Abstract
MR technology is constantly improving. Functional imaging techniques such as MR spectroscopy, perfusion imaging, diffusion imaging and diffusion tensor imaging are increasingly utilized in the pediatric patient with a brain tumor. However estimate of tumor size remains the primary imaging endpoint in the evaluation of response to treatment; validation across institutions and vendor platforms of MRI functional parameters is necessary given the relative uncommon occurrence of brain tumors in children. Pediatric neuroimaging can be challenging, and the optimal way to image children with CNS tumors is not uniformly applied across all centers. Application of proper scanning techniques and validation of functional imaging techniques should lead to improved care of children with CNS tumors
Throughout the past century, great strides have been made in the use of imaging techniques to localize and characterize brain tumors. Major milestones include the first reported injection of air into the lateral ventricles by Dandy in 19181; the routine use of pneumoencephalography and angiography starting in the 1940s; the arrival of computed tomography (CT) starting in the early 1970s; and, in the mid 1980s, the advent of magnetic resonance imaging (MRI) techniques. Our ability to evaluate pediatric patients with tumors of the central nervous system (CNS) continues to evolve.
MRI is by far the most useful and employed technique nowadays, due to its exquisite intrinsic ability to differentiate tissue character. In addition, MRI techniques can estimate multiple tissue parameters –based on anatomy and functional/metabolic features of cerebral tissue. This paper will focus on recent advances in the field of MRI, and will illustrate many of the multiparametric capabilities of MRI. The technical and logistical challenges we face in increasing the application of these new techniques to the pediatric patient will be outlined.
Tumor definition, staging
In the majority of children with CNS neoplasms, MRI can clearly define the location, size and extent of a tumor. With the use of multichannel phased array coils, images of very high anatomical resolution can now routinely be obtained. These high quality images can be put to use in the operative setting, allowing the neurosurgeon the ability to perform image-guided surgical navigation. MR images are acquired intraoperatively (intraoperative MR) in an increasing number of medical centers.
In most centers, tumor size is assessed manually and reported using linear scalar measurements; to report tumor size and assess tumor response, bi-directional measurements (maximal length and perpendicular width) are preferred over unidimentional approaches (e.g. RECIST criteria). At many research centers, software programs are available to determine tumor volumes (3D) in a semiautomatic fashion. These techniques depend on clear margins between tumor tissue and surrounding parenchyma; this is simple for focal, well marginated tumors. However, in the case of non-focal tumors (such as malignant gliomas, optic pathway gliomas and brainstem gliomas) conventional MRI techniques do not reliably define the extent of tumor and surrounding infiltration.
The MR imaging features of most pediatric brain tumors have been presented in the literature and are beyond the scope of this publication. At times these features are such that the tumor-type can be reliably suggested pre-operatively – for example dysembryoplastic neuroepithelial tumor, pilocytic astrocytoma and pleomorphic xanthoastrocytoma among others2. Specific features have been described for medulloblastoma and PNET using FLAIR and diffusion imaging3. The various types of gliomas arising within the brainstem have been categorized, with clear impact on choice of treatment and outcome prediction4. In some cases, MR imaging characteristics are deemed sufficient to determine a specific tumor diagnosis, obviating the need for surgical biopsy prior to treatment - as in the case of infiltrative brainstem gliomas, optic pathway gliomas (especially in NF-1 patients) and certain tectal tumors.
In most instances, the ability to stage the extent of a tumor, and especially to detect the presence of subarachnoid metastatic disease, is more important than the pre-operative determination of a particular tumor type. Pediatric brain tumors have a propensity to disseminate throughout the subarachnoid spaces, often at the time of presentation; this is reliably demonstrated on high quality screening MRI studies of the CNS. Clear outcome differences have been demonstrated in regards to pediatric brain tumors depending on the presence or absence of dissemination. Following a resection or treatment, MRI can detect recurrences prior to symptoms. The impact of such surveillance scanning on outcome has been questioned, especially in regards to the surveillance of medulloblastoma following treatment; however, with improved surgical techniques and novel therapeutic regiments available for the salvage of recurrent tumors, the detection of smaller, pre symptomatic recurrent tumor leads to improved outcomes5,6.
Anatomic imaging features such as the ones outlined so far in this text do not address many questions that come up in the care of a patient with a neoplasm of the CNS - issues such as histological grading of a tumor; selection of optimal biopsy sites and/or surgical approach; assessment of response to novel therapeutic approaches such as antiangiogenic agents; assessment of the long-term effects of treatment on cognition. For these reasons, novel functional imaging techniques are increasingly applied to the patient with a brain tumor. These functional imaging techniques can reflect tissue biochemistry (MR spectroscopy), capillary density (susceptibility perfusion techniques), capillary permeability (relaxivity imaging), and cellular density / tissue microarchitecture (diffusion imaging). In addition, cortical activation imaging (fMRI) using blood oxygen level dependent techniques can identify various loci of eloquent cerebral cortical function. fMRI is increasingly used in preoperative tumor assessment - to guide the surgeon away from vital cortical regions7; fMRI, in conjunction with MR Spectroscopy, has also been used in the treatment planning of malignant gliomas to improve radiation delivery using intensity-modulated radiation therapy7. Finally, molecular imaging, long the domain of PET and SPECT imaging, is finding increasing applications with MR imaging techniques. Thus, the portfolio of MR functional imaging techniques is constantly expanding. A discussion of principles, applications and current limitations of these MRI techniques follows, with the exclusion of BOLD fMRI and molecular imaging
MR Spectroscopy
MR spectroscopy (MRS) provides information about the presence and amount of hydrogen molecules attached to different cerebral molecular compounds. Hydrogen atoms located on different molecular compounds display intrinsic differences in resonant frequencies due to their differing molecular environment. A spectrum of resonant frequencies (or chemical shift) vs. amplitude (concentration) can be generated that reflects the presence and concentration of these molecular compounds. The four most relevant compounds identified within cerebral tissue include N-acetyl-aspartate (NAA, a neuronal marker), choline (a marker of membrane-associated compounds), creatine and phosphocreatine (energy metabolites), and lactate (a by-product of cerebral metabolism). MRS can differentiate normal from tumor tissue, as tumor tissue in general shows elevation of choline and decrease in NAA.
MR spectroscopy has shown potential to differentiate tumor grades: in a study of 21 adult patients with grade II and III gliomas, choline and NAA levels correlated with both the degree of cellular density and the proliferative index9. Pre-operative differentiation of grade II astrocytomas and oligodendrogliomas vs III astrocytomas was also feasible in a different study of 26 adult patients10. In children, MRS is able to differentiate tumor from radiation necrosis (figure 1); monitor tumor response versus progression, and prognosticate overall survival11,12,13. In a study of 60 children with brain tumors, the metabolic profiles defined with quantitative short echo time MRS revealed features of pediatric brain tumors that improve preoperative diagnosis14.
Figure 1.
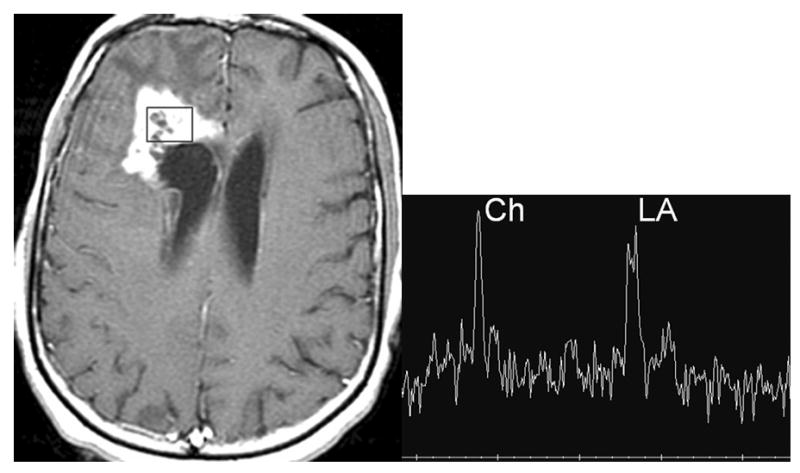
A patient treated for a malignant frontal glioma with suspected radiation necrosis. Axial contrast enhanced T1 image (A) shows an enhancing lesion next to the ventricle. Single voxel long echo MRS (B) shows marked elevation of choline (peak on the left) and lactate (double peak on the right) indicative of tumor rather than radiation necrosis. The box in A indicates the position on the MRS.
Spectroscopy can be performed with single-voxel or multi-voxel techniques. Single voxel studies have faster acquisition times and better spectral resolution, and require a fairly large voxel size (6-8 cm3); tissue composition within the voxel must be homogeneous, otherwise partial volume averaging of tissue of various compositions (normal tissue, tumor, necrotic tissue…) occurs and the spectrum has little value. In single voxel imaging the prescription (choosing the localization) of the voxel is totally operator dependent (therefore not very reproducible). Spectroscopic analysis of a large volume of cerebral tissue with multiple small voxels (1 cm3) is feasible with multi-voxel studies (or magnetic resonance spectroscopic imaging, MRSI). MRSI allows for a more comprehensive examination of specific areas or regions of interest. MRSI techniques are available in both 2 dimensions (single slice) and 3 dimensions (multi slice or whole brain), and can be prescribed in a fairly standardized fashion. MRSI of adult cerebral malignant gliomas can separate areas of high tumor grade from regions of lower grade15. With MRSI, MR spectroscopy becomes much more operator independent, therefore can generate more reproducible data. Given the increased commercial availability of MRSI software, the reliability and value of MRSI in the evaluation and monitoring of pediatric CNS tumors could realistically be addressed in a multicenter study.
Perfusion Imaging
Perfusion imaging can be performed with techniques based on dynamic susceptibility contrast (DSC) or based on vascular permeability. DSC techniques assess cerebral tissue perfusion following a dynamic injection of gadolinium. During the first past transit of contrast through cerebral capillaries, which lasts anywhere from 5 to 15 seconds, gadolinium is restricted to the intravascular space. This intravascular restriction gives rise to signal inhomogeneity within image voxels (i.e. a susceptibility effect), which is reflected as signal loss on susceptibility sensitive T2 (T2*) imaging techniques. A curve showing concentration of gadolinium over time can be generated. The concentration of gadolinium is a direct reflection of the capillary density. From this, the regional cerebral blood volume (rCBV) can be determined, which corresponds to the volume of blood within brain tissue (figure 2). rCBV can reflect the neovascularization associated with tumor growth (tumor angiogenesis); in adults with glial tumors, angiogenesis is highly correlated to tumor grade, and the rCBV of most high-grade glial tumors is greater than that of low grade tumors16. Using a combined first pass gradient-echo and spin-echo method, tumor grade was predicted with high accuracy in a study of 73 adult patients with gliomas17; in addition, the mean vessel diameter mVD can be determined with this combined gradient-spin echo approach (mVD and tumor grade were highly correlated17). In a study of 35 patients (including 5 children) with low grade gliomas (excluding pilocytic astrocytoma), DSC perfusion imaging helped to identify those tumors that progressed rapidly and a subset of gliomas that had a propensity for malignant transformation18.
Figure 2.
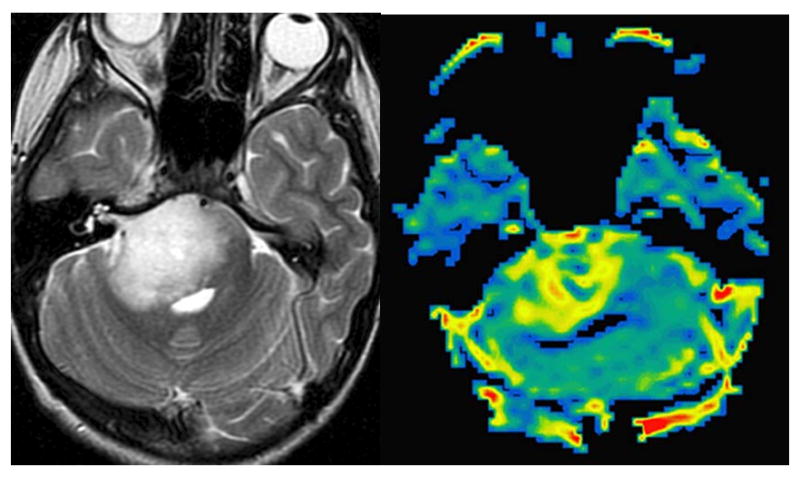
Axial T2 weighted image (A) of a patient with an infiltrative brain stem glioma. rCBV map of a DSC perfusion study (B) reveals significant increase in signal, reflective of high capillary density.
DSC perfusion imaging can be used to select stereotactic biopsy sites by guiding the surgeon to the more capillary dense (i.e. malignant) portion of a tumor. Tumor response to treatment can be assessed; and the differentiation of tumor from radiation necrosis/scar tissue can be made, as the latter has low capillary density, versus high density for a malignant tumor. The application of perfusion imaging to evaluate children on antiangiogenic agents is of great interest: when effective, these cytostatic agents may not cause a decrease in tumor size, but rather prevent progression. So the standard anatomic measurements of tumor size used to assess the efficacy of cytotoxic regimens may not apply to antiangiogenic regimens; however, in response to an antiangiogenic agent, a decrease in capillary density / rCBV demonstrable by DSC imaging is expected19.
For many reasons, DSC perfusion studies do not consistently generate usable data. First, a larger bore intravenous catheter must be placed (preferably in the antecubital fossa) to safely deliver the injection of 3 to 4 cc/sec of gadolinium necessary to generate a well-defined first pass curve; that is not always easy, especially in sicker children, or those on steroids. Second, susceptibility artifacts are not negligible, as DSC perfusion utilizes an echo planar technique (echo planar images are prone to image distortion by susceptibility artifacts); studies preformed on children with dental braces or in the presence of significant amounts of intracranial/intratumoral hemorrhage are often useless. Finally, gadolinium contrast can leak through tumor capillaries; such leakage can result in an under estimation of rCBV, as the computations performed to process the susceptibility T2* signal assumes that the contrast remains confined to the capillary20.
Permeability based perfusion techniques take advantage of leakage of gadolinium through tumor vessel walls. In healthy cerebral capillaries, the blood brain barrier prevents gadolinium leakage and the injected contrast does not leak into the interstitium. Tumor capillaries have disrupted or absent blood brain barriers, which allows contrast to leak outside the vessel into tissue interstitium. The rate of leakage is proportional to the permeability of the capillary wall. Permeability imaging, also known as relaxivity imaging, is a T1-weighted technique in which a curve of dynamic signal intensity (i.e. enhancement) versus time is generated. The slope of enhancement reflects the degree of permeability of capillaries. The intensity of initial enhancement reflects tissue vascularity: fractional blood volume (fBV) can be extrapolated21. fVB is a fairly good estimate of the rCBV data obtained with susceptibility perfusion22. In adult patients with malignant glial tumors, this technique has been shown of use in grading gliomas and in assessment of response to anti-angiogenic therapies21.
Permeability-based perfusion techniques have many advantages over DSC techniques: studies have superior anatomic resolution, and do not suffer from the drawback of T2* susceptibility artifacts observed on the echo planar images. Further, injection rates are much more acceptable, 1 to 2 cc/sec, thus more tolerable to the patient; central lines or PICC lines can be used for venous access. As permeability based approaches can be more consistently performed, they may become the perfusion technique of choice for multicenter CNS tumor evaluations.
Diffusion Imaging
Diffusion imaging (DI) investigates molecular translational movements (i.e. Brownian motion) of water molecules. Brownian motion produces signal loss proportional to the degree of molecular translation/diffusion. Within the cerebral milieu, cellular membranes and other macromolecular structures restrict water diffusion, which results in increased signal on DI (less diffusion means less signal loss secondary to spin dephasing). Thus signal on diffusion images can reflect cellular density and microarchitecture. The apparent diffusion coefficient (ADC) is a measure of the ability of tissue to restrict water diffusion. ADC decreases with increasing cellularity (figure 3); in a study of 37 adult patients with malignant cerebral gliomas (grade III and IV), the ADC was shown to adversely correlate with the grade of tumor and the cellular proliferation Ki-67 labeling index23. Further, increase in ADC, reflecting decreased in cellularity, has been observed as tumors that respond to treatment.
Figure 3.
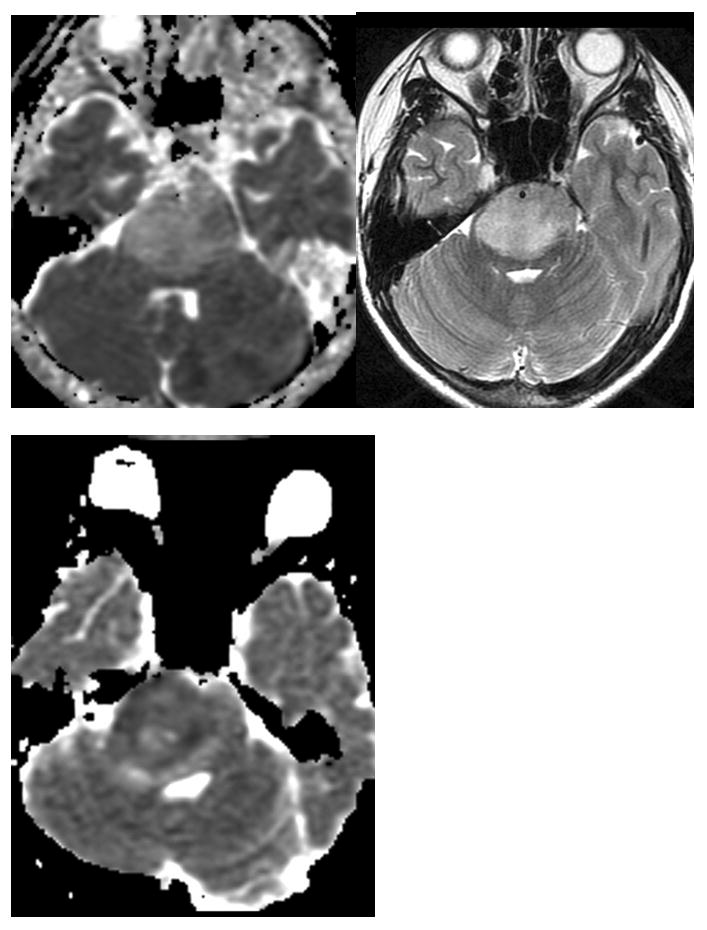
Assessment of tumor cellularity with DI. Axial T2 image (A) of a patient with a brain stem infiltrative glioma. The ADC image of this patient (B) shows elevated signal consistent with unrestricted diffusion and low cellularity. ADC image (C) of a different patient (same patient as figure 2) shows low signal consistent with restricted diffusion and higher cellularity.
DI can also be used to assess the microscopic structural integrity of white matter tracts as the movement of water is restricted by the myelin sheath surrounding the axon and by the cell membrane. Diffusion of water molecules is less restricted in a direction parallel to the long axis of axons, and more restricted in the direction perpendicular to the axon. Anisotropy, an aggregate measure of the magnitude of displacement across all three dimensions, is directly correlated with the degree of myelination as assessed using myelin-sensitive histological staining24. Using information gleaned from the size, shape, orientation, and distribution (or special pattern) of diffusion ellipsoids within an imaged volume, one can characterize features of water diffusion within a voxel – the diffusion tensor. A voxel by voxel analysis of anisotropy can be performed: Diffusion Tensor Imaging (DTI). Connectivity maps (i.e. tractography) can also be generated, which demonstrate the long association (axonal) pathways that convey cortical connections as track like structures.
DTI tractography is used to plan the surgical approach to a focal tumor: important association pathways (e.g. pyramidal, optic…) can be localized in relation to a tumor; this helps the surgeon to avoid injury to these white matter tracts. DTI also shows promise as tool to evaluate for the presence and extent of tumoral invasion: whereas focal (well-marginated) tumors should displace but not disrupt axons outside of their borders, the microscopic tumor extension of infiltrative tumors is expected to disrupt local axonal structures25,26,27.
DTI evaluation of axonal integrity has also been used in the assessment of global white matter injury in children who have received whole brain radiation. In a study of survivors of acute lymphoblastic leukemia, loss of anisotropy within cerebral white matter was highly correlated with IQ scores, independent of age28.
Current DTI tractography techniques have their limitations. The exact nature of the tract like structures depicted is not known (they surely do not represent axons proper). Resolution is limited, as only the larger association pathways can be identified. Resolution is lost when fibres with intersecting trajectories (crossing fibres) are present within a voxel. More powerful diffusion techniques are currently being investigated, including diffusion spectrum imaging (DSI), which can resolve crossing fibres at the scale of single MRI voxels; DSI should vastly increase the intrinsic resolution of diffusion imaging techniques, and lead to improved applications in surgical planning, evaluation of tumor infiltration and assessment of white matter damage29.
Best practice in pediatric neurooncologic imaging
Tumors of the CNS are relatively uncommon in infants and children. In addition, a wide variety of histologies is encountered, including unique pathological entities seen in infants and young children. This wide histological variety is reflected by a wide range of imaging appearances of the lesions at MRI. As a result, it is difficult for individual centers to collect a significant experience for a particular tumor type. Multicenter collaboration is clearly needed in pediatric neurooncology to advance the field and improve outcomes. The Children's Oncology Group (COG) includes most pediatric oncologic centers in North America and many beyond; large numbers of patients can be entered in treatment protocols - in which imaging research questions can be addressed. A smaller collaborative croup, the Pediatric Brain Tumor Consortium (PBTC), has been in existence for approximately a decade, and currently includes 11 centers. Uniform imaging protocols are now required as part of the evaluation and follow up of patients entered on COG and PBTC treatment protocols. Uniformity of imaging acquisition parameters allows for more rigorous central review of studies.
Central radiographic reviews by designated radiologists are mandated in most COG and PBTC study protocols of children with CNS tumors. While central analysis of anatomic images is fairly straightforward, analysis of functional imaging studies is more problematic: some functional techniques are operator dependent (e.g. single voxel spectroscopy, susceptibility perfusion); not all techniques are DICOM compliant, so compatibility across vendor platforms becomes an issue. Further, there are regular upgrades by MRI vendors which can render prior study results obsolete, therefore historical data is difficult to accumulate.
Centralized reviews of MR studies performed and interpreted across a broad range of academic and regional/community centers have also demonstrated that there exists a wide range in the quality of imaging studies performed on children, with demonstrable impact on patient outcome. For example, in the COG 9961 study (a study of over 400 children with average risk medulloblastoma requiring a clear demonstration by MR imaging of small (< 1.5 cm2) or absent tumor residual following surgery and absence of tumor dissemination / metastatic disease), a central review of all the CNS imaging studies was performed by 2 pediatric neuroradiologists. At central review, 15% of MRI studies acquired at the time of patient entry to the treatment protocol were judged to be of inadequate quality to properly evaluate for residual tumor and tumor dissemination; poor technique or excessive motion were the most common causes. Further, 7% of registered patients were declared not eligible due to the presence of significant residual tumor or presence of tumor dissemination not identified at the time of initial interpretation. The event free survival for these patient groups was significantly poorer than for the patients who were properly evaluated and entered on study. The five-year event free survival was 83% for the appropriately entered patients; 73% for the patients with study entry MRI scans judged to be of inadequate quality; 75% for patients with local residual tumor > 1.5 cm2; and 36% for the patients with disseminated disease30.
Technically well performed studies that are correctly interpreted by physicians who have a good understanding of study eligibility criteria are integral to collaborative studies. With greater understanding of these issues by the imaging and oncologic communities, improved patient outcome and more rapid completion of treatment studies can be expected (as fewer patients with inadequate staging studies or patients who are not eligible would be entered on study). Upfront, rapid centralized assessment (rapid review) by designated study radiologist of imaging studies acquired on patients entered in collaborative studies may even be indicated.
Conclusion
MR technology is improving, with faster gradients, more sensitive coils and improved software. Evaluation of tumor size remains the primary imaging endpoint in the evaluation of response for most pediatric patients with CNS neoplasms. As evident from the literature, multiple MRI functional parameters have been validated, primarily in adult CNS tumor studies; these techniques need validation across institutions and vendor platforms in the pediatric population. Greater use of these functional parameters will increase the impact of imaging on patient care. More powerful magnets (3 Tesla instead of 1.5 Tesla) will further advance the field of imaging; advantages of the 3 Tesla systems include greater speed and resolution, increased sensitivity to gadolinium enhancement and improved spectral resolution (with MRS).
The optimal way to image children with CNS tumor is not uniformly applied across all centers. Pediatric neuroimaging can be challenging, primarily because of issues of sedation, patient size and physiologic motion. Dissemination throughout the subarachnoid spaces is not always recognized. Criteria for study eligibility and response determination are not always understood. Increased awareness of these factors, validation of functional imaging techniques and the increased availability of electronic distribution of imaging studies should lead to improved care of children with CNS tumors.
References
- 1.Dandy WE. Ventriculography following the injection of air into the cerebral ventricles. Ann Surg. 1918;68:5–11. doi: 10.1097/00000658-191807000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koeller KK, Henry JM. Superficial gliomas: radiologic-pathologic correlation. Radiographics. 2001;21:1533–1556. doi: 10.1148/radiographics.21.6.g01nv051533. [DOI] [PubMed] [Google Scholar]
- 3.Zimmerman RA, Haselgrove JC, Bilaniuk LT, Hunter JV. Diffusion-weighted imaging and fluid attenuated inversion recovery imaging in the evaluation of primitive neuroectodermal tumors. Neuroradiology. 2001;43:927–933. doi: 10.1007/s002340100603. [DOI] [PubMed] [Google Scholar]
- 4.Fischbein NJ, Prados MD, Wara W, et al. Radiologic classification of brain stem tumors: correlation of magnetic resonance imaging appearance with clinical outcome. Pediatr Neurosurg. 1996;24:9–23. doi: 10.1159/000121010. [DOI] [PubMed] [Google Scholar]
- 5.Shaw DW, Geyer JR, Berger MS, et al. Asymptomatic Recurrence Detection With Surveillance Scanning in Children With Medulloblastoma. J Clin Oncol. 1997;15:1811–1813. doi: 10.1200/JCO.1997.15.5.1811. [DOI] [PubMed] [Google Scholar]
- 6.Saunders DE, Hayward RD, Phipps KP, et al. Surveillance neuroimaging of intracranial medulloblastoma in children: how effective, how often, and for how long? J Neurosurg. 2003;99:280–286. doi: 10.3171/jns.2003.99.2.0280. [DOI] [PubMed] [Google Scholar]
- 7.Bogomolny DL, Petrovich NM, Hou BL, et al. Functional MRI in the brain tumor patient. Top Magn Reson Imaging. 2004;15:325–335. doi: 10.1097/00002142-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Narayana A, Chang J, Thakur S, et al. Use of MR spectroscopy and functional imaging in the treatment planning of gliomas. Br J Radiol. 2007;80:347–354. doi: 10.1259/bjr/65349468. [DOI] [PubMed] [Google Scholar]
- 9.McKnight TR, Lamborn KR, Love TD, et al. Correlation of magnetic resonance spectroscopic and growth characteristics within Grades II and III gliomas. J Neurosurg. 2007;106:660–666. doi: 10.3171/jns.2007.106.4.660. [DOI] [PubMed] [Google Scholar]
- 10.Stadlbauer A, Gruber S, Nimsky C, et al. Preoperative grading of gliomas by using metabolite quantification with high-spatial-resolution proton MR spectroscopic imaging. Radiology. 2006;238:958–969. doi: 10.1148/radiol.2382041896. [DOI] [PubMed] [Google Scholar]
- 11.Tzika A, Zurakowski D, Poussaint TY, et al. Proton magnetic spectroscopic imaging of the child's brain: the response of tumors to treatment. Neuroradiology. 2001;43:169–177. doi: 10.1007/s002340000454. [DOI] [PubMed] [Google Scholar]
- 12.Warren KE, Frank JA, Black JL, et al. Proton magnetic resonance spectroscopic imaging in children with recurrent primary brain tumors. J Clin Oncol. 2000;18:1020–1026. doi: 10.1200/JCO.2000.18.5.1020. [DOI] [PubMed] [Google Scholar]
- 13.Tzika AA, Astrakas LG, Zarifi MK, et al. Spectroscopic and Perfusion Magnetic Resonance Imaging Predictors of Progression in Pediatric Brain Tumors. Cancer. 2004;100:1246–56. doi: 10.1002/cncr.20096. [DOI] [PubMed] [Google Scholar]
- 14.Panigrahy A, Krieger MD, Gonzalez-Gomez I, et al. Quantitative short echo time 1H-MR spectroscopy of untreated pediatric brain tumors: preoperative diagnosis and characterization. AJNR Am J Neuroradiol. 2006;27:560–572. [PMC free article] [PubMed] [Google Scholar]
- 15.Pirzkall A, McKnight TR, Graves EE, et al. MR-spectroscopy guided target delineation for high-grade gliomas. Int J Radiat Oncol Biol Phys. 2001;50:915–928. doi: 10.1016/s0360-3016(01)01548-6. [DOI] [PubMed] [Google Scholar]
- 16.Aronen HJ, Gazit IE, Louis DN, et al. Cerebral Blood Volume Maps of Gliomas: Comparison with Tumor Grade and Histologic Findings. Radiology. 1994;191:41–51. doi: 10.1148/radiology.191.1.8134596. [DOI] [PubMed] [Google Scholar]
- 17.Schmainda KM, Rand SD, Joseph AM, et al. Characterization of a first-pass gradient-echo spin-echo method to predict brain tumor grade and angiogenesis. AJNR Am J Neuroradiol. 2004;25:1524–1532. [PMC free article] [PubMed] [Google Scholar]
- 18.Law M, Oh S, Babb JS, et al. Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging--prediction of patient clinical response. Radiology. 2006;238:658–67. doi: 10.1148/radiol.2382042180. [DOI] [PubMed] [Google Scholar]
- 19.Pollack IF, Jakacki RI, Blaney SM, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro Oncol. 2007;9:145–60. doi: 10.1215/15228517-2006-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cha S, Knopp EA, Johnson G, et al. Intracranial Mass Lesions: Dynamic Contrast-enhanced Susceptibility-weighted Echo-planar Perfusion MR Imaging. Radiology. 2002;223:11–29. doi: 10.1148/radiol.2231010594. [DOI] [PubMed] [Google Scholar]
- 21.Roberts HC, Roberts TP, Brasch RC, Dillon WP. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol. 2000;21:891–899. [PMC free article] [PubMed] [Google Scholar]
- 22.Haroon HA, Patankar TF, Zhu XP, et al. Comparison of cerebral blood volume maps generated from T2* and T1 weighted MRI data in intra-axial cerebral tumours. Br J Radiol. 2007;80:161–168. doi: 10.1259/bjr/17112059. [DOI] [PubMed] [Google Scholar]
- 23.Higano S, Yun X, Kumabe T, et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241:839–46. doi: 10.1148/radiol.2413051276. [DOI] [PubMed] [Google Scholar]
- 24.Miller JH, McKinstry RC, Philip JV, et al. Diffusion-tensor MR imaging of normal brain maturation: a guide to structural development and myelination. AJR am J Roentgenol. 2003;180:851–859. doi: 10.2214/ajr.180.3.1800851. [DOI] [PubMed] [Google Scholar]
- 25.Jellison BJ, Field AS, Medow J, et al. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004;25:356–69. [PMC free article] [PubMed] [Google Scholar]
- 26.Talos IF, Zou KH, Kikinis R, Jolesz FA. Volumetric assessment of tumor infiltration of adjacent white matter based on anatomic MRI and diffusion tensor tractography. Acad Radiol. 2007;14:431–436. doi: 10.1016/j.acra.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helton KJ, Phillips NS, Khan RB, et al. Diffusion tensor imaging of tract involvement in children with pontine tumors. AJNR Am J Neuroradiol. 2006;27:786–93. [PMC free article] [PubMed] [Google Scholar]
- 28.Khong PL, Leung LH, Chan GC, et al. White matter anisotropy in childhood medulloblastoma survivors: association with neurotoxicity risk factors. Radiology. 2005;236:647–652. doi: 10.1148/radiol.2362041066. [DOI] [PubMed] [Google Scholar]
- 29.Schmahmann JD, Pandya DN, Wang R, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–53. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 30.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]


