Abstract
The prevalence of diabetes, hypertension, and cardiovascular disease (CVD) and chronic kidney disease (CKD) is increasing in concert with obesity. Insulin resistance, metabolic dyslipidemia, central obesity, albuminuria and hypertension commonly cluster to comprise the cardiometabolic syndrome. Emerging evidence supports a shift in our understanding of the crucial role of elevated serum aldosterone in promoting insulin resistance and resistant hypertension. Aldosterone enhances tissue generation of oxygen free radicals and systemic inflammation. This increase in oxidative stress and inflammation, in turn, contributes to impaired insulin metabolic signaling, reduced endothelial-mediated vasorelaxation and associated cardiovascular and renal structural and functional abnormalities. In this context, recent investigation indicates that hyperaldosteronism, which is often associated with obesity, contributes to impaired pancreatic beta-cell function as well as diminished skeletal muscle insulin metabolic signaling. Accumulating evidence indicates that the cardiovascular and renal abnormalities associated with insulin resistance are mediated, in part, by aldosterone's non-genomic as well as genomic signaling through the mineralocorticoid receptor (MR). In the cardiometabolic syndrome there are increased circulating levels of glucocorticoids, which can also activate MR signaling in cardiovascular, adipose, skeletal muscle, neuronal, and liver tissue. Further, there is increasing evidence that fat tissue produces a lipid soluble factor that stimulates aldosterone production from the adrenal zona glomerulosa. Recently, have we learned that MR blockade improves pancreatic insulin release, insulin-mediated glucose utilization, endothelium-dependent vasorelaxation as well as reducing the progression of CVD and CKD. In summary, aldosterone excess exerts detrimental metabolic effects that contribute to the development of the CMS and resistant hypertension as well as CVD and CKD.
Keywords: Aldosterone, Insulin Resistance, Hypertension, Cardiometabolic Syndrome
Introduction
The prevalence of hypertension, cardiovascular disease (CVD) and chronic kidney disease (CKD) is progressively increasing in the United States, a phenomenon that closely parallels the burgeoning epidemics of obesity and the cardiometabolic syndrome (CMS).1–12 Approximately 70 million adults in the United States are obese and another 70 million have hypertension.1,6,13 Data from the National Health and Nutrition Examination Survey (NHANES) indicate that the prevalence of hypertension increases progressively with increasing body mass index (BMI) from 15% among persons with a BMI of 25 kg/m2 to approximately 40% among those who are obese, with a BMI of 30kg/m2 or more.2 Recent work supports the notion that hypertensive patients exhibit more frequent impairments of insulin metabolic signaling, dyslipidemia, microalbuminuria, and obesity, all components of the CMS.4–7
The pathogenesis of the CMS is complex and not fully understood.8,17 Increasing evidence reveals that the renin-angiotensin-aldosterone system is inextricably involved in linking obesity, metabolic dyslipidemia, insulin resistance, CKD, and hypertension,10–29 and a number of successful compounds have been developed around molecular targets of this pathway. Indeed, emerging evidence supports a crucial role for aldosterone in the pathogenesis and progression of the CMS. As recently summarized,17 elevated plasma aldosterone levels directly contribute to insulin resistance, endothelial dysfunction, glomerular hyperfiltration, and excess glomerular and tubular leakage of albumin; processes that lead to maladaptive cardiovascular and renal remodeling. It is increasingly recognized that obesity, which is often associated with elevated plasma levels of aldosterone, is a major factor for the development of albuminuria and CKD, in concert with other components of the CMS.7, 17 Data are also emerging that suggest patients with resistant hypertension, those not controlled to goal on three antihypertensive medications, tend to be overweight, often have elevated plasma and urine levels of aldosterone, and have salutary blood pressure responses to mineralocorticoid receptor (MR) blockers.30–56
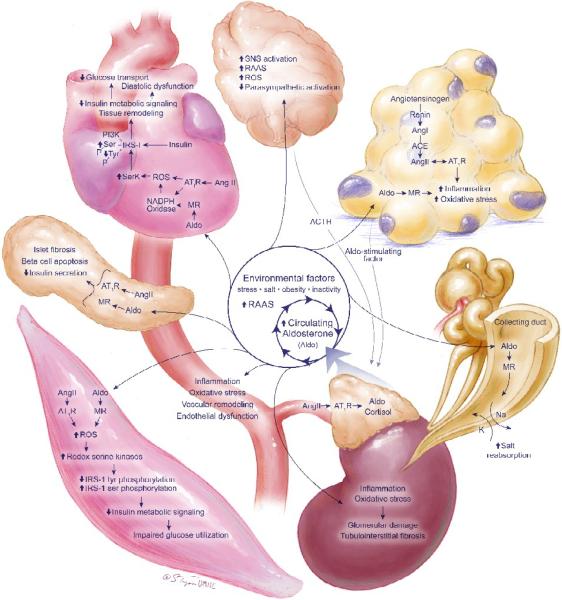
Aldosterone is synthesized in the zona glomerulosa of the adrenal gland in response to angiotensin II (Ang II), adrenocorticotropin (ACTH), potassium, and lipid soluble factor(s) produced in fat tissue (Fig 1).17,30 The classical genomic pathway of aldosterone action involves binding to cytosolic MRs and subsequent translocation to the nucleus, gene transcription, and translation of effector proteins involved in regulating sodium and potassium balance across renal tubular epithelial cells.17,23 Aldosterone also exerts rapid, non-genomic effects that mediate maladaptive tissue remodeling throughout the cardiovascular and central nervous system, further perpetuating the CMS, insulin resistance, and the hypertensive state. The non-genomic pathways of aldosterone are independent of renal tubular reabsorption of sodium and volume expansion and utilize rapid activation of the tyrosine kinase signaling and subsequent downstream activation of extracellular receptor kinase (ERK1/2), Rho kinase, and protein kinase (PKC) in association with increased cytosolic calcium and generation of reactive oxygen species (ROS).8,16–18 The consequences of activating these pathways are increased NADPH oxidase activation/ROS generation, mitochondrial electron transport uncoupling, and downstream activation of redox-sensitive serine kinases (Fig 1–4).8,17,23,24 As MRs are expressed in numerous tissues, elevated circulating aldosterone levels signaling via nongenomic as well as genomic mechanisms result in a number of maladaptive tissuespecific effects (Fig 1).
The Contribution of Aldosterone to the Pathogenesis of CMS
Aldosterone secretion from the adrenal gland has been classically considered to be regulated by renin-angiotensin system activation in response to intravascular volume contraction.5,6 When this axis is perturbed, as seen in diverse disease states including the CMS, heart failure and CKD, inappropriate aldosterone secretion occurs despite high salt and volume retention and contributes to a state of hyperaldosteronism.6,16,22,23 Recent evidence suggests that increased non-genomic MR signaling, in response to these elevated levels of aldosterone, is involved in the pathophysiology of insulin resistance and other components of the CMS.17 Indeed, the MR has a high affinity for both aldosterone as well as 11-beta-hydroxyglucocorticoids, the levels of which are often elevated in clinical states characterized by central obesity, such as the CMS.17 The enzyme 11-beta-hydroxysteroid dehydrogenase, which prevents glucocorticoids from signaling through the MR, is present at much lower levels in cardiovascular and metabolic tissue such as skeletal muscle, liver and fat,23,24 thereby allowing for both aldosterone as well as 11-beta-hydroxyglucocorticoids to act through the MR to impact insulin metabolic signaling with consequent maladaptive tissue remodeling.17 This is of particular significance in the CMS wherein circulating glucocorticoid concentrations may be several orders of magnitude greater than aldosterone.
As summarized in a recent review,17 there is emerging evidence that adipose tissue produces a lipid soluble factor that stimulates aldosterone secretion.30 There is also emerging evidence that both aldosterone and glucocorticoids can interact via MRs to promote adipogenesis and increases in fat macrophage infiltration.29,30 Thus, the interaction of fat, the adrenal cortex, and aldosterone/glucocorticoids is a positive servoregulatory relationship whereby fat increases aldosterone and glucocorticoid production, and these hormones, in turn, promote further adipogenesis and inflammation in fat tissue (Fig 1). In this context, MR blockade has been shown to reverse obesity-related increases in the pro-inflammatory adipokines TNF-alpha, MCP-1, and IL-6 and improve expression of adiponectin.31,32 Thus, in clinical conditions characterized by increased obesity, MR activation by glucocorticoids, in addition to aldosterone, further potentiates inflammation, oxidative stress, fibrosis, and insulin resistance.22–24
Elevations in plasma aldosterone levels are associated with the CMS independent of other renin-angiotensin-aldosterone (RAAS) components such as Ang II.17,26,27 For example, it has been observed that in primary hyperaldosteronism, in which there are low levels of plasma renin activity and Ang II, there are higher blood glucose levels and a higher prevalence of CMS compared to that observed in individuals with essential hypertension.27 In another study, insulin resistance as measured by homeostatic model assessment (HOMA) was higher, and adiponectin levels were lower in primary hyperaldosteronism than in essential hypertension.28 The relationship between elevated plasma levels of aldosterone and insulin resistance is further strengthened by the observation that resection of aldosterone-producing tumors improves insulin sensitivity.33
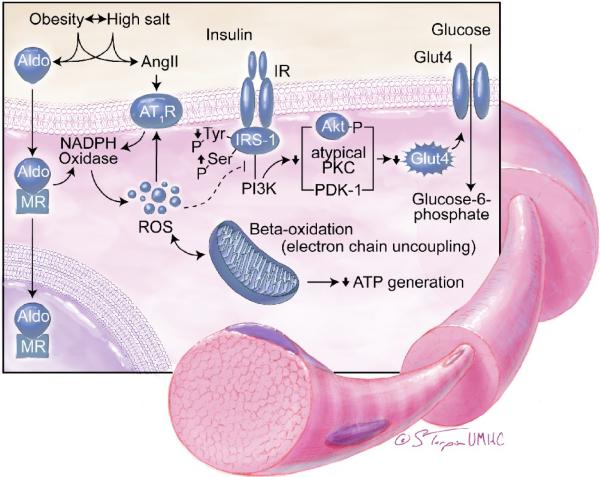
Recent investigation has helped delineate the mechanism by which aldosterone negatively impacts insulin metabolic signaling (Fig 2). For example, in murine brown fat tissue, aldosterone dose-dependently impaired insulin-induced glucose uptake by about 25% and increased mRNA of the proinflammatory adipokines.14 Data from our laboratory in a transgenic rodent model of RAAS activation and insulin resistance show that in-vivo MR blockade improves system insulin sensitivity as well as ex-vivo skeletal muscle glucose uptake.11,17 This improvement in skeletal muscle insulin metabolic signaling was associated with decreased NADPH oxidase activity and the attenuation of ROS (Fig 2).
Several other mechanisms of aldosterone-induced insulin resistance and impaired glucose sensitivity have been suggested, including negative effects on pancreatic beta cell function and stimulation of hepatic gluconeogenesis.17,34 In this context, hypokalemia has been shown to have a direct impact on pancreatic beta-cell function.17 Aldosterone also causes an impairment in hepatic insulin metabolic signaling which contributes, in part, to increased hepatic gluconeogenesis.34,35 In studies demonstrating an association between hyperaldosteronism and decreased pancreatic beta-cell mass, there were negative relationships between serum aldosterone, c-peptide levels and HOMA sensitivity.34 In that study, these abnormalities of beta-cell function and insulin sensitivity, occurred largely independent of decreased serum potassium, suggesting that aldosterone exerted negative effects on directly on beta-cell function. There is emerging data that aldosterone exerts these detrimental effects on beta-cell structural and functional integrity though increases in islet cell inflammation and oxidative stress.17,34
The Role of Aldosterone in Endothelial Dysfunction
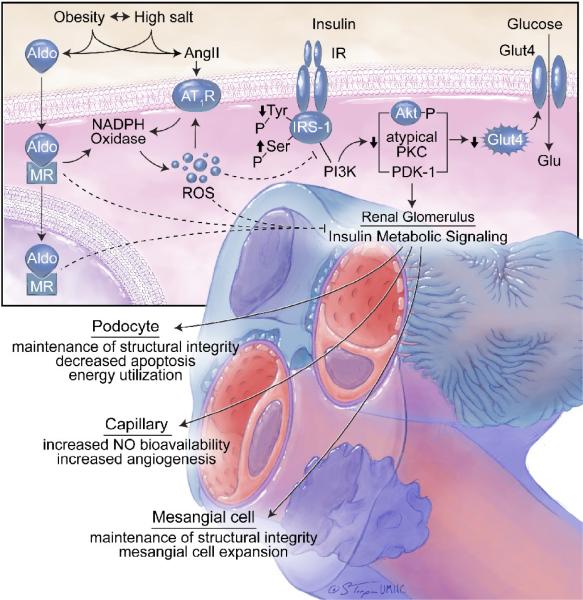
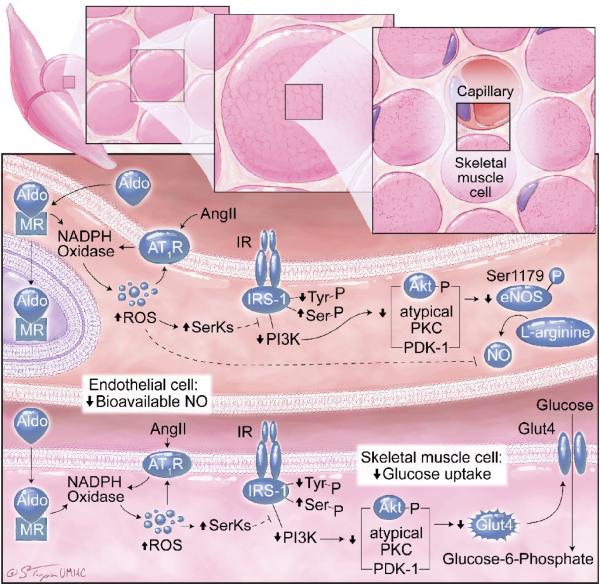
Endothelial dysfunction is commonly present in concert with insulin resistance and other components of the CMS (Fig 3).15–19 Several vascular metabolic abnormalities have been documented in obese, insulin resistant subjects. These abnormalities include impaired insulin-stimulated glucose uptake and reduced bioavailable nitric oxide (NO).17 In this context, insulin-dependent glucose utilization is partly dependent on insulin-mediated increases in blood flow and substrate delivery to tissues (Fig 3). In insulin resistance, there is decreased insulin stimulation of NO bioactivity (decreased endothelial NOS (eNOS) activation and increased NO destruction), diminished vasodilatation, and impaired substrate delivery. Increasing evidence demonstrates that elevated plasma levels of aldosterone contribute to this decrease in insulin metabolic signaling in vascular tissue.15–19 Increased generation of ROS plays an important role in aldosterone- and Ang II-mediated decreases in insulin metabolic signaling. Increases in RAAS generation of ROS activate redox-sensitive serine kinases, which promote serine phosphorylation of insulin receptor-1 (IRS-1) levels. This increase in serine phosphorylation of IRS-1 reduces engagement with phosphoinositol 3-kinase (PI3-K), with resulting diminution of protein kinase B (Akt) and atypical protein kinase activation of eNOS phosphorylation/activation (Fig 3).17 As a result, insulin-resistant individuals with obesity and elevated plasma levels of aldosterone are more prone to endothelial dysfunction and subsequent development of hypertension.
Aldosterone and Hypertension
Aldosterone mediates several maladaptive changes in the nervous and cardiovascular systems that promote hypertension in addition to CVD and CKD (Fig 1). Elevated plasma aldosterone levels are reported both in hypertensive patients and animal models of hypertension23,36–39,47 and have been correlated with increased left ventricular mass45,46,67 as well as established as a risk factor for developing hypertension.47,48 Primary aldosteronism, resulting from bilateral adrenal hyperplasia or an aldosterone-producing adenoma, occurs with prevalence estimated at 0.5% to 4.8% of the population with general hypertension, and 4.5% to 22% of those with resistant hypertension.40–44 Importantly, primary hyperaldosteronism leads to a greater frequency of resistant hypertension, as well as CVD and CKD morbidity and mortality, compared to essential hypertension.17,49,50 Primary hyperaldosteronism has also been linked to hypertension-related atrial fibrillation.70
Elevated levels of aldosterone, in association with obesity and insulin resistance, promote non-genomic inflammation and oxidative stress pathways that advance the development of resistant hypertension through a number of mechanisms.15–19 As previously noted, aldosterone has been demonstrated to inhibit endothelium-dependent relaxation by decreasing NO bioavailability, a consequence of increased ROS generation (Fig 3). In addition, aldosterone-induced perivascular fibrosis reduces vascular compliance and increases stiffness, while increased Na+/H+ exchange promotes vascular smooth muscle cell proliferation.55,56 These actions potentiate the elevation of blood pressure that occurs from the classical effects of aldosterone to promote salt retention and volume expansion, collectively causing severe hypertension that is resistant to treatment unless an MR antagonist such as spironolactone or epleronone is employed as part of the therapeutic regime.17,20
As in the heart, vasculature, pancreas, skeletal muscle, and fat, high levels of circulating aldosterone (as well as centrally-administered aldosterone) can also increase local RAAS activation in brain regions that contribute to increased sympathetic tone in hypertension.17,65,66 For example, brain MR blockade reduces NADPH-induced superoxide in the paraventricular nucleus (PVN) of the hypothalamus and reduces descending sympathetic PVN output.66 In addition to circulating levels, aldosterone is also synthesized in the brain67,68 and serves as a MR ligand to increase sympathetic drive to the heart, kidneys, and vascular smooth muscle. Sympathetic pathways are also activated by aldosterone-MR actionin the central nervous system.69 Finally, aldosterone-induced increases in salt appetite and sodium intake in part, via MR activation in the amygdala, further promote hypertension.67,70
Aldosterone Effects on Heart and Kidney
Aldosterone has also been shown to mediate maladaptive remodeling in the heart. Left ventricular hypertrophy, cardiac fibrosis, and diastolic dysfunction are all associated with high aldosterone (Fig 1).57–59 In a feed-forward mechanism, cardiac MR activation potentiates the local RAAS by increasing angiotensin type 1 receptor (AT1R) and angiotensin converting enzyme (ACE) expression and enhancing Ang II-induced oxidative stress.8,54,61–64 MR antagonism reduces Ang II-mediated increases in NADPH oxidase subunit expression and ROS generation in hypertensive rats60 and abrogates left ventricular hypertrophy, collagen synthesis, and cardiac arrythmias in human hypertensive patients.
Aldosterone has a number of adverse effects in the renal axis of the CMS as well. High circulating levels of aldosterone cause renal hyperfiltration and promote both glomerular and tubulointerstitial disease, as discussed in detail in a previous review.17 Aldosterone has been shown to induce hypertrophy of the glomerular mesangium in kidneys of hypertensive rats, leading to podocyte damage, glomerulosclerosis, and proteinuria.14,17,21 Aldosterone-induced renal damage is likely via redox-mediated deficits in insulin signaling (Fig 4), as MR antagonism abrogates local RAAS signaling, attenuates glomerular remodeling, and improves insulin signaling while decreasing NADPH oxidase activity and ROS generation. Additionally, in human patients with kidney disease, the addition of MR blockade to treatment with ACE inhibitors or AT1R blockers (ARB)s dramatically amplifies the improvements in proteinuria and albuminuria.
Current and Future Clinical Perspectives
While the evidence to date to support a role for MR antagonism on insulin sensitivity is best validated in preclinical models, there is observational data to drive future work. The bulk of current evidence supports a role for MR antagonism on CVD and CKD end-points. Current standards of practice advocate RAAS inhibition with an ACE inhibitor or AT1R blockade to retard progression of CVD and renal disease; however, neither ensures optimal control of cardiovascular morbidity and mortality. Optimal RAAS suppression is difficult to achieve with currently available antihypertensive agents, partly because ACE inhibition and AT1R blockade both activate compensatory feedback mechanisms that result in increased plasma renin activity, active Ang II, and aldosterone escape mechanisms.
Aldosterone breakthrough has been estimated to occur in 10–53% of patients on chronic ACE inhibitor or ARB therapy and can be associated with negative cardiovascular and renal consequences.14 To the best of our knowledge, at least fourteen clinical investigations have confirmed an incremental renal and cardiovascular benefit when MR blockade is added to a regimen comprising ACE inhibitors or ARBs.21 Indeed, mounting evidence suggests combination treatment with MR blockade may improve end organ disease outcomes and provide additional blood pressure-lowering effects in settings of resistant hypertension.7,16,17,21,72–79
Conclusions
Increasing data suggests that excess circulating aldosterone promotes the development of impaired insulin metabolic signaling and endothelial function, which in turn contribute to hypertension and associated cardiovascular and renal structural and functional abnormalities. Central to the CMS is obesity, a condition that stimulates adrenal production of aldosterone which, in turn, is associated with insulin resistance, the metabolic syndrome and an increased propensity for development of type 2 diabetes (Fig 1). Many of these adverse effects of aldosterone are mediated through rapid membrane actions of this hormone. Accumulating evidence indicates that therapy with MR antagonists has considerable clinical utility in the treatment of resistant hypertension, and in the prevention of CVD and CKD in patients with the metabolic syndrome and diabetes. Future investigative efforts should focus on further delineation of the role of MR blockade in the management of the metabolic syndrome and resistant hypertension.
Acknowledgements
National Institutes of Health (R01 HL73101-01A1 NIH/NHLBI) and the Veterans Affairs Research Service (VA Merit Review) support Dr. Sowers' research. Dr Whaley-Connell is supported by the Veterans Affairs Research Service (VA CDA-2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ginsberg HN, MacCallum PR. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr. 2009;4(2):113–9. doi: 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The Metabolic Syndrome: prevalence and associated risk factor findings in the US Population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik S, Wong ND, Franklin SS, Kamath TV, L'Italien GJ, Pio JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 4.Manrique C, Lastra G, Whaley-Connell A, Sowers JR. Hypertension and the cardiometabolic syndrome. J Clin Hypertens (Greenwich) 2005;7:471–476. doi: 10.1111/j.1524-6175.2005.04617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sowers JR. Obesity as a cardiovascular risk factor. Am J Med. 2003;115(Supp 8A):37S–41S. doi: 10.1016/j.amjmed.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Van Gaal LF, Mertens IL, DeBlock CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–80. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 7.Sowers JR. Metabolic risk factors and renal disease. Kidney Int. 2007;71(8):719–20. doi: 10.1038/sj.ki.5002006. [DOI] [PubMed] [Google Scholar]
- 8.Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, et al. Renin- -aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293(4):H2009–23. doi: 10.1152/ajpheart.00522.2007. [DOI] [PubMed] [Google Scholar]
- 9.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 10.Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R673–80. doi: 10.1152/ajpregu.00561.2007. [DOI] [PubMed] [Google Scholar]
- 11.Lastra G, Whaley-Connell A, Manrique C, Habibi J, Gutweiler AA, Appesh L, et al. Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2) rat. Am J Physiol Endocrinol Metab. 2008;295:E110–E116. doi: 10.1152/ajpendo.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakris GL, Sowers JR, American Society of Hypertension Writing Group ASH position paper: treatment of hypertension in patients with diabetes-an update. J Clin Hypertens (Greenwich) 2008;10(9):707–13. doi: 10.1111/j.1751-7176.2008.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden MR, Whaley-Connell A, Sowers JR. Renal redox stress and remodeling in metabolic syndrome, type 2 diabetes mellitus, and diabetic nephropathy. Am J Nephrol. 2005;25(6):553–69. doi: 10.1159/000088810. [DOI] [PubMed] [Google Scholar]
- 14.Bomback AS, Klemmer PJ. Interaction of Aldosterone and Extracellular Volume in the Pathogenesis of Obesity-Associated Kidney Disease. Am J Nephrol. 2009;30(2):140–146. doi: 10.1159/000209744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–318. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- 16.Wei Y, Whaley-Connell AT, Habibi J, Rehmer J, Rehmer N, Patel K, et al. Mineralocorticoid receptor antagonism attenuates vascular apoptosis and injury via rescuing protein kinase B activation. Hypertension. 2009;53(2):158–65. doi: 10.1161/HYPERTENSIONAHA.108.121954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sowers JR, Whaley-Connell A, Epstein M. The emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150(11):776–83. doi: 10.7326/0003-4819-150-11-200906020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hitomi H, Kiyomoto H, Nishiyama A, Hara T, Moriwaki K, Kaifu K, et al. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension. 2007;50:750–755. doi: 10.1161/HYPERTENSIONAHA.107.093955. [DOI] [PubMed] [Google Scholar]
- 19.Brown NJ. Aldosterone and vascular inflammation. Hypertension. 2008;51:161–167. doi: 10.1161/HYPERTENSIONAHA.107.095489. [DOI] [PubMed] [Google Scholar]
- 20.Gaddam KK, Nishizaka MK, Pratt-Ubunama MN, Pimenta E, Aban I, Oparil S, et al. Characterization of resistant hypertension: association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch Intern Med. 2008;168(11):1159–1164. doi: 10.1001/archinte.168.11.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whaley-Connell A, Habibi J, Wei Y, Gutweiler A, Jellison J, Wiedmeyer CE, Ferrario CM, Sowers JR. Mineralcorticoid receptor antagonism attenuates kidney renin-angiotensin-aldosterone system mediated filtration barrier remodeling in the transgenic Ren2 rat. Am J of Physiol Renal Physiol. 2009;296(5):F1013–22. doi: 10.1152/ajprenal.90646.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, et al. Enhanced aldosterone signaling in the early nephrology of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17:3438–46. doi: 10.1681/ASN.2006080944. [DOI] [PubMed] [Google Scholar]
- 23.Bochud M, Nussberger J, Bovet P, Maillard MR, Elston RC, Paccaud F, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension. 2006;48(2):239–45. doi: 10.1161/01.HYP.0000231338.41548.fc. [DOI] [PubMed] [Google Scholar]
- 24.Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, et al. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrin Metab. 2006;91(2):454–9. doi: 10.1210/jc.2005-1733. [DOI] [PubMed] [Google Scholar]
- 25.Fallo F, Mea PD, Sonino N, Bertello C, Ermani M, Vettor R, et al. Adiponectin and insulin sensitivity in primary aldosteronism. Am J Hypertens. 2007;20(8):855–61. doi: 10.1016/j.amjhyper.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Giacchetti G, Ronconi V, Turchi F, Agostinelli L, Mantero F, Rilli S, et al. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens. 2007;25(1):177–186. doi: 10.1097/HJH.0b013e3280108e6f. [DOI] [PubMed] [Google Scholar]
- 27.Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab. 2006;91:3457–63. doi: 10.1210/jc.2006-0736. [DOI] [PubMed] [Google Scholar]
- 28.Ehrhart-Bornstein M, Arakelyan K, Krug AW, Scherbaum WA, Bornstein SR. Fat cells may be the obesity-hypertension link: human adipogenic factors stimulate aldosterone secretion from adrenocortical cells. Endocr Res. 2004;30(4):865–70. doi: 10.1081/erc-200044122. [DOI] [PubMed] [Google Scholar]
- 29.Rondinone CM, Rodbard D, Baker ME. Aldosterone stimulated differentiation of mouse 3T3-L1 cells into adipocytes. Endocrinology. 1993;132(6):2421–6. doi: 10.1210/endo.132.6.8504747. [DOI] [PubMed] [Google Scholar]
- 30.Caprio M, Feve B, Claes A, Viengchareun S, Lombes M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007;21:2185–94. doi: 10.1096/fj.06-7970com. [DOI] [PubMed] [Google Scholar]
- 31.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, et al. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117:2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campión J, Lahera V, Cachofeiro V, Maestro B, Dávila N, Carranza MC, et al. In vivo tissue specific modulation of rat insulin receptor gene expression in an experimental model of mineralocorticoid excess. Mol Cell Biochem. 1998;185:177–182. doi: 10.1023/a:1006871309864. [DOI] [PubMed] [Google Scholar]
- 33.Fallo F, Mea PD, Sonino N, Bertello C, Ermani M, Vettor R, et al. Adiponectin and insulin sensitivity in primary aldosteronism. Am J Hypertens. 2007;20(8):855–61. doi: 10.1016/j.amjhyper.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Mosso LM, Carvajal CA, Fardella CE. A possible association between primary aldosteronism and lower [beta]-cell function. J Hypertens. 2008;26(3):609–10. doi: 10.1097/HJH.0b013e3282861fa4. [DOI] [PubMed] [Google Scholar]
- 35.Hayden MR, Sowers JR. Pancreatic Renin-Angiotensin-Aldosterone System in the Cardiometabolic Syndrome and Type-2 Diabetes. J Cardiometab Synd. 2008;3:129–131. doi: 10.1111/j.1559-4572.2008.00006.x. [DOI] [PubMed] [Google Scholar]
- 36.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissman P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40(6):892–96. doi: 10.1161/01.hyp.0000040261.30455.b6. [DOI] [PubMed] [Google Scholar]
- 37.Strauch B, Zelinka T, Hampf M, Bernhardt R, Widimsky J., Jr. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in Central Europe region. J Hum Hypertens. 2003;17(5):349–352. doi: 10.1038/sj.jhh.1001554. [DOI] [PubMed] [Google Scholar]
- 38.Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22(11):2217–2226. doi: 10.1097/00004872-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 39.Kidambi S, Kotchen JM, Grim CE, Raff H, Mao J, Singh RJ, et al. Association of adrenal steroids with hypertension and the metabolic syndrome in blacks. Hypertension. 2007;49(3):704–711. doi: 10.1161/01.HYP.0000253258.36141.c7. [DOI] [PubMed] [Google Scholar]
- 40.Douma S, Petidis K, Doumas M, Papaefthimiou P, Triantafyllou A, Kartali N, Papadopoulos N, Vogiatzis K, Zamboulis C. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371(9628):1921–6. doi: 10.1016/S0140-6736(08)60834-X. [DOI] [PubMed] [Google Scholar]
- 41.Strauch B, Zelinka T, Hampf M, Bernhardt R, Widimsky J., Jr. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens. 2003;17(5):349–52. doi: 10.1038/sj.jhh.1001554. [DOI] [PubMed] [Google Scholar]
- 42.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F, PAPY Study Investigators A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293–300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 43.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM, American Heart Association Professional Education Committee Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510–26. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 44.Pimenta E, Calhoun DA. Aldosterone and metabolic dysfunction: an unresolved issue. Hypertension. 2009;53(4):585–6. doi: 10.1161/HYPERTENSIONAHA.108.123406. [DOI] [PubMed] [Google Scholar]
- 45.Duprez DA, Bauwens FR, De Buyzere ML, De Backer TL, Kaufman JM, Van Hoecke J, Vermeulen A, Clement DL. Influence of arterial blood pressure and aldosterone on left ventricular hypertrophy in moderate essential hypertension. Am J Cardiol. 1993;71(3):17A–20A. doi: 10.1016/0002-9149(93)90240-d. [DOI] [PubMed] [Google Scholar]
- 46.Schunkert H, Hense HW, Muscholl M, Luchner A, Kürzinger S, Danser AH, Riegger GA. Associations between circulating components of the renin-angiotensin-aldosterone system and left ventricular mass. Heart. 1997;77(1):24–31. doi: 10.1136/hrt.77.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, Benjamin EJ, Levy D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351(1):33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- 48.Newton-Cheh C, Guo CY, Gona P, Larson MG, Benjamin EJ, Wang TJ, Kathiresan S, O'Donnell CJ, Musone SL, Camargo AL, Drake JA, Levy D, Hirschhorn JN, Vasan RS. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension. 2007;49(4):846–56. doi: 10.1161/01.HYP.0000258554.87444.91. [DOI] [PubMed] [Google Scholar]
- 49.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–8. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Ronconi V, Turchi F, Rilli S, Di Mattia D, Agostinelli L, Boscaro M, Giacchetti G. Metabolic syndrome in primary aldosteronism and essential hypertension: Relationship to adiponectin gene variants. Nutr Metab Cardiovasc Dis. 2009 May 28; doi: 10.1016/j.numecd.2009.03.007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci U S A. 1999;96(5):2514–9. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shigaev A, Asher C, Latter H, Garty H, Reuveny E. Regulation of sgk by aldosterone and its effects on the epithelial Na(+) channel. Am J Physiol Renal Physiol. 2000;278(4):F613–9. doi: 10.1152/ajprenal.2000.278.4.F613. [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am J Pathol. 2002;161(5):1773–81. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao W, Ahokas RA, Weber KT, Sun Y. ANG II-induced cardiac molecular and cellular events: role of aldosterone. Am J Physiol Heart Circ Physiol. 2006;291(1):H336–43. doi: 10.1152/ajpheart.01307.2005. [DOI] [PubMed] [Google Scholar]
- 55.Miyata Y, Muto S, Kusano E. Mechanisms for nongenomic and genomic effects of aldosterone on Na+/H+ exchange in vascular smooth muscle cells. J Hypertens. 2005;23(12):2237–50. doi: 10.1097/01.hjh.0000194122.27475.6c. [DOI] [PubMed] [Google Scholar]
- 56.Ebata S, Muto S, Okada K, Nemoto J, Amemiya M, Saito T, Asano Y. Aldosterone activates Na+/H+ exchange in vascular smooth muscle cells by nongenomic and genomic mechanisms. Kidney Int. 1999;56(4):1400–12. doi: 10.1046/j.1523-1755.1999.00674.x. [DOI] [PubMed] [Google Scholar]
- 57.Choi EY, Ha JW, Yoon SJ, Shim CY, Seo HS, Park S, Ko YG, Kang SM, Choi D, Rim SJ, Jang Y, Chung N. Increased plasma aldosterone-to-renin ratio is associated with impaired left ventricular longitudinal functional reserve in patients with uncomplicated hypertension. J Am Soc Echocardiogr. 2008;21(3):251–6. doi: 10.1016/j.echo.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Galetta F, Bernini G, Franzoni F, Bacca A, Fivizzani I, Tocchini L, Bernini M, Fallahi P, Antonelli A, Santoro G. Cardiac remodeling in patients with primary aldosteronism. J Endocrinol Invest. 2009 Jun 24; doi: 10.1007/BF03346529. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 59.Li R, Richey PA, DiSessa TG, Alpert BS, Jones DP. Blood aldosterone-to-renin ratio, ambulatory blood pressure, and left ventricular mass in children. J Pediatr. 2009;155(2):170–5. doi: 10.1016/j.jpeds.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology. 2007;148(8):3773–80. doi: 10.1210/en.2006-1691. [DOI] [PubMed] [Google Scholar]
- 61.Harada E, Yoshimura M, Yasue H, Nakagawa O, Nakagawa M, Harada M, Mizuno Y, Nakayama M, Shimasaki Y, Ito T, Nakamura S, Kuwahara K, Saito Y, Nakao K, Ogawa H. Aldosterone induces angiotensin-converting-enzyme gene expression in cultured neonatal rat cardiocytes. Circulation. 2001;104(2):137–9. doi: 10.1161/01.cir.104.2.137. [DOI] [PubMed] [Google Scholar]
- 62.Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani J, McMahon E. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology. 2002;143(12):4828–36. doi: 10.1210/en.2002-220120. [DOI] [PubMed] [Google Scholar]
- 63.Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20(9):1546–8. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 64.Hirono Y, Yoshimoto T, Suzuki N, Sugiyama T, Sakurada M, Takai S, Kobayashi N, Shichiri M, Hirata Y. Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system. Endocrinology. 2007;148(4):1688–96. doi: 10.1210/en.2006-1157. [DOI] [PubMed] [Google Scholar]
- 65.Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol. 2003;284(4):R916–27. doi: 10.1152/ajpregu.00406.2002. [DOI] [PubMed] [Google Scholar]
- 66.Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain rennin-angiotensin system in rats with heart failure? Hypertension. 2008;51(3):727–33. doi: 10.1161/HYPERTENSIONAHA.107.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez-Sanchez EP. Brain mineralocorticoid receptors: orchestrators of hypertension and end-organ disease. Curr Opin Nephrol Hypertens. 2004;13(2):191–6. doi: 10.1097/00041552-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 68.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab. 2005;288(2):E342–6. doi: 10.1152/ajpendo.00355.2004. [DOI] [PubMed] [Google Scholar]
- 69.Huang BS, Amin MS, Leenen FH. The central role of the brain in salt-sensitive hypertension. Curr Opin Cardiol. 2006;21(4):295–304. doi: 10.1097/01.hco.0000231398.64362.94. [DOI] [PubMed] [Google Scholar]
- 70.Sakai RR, McEwen BS, Fluharty SJ, Ma LY. The amygdala: site of genomic and nongenomic arousal of aldosterone-induced sodium intake. Kidney Int. 2000;57(4):1337–45. doi: 10.1046/j.1523-1755.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura T, Kawachi K, Saito Y, Saito T, Morishita K, Hoshino J, Hosoi T, Iwasaki T, Ohyama Y, Kurabayashi M. Effects of ARB or ACE-Inhibitor Administration on Plasma Levels of Aldosterone and Adiponectin in Hypertension. Int Heart J. 2009;50(4):501–12. doi: 10.1536/ihj.50.501. [DOI] [PubMed] [Google Scholar]
- 72.Furumatsu Y, Nagasawa Y, Tomida K, Mikami S, Kaneko T, Okada N, Tsubakihara Y, Imai E, Shoji T. Effect of renin-angiotensin-aldosterone system triple blockade on non-diabetic renal disease: addition of an aldosterone blocker, spironolactone, to combination treatment with an angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker. Hypertens Res. 2008;31(1):59–67. doi: 10.1291/hypres.31.59. [DOI] [PubMed] [Google Scholar]
- 73.Sato A, Hayashi K, Saruta T. Antiproteinuric effects of mineralocorticoid receptor blockade in patients with chronic renal disease. Am J Hypertens. 2005;18(1):44–9. doi: 10.1016/j.amjhyper.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 74.Sato A, Saruta T, Funder JW. Combination therapy with aldosterone blockade and renin-angiotensin inhibitors confers organ protection. Hypertens Res. 2006;29(4):211–6. doi: 10.1291/hypres.29.211. [DOI] [PubMed] [Google Scholar]
- 75.Epstein M. Aldosterone blockade: an emerging strategy for abrogating progressive renal disease. Am J Med. 2006;119(11):912–9. doi: 10.1016/j.amjmed.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 76.Bauersachs J, Fraccarollo D. Aldosterone antagonism in addition to angiotensin-converting enzyme inhibitors in heart failure. Minerva Cardioangiol. 2003;51(2):155–64. [PubMed] [Google Scholar]
- 77.Takeda Y, Zhu A, Yoneda T, Usukura M, Takata H, Yamagishi M. Effects of aldosterone and angiotensin II receptor blockade on cardiac angiotensinogen and angiotensin-converting enzyme 2 expression in Dahl salt-sensitive hypertensive rats. Am J Hypertens. 2007;20(10):1119–24. doi: 10.1016/j.amjhyper.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 78.Jansen PM, Danser AH, Imholz BP, van den Meiracker AH. Aldosterone-receptor antagonism in hypertension. J Hypertens. 2009;27(4):680–91. doi: 10.1097/HJH.0b013e32832810ed. [DOI] [PubMed] [Google Scholar]
- 79.Tylicki L, Rutkowski P, Renke M, Larczyński W, Aleksandrowicz E, Lysiak-Szydlowska W, Rutkowski B. Triple pharmacological blockade of the reninangiotensin-aldosterone system in nondiabetic CKD: an open-label crossover randomized controlled trial. Am J Kidney Dis. 2008;52(3):486–93. doi: 10.1053/j.ajkd.2008.02.297. [DOI] [PubMed] [Google Scholar]