Abstract
CD4+CD25+ regulatory T cells (TRegs) are critical for the acquisition of peripheral allograft tolerance. However, it is unclear whether TRegs are capable of mediating alloantigen-specific suppressive effects and, hence, contributing to the specificity of the tolerant state. In the current report we have used the ABM TCR transgenic (Tg) system, a C57BL/6-derived strain in which CD4+ T cells directly recognize the allogeneic MHC-II molecule I-Abm12, to assess the capacity of TRegs to mediate allospecific effects. In these mice, 5–6% of Tg CD4+ T cells exhibit conventional markers of the TReg phenotype. ABM TRegs are more effective than wild-type polyclonal TRegs at suppressing effector immune responses directed against I-Abm12 alloantigen both in vitro and in vivo. In contrast, they are incapable of suppressing responses directed against third-party alloantigens unless these are expressed in the same allograft as I-Abm12. Taken together, our results indicate that in transplantation, TReg function is dependent on TCR stimulation, providing definitive evidence for their specificity in the regulation of alloimmune responses.
The emergence of T cell immunoregulation is considered the hallmark of peripheral allograft tolerance (1–5). Immunoregulatory networks active in tolerant recipients are characterized by donor specificity, capacity to mediate linked suppression, and dependence on the indirect pathway of allorecognition. Multiple reports have established that activation of CD4+CD25+ regulatory T cells (TRegs)5 constitutes an essential element of the immunoregulatory pathways that create peripheral allograft tolerance (6–8). In the absence of this T cell subset, a variety of potent tolerizing therapies lose their ability to induce tolerance (9, 10). Indeed, some of these therapies appear to be acting, at least in part, by directly modulating the function of TRegs (10). Despite the pre-eminence of TRegs in transplant tolerance models, our understanding of how these cells account for the major features of the tolerant state is still incomplete.
Bulk TReg populations are capable of establishing a variety of interactions with both self and foreign MHC:peptide complexes (11–13). Given the heterogeneity of TReg Ag recognition, mono-specific TCR transgenic (Tg) systems have been critical to understanding the specificity of TReg function in response to nominal Ags. For instance, the demonstration that after specific TCR stimulation, TReg suppression in vitro can be extended to bystander effector T cells (TEff) bearing different specificities was facilitated by the use of influenza hemagglutinin-specific TCR Tg TRegs (14). Similarly, the Ag-specific nature of TReg proliferation (15, 16) and suppressive function (17) in vivo was also demonstrated through the use of TCR Tg systems. In contrast to immune responses against nominal Ags, in the absence of a suitable TCR Tg allo-reactive system, the elucidation of TReg specificity in transplantation has been more difficult to achieve and is still controversial. A source for confusion has been the widespread use of lymphopenic adoptive transfer systems in which nonspecific suppression of homeostatic proliferation can mask the regulatory effects of polyclonal TRegs (18). In addition, in these models TRegs harvested from naive, alloantigen-inexperienced mice are capable of preventing TEff from rejecting MHC-mismatched allografts when cell transfer is performed at high ratios of TReg to TEff (10, 19, 20). This finding, which most likely reflects the inherent alloantigen cross-reactivity of TReg TCRs, can also be interpreted as indicating that alloantigen-specific TRegs are not required in transplantation tolerance. In contrast, we and others have shown that TRegs exhibit donor specificity, but only after alloantigen exposure in the presence of a tolerizing regimen (6, 8, 10), a phenomenon that is crucial for the induction of transplantation tolerance. It must be acknowledged, however, that these later experiments were not performed using a criss-cross design (21) and therefore cannot be considered unambiguous proof of specificity. Thus, elucidation of whether TRegs can mediate alloantigen-specific suppressive effects, which would be critical as a first step to understanding the mechanisms of donor specificity in transplantation tolerance, remains an unsolved question.
The ABM TCR Tg mouse is a C57BL/6 (I-Ab)-derived strain that expresses a Vα2.1 and a Vβ8.1 TCR specific for the intact class II molecule I-Abm12 (expressed on a variant strain of C57BL/6 called B6.C-H2bm12/KhEg, hereafter referred to as bm12) and does not recognize other alloantigens (22–24). This is, therefore, a CD4+ TCR Tg model of direct alloantigen presentation. I-Abm12 and I-Ab differ only at three amino acids in a span of five amino acids (25). Hence, bm12 and C57BL/6 mice have only a limited MHC class II mismatch, which is, nonetheless, sufficient to prompt rejection of bm12 skin allografts by C57BL/6 mice (26). In contrast, bm12 hearts are not acutely rejected by C57BL/6 recipients, although the grafts eventually develop severe arterial disease (chronic rejection) (24).
We have previously determined that ABM mice, in which 90–95% of peripheral CD4+ T cells express the Vα2.1/Vβ8.1 TCR Tg (24), spontaneously accept bm12 heart allografts, provided recipients have not been previously sensitized by the placement of bm12 skin allografts (24). In addition, long-term surviving bm12 heart allografts from ABM recipients exhibit only minimal signs of chronic rejection. Thus, despite the very high frequency of allo-reactive T cells, ABM recipients fail to acutely or chronically reject bm12 heart allografts. We report in this article that in ABM mice a small fraction of TCR Tg CD4+ T cells constitutively express CD25 and are bona fide TRegs. These allospecific regulatory T cells are powerfully suppressive both in vitro and in vivo and are responsible for the capacity of ABM mice to spontaneously accept bm12 hearts. Using this system we show that during alloimmune responses, TReg suppressive function is dependent on specific TCR stimulation. This suggests that one of the mechanisms contributing to the exquisite specificity of allograft tolerance could be the preferential activation of alloantigen-specific TRegs.
Materials and Methods
Mice
The ABM (anti-bm12) TCR Tg mice were generated by Dr. E. Palmer (University Hospital, Basel, Switzerland) (22). TEa CD4+ TCR Tg mice were provided by Dr. R. J. Noelle (Dartmouth Medical School, Lebanon, NH). The TEa TCR recognizes the I-E-derived peptide ASFEAQGLA NIAVDKA in the context of I-Ab, which is expressed in all APCs from H-2b/I-E+ strains (e.g., CB6F1, an F1 hybrid of C57BL/6 and BALB/c) (27). Bm12, CB6F1, BALB/c, C57BL/6, and C57BL/6 nude mice were purchased from The Jackson Laboratory. F1 (BALB/c × bm12) hybrids and ABM backcrossed into the Rag-2-deficient (Rag−/−) background were generated in our laboratory. Only Rag+/+ ABM mice were used to isolate TCR Tg CD4+CD25+ T cells. Mice were maintained under pathogen-free conditions at Beth Israel Deaconess Medical Center and were used at 6–8 wk of age. Animal experiments were approved by the Beth Israel Deaconess Medical Center institutional animal care committee.
Cell sorting
Single-cell suspensions, prepared from lymph nodes and spleens, were enriched for T cells using T cell enrichment columns (R&D Systems), and T cell subset sorting was achieved using a MoFlo cell sorter (DakoCytomation) after staining with fluorochrome-conjugated anti-CD25, anti-CD4, anti-Vα2.1, and anti-Vβ8.1 mAbs (all mAbs from BD Pharmingen). Purity was consistently >95% for CD4+CD25− and CD4+CD25+ T cell preparations and >90% for Vα2.1 and Vβ8.1 double-positive cells. T cell subsets from C57BL/6 or TEa mice were sorted based on CD4 and CD25 markers only.
Cell culture experiments
CD4+CD25− T cells (5 × 104) were cultured with 3 × 105 irradiated allogeneic splenocytes or 104 allogeneic bone marrow-derived mature DCs, with or without 5 × 104 of CD4+CD25+ T cells, and proliferation was measured by [3H]TdR incorporation. DCs were derived from bone marrow by culture for 6 days in RPMI 1640 plus 10% FCS, antibiotics, 50 μM 2-ME, and 10 ng/ml GM-CSF, with addition of LPS during the last 12 h, and were sorted based on high CD86 expression.
Real-time PCR
Real-time PCR was performed with the ABI 7700 sequence detector system using commercially designed primer/probe sets (Applied Biosystems). The expression of the target genes was normalized to that of the housekeeping gene GAPDH, and data were expressed as the relative fold difference between cDNA from the study samples and that from a calibrated sample.
Heterotropic cardiac transplantation
Cardiac transplants were performed in ABM recipients as previously described (28). In some cases thymectomized recipients were given 200 μg of rat anti-mouse CD25 mAb (PC61, 5.3, IgG1; ATCC TB222) i.p. 4 wk before transplantation. We have previously determined that at such doses, anti-CD25 mAb eliminates >80% of CD4+CD25+ T cells in secondary lymphoid tissues.
Adoptive cell transfer and skin transplantation
Lymphopenic C57BL/6 nude mice were injected with sorted CD4+CD25+ and/or CD4+CD25− T cells transferred at different cell ratios 1 day before skin allograft transplantation. Full-thickness trunk skin grafts from donor mice were then grafted onto the dorsum of adoptively transferred recipient mice.
Results
TCR Tg TRegs can be identified in the ABM Tg model
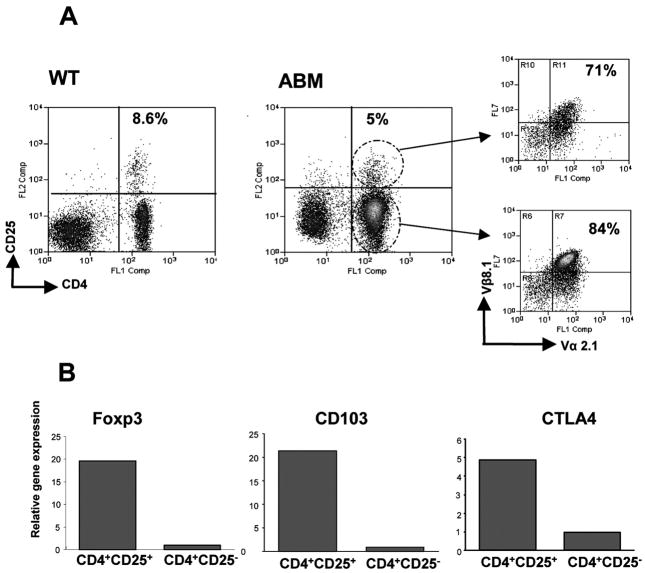
In ABM mice, a small fraction (mean, 5%; n > 20) of CD4+ T cells from secondary lymphoid tissues constitutively expressed CD25 as well as other markers typical of the TReg phenotype (Fig. 1). The proportion of CD4+CD25+ T cells was smaller than that observed in C57BL/6 controls (mean, 8.6%; n > 20; p < 0.005). Most of the CD4+CD25+ T cells present in ABM mice bore the Vα2.1+/Vβ8.1+ TCR (mean, 71%; n > 20), indicating that they expressed the anti-bm12 TCR Tg, although this proportion was lower than that in ABM CD4+CD25− T cells (mean, 84%; n > 20; p < 0.004; Fig. 1A). As previously reported for other mice carrying Tg TCRs (14, 29, 30), in ABM mice, CD4+CD25+ T cells were only found in conventional, not in Rag−/−, backgrounds (data not shown). This presumably reflects the need for endogenous TCR α-chain rearrangement for the thymic development of CD4+CD25+ Tg cells (30). To characterize ABM Tg CD4+CD25+ T cells, we quantified the expression of genes associated with TReg function. Resting CD4+CD25+, but not CD4+CD25−, ABM Tg T cells expressed high levels of CTLA4, Forkhead/winged helix transcription factor gene (FoxP3), and CD103 (Fig. 1B). No significant differences were found in the expression of these genes between Tg and control C57BL/6 CD4+CD25+ T cells (data not shown). Together, our results indicate that allospecific anti-bm12 Tg CD4+CD25+ T cells are present in ABM mice, and that these cells exhibit a similar phenotype to conventional TRegs.
FIGURE 1.
TCR Tg CD4+CD25+ T cells expressing markers associated with the regulatory phenotype can be found in ABM Tg mice. A, In ABM Tg mice, a small fraction of CD4+ T cells constitutively express CD25 together with the ABM Tg defined by Vα2.1 and Vβ8.1. Values represent the mean of >20 mice analyzed/group. B, Among ABM Tg CD4+ T cells, only CD4+CD25+ T cells exhibit up-regulation of genes associated with the TReg pheno-type. Data are expressed as the relative fold difference between target samples and a calibrator. Values plotted represent the mean of one experiment that is representative of four performed.
ABM Tg CD4+CD25+ T cells are alloantigen specific and mediate powerful suppressive effects in vitro
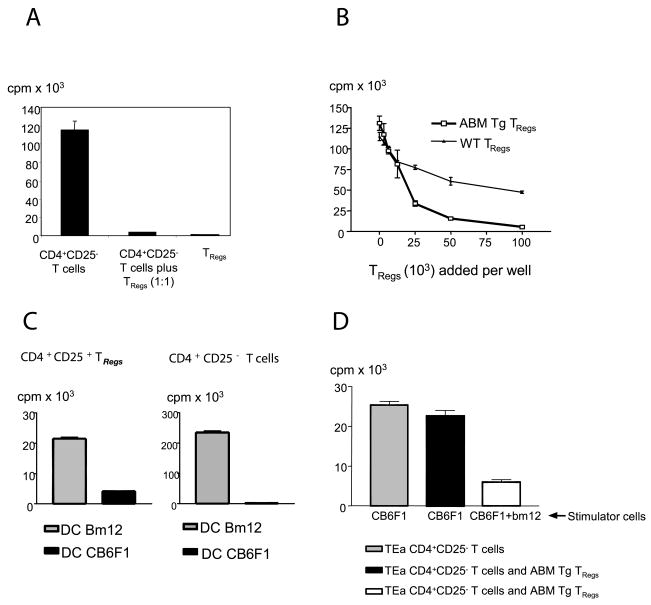
ABM Tg TRegs, but not ABM Tg CD4+CD25− TRegs, were anergic in vitro after direct stimulation with bm12 splenocytes (Fig. 2A). In addition, ABM Tg TRegs, but not wild-type C57BL/6 TRegs, powerfully suppressed the allospecific proliferation of ABM Tg CD4+CD25− TEff (Fig. 2B). To study the allospecificity of ABM Tg TRegs in vitro, we took advantage of the capacity of TRegs to proliferate if cultured with mature DCs (16). ABM Tg TRegs proliferated in response to mature bm12, but not third-party, bone marrow-derived mature DCs (Fig. 2C). Highly specific effects were also elicited when ABM Tg CD4+CD25− T cells were challenged with the two populations of mature DCs, albeit the effector T cell proliferation was significantly higher than that of TRegs (Fig. 2C). To determine whether ABM Tg TRegs could suppress the proliferation of T cells bearing different TCR specificities, we used TEa Tg CD4+CD25− T cells, which mount strong proliferative responses when cultured with CB6F1, but not with bm12, irradiated splenocytes. ABM Tg TRegs did not suppress the proliferationof TEa Tg CD4+CD25− T cells in response to CB6F1 stimulators (Fig. 2D, center column). In contrast, in the presence of mixed CB6F1 and bm12 stimulators, ABM Tg TRegs markedly inhibited TEa Tg CD4+CD25− T cell proliferation (Fig. 2D, right column). Taken together, our results indicate that ABM Tg TRegs are absolutely dependent on their cognate alloantigen for activation and proliferation. However, once activated, they can suppress the proliferation of TEff specific for alloantigens expressed on different APCs (bystander suppression).
FIGURE 2.
ABM Tg CD4+CD25+ T cells exhibit powerful, TCR-restricted, suppressive properties in vitro. A, ABM Tg CD4+CD25+ T cells (5 × 104) do not proliferate in response to 3 × 105 irradiated bm12 splenocytes and suppress the proliferation of 5 × 104 CD4+CD25− T cells. B, The proliferation of 5 × 104 ABM Tg CD4+CD25− T cells in response to 3 × 105 irradiated bm12 splenocytes is powerfully suppressed by increasing numbers of ABM Tg, but not polyclonal C57BL/6, TRegs. C, Both 5 × 104 ABM Tg CD4+CD25− and CD4+CD25+ T cells proliferate in response to 104 bm12, but not CB6F1, bone marrow-derived mature dendritic cells. Cell proliferation was estimated in all cases by [3H]TdR incorporation. Data are expressed as the mean cpm of triplicate cultures ± SE. Data portrayed in all panels are representative of at least three independent experiments. D, ABM Tg TRegs (5 × 104) suppress the proliferation of 5 × 104 TEa CD4+CD25− T cells only when bm12 irradiated splenocytes are added to the stimulator CB6F1 cell population.
ABM mice reject bm12 heart allografts in the absence of TRegs
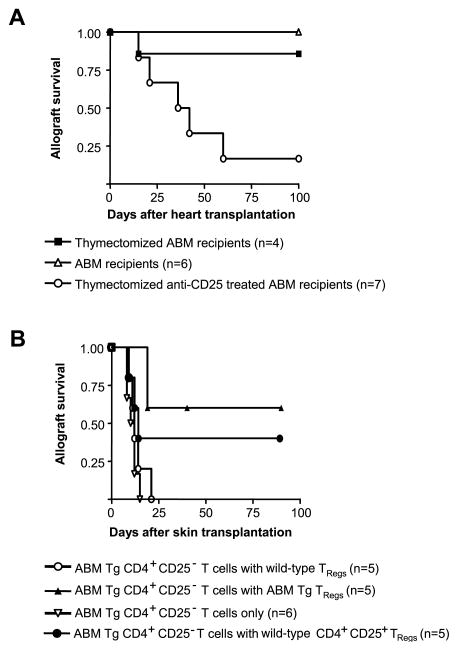
To determine the role of ABM TRegs in the prevention of both acute and chronic bm12 heart allograft rejection, we performed a set of heart transplants in ABM Tg recipients that had been thymectomized and depleted of CD4+CD25+ TRegs by anti-CD25 mAb treatment. ABM mice universally rejected bm12 heart allografts in the absence of TRegs (Fig. 3A). Thus, allospecific TRegs powerfully suppress cytopathic alloreactive T cells in vivo and prevent both acute and chronic allograft rejection.
FIGURE 3.
ABM Tg TRegs exhibit powerful suppressive properties in both heart and skin allograft models. A, Thymectomized ABM mice fail to spontaneously accept bm12 heart allografts in the absence of CD4+CD25+ TRegs (p < 0.0117). B, ABM Tg CD4+CD25− T cells (105) adoptively transferred into syngeneic nude C57BL/6 hosts rapidly induce rejection of bm12 skin allografts. In contrast, the cotransfer of 105 ABM Tg, but not wild-type, TRegs precludes TEff from rejecting the allografts (p < 0.0198). To prevent 105 ABM Tg CD4+CD25− T cells from rejecting bm12 skin allografts, 3 × 105 wild-type C57BL/6 TRegs have to be cotransferred into nude recipients (p < 0.0025).
ABM Tg TRegs exhibit more powerful suppressive properties than C57BL/6 TRegs after transfer into bm12 skin allograft recipients
To test the capacity of ABM Tg TRegs to mediate alloantigen-specific effects in vivo, we conducted adoptive transfer experiments using lymphopenic skin allograft recipients. In this model, the transfer of as few as 1 × 105 CD4+CD25− or CD8+ wild-type naive T cells into skin allograft recipients results in rapid graft rejection, whereas transferred TRegs cells do not induce rejection and prevent CD4+CD25− TEff populations from destroying the grafts (10). The median survival time of bm12 skin grafts challenged with 105 ABM Tg CD4+CD25− T cells was 10 days (Fig. 3B). C57BL/6 TRegs (105) did not prevent 105 ABM Tg CD4+CD25− T cells from rapidly rejecting bm12 allografts. In contrast, ABM Tg TRegs significantly delayed the occurrence of graft rejection (median survival time, 12 vs 40 days; p < 0.01; Fig. 3B). C57BL/6 TRegs only prevented skin allograft rejection when transferred at a high ratio (3:1) of TReg to ABM Tg CD4+CD25− T cell, albeit this protective effect was less marked than after administering an equivalent number of ABM Tg TRegs (data not shown). The need to transfer very high TReg to TEff ratios to ensure effective suppression when using naive polyclonal TRegs has been previously reported (10). ABM mice have a 30-fold higher frequency of I-Abm12-reactive CD4+ T cells than polyclonal C57BL/6 mice (24). Hence, our data indicate that the net suppressive effects exerted by bulk TReg populations in transplantation critically depend on the frequency of alloreactive TRegs among them.
ABM Tg TRegs do not prevent the rejection of third-party skin allografts
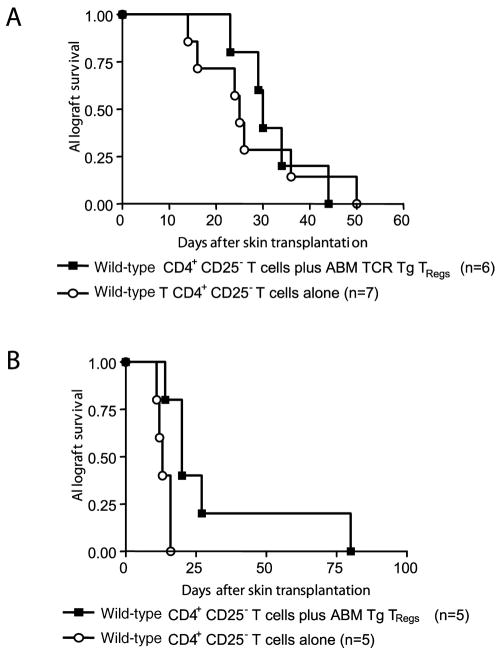
To elucidate the fine specificity of TReg function in vivo, we performed additional experiments transferring C57BL/6 CD4+CD25− T cells together with ABM Tg TRegs into recipients of bm12 or third-party (BALB/c) skin allografts. Polyclonal CD4+CD25− T cells are capable of rejecting any MHC-mis-matched allogeneic skin allograft (10). ABM Tg TRegs, in contrast, do not mediate immunosuppressive effects in vitro unless direct recognition of intact bm12 alloantigens takes place (Fig. 2). Furthermore, ABM Tg CD4+CD25− T cells fail to reject third-party BALB/c skin allografts (our unpublished observations). Hence, we hypothesized that ABM Tg TRegs would prevent wild-type CD4+CD25− T cells from rejecting bm12, but not third-party strain, allografts. As predicted, the cotransfer of ABM Tg TRegs had no effect on the capacity of C57BL/6 CD4+CD25− T cells to reject BALB/c skin allografts (Fig. 4A), whereas a protective effect was exerted upon bm12 allografts (Fig. 4B). The failure of transferred ABM Tg TRegs to delay BALB/c skin allograft rejection persisted even after markedly increasing the ratio of TReg to TEff, at variance with the effect of transferring polyclonal C57BL/6 TRegs (Fig. 5C). These findings indicate that adoptively transferred TRegs suppress cytopathic alloimmune responses only when TRegs are stimulated by allografts expressing their cognate Ags.
FIGURE 4.
ABM Tg TRegs do not suppress the rejection of third-party skin allografts mediated by C57BL/6 CD4+CD25− T cells. A, ABM Tg TRegs (105) cannot prevent 105 wild-type CD4+CD25− T cells from rejecting BALB/c skin allografts after adoptive transfer into lymphopenic recipients. B, In contrast, 105 ABM Tg TRegs significantly delay the rejection of bm12 skin allografts when transferred together with an equal number of C57BL/6 CD4+CD25− T cells (p < 0.0163).
FIGURE 5.
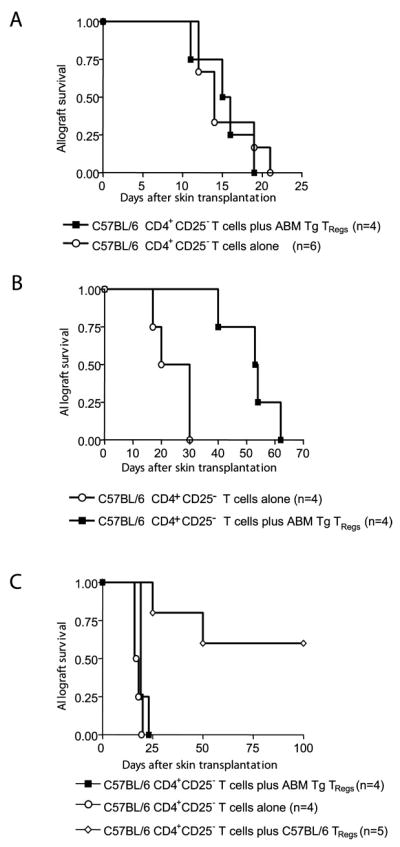
ABM Tg TRegs can mediate linked suppression if administered at a high TReg to TEff ratio. A, ABM Tg TRegs (105) do not delay rejection of F1 (bm12 × BALB/c) skin allografts when transferred together with 105 C57BL/6 CD4+CD25− T cells. B, In contrast, ABM Tg TRegs significantly (p < 0.0091) delay allograft rejection if transferred at a 4:1 ratio of TReg to TEff ratio (2 × 105 ABM Tg TRegs vs 0.5 × 105 C57BL/6 CD4+CD25− T cells). C, ABM Tg TRegs cannot protect BALB/c skin allografts even when they are transferred at a high TReg to TEff ratio (2 ×105 Tg TRegs vs 0.5 × 105 C57BL/6 CD4+CD25− T cells). In contrast, 2 × 105 C57BL/6 TRegs neutralize the capacity of 0.5 × 105 C57BL/6 CD4+CD25 to reject BALB/c allografts.
ABM Tg TRegs can mediate linked suppression
Linked suppression, a phenomenon in which TRegs can suppress the rejection of third-party alloantigens provided they are expressed on the same APC as the tolerated Ags (4), is considered one of the hallmarks of peripheral allograft tolerance. To test the capacity of TRegs to mediate this process, we used F1 (BALB/c × bm12) allografts as a source of APCs expressing both bm12 and third-party (BALB/c) alloantigens. The cotransfer of ABM Tg 3 TRegs and C57BL/6 CD4+CD25− T cells at a 1:1 ratio into hosts grafted with F1 skin did not delay the occurrence of allograft rejection (Fig. 5A). Nonetheless, the administration of a higher TReg to CD4+CD25− T cell ratio resulted in significant prolongation of F1 allograft survival (Fig. 5B). These results are at variance with those of experiments performed using BALB/c allografts (Fig. 5C). Taken together, our findings indicate that TReg suppressive effects can extend to TEff responding to third-party alloantigens present on the same graft that stimulates the TRegs (linked suppression).
Discussion
In the current study we have focused on the elucidation of the capacity of natural TRegs to mediate both alloantigen-specific and linked suppressive effects in transplantation. To do so we have used a unique TCR Tg system in which CD4+ T cells directly recognize the allogeneic MHC-II molecule, I-Abm12. We report in this paper for the first time that in ABM TCR Tg mice, a fraction of anti-I-Abm12-specific CD4+ T cells are bona fide TRegs capable of mediating alloantigen-specific suppressive effects both in vitro and in vivo. In addition, the regulatory properties of ABM TCR Tg TRegs are not restricted to bm12 allografts, but can also extend to F1 (BALB/c ×bm12) allografts (linked suppression), although in this case effective prevention of graft rejection requires transfer of a high TReg to TEff cell ratio. We have previously determined that bm12 alloantigens placed into C57BL/6 hosts are not effectively processed by host APCs (28). Hence, bm12 allografts are only capable of stimulating polyclonal C57BL/6 T cells through direct alloantigen presentation. In contrast, the use of grafts with multiple mismatches at both major and minor histocompatibility Ags (e.g., BALB/c or F1 grafts into B6 recipients) results in direct and indirect allorecognition events. These data indicate that the requirement for a high ABM Tg TReg to wild-type CD4+CD25− T cell ratio to ensure effective protection of F1, but not bm12, grafts may be due to 1) stimulation of a higher frequency of effector T cells by F1 than by bm12 grafts, and/or 2) inefficient suppression by TRegs of CD4+CD25− T cells activated through the indirect allorecognition pathway. The latter would imply that effective suppression of alloimmune responses requires TRegs and TEff sharing a common APC.
Our data also show that the spontaneous acceptance of bm12 heart allografts by ABM TCR Tg recipients is absolutely dependent on the presence of anti-I-Abm12 TCR Tg TRegs. This is remarkable given that in ABM mice, the overall anti-I-Abm12 TEff responder frequency is ~70% (24), whereas only 5% of TCR Tg CD4+ T cells express TReg markers. These findings indicate that in nonlymphopenic situations, natural TRegs with a defined specificity exhibit a very strong capacity to prevent allograft rejection when their cognate Ag is expressed on the graft. This is in keeping with a recent report using mice expressing a Tg TCR directed against the minor histocompatibility Ag, HY (31). Taken together, these studies, using nonlymphopenic hosts, suggest that both in vitro assays and in vivo adoptive transfer systems, in which unphysiologic ratios of TReg to TEff are commonly required to ensure effective suppression, most likely underestimate the regulatory properties of natural TRegs. These are clinically relevant observations, suggesting that administration of a limited number of allospecific TRegs to nonlymphopenic transplant recipients might be an effective strategy to induce graft acceptance.
The use in our experiments of natural, alloantigen-inexperienced, ABM TCR Tg TRegs (i.e., TRegs obtained from naive unmanipulated ABM mice) precludes us from directly addressing the current controversy of whether tolerizing regimens result in the generation of allospecific TRegs (5, 6, 10, 21). Our observation that regulation of transplant rejection by TRegs critically depends on specific TCR stimulation raises the possibility that tolerance-inducing strategies might be acting, at least in part, by preferentially expanding alloantigen-specific TRegs. However, the use of polyclonal TRegs harvested from tolerized recipients to assess TReg specificity has resulted in much less clear-cut results (5, 6, 10, 21, 32). In a polyclonal population of T cells, the expression of two TCR heterodimers by a single cell or Ag cross-reactivity by a given TCR may create a situation in which a TReg with alloantigen-specificity may be activated by another Ag. Moreover, the use of adoptive transfer systems involving lymphopenic hosts may exacerbate these effects. Alternatively, other regulatory T cell subsets might also be participating in ensuring transplantation tolerance allospecificity. Additional studies are required to completely elucidate these hypotheses.
Footnotes
Abbreviations used in this paper: TReg, regulatory T cell; TEff, effector T cell; Tg, transgenic.
Disclosures
The authors have no financial conflict of interest.
This work was supported by grants from the National Institutes of Health: RO1AI51559 (to M.H.S.), R21HL079450 (to L.A.T. and M.H.S.), R01AI37691 (to M.H.S. and L.A.T.), and PO1AI41521 (to L.A.T., M.H.S., and T.B.S.).
References
- 1.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, Waldmann H. “Infectious” transplantation tolerance. Science. 1993;259:974–977. doi: 10.1126/science.8094901. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann H, Cobbold S. Regulating the immune response to transplants: a role for CD4+ regulatory cells? Immunity. 2001;14:399–406. doi: 10.1016/s1074-7613(01)00120-0. [DOI] [PubMed] [Google Scholar]
- 3.Zheng XX, Sanchez-Fueyo A, Domenig C, Strom TB. The balance of deletion and regulation in allograft tolerance. Immunol Rev. 2003;196:75–84. doi: 10.1046/j.1600-065x.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 4.Davies JD, Leong LY, Mellor A, Cobbold SP, Waldmann H. T cell suppression in transplantation tolerance through linked recognition. J Immunol. 1996;156:3602–3607. [PubMed] [Google Scholar]
- 5.Wood KJ, Sakaguchi S. Regulatory lymphocytes: regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 6.Hara M, Kingsley CI, Niimi M, Read S, Turvey SE, Bushell AR, Morris PJ, Powrie F, Wood KJ. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Fueyo A, Weber M, Domenig C, Strom TB, Zheng XX. Tracking the immunoregulatory mechanisms active during allograft tolerance. J Immunol. 2002;168:2274–2281. doi: 10.4049/jimmunol.168.5.2274. [DOI] [PubMed] [Google Scholar]
- 8.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 9.Zheng XX, Sanchez-Fueyo A, Sho M, Domenig C, Sayegh MH, Strom TB. Favorably tipping the balance between cytopathic and regulatory T cells to create transplantation tolerance. Immunity. 2003;19:503–514. doi: 10.1016/s1074-7613(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 13.Montagnoli C, Bacci A, Bozza S, Gaziano R, Mosci P, Sharpe AH, Romani L. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J Immunol. 2002;169:6298–6308. doi: 10.4049/jimmunol.169.11.6298. [DOI] [PubMed] [Google Scholar]
- 14.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 15.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA. 2003;100:8886–8891. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barthlott T, Kassiotis G, Stockinger B. T cell regulation as a side effect of homeostasis and competition. J Exp Med. 2003;197:451–460. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies JD, O’Connor E, Hall D, Krahl T, Trotter J, Sarvetnick N. CD4+ CD45RB low-density cells from untreated mice prevent acute allograft rejection. J Immunol. 1999;163:5353–5357. [PubMed] [Google Scholar]
- 20.Graca L, Thompson S, Lin CY, Adams E, Cobbold SP, Waldmann H. Both CD4+CD25+ and CD4+CD25− regulatory cells mediate dominant transplantation tolerance. J Immunol. 2002;168:5558–5565. doi: 10.4049/jimmunol.168.11.5558. [DOI] [PubMed] [Google Scholar]
- 21.Graca L, Le Moine A, Cobbold SP, Waldmann H. Dominant transplantation tolerance: opinion. Curr Opin Immunol. 2003;15:499–506. doi: 10.1016/s0952-7915(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 22.Backstrom BT, Muller U, Hausmann B, Palmer E. Positive selection through a motif in the αβ T cell receptor. Science. 1998;281:835–838. doi: 10.1126/science.281.5378.835. [DOI] [PubMed] [Google Scholar]
- 23.Rulifson IC, Szot GL, Palmer E, Bluestone JA. Inability to induce tolerance through direct antigen presentation. Am J Transplant. 2002;2:510–519. doi: 10.1034/j.1600-6143.2002.20604.x. [DOI] [PubMed] [Google Scholar]
- 24.Sayegh MH, Wu Z, Hancock WW, Langmuir PB, Mata M, Sandner S, Kishimoto K, Sho M, Palmer E, Mitchell RN, et al. Allograft rejection in a new allospecific CD4+ TCR transgenic mouse. Am J Transplant. 2003;3:381–389. doi: 10.1034/j.1600-6143.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 25.McIntyre KR, Seidman JG. Nucleotide sequence of mutant I-Aβbm12 gene is evidence for genetic exchange between mouse immune response genes. Nature. 1984;308:551–553. doi: 10.1038/308551a0. [DOI] [PubMed] [Google Scholar]
- 26.Stuart PM, Beck-Maier B, Melvold RW. Provocation of skin graft rejection across murine class II differences by non-bone-marrow-derived cells. Transplantation. 1984;37:393–396. doi: 10.1097/00007890-198404000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez M, Quezada SA, Blazar BR, Panoskaltsis-Mortari A, Rudensky AY, Noelle RJ. The balance between donor T cell anergy and suppression versus lethal graft-versus-host disease is determined by host conditioning. J Immunol. 2002;169:5581–5589. doi: 10.4049/jimmunol.169.10.5581. [DOI] [PubMed] [Google Scholar]
- 28.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 29.Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc Natl Acad Sci USA. 2002;99:8213–8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schenk S, Kish DD, He C, El-Sawy T, Chiffoleau E, Chen C, Wu Z, Sandner S, Gorbachev AV, Fukamachi K, et al. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+CD25+ T cells. J Immunol. 2005;174:3741–3748. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 31.Benghiat FS, Graca L, Braun MY, Detienne S, Moore F, Buonocore S, Flamand V, Waldmann H, Goldman M, Le Moine A. Critical influence of natural regulatory CD25+ T cells on the fate of allografts in the absence of immunosuppression. Transplantation. 2005;79:648–654. doi: 10.1097/01.tp.0000155179.61445.78. [DOI] [PubMed] [Google Scholar]
- 32.Graca L, Le Moine A, Lin CY, Fairchild PJ, Cobbold SP, Waldmann H. Donor-specific transplantation tolerance: the paradoxical behavior of CD4+CD25+ T cells. Proc Natl Acad Sci USA. 2004;101:10122–10126. doi: 10.1073/pnas.0400084101. [DOI] [PMC free article] [PubMed] [Google Scholar]