicz DP. Deconstructing the vanilla milkshake: the dominant effect of sucrose on self-administration of nutrient-flavor mixtures. Appetite. 2008;50:128–138. doi: 10.1016/j.appet.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academy Press; 1995. [Google Scholar]
- Patel SM, Ebenezer IS. The effects of chronic intraperitoneal administration of the GABAB receptor agonist baclofen on food intake in rats. Eur J Pharmacol. 2008;593:68–72. doi: 10.1016/j.ejphar.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Phillips G, Willner P, Muscat R. Reward-dependent suppression or facilitation of consummatory behaviour by raclopride. Psychopharmacology (Berl) 1991a;105(3):355–360. doi: 10.1007/BF02244430. [DOI] [PubMed] [Google Scholar]
- Phillips G, Willner P, Muscat R. Suppression or facilitation of operant behaviour by raclopride dependent on concentration of sucrose reward. Psychopharmacology (Berl) 1991b;105(2):239–246. doi: 10.1007/BF02244316. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Rao RE, Wojnicki FH, Coupland J, Ghosh S, Corwin RL. Baclofen, raclopride, and naltrexone differentially reduce solid fat emulsion intake under limited access conditions. Pharmacol Biochem Behav. 2008;89:581–590. doi: 10.1016/j.pbb.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reas DL, Grilo CM. Review and meta-analysis of pharmacotherapy for binge-eating disorder. Obesity. 2008;16:2024–2038. doi: 10.1038/oby.2008.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DR, Friedman MI. Diet composition alters the acceptance of fat by rats. Appetite. 1990;14:219–230. doi: 10.1016/0195-6663(90)90089-q. [DOI] [PubMed] [Google Scholar]
- Rockwood GA, Reid LD. Naloxone modifies sugar-water intake in rats drinking with open gastric fistulas. Physiol Behav. 1982;29(6):1175–1178. doi: 10.1016/0031-9384(82)90316-x. [DOI] [PubMed] [Google Scholar]
- Sato I, Arima H, Ozaki N, Ozaki N, Watanabe M, Goto M, Shimizu H, Hayashi M, Banno R, Nagasaki H, Oiso Y. Peripherally administered baclofen reduced food intake and body weight in db/db as well as diet-induced obese mice. FEBS Lett. 2007;581:4857–4864. doi: 10.1016/j.febslet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Schneider LH, Gibbs J, Smith GP. D-2 selective receptor antagonists suppress sucrose sham feeding in rats. Brain Res Bull. 1986;17(4):605–611. doi: 10.1016/0361-9230(86)90231-5. [DOI] [PubMed] [Google Scholar]
- Schneider LH, Davis JD, Watson CA, Smith GP. Similar effect of raclopride and reduced sucrose concentration on the microstructure of sucrose sham feeding. Eur J Pharmacol. 1990;186(1):61–70. doi: 10.1016/0014-2999(90)94060-b. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Reinforcement value of sucrose measured by progressive ratio operant licking in the rat. Phys Behav. 2003;79:663–670. doi: 10.1016/s0031-9384(03)00143-4. [DOI] [PubMed] [Google Scholar]
- Smith GP. Dopamine and food reward. Prog Psychobiol Physiol Psychol. 1995;16:83–144. [PubMed] [Google Scholar]
- Thomas MA, Rice HB, Weinstock D, Corwin RL. Effects of aging on food intake and body composition in rats. Physiol Behav. 2002;76:487–500. doi: 10.1016/s0031-9384(02)00800-4. [DOI] [PubMed] [Google Scholar]
- Weatherford SC, Smith GP, Melville LD. D-1 and D-2 receptor antagonists decrease corn oil sham feeding in rats. Physiol Behav. 1988;44:569–572. doi: 10.1016/0031-9384(88)90320-4. [DOI] [PubMed] [Google Scholar]
- Weatherford SC, Greenberg D, Gibbs J, Smith JD. The potency of D-1 and D-2 receptor antagonists is inversely related to the reward value of sham-fed corn oil and sucrose in rats. Pharmcol Biochem Behav. 1990;37:317–323. doi: 10.1016/0091-3057(90)90341-e. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FHE, Roberts DCS, Corwin RLW. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food deprived rats. Pharmacol Biochem Behav. 2006;84:197–206. doi: 10.1016/j.pbb.2006.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FHE, Stine JG, Corwin RL. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Phys Behav. 2007;92:566–574. doi: 10.1016/j.physbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Wojnicki FHE, Johnson DS, Corwin RLW. Access conditions affect binge-type shortening consumption in rats. Physiol Behav. 2008a;95:649–657. doi: 10.1016/j.physbeh.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnicki FHE, Charny G, Corwin RL. Binge-type behavior in rats consuming trans-fat-free shortening. Physiol Behav. 2008b;94:627–629. doi: 10.1016/j.physbeh.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WZ, Silva RM, Sclafani A, Delamater AR, Bodnar RJ. Role of D(1) and D(2) dopamine receptors in the acquisition and expression of flavor-preference conditioning in sham-feeding rats. Pharmacol Biochem Behav. 2000;67(3):537–544. doi: 10.1016/s0091-3057(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99(2):267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Walsh BT. Pharmacologic treatment of eating disorders. Can. J. Psychiatry. 2002;47:227–234. doi: 10.1177/070674370204700302. [DOI] [PubMed] [Google Scholar]