Abstract
Background
Hypertensive disorders of pregnancy are more frequent in primiparous women, but may be more severe in multiparas. We examined trends in pregnancy-induced hypertension (PIH)-related stillbirth and neonatal mortality and explored whether mortality varied by parity and maternal race.
Methods
We carried out a population-based study of 57 million singleton live- and stillbirths (24-46 weeks) in the United States between 1990 and 2004. We estimated rates and adjusted odds ratio (OR) of stillbirth and neonatal death in relation to PIH, comparing births in 1990-91 with 2003-04.
Results
PIH increased from 3.0% in 1990 to 3.9% in 2004. In both 1990-91 and 2003-04 periods, PIH was associated with an increased risk of stillbirth and neonatal death. We explored this in more detail in 2003-04, and observed that the increased risk of stillbirth was higher in women having their second or higher order births (OR=2.24, 95% confidence interval (CI)=2.11-2.37) compared with women having their first birth (OR=1.52, 95% CI=1.40-1.64). Patterns were similar for neonatal death (OR=1.30, 95% CI=1.18-1.43 in first and OR=1.64, 95% CI=1.51-1.78 in second or higher order births). Among multiparas, the association between PIH and stillbirth was stronger in Blacks (OR=2.93, 95% CI=2.66-3.22) than Whites (OR=1.98, 95% CI=1.83-2.14).
Conclusions
A substantial burden of stillbirth and neonatal mortality is associated with PIH, especially among multiparas women, which may be due to more severe disease women, or to a higher burden of underlying disease.
Hypertensive disorders of pregnancy complicate 5-8% of pregnancies and are associated with increased risks of perinatal morbidity and mortality,1-3 and maternal morbidity.4 Preeclampsia, part of the spectrum of pregnancy-induced hypertension (PIH), is typically a disease of the first pregnancy, with a reduction in incidence among multiparas.5 The occurrence of PIH in one pregnancy is a strong predictor of recurrence in the next pregnancy,6-9 and recurrent hypertensive disorders is associated with substantially higher risks of adverse perinatal outcomes.10
A study of first births in Norway between 1967-03 showed that the risk of stillbirth in relation to preeclampsia declined substantially between the periods 1967-78 (odds ratio 4.4) and 1991-03 (odds ratio 1.4).11 The rate of births at <32 weeks among preeclamptic mothers tripled during the study period (from 1.6% in 1967-78 to 5.0% in 1991-03), but the decline in stillbirth was not paralleled by a substantial increase in post-natal death.11 Whether similar patterns are evident among multiparous women remains unexplored.
Rates of both preeclampsia and gestational hypertension have increased in the United States,12 which underscores the importance of evaluating the burden of perinatal mortality associated with these conditions. Among women who had had preeclampsia in a previous pregnancy, severe gestational hypertension without proteinuria in the next pregnancy was associated with increased risk of preterm birth and small for gestational babies than among women who developed recurrent mild preeclampsia.13 Hauth et al.14 reported higher rates of infant and maternal morbidities in healthy nulliparas who developed severe hypertension or preeclampsia. These studies were, however, relatively small, and mortality could not be properly assessed.
Because limited attention has been devoted to pregnancy outcomes in multiparous women with hypertensive disorders of pregnancy, we investigated fetal and neonatal mortality in first and higher order singleton births in the United States. We compared births in 1990-91 to those in 2003-04, among Black and White women. These data, however, do not allow a distinction between preeclampsia and hypertension without proteinuria, and we thus used pregnancy-induced hypertension, comprising hypertension with and without proteinuria, as the entity of interest.
METHODS
Data source and composition of the analytic sample
We used the United States linked natality and infant mortality data for the period 1990-2004.15 These data are based on birth and infant death certificates, and include data on maternal characteristics, medical and obstetrical complications, and fetal and infant outcomes. Infant deaths were not linked to the corresponding live births in the 1992-94 periods. To provide the most recent estimates, we focused our analysis on the period 2003-04, using 1990-91 as comparison.
Pregnancy-induced hypertension and perinatal mortality
The data include a check-box diagnoses of hypertension with or without proteinuria (i.e., PIH), as well as diagnoses of chronic hypertension and eclampsia, but a separate diagnosis of preeclampsia was unavailable. The 0.2% of women with a diagnosis of eclampsia was combined with the PIH group. Classification of preeclampsia diagnosis was modified in 1996 by the American College of Obstetricians and Gynecologists. The revision required that all cases meet criteria for hypertension in the presence of proteinuria after the 20th week of gestation.16
As PIH is rare in early pregnancy, we considered as stillbirths all registered fetal deaths with a gestational age of 24 weeks or higher. Neonatal deaths were defined as live births (from week 24 of gestation and onward) registered as having died within the first 28 days of life. Gestational age was based on the date of last menstrual period. If only the day of the menstrual period was missing, gestational age was statistically imputed by the National Center for Health Statistics prior to releasing the data.17
Data exclusions
We restricted this analysis to singleton births. We excluded 1.1% (n=651,522) pregnancies with missing gestational age (i.e., missing month or year of menstrual date.) We also excluded 1.3% (n=773,935) of births with a gestational age <24 weeks or ≥47 weeks (n=104,724, 0.2%), and an additional 1.8% (n=972,718) of births with missing PIH status. After all exclusions, 56,987,638 singleton live births and 221,321 stillbirths remained.
Statistical analysis
We used logistic regression models to estimate odds ratio (OR) of stillbirth and neonatal death in relation to PIH, adjusted for geographic region, maternal age, education, race, and marital status, chronic hypertension and diabetes (categorized as shown in table 1.) To evaluate the recent impact of PIH on stillbirth and neonatal deaths, we restricted the majority of the analyses to the 2003-04 period. We performed all analyses separately for first and higher order births. In most analyses, we further categorized the latter group as second, third or fourth of higher order births. For 2003-04, we additionally estimated the OR of mortality following PIH in White and Black women.
Table 1.
Maternal socio-demographic characteristics and pregnancy-induced hypertension (PIH): United States 1990 to 2004
| Maternal characteristics |
Total population |
Preg-induced hypertension |
Normotensive Women |
Missing data on PIH |
|---|---|---|---|---|
| Number of births | 57,208,959 | 2,053,497 | 55,155,462 | 972,718 |
| Geographic region | ||||
| Northeast | 17.0 | 15.7 | 17.1 | 21.7 |
| Midwest | 22.7 | 23.9 | 22.7 | 10.8 |
| South | 35.9 | 41.1 | 35.7 | 53.3 |
| West | 24.4 | 19.4 | 24.6 | 14.2 |
| Maternal age (years) | ||||
| <20 | 12.3 | 14.6 | 12.2 | 12.9 |
| 20-24 | 25.5 | 25.8 | 25.5 | 25.5 |
| 25-29 | 27.7 | 26.6 | 27.8 | 28.0 |
| 30-34 | 22.6 | 20.5 | 22.7 | 22.4 |
| 35-39 | 10.0 | 10.0 | 10.0 | 9.5 |
| ≥40 | 2.0 | 2.5 | 1.9 | 1.8 |
| Education (years) | ||||
| <8 | 6.1 | 4.2 | 6.2 | 5.7 |
| 8-12 | 49.9 | 50.0 | 49.9 | 51.9 |
| 13-15 | 35.6 | 37.9 | 35.5 | 34.4 |
| ≥16 | 8.4 | 7.9 | 8.4 | 8.1 |
| Primiparity | 33.6 | 49.1 | 33.0 | 33.6 |
| Single marital status | 32.4 | 33.4 | 32.4 | 29.7 |
| Maternal smoking† | 13.7 | 11.0 | 13.8 | 15.3 |
| Chronic hypertension | 0.7 | 0.7 | 0.7 | 0.7 |
| Diabetes | 2.8 | 6.7 | 2.6 | 2.5 |
| Maternal race | ||||
| Whites | 79.0 | 79.2 | 79.0 | 78.4 |
| Blacks | 15.6 | 17.0 | 15.5 | 15.3 |
| Other races | 5.5 | 3.8 | 5.5 | 6.3 |
Data from the state of California excluded since this state did not report smoking data Geographic regions were classified as Northeast (Maine, New Hampshire, Vermont, Rhode Island, Massachusetts, Connecticut, New York, New Jersey, and Pennsylvania), Midwest (Michigan, Ohio, Illinois, Indiana, Wisconsin, Minnesota, Iowa, Missouri, North Dakota, South Dakota, Nebraska, and Kansas), South (Delaware, Maryland, District of Columbia, Virginia, West Virginia, North Carolina, South Carolina, Georgia, Florida, Kentucky, Tennessee, Alabama, Mississippi, Arkansas, Louisiana, Oklahoma, and Texas) and West (Montana, Idaho, Wyoming, Colorado, New Mexico, Arizona, Utah, Nevada, Washington, Oregon, California, Hawaii, and Alaska.)
Since maternal smoking, associated with a lower risk of preeclampsia18-20 and higher risk of perinatal mortality,21 is not reported in the California birth certificates, we performed a sub-analysis excluding California for the 2003-04 period, , to assess whether further adjustment for smoking altered the associations between PIH and mortality.
RESULTS
Women with PIH were more frequently younger (<20 years) or older (≥35 years) than women without PIH (Table 1). Rates of smoking were lower among women diagnosed with PIH, and a slightly higher proportion of black women than white women were diagnosed with PIH. PIH was reported in 5.3% and 2.8% of primiparous and multiparous women, respectively.
Differences in rates of stillbirth and neonatal mortality between women with and without PIH were greater in 1990-91 than in 2003-04 among both first and higher-order births (Table 2.) There was a higher rate of obstetrical interventions at preterm gestations in women diagnosed with PIH than in women without such diagnosis.
Table 2.
Pregnancy outcomes by pregnancy-induced hypertension (PIH) status: United States 1990-91 and 2003-04
| First births |
Second or higher-order births |
|||
|---|---|---|---|---|
| PIH | Non-PIH | PIH | Non-PIH | |
| Total number of births | ||||
| 1990-91 | 116,555 | 2,464,328 | 110,617 | 4,994,079 |
| 2003-04 | 142,342 | 2,483,383 | 158,094 | 5,031,247 |
| Stillbirths per 1,000 total births | ||||
| 1990-91 | 6.1 | 3.9 | 10.2 | 4.0 |
| 2003-04 | 4.8 | 3.2 | 7.8 | 3.4 |
| Number of live births | ||||
| 1990-91 | 115,840 | 2,454,658 | 109,493 | 4,974,191 |
| 2003-04 | 141,663 | 2,475,488 | 156,859 | 5,014,180 |
| Neonatal deaths per 1,000 live births | ||||
| 1990-91 | 4.2 | 3.1 | 4.7 | 3.1 |
| 2003-04 | 3.0 | 2.3 | 3.7 | 2.2 |
| Birthweight (g), mean (standard dev)† | ||||
| 1990-91 | 3,152 (729) | 3,324 (556) | 3,196 (768) | 3,391 (577) |
| 2003-04 | 3,034 (728) | 3,284 (552) | 3,083 (759) | 3,356 (555) |
| Live births delivered at <37 wks (%) | ||||
| 1990-91 | 17.0 | 9.4 | 18.0 | 9.6 |
| 2003-04 | 22.4 | 9.8 | 24.6 | 10.4 |
| All births delivered at <37 wks (%) | ||||
| 1990-91 | 17.3 | 9.6 | 18.5 | 9.8 |
| 2003-04 | 22.6 | 10.0 | 25.0 | 10.6 |
| Live births delivered at <32 wks (%) | ||||
| 1990-91 | 3.2 | 1.6 | 3.5 | 1.6 |
| 2003-04 | 4.0 | 1.5 | 4.3 | 1.4 |
| All births delivered at <32 wks (%) | ||||
| 1990-91 | 3.4 | 1.7 | 3.9 | 1.8 |
| 2003-04 | 4.2 | 1.6 | 4.6 | 1.5 |
| Cesarean delivery (%)‡ | ||||
| 1990-91 | 42.5 | 22.5 | 38.1 | 20.6 |
| 2003-04 | 43.0 | 26.1 | 40.3 | 26.3 |
| Labor induction (%)‡ | ||||
| 1990-91 | 36.4 | 10.3 | 30.0 | 9.1 |
| 2003-04 | 52.2 | 22.4 | 41.6 | 19.5 |
| Preterm obstetrical intervention (%)* | ||||
| 1990-91 | 12.4 | 2.5 | 12.8 | 2.9 |
| 2003-04 | 18.1 | 3.8 | 19.6 | 4.6 |
Cesarean delivery and labor induction includes interventions at all gestational ages
Preterm obstetrical intervention includes labor induction, cesarean or both at preterm gestational ages
PIH was associated with a higher risk of stillbirth, especially among second and higher order births, both in the 1990-91 and 2003-04 periods (Table 3.) When birth order was more finely stratified, the risk of stillbirth in relation to PIH was fairly similar among women with their second, third or fourth or higher-order births. The risk of neonatal death following PIH was also elevated, although the difference between first and higher order births was less marked (Table 3.)
Table 3.
Association between pregnancy-induced hypertension and risks of stillbirth and neonatal death by birth order and period: United States 1990-91 and 2003-04
| Stillbirth per 1,000 total births |
Neonatal deaths per 1,000 live births |
|||||
|---|---|---|---|---|---|---|
| PIH | No PIH | OR (95% CI) | PIH | No PIH | OR (95% CI) | |
| 1990-91 period | ||||||
| Number of births | n=227,172 | n=7,458,407 | n=225,333 | n=7,428,849 | ||
| First births | 6.1 | 3.9 | 1.37 (1.24-1.50) | 4.2 | 3.1 | 1.32 (1.20-1.44) |
| Second or higher order births | 10.2 | 4.0 | 2.20 (2.03-2.37) | 4.7 | 3.1 | 1.46 (1.43-1.59) |
| Second births | 8.6 | 3.3 | 2.19 (1.95-2.46) | 4.2 | 2.7 | 1.49 (1.31-1.71) |
| Third births | 9.8 | 3.7 | 2.31 (1.98-2.68) | 4.5 | 3.0 | 1.46 (1.22-1.74) |
| Fourth or higher-order births | 13.2 | 5.2 | 2.20 (1.19-2.51) | 5.8 | 3.8 | 1.47 (1.25-1.71) |
| P-value for linear trend | <0.001 | 0.006 | 0.249 | <0.001 | ||
| 2003-04 period | ||||||
| Number of births | n=300,436 | n=7,514,630 | n=298,522 | n=7,489,668 | ||
| First births | 4.8 | 3.2 | 1.52 (1.40-1.64) | 3.0 | 2.3 | 1.30 (1.18-1.43) |
| Second or higher order births | 7.8 | 3.4 | 2.24 (2.11-2.37) | 3.7 | 2.2 | 1.64 (1.51-1.78) |
| Second births | 6.1 | 2.7 | 2.26 (2.05-2.49) | 3.3 | 1.9 | 1.68 (1.47-1.91) |
| Third births | 7.4 | 3.2 | 2.29 (2.04-2.58) | 3.3 | 2.0 | 1.56 (1.31-1.85) |
| Fourth or higher-order births | 12.7 | 5.4 | 2.30 (2.11-2.51) | 5.0 | 2.9 | 1.68 (1.46-1.92) |
| P-value for linear trend | <0.001 | <0.001 | 0.020 | <0.001 | ||
PIH, pregnancy-induced hypertension; OR, odds ratio; CI, confidence interval
Odds ratios are adjusted for geographic region, maternal age, maternal education, maternal race, marital status, diabetes, and chronic hypertension
Blacks had consistently higher mortality rates, and multiparous Blacks had a higher OR of stillbirth following PIH compared with multiparous Whites (Table 4.) In 2003-04, rates of preterm obstetrical interventions among primiparous White and Black women without PIH were 3.6% and 5.4%, respectively, and 4.3% and 6.7% among multiparous Whites and Blacks, respectively. These rates were 4 to 5-fold higher among women diagnosed with PIH.
Table 4.
Odds ratios of stillbirth and neonatal death following pregnancy-induced hypertension by birth order and maternal race: United States, 2003-04
| Stillbirth per 1,000 total births |
Neonatal deaths per 1,000 live births |
|||||
|---|---|---|---|---|---|---|
| PIH | No PIH | OR (95% CI) | PIH | No PIH | OR (95% CI) | |
| White women | ||||||
| Number of births | n=235,629 | n=5,873,271 | n=234,513 | n=5,856,238 | ||
| First births | 3.7 | 2.8 | 1.35 (1.22-1.49) | 2.7 | 2.2 | 1.32 (1.18-1.48) |
| Second or higher order births | 5.7 | 2.9 | 1.98 (1.83-2.14) | 3.2 | 2.0 | 1.62 (1.46-1.80) |
| Second births | 4.5 | 2.3 | 1.98 (1.75-2.24) | 2.8 | 1.7 | 1.61 (1.38-1.89) |
| Third births | 5.6 | 2.8 | 2.02 (1.74-2.35) | 2.9 | 1.8 | 1.63 (1.32-2.01) |
| Fourth or higher-order births | 9.1 | 4.6 | 1.94 (1.72-2.19) | 4.1 | 2.5 | 1.63 (1.36-1.94) |
| P-value for linear trend | <0.001 | <0.001 | <0.001 | 0.099 | ||
| Black women | ||||||
| Number of births | n=50,484 | n=1,101,405 | n=49,779 | n=1,095,283 | ||
| First births | 10.9 | 5.3 | 2.11 (1.83-2.41) | 4.5 | 3.7 | 1.20 (0.98-1.48) |
| Second or higher order births | 16.1 | 5.7 | 2.93 (2.66-3.22) | 6.1 | 3.6 | 1.64 (1.41-1.91) |
| Second births | 13.0 | 4.8 | 2.68 (2.26-3.19) | 5.6 | 3.3 | 1.67 (1.29-2.17) |
| Third births | 15.5 | 5.2 | 3.14 (2.59-3.80) | 5.2 | 3.4 | 1.48 (1.07-2.05) |
| Fourth or higher-order births | 23.0 | 7.9 | 2.95 (2.58-3.37) | 7.4 | 4.3 | 1.74 (1.39-2.19) |
| P-value for linear trend | <0.001 | <0.001 | <0.001 | 0.125 | ||
PIH, pregnancy-induced hypertension; OR, odds ratio; CI, confidence interval
Odds ratios are adjusted for geographic region, maternal age, maternal education, marital status, diabetes, and chronic hypertension
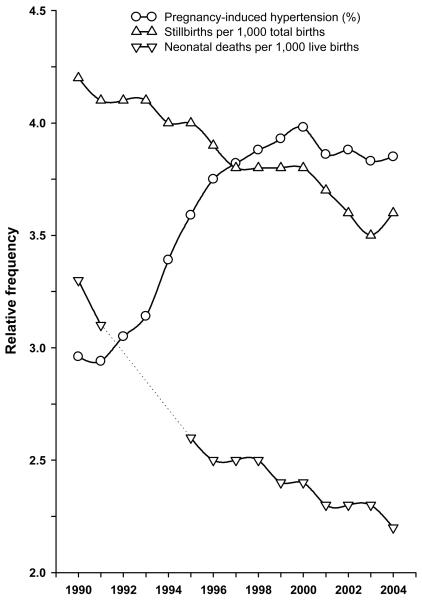
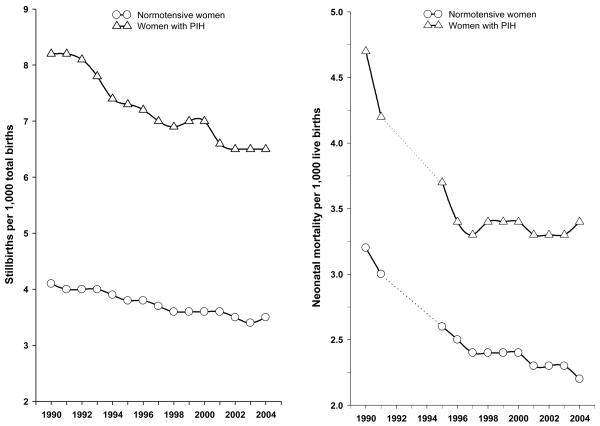
Trends in PIH and risks of stillbirth and neonatal mortality between 1990 and 2004 are shown in Figure 1 (appendix). When stratified by PIH status (Fig 2, appendix), the patterns of declining stillbirths and neonatal deaths were similar, although stillbirth showed a steeper decline among women with PIH.
DISCUSSION
In this study, pregnancy-induced hypertension was associated with higher risks of stillbirth and neonatal mortality, especially in second and higher order births. Among normotensive births, on the other hand, rates of both stillbirth and neonatal death were similar between first and higher order births although, as expected, the risk was lowest in second births and highest in fourth or higher order births. Although absolute mortality rates have declined since 1990-91, odds ratios for mortality in relation to PIH changed little. Black women had higher absolute rates of stillbirth and neonatal death compared with Whites, but similar odds ratios of neonatal death. Among multiparas, Blacks had a higher odds ratio for stillbirth than Whites.
The substantial difference in PIH-associated mortality between first and later births may be due to more severe disease in multiparas, or to underlying maternal characteristics, including chronic diseases and, importantly, obesity.22-25 For instance, Catov and colleagues26 reported that these conditions, plus multiple pregnancy, obesity, and having had preeclampsia in the previous pregnancy accounted for over 50% of preeclampsia among multiparas.
It has been estimated that about 25% of preeclampsia cases in multiparous women occur in those with a prior preeclampsia,27,28 and this proportion may be as high as 38% among women who experienced severe preeclampsia in a previous pregnancy.26 Overweight and obesity contribute significantly to preeclampsia,26,29-32 and prepregnancy body-mass index (BMI) is associated with increased blood pressure throughout pregnancy.33 High BMI is also a risk factor for perinatal death,31,34,35 and women with the highest risk of stillbirth may thus not be those with a previous diagnosis of PIH but, instead, women with other risk factors, or with chronic diseases that were not evident in the first pregnancy. Type 2 diabetes, a risk factor for hypertensive disorders in pregnancy36,37 and stillbirth38 may have also contributed to our results. Findings from a randomized trial showed that untreated gestational diabetes was associated with increased preeclampsia risk, but the association with perinatal death did not reach statistical significance.39 Obesity, diabetes, and subclinical maternal disease may be more common among multiparous women and could thus result in a different etiology and severity of preeclampsia in multiparas compared with primiparas.40 In this analysis, despite adjustments for pre-existing hypertension and diabetes, the increased risk of stillbirth among multiparas with PIH persisted. It is possible that these conditions are not well registered in the US birth certificates, rendering adjustment ineffectual. In an analysis restricted to women with PIH, we also examined whether the increased mortality among multiparas could be explained by gestational age and birth weight; adjustment for these factors did not explain the findings (not shown).
Strengths and limitations of the data and consistency of associations
This analysis includes virtually all singleton births in the US, and we were able to adjust for a number of covariates. Although we only used smoking in a sub-analysis (due to sizeable missing data), the consistency in associations between the full analysis and those restricted to states that reported smoking data is reassuring. When we included a missing indicator for smoking, the results was again essentially unchanged (not shown.)
The main limitation of this analysis is that the diagnosis of PIH in our data did not permit distinctions between preeclampsia and gestational hypertension. The overall rates of PIH in our analysis were lower than those reported in a recent study,12 suggesting the possibility of some under-reporting of PIH. A diagnosis of PIH is more likely to be accurate for babies who died than for babies who survived, and this may have biased the estimates away from the null. However, this mechanism is unlikely to explain the differences we observed between primiparas and multiparas. Furthermore, among primiparas, the estimated ORs were lower than those seen in Norway.11 This may be because while the Norwegian study was based on preeclampsia, ours included women diagnosed with either preeclampsia or non-proteinuric hypertension. Thus, some attenuation of the association between PIH and mortality, given that PIH is likely a less severe disease than preeclampsia, is likely in our study.. If misclassification of PIH were non-differential, even a moderately low specificity would bias estimates towards the null, while low sensitivity would have little effect in case of a rare disease such as PIH. If severity of PIH influences the likelihood that a complication is recorded, the diagnosis we used may more closely reflect preeclampsia than gestational hypertension.
Errors in menstrual estimate of gestational age could have affected our comparisons to some extent.41,42 However, we used gestational age for descriptive purposes, except when trying to assess whether it mediated the risk among strata of parity. The main analyses were not adjusted for gestational age, as it is an intermediate variable in the association between PIH and mortality.11
Deaths are generally accurately reported, although a small proportion remained unlinked in these data (<2%), and the distinction between stillbirth and (early) neonatal death may not always be clear-cut. However, these mechanisms are unlikely to have any substantial impact on our estimates. Residual confounding due to unmeasured factors may also have influenced our estimates to some extent.
Conclusions
Our findings suggest that women with second and higher order births following PIH have a higher risk of stillbirth than first-order births following PIH, particularly among Blacks. The elevated risk of mortality in multiparous women may be due to more severe disease or to the underlying characteristics of multiparas, and attempts should be made to explore this in studies where these predictors are available.
ACKNOWLEDGMENTS
We thank Aimee D'Aloisio and Mathew Longnecker from the National Institute of Environmental Health Sciences, National Institutes of Health, NC; and Anthony Vintzileos from the Department of Obstetrics and Gynecology, Winthrop-University Hospital, NY for their review and comments on an earlier draft of the manuscript.
Figure 1.
Temporal trend in rates of pregnancy-induced hypertension, stillbirth, and neonatal mortality in the United States, 1990 to 2004 (neonatal deaths were not linked to the corresponding live births data file in 1992-94, and so are represented by dashed lines.)
Figure 2.
Temporal trends in stillbirth (left panel) and neonatal mortality (right panel) in relation to pregnancy-induced hypertension status in the United States, 1990 to 2004 (neonatal deaths were not linked to the corresponding live births data file in 1992-94, and so are represented by dashed lines.)
REFERENCES
- 1.Ananth CV, Peedicayil A, Savitz DA. Effect of hypertensive diseases in pregnancy on birthweight, gestational duration, and small-for-gestational-age births. Epidemiology. 1995;6(4):391–5. doi: 10.1097/00001648-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Xiao R, Sorensen TK, Williams MA, Luthy DA. Influence of pre-eclampsia on fetal growth. J Matern Fetal Neonatal Med. 2003;13(3):157–62. doi: 10.1080/jmf.13.3.157.162. [DOI] [PubMed] [Google Scholar]
- 3.Xiong X, Buekens P, Pridjian G, Fraser WD. Pregnancy-induced hypertension and perinatal mortality. J Reprod Med. 2007;52(5):402–6. [PubMed] [Google Scholar]
- 4.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22(2):203–12. doi: 10.1081/PRG-120021066. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Zeisler J, Hatch MC, Berkowitz G. Epidemiology of pregnancy-induced hypertension. Epidemiol Rev. 1997;19(2):218–32. doi: 10.1093/oxfordjournals.epirev.a017954. [DOI] [PubMed] [Google Scholar]
- 6.Ananth CV, Peltier MR, Chavez MR, Kirby RS, Getahun D, Vintzileos AM. Recurrence of ischemic placental disease. Obstet Gynecol. 2007;110(1):128–33. doi: 10.1097/01.AOG.0000266983.77458.71. [DOI] [PubMed] [Google Scholar]
- 7.Basso O, Christensen K, Olsen J. Higher risk of pre-eclampsia after change of partner. An effect of longer interpregnancy intervals? Epidemiology. 2001;12(6):624–9. doi: 10.1097/00001648-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Lie RT, Rasmussen S, Brunborg H, Gjessing HK, Lie-Nielsen E, Irgens LM. Fetal and maternal contributions to risk of pre-eclampsia: population based study. BMJ. 1998;316(7141):1343–7. doi: 10.1136/bmj.316.7141.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostello D, Kallogjeri D, Tungsiripat R, Leet T. Recurrence of preeclampsia: effects of gestational age at delivery of the first pregnancy, body mass index, paternity, and interval between births. Am J Obstet Gynecol. 2008;199(1):55 e1–7. doi: 10.1016/j.ajog.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 10.Hnat MD, Sibai BM, Caritis S, Hauth J, Lindheimer MD, MacPherson C, VanDorsten JP, Landon M, Miodovnik M, Paul R, Meis P, Thurnau G, Dombrowski M. Perinatal outcome in women with recurrent preeclampsia compared with women who develop preeclampsia as nulliparas. Am J Obstet Gynecol. 2002;186(3):422–6. doi: 10.1067/mob.2002.120280. [DOI] [PubMed] [Google Scholar]
- 11.Basso O, Rasmussen S, Weinberg CR, Wilcox AJ, Irgens LM, Skjaerven R. Trends in fetal and infant survival following preeclampsia. JAMA. 2006;296(11):1357–62. doi: 10.1001/jama.296.11.1357. [DOI] [PubMed] [Google Scholar]
- 12.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens. 2008;21(5):521–6. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- 13.Buchbinder A, Sibai BM, Caritis S, Macpherson C, Hauth J, Lindheimer MD, Klebanoff M, Vandorsten P, Landon M, Paul R, Miodovnik M, Meis P, Thurnau G. Adverse perinatal outcomes are significantly higher in severe gestational hypertension than in mild preeclampsia. Am J Obstet Gynecol. 2002;186(1):66–71. doi: 10.1067/mob.2002.120080. [DOI] [PubMed] [Google Scholar]
- 14.Hauth JC, Ewell MG, Levine RJ, Esterlitz JR, Sibai B, Curet LB, Catalano PM, Morris CD. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95(1):24–8. doi: 10.1016/s0029-7844(99)00462-7. [DOI] [PubMed] [Google Scholar]
- 15.Taffel SM, Ventura SJ, Gay GA. Revised U.S. certificate of birth--new opportunities for research on birth outcome. Birth. 1989;16(4):188–93. doi: 10.1111/j.1523-536x.1989.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 16.ACOG technical bulletin Hypertension in pregnancy. Number 219--January 1996 (replaces no. 91, February 1986). Committee on Technical Bulletins of the American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1996;53(2):175–83. [PubMed] [Google Scholar]
- 17.Taffel S, Johnson D, Heuser R. A method of imputing length of gestation on birth certificates. Vital Health Stat. 1982;2(93):1–11. [PubMed] [Google Scholar]
- 18.Conde-Agudelo A, Althabe F, Belizan JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am J Obstet Gynecol. 1999;181(4):1026–35. doi: 10.1016/s0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- 19.England L, Zhang J. Smoking and risk of preeclampsia: a systematic review. Front Biosci. 2007;12:2471–83. doi: 10.2741/2248. [DOI] [PubMed] [Google Scholar]
- 20.Peltier MR, Ananth CV. Is the association of maternal smoking and pregnancy-induced hypertension dependent on fetal growth? Am J Obstet Gynecol. 2007;196(6):532 e1–6. doi: 10.1016/j.ajog.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 21.Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–40. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 22.Sibai BM, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155(5):1011–6. doi: 10.1016/0002-9378(86)90336-4. [DOI] [PubMed] [Google Scholar]
- 23.Eskenazi B, Fenster L, Sidney S. A multivariate analysis of risk factors for preeclampsia. JAMA. 1991;266(2):237–41. [PubMed] [Google Scholar]
- 24.Eskenazi B, Fenster L, Sidney S, Elkin EP. Fetal growth retardation in infants of multiparous and nulliparous women with preeclampsia. Am J Obstet Gynecol. 1993;169(5):1112–8. doi: 10.1016/0002-9378(93)90265-k. [DOI] [PubMed] [Google Scholar]
- 25.Suhonen L, Teramo K. Hypertension and pre-eclampsia in women with gestational glucose intolerance. Acta Obstet Gynecol Scand. 1993;72(4):269–72. doi: 10.3109/00016349309068036. [DOI] [PubMed] [Google Scholar]
- 26.Catov JM, Ness RB, Kip KE, Olsen J. Risk of early or severe pre-eclampsia related to pre-existing conditions. Int J Epidemiol. 2007;36(2):412–9. doi: 10.1093/ije/dyl271. [DOI] [PubMed] [Google Scholar]
- 27.Chesley LC, Annitto JE, Cosgrove RA. The remote prognosis of eclamptic women. Sixth periodic report. Am J Obstet Gynecol. 1976;124(1 Pt 1):446–459. doi: 10.1016/0002-9378(76)90168-x. [DOI] [PubMed] [Google Scholar]
- 28.Singh MM, Macgillivray I, Mahaffy RG. A study of the long-term effects of pre-eclampsia on blood pressure and renal function. J Obstet Gynaecol Br Commonw. 1974;81(11):903–6. doi: 10.1111/j.1471-0528.1974.tb00404.x. [DOI] [PubMed] [Google Scholar]
- 29.Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007;18(2):234–9. doi: 10.1097/01.ede.0000254119.99660.e7. [DOI] [PubMed] [Google Scholar]
- 30.Getahun D, Ananth CV, Oyelese Y, Chavez MR, Kirby RS, Smulian JC. Primary preeclampsia in the second pregnancy: effects of changes in prepregnancy body mass index between pregnancies. Obstet Gynecol. 2007;110(6):1319–25. doi: 10.1097/01.AOG.0000292090.40351.30. [DOI] [PubMed] [Google Scholar]
- 31.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103(2):219–24. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 32.Walsh SW. Obesity: a risk factor for preeclampsia. Trends Endocrinol Metab. 2007;18(10):365–70. doi: 10.1016/j.tem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Miller RS, Thompson ML, Williams MA. Trimester-specific blood pressure levels in relation to maternal pre-pregnancy body mass index. Paediatr Perinat Epidemiol. 2007;21(6):487–94. doi: 10.1111/j.1365-3016.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 34.Chu SY, Kim SY, Lau J, Schmid CH, Dietz PM, Callaghan WM, Curtis KM. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. 2007;197(3):223–8. doi: 10.1016/j.ajog.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 35.Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG. 2005;112(4):403–8. doi: 10.1111/j.1471-0528.2005.00437.x. [DOI] [PubMed] [Google Scholar]
- 36.Solomon CG, Graves SW, Greene MF, Seely EW. Glucose intolerance as a predictor of hypertension in pregnancy. Hypertension. 1994;23(6 Pt 1):717–21. doi: 10.1161/01.hyp.23.6.717. [DOI] [PubMed] [Google Scholar]
- 37.Sowers JR, Saleh AA, Sokol RJ. Hyperinsulinemia and insulin resistance are associated with preeclampsia in African-Americans. Am J Hypertens. 1995;8(1):1–4. doi: 10.1016/0895-7061(94)00166-9. [DOI] [PubMed] [Google Scholar]
- 38.Silver RM. Fetal death. Obstet Gynecol. 2007;109(1):153–67. doi: 10.1097/01.AOG.0000248537.89739.96. [DOI] [PubMed] [Google Scholar]
- 39.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 40.Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175(5):1365–70. doi: 10.1016/s0002-9378(96)70056-x. [DOI] [PubMed] [Google Scholar]
- 41.Gjessing HK, Skjaerven R, Wilcox AJ. Errors in gestational age: evidence of bleeding early in pregnancy. Am J Public Health. 1999;89(2):213–8. doi: 10.2105/ajph.89.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savitz DA, Terry JW, Jr., Dole N, Thorp JM, Jr., Siega-Riz AM, Herring AH. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187(6):1660–6. doi: 10.1067/mob.2002.127601. [DOI] [PubMed] [Google Scholar]