Abstract
During baroreceptor unloading, sympathoexcitation is attenuated in near-term pregnant compared with nonpregnant rats. Alterations in balance among different excitatory and inhibitory inputs within central autonomic pathways likely contribute to changes in regulation of sympathetic outflow in pregnancy. Both baroreflex-dependent and baroreflex-independent GABAergic inputs inhibit sympathoexcitatory neurons within rostral ventrolateral medulla (RVLM). The present experiments tested the hypothesis that influence of baroreflex-independent GABAergic inhibition of RVLM is greater in pregnant compared with nonpregnant rats. Afferent baroreceptor inputs were eliminated by bilateral sinoaortic denervation in inactin-anesthetized rats. In pregnant compared with nonpregnant rats, baseline mean arterial pressure (MAP) was lower (pregnant = 75 ± 6 mmHg, nonpregnant = 115 ± 7 mmHg) and heart rate was higher (pregnant = 381 ± 10 beats/min, nonpregnant = 308 ± 10 beats/min). Pressor and sympathoexcitatory [renal sympathetic nerve activity, (RSNA)] responses due to bilateral GABAA receptor blockade (bicuculline, 4 mM, 100 nl) of the RVLM were greater in pregnant rats (ΔMAP: pregnant = 101 ± 4 mmHg, nonpregnant = 80 ± 6 mmHg; ΔRSNA: pregnant = 182 ± 23% control, nonpregnant = 133 ± 10% control). Unexpected transient sympathoexcitatory effects of angiotensin AT1 receptor blockade in the RVLM were greater in pregnant rats. Although excitatory responses to bicuculline were attenuated by prior RVLM AT1 receptor blockade in both groups, pressor responses to disinhibition of the RVLM remained augmented in pregnant rats. Increased influence of baroreflex-independent GABAergic inhibition in RVLM could contribute to suppressed sympathoexcitation during withdrawal of arterial baroreceptor input in pregnant animals.
Keywords: sympathetic nerve activity, brain stem, cardiovascular regulation, angiotensin II
Pregnancy is characterized by increased blood volume and cardiac output, mild tachycardia, and decreased arterial blood pressure due to a significant decrease in total peripheral resistance (21, 47). Pregnant women and animals are more susceptible to orthostatic (3) and hemorrhagic hypotension (6, 8). Although decreased vascular sensitivity to endogenous vasoconstrictors likely contributes to decreased compensatory responses (21), there is evidence that alterations in central nervous system (CNS) autonomic control mechanisms play a major role in regulation of sympathetic outflow and blood pressure during pregnancy.
Attenuated arterial baroreflex control of heart rate (HR) has been reported in pregnant animals (5, 50) and women (28, 49) and is partly due to suppression of the sympathetic component of the reflex. Direct assessment of arterial baroreflex control of renal sympathetic nerve activity (RSNA) in near-term pregnant rats (14, 43, 51) and rabbits (7, 54, 55) revealed that maximum baroreflex gain is decreased, largely due to an attenuated ability to increase RSNA above baseline levels in response to a hypotensive challenge. However, arterial baroreflex sympathoinhibitory responses are well maintained (14) or augmented (43, 51) in pregnant rats. Although adaptation of afferent baroreceptor discharge during prolonged pressure stimuli (10 min) appears to be more pronounced in pregnant rats (33) when afferent arterial baroreceptor discharge is assessed by using methods similar to those used in the above-mentioned baroreflex studies, the gain of arterial baroreceptor afferent discharge is not altered by pregnancy (33, 43). Therefore, it is likely that alterations in central autonomic integration contribute to the suppressed baroreflex sympathoexcitation that has been reported in pregnant animals.
Using c-fos immunoreactivity as an indicator of neuronal activation, our laboratory demonstrated that arterial baroreflex-mediated activation of the rostral ventrolateral medulla (RVLM) in response to a hypotensive challenge is attenuated in near-term pregnant rats (17). Tonically active sympathetic premotor neurons located in the RVLM project to the intermediolateral cell column of the spinal cord and provide an essential excitatory input to preganglionic sympathetic neurons (29). Although synaptic input appears to provide tonic excitation of RVLM neurons (48, 66), the source of this input and the transmitters involved are not thoroughly characterized. Under certain experimental conditions, a role for excitatory amino acid inputs to the RVLM has been demonstrated (37, 53). In addition, there is evidence for a nonexcitatory amino acid excitatory influence in the RVLM (67). Angiotensin II (ANG II)-type 1 (AT1) receptors are present (36, 70), and most studies support an overall excitatory effect of ANG II in the RVLM (19, 36, 68).
The major source of baseline inhibition of the RVLM is through GABAergic projections from the caudal ventrolateral medulla (CVLM) (60), although other GABAergic influences including GABA interneurons within the RVLM may also contribute (19, 29). In regard to arterial baroreflex-mediated sympathoinhibition, baroreceptor afferent nerves project to and excite second order neurons in the nucleus tractus solitarius (NTS), which ultimately results in inhibition of presympathetic neurons in the RVLM through an obligatory synapse in the CVLM and release of GABA in the RVLM (29, 60). In addition to baroreflex-dependent inhibition of the RVLM, a substantial baroreflex-independent inhibition of the RVLM has been described (15, 16, 60). When arterial pressure is low and therefore arterial baroreceptor input is minimal, iontophoretic application of the GABAA antagonist bicuculline results in increased firing of spinally projecting RVLM neurons (65). Following elimination of arterial baroreceptor input, either by NTS lesions or sinoaortic denervation (SAD) (18, 61), microinjection of bicuculline into the RVLM results in large sympathoexcitiatory responses. In sinoaortic-denervated rats, neuronal blockade of the CVLM results in increased arterial pressure and sympathetic nerve activity (15, 61). Taken together, these data indicate that there is a substantial baroreflex-independent inhibitory influence mediated by GABAA receptors in the RVLM.
The difference in arterial baroreflex-mediated sympathoexcitation in pregnant rats is most evident at low arterial pressures, when baroreceptor input is minimal. One possible explanation for this observation is that in the absence of baroreceptor input baroreflex-independent GABA influences result in decreased activation of RVLM presympathetic neurons. The present experiments were performed to test the hypothesis that the influence of arterial baroreflex-independent GABAergic inhibition of the RVLM is greater in near-term pregnant compared with nonpregnant rats. In addition, since an interaction has been shown between ANG II and GABA in the RVLM (63, 68) and pregnancy is associated with a generalized up-regulation of the renin-angiotensin system (47), the potential contribution of AT1 receptor activation in the RVLM to differences between pregnant and nonpregnant rats was evaluated.
MATERIALS AND METHODS
Animal Model and Surgical Preparation
Experiments were performed in 26 SAD female Sprague-Dawley rats (Harlan, Indianapolis, IN). Nonpregnant rats 3 to 5 mo of age were monitored for at least two regular 4-day estrous cycles before they were entered into an experiment. Stage of the cycle was determined by daily vaginal smear cytology. To avoid confounding effects of fluctuating hormone levels, experiments in nonpregnant rats were performed in the estrus stage of the cycle when circulating levels of ovarian hormones (estrogen and progesterone) were consistent and low (11). Timed, pregnant Sprague-Dawley rats were purchased from Harlan. Presence of a vaginal plug in the cage was defined as day 1 of pregnancy and experiments were performed on day 21 in near-term pregnant rats. Rat gestation is ~22 days. All surgical procedures and experimental protocols were performed according to the guidelines of the American Physiological Society for research involving animals (1) and were approved by the Institutional Laboratory Animal Care and Use Committee at the University of Missouri.
General Surgical Procedure
Rats were initially anesthetized with inactin (100 mg/kg ip). Depth of anesthesia was evaluated periodically, and supplemental doses of inactin (10 mg/kg iv) were administered as needed to maintain blockade of the pedal and palpibral reflexes and to block changes in mean arterial pressure (MAP) and HR during tail pinch. Our laboratory has found that arterial baroreflex sympathoexcitatory responses are well maintained with inactin anesthesia. Compared with chloralose-anesthetized rats (14), baroreflex responses in nonpregnant and pregnant inactin-anesthetized rats (43) more closely resemble responses seen in conscious rats (51). Rectal temperature was monitored (telethermometer, Yellow Springs Instruments) and maintained at 37–38°C with a water-circulating heating pad beneath the animal. Catheters were implanted in a femoral artery and vein for measurement of arterial pressure and injection of drugs. The trachea was cannulated, and the rats were artificially ventilated with oxygen-enriched room air by using a small animal ventilator (Harvard Apparatus, South Natick, MA). A renal sympathetic nerve was isolated retroperitoneally, placed on a bipolar platinum electrode, and sealed with dental impression material (Coltene President polyvinylsiloxane) for measurement of RSNA. The nerve signal was monitored on a dual-beam oscilloscope (Hitachi VC-6523) and audio amplifier (Winston Electronic). A band-pass filter of 30 Hz-3 KHz was used, and the signal was amplified 5,000 –10,000 with a RPS 107 amplifier (Grass Instruments, Quincy, MA). Nerve activity was converted from an analog to a digital signal with Powerlab Data Acquisition System (ADInstruments) and stored on a computer hard drive along with arterial blood pressure. MAP and HR were calculated by Powerlab software. The raw nerve activity signal was rectified, integrated (time constant = 28 ms), and smoothed using Powerlab software. Electrical noise was determined by measuring the signal 30–60 min after euthanasia (0.25 ml beuthanasia solution intravenously: 97 mg/kg pentobarbital + 12.5 mg/kg phenytoin sodium) and this value was subtracted from all nerve activity values. Nerve activity was standardized as a percent of the initial baseline value preceding initiation of the protocol.
SAD
Afferent input from arterial baroreceptors was eliminated by surgical SAD as described previously (40, 62). Briefly, following a midline incision, we sectioned bilaterally the superior laryngeal nerves as they merge with the vagi, the sympathetic nerve trunks immediately caudal to the superior cervical ganglia, and the aortic depressor nerves. Carotid sinus baroreceptor input was eliminated by stripping the carotid bifurcation regions of connective tissue and then painting the area with 10% phenol in ethanol. Reflex responses to an increase in MAP (phenylephrine, 5 µg iv) were tested before and after SAD. Following SAD surgery, an animal was accepted as baroreceptor denervated if the decrease in RSNA was no more than 20% and the decrease in HR was no more than six beats/min in response to phenylephrine.
Drugs and Solutions
Inactin, l-glutamic acid, bicuculline methiodide, ANG II, and gallamine triethiodide were purchased from Sigma (St. Louis, MO). L-158,809 was kindly provided by Merck Research Laboratories (Rahway, NJ). Phenylephrine hydrochloride was purchased from Baxter Healthcare (Irvine, CA). Inactin and gallamine triethiodide were dissolved in sterile isotonic saline. All other chemicals were dissolved in PBS. Beuthanasia-D solution was purchased from Schering Plough Animal Health (Canada).
Brain Stem Microinjections
Following catheter and nerve electrode placement, anesthetized animals were positioned prone in a stereotaxic frame (David Kopf). Rats were paralyzed with gallamine triethiodide (25 mg·kg−1 ·h−1 iv). A dorsal skin incision was made, the muscle was blunt dissected, occipital craniotomy was performed, and the dura was cut and retracted laterally to expose the brain stem. The rat’s head was deflected downward until the tip of calamus scriptorius was positioned 2.4 mm caudal to the intra-aural line (39).
Triple-barrel microinjection pipettes were used for microinjections into the RVLM. The tip diameter of pipettes was ~10 µm per barrel. Depending on the protocol, each of three individual barrels contained one of the following: l-glutamate (10 mM), bicuculline (4 mM), ANG II (20 µM), or the AT1 receptor antagonist L-158,809 (10 mM). The pipette barrels were connected with silicone tubing to pressure injection modules of a custom-built micropressure control unit. Volume of microinjections was determined by observing movement of the solution meniscus in the pipette barrel with a × 175 microscope supplied with a scaled reticule (Rolyn Optics, Germany). Relative to calamus scriptorius, the stereotaxic coordinates for the RVLM were anterior-posterior = 0.7 mm, medial-lateral = 2.00 mm, and dorsal-ventral =−3.6 –3.7 mm. These coordinates are within the range reported in other studies that used a similar method for positioning the rat in the stereotaxic frame and subsequently identified the RVLM (30, 39, 44). At the time of the experiment, the RVLM was functionally defined as the site that produced an increase in MAP ≥ 15 mmHg and an increase in RSNA in response to microinjection of l-glutamate (10 mM, 30 nl). The RVLM was identified within one to two penetrations (2 to 6 injections) per side.
At the end of all experiments, the injection site was marked by microinjection of Chicago Sky Blue dye (2%, 100 nl), and the site within the RVLM was verified by standard histological evaluation. Location of injection sites was estimated by comparison to a rat brain atlas (57). Following euthanasia, the number of fetuses was counted in the pregnant rats. Three to four fetuses were randomly selected, weighed, and an approximate average fetal weight was determined for each pregnant rat to verify normal pregnancy.
Control Experiments in Nonpregnant Rats
Preliminary experiments were performed in 11 nonpregnant SAD rats (227 ± 4.3 g). SAD was performed, and the RVLM was functionally identified as described above. In six nonpregnant rats, responses to unilateral injection of ANG II (20 µM, 50 nl) into the RVLM were determined. In three of these rats, two injections of ANG II were separated by 50 min. In the other three rats, 30 min after the first ANG II injection, the AT1 receptor antagonist L-158,809 (10 mM, 100 nl) was microinjected into the ipsilateral RVLM, and 20 min later responses to microinjection of ANG II in the RVLM were tested again. In five additional nonpregnant rats, responses to repeated bilateral microinjections of bicuculline (4 mM, 100 nl) in the RVLM were determined to test for effects of time. To approximate the time course of the experimental protocol in which AT1 receptor blockade was performed, 70–90 min were allowed before responses to bilateral bicuculline in the RVLM were again determined.
Experimental Protocol: Effects of Pregnancy and AT1 Receptor Blockade on Baroreflex-Independent GABAA Inhibition of the RVLM
Approximately 1 h following SAD, in nine estrus-stage nonpregnant and six pregnant rats, a triple-barrel glass micropipette, filled with l-glutamate, the GABAA receptor antagonist, bicuculline, and the AT1 receptor antagonist (AT1X) L-158,809 in separate barrels was used for microinjections into the RVLM. The RVLM was functionally identified bilaterally based on pressor and sympathoexcitatory responses to l-glutamate (10 mM, 30 nl). Stereotaxic coordinates were noted and 10–15 min after MAP, HR, and RSNA had returned to control levels, bicuculline (4 mM, 100 nl) was first injected into the right RVLM and then within 5 min into the left RVLM. Following a 45- to 50-min recovery period, L-158,809 (10 mM, 100 nl) was microinjected into the RVLM bilaterally. After 20 min, bilateral disinhibition of the RVLM with bicuculline (4 mM, 100 nl) was repeated.
Statistical Analysis
Student’s t-tests were used to compare baseline MAP and HR between nonpregnant and pregnant rats. Paired t-tests were used to compare values before and after intravenous phenylephrine or microinjection of l-glutamate, bicuculline, and ANG II into the RVLM, changes due to the first and second ANG II injection into the RVLM, and changes to the first and second microinjection of bicuculline in the RVLM in time control experiments. Responses to phenylephrine before and after SAD and responses to microinjection of bicuculline into the RVLM before and after RVLM AT1X in nonpregnant and pregnant rats were compared by two-way ANOVA with repeated measures on one factor. Student-Newman-Keuls post hoc test was performed following ANOVA. P ≤ 0.05 was considered significant. Values are presented as means ± SE.
RESULTS
Control Experiments in Nonpregnant Rats
Baseline hemodynamic values for 11 nonpregnant female rats in which preliminary experiments were performed are in Table 1. Phenylephrine increased MAP and reflexly decreased RSNA and HR prior to SAD (Table 2). Shortly after SAD (10–15 min) both MAP and HR were elevated, and reflex responses to phenylephrine-induced increases in MAP were attenuated by 90% (Table 1 and Table 2). Following surgical exposure of the brain stem, the RVLM was identified bilaterally with microinjections of l-glutamate, which significantly increased MAP, HR, and RSNA (Table 3). Immediately prior to the experiment (90–120 min after SAD), baseline MAP and HR had decreased to levels not different from pre-SAD values (Table 1).
Table 1.
Baseline values
| Initial | SAD, 10–15 min | SAD, 90–120 min | |
|---|---|---|---|
| Control Experiments | |||
| NP (n = 11) | |||
| MAP, mmHg | 101±2 | 147±6* | 109±6† |
| HR, beats/min | 296±8 | 392±8* | 289±11† |
| Experimental Protocol | |||
| NP (n = 9) | |||
| MAP, mmHg | 99±4 | 139±5* | 115±7*† |
| HR, beats/min | 307±12 | 383±9* | 308±10† |
| P (n = 6) | |||
| MAP, mmHg‡§ | 86±3 | 107±5* | 75±6† |
| HR, beats/min‡§ | 354±12 | 451±10* | 381±10† |
Values are means ± SE; n = number of rats. SAD, sinoaortic denervation; NP, nonpregnant; MAP, mean arterial pressure; HR, heart rate; P, pregnant.
Within group, different from initial;
within group, different from SAD (10–15 min);
main effect of pregnancy;
main effect of SAD.
Table 2.
Response to intravenous phenylephrine
| ΔMAP, mmHg | Δ RSNA, %control | Δ HR, beats/min | |
|---|---|---|---|
| Control Experiments | |||
| NP (n = 11) | |||
| Before SAD | 46±2 | −68±2 | −31±6 |
| After SAD | 36±2* | −7±2* | −3±0* |
| Experimental Protocol | |||
| NP (n = 9) | |||
| Before SAD | 49±3 | −52±8 | −30±3 |
| After SAD | 40±2* | −4±2* | −3±1* |
| P (n = 6) | |||
| Before SAD | 32±2† | −64±6 | −8±2† |
| After SAD | 48±2*‡ | −4±1* | −3±0 |
Values are means ± SE; n = number of rats. RSNA, renal sympathetic nerve activity.
Within group, different from before SAD;
different from NP before SAD;
different from NP after SAD.
Table 3.
Responses to l-glutamate in RVLM
| NP | P | |||||
|---|---|---|---|---|---|---|
| Δ MAP, mmHg | Δ HR, beats/ min | Δ RSNA, %control | Δ MAP, mmHg | Δ HR, beats/min | Δ RSNA, %control | |
| Control experiments (n = 11) | 49±5 | 13±2 | 152±18 | |||
| Experimental protocol† | 43±7 | 14±4 | 141±12 | 49±6 | 9±7 | 155±24 |
Values are means ± SE;
NP, n = 9; P, n = 6.
AT1 Receptor Blockade in RVLM
MAP, RSNA, and HR responses to repeated microinjection of ANG II into the RVLM with and without AT1 receptor blockade (AT1X) are shown in Table 4. In the control group, unilateral injection of ANG II into the RVLM resulted in significant increases in MAP, RSNA, and HR. Responses to the first and second ANG II microinjection 50 min later were not different. In the AT1X group (n = 3), microinjection of ANG II prior to AT1X increased MAP and RSNA. Microinjection of L-158,809 (AT1X) in the RVLM resulted in an initial increase in MAP, HR, and RSNA, but these parameters returned to levels not different from baseline values within 20 min at which time ANG II was administered a second time. Following AT1X changes in MAP and RSNA in response to microinjection of ANG II in the RVLM were not statistically significant. Microinjection of ANG II into the RVLM did not significantly change HR either before or after AT1X in this group of rats. These data verified that the dose of L-158,809 used in these experiments significantly attenuated responses to AT1 receptor activation in the RVLM.
Table 4.
Response to ANG II in the RVLM
| Control Group, n =3 | AT1X Group, n = 3 | |||
|---|---|---|---|---|
| ANG II-1 | ANG II-2 | ANG II Before AT1X |
ANG II After AT1X |
|
| Δ MAP, mmHg | 29±3 | 32±2 | 37±4 | 5±3* |
| Δ RSNA. %control | 116±40 | 107±33 | 141±29 | 21±8* |
| Δ HR, beats/min | 10±0 | 9±0 | 8±2 | 2±0 |
Values are means ± SE; n = number of rats. AT1X = L-158,809 in RVLM.
Different from ANG II response before AT1X.
Bicuculline Time Control Experiments: Responses to Repeated Bilateral GABAA Receptor Blockade in the RVLM
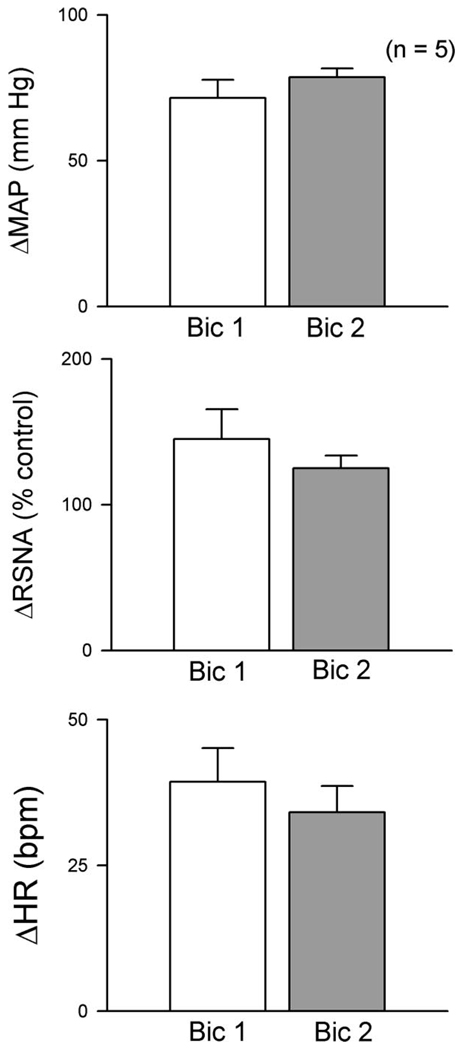
In five rats, bilateral microinjection of bicuculline into the RVLM significantly increased MAP, RSNA, and HR. Left and right bicuculline injections were separated by less than 5 min and responses were maximal within 1 to 2 min following the last injection. All values returned to control levels within 30–45 min. Mean responses to bilateral bicuculline in the RVLM are shown in Fig. 1. Seventy to ninety minutes after the first bicuculline response (Bic1), bicuculline was again microinjected into the RVLM bilaterally (Bic2) and increases in MAP, RSNA, and HR were not different from the initial response. Thus excitatory responses to disinhibition of the RVLM were reproducible for the length of the experimental protocol.
Fig. 1.
Responses to repeated bilateral GABAA receptor blockade in the rostral ventrolateral medulla (RVLM). Bilateral microinjection of bicuculline (Bic1; 4 mM, 100 nl) into the RVLM of 5 nonpregnant (NP) sinoaortic denervatied (SAD) rats significantly increased mean arterial pressure (MAP), renal sympathetic nerve activity (RSNA), and heart rate (HR). All values returned to baseline within ~45 min (not shown). Bic was microinjected again into the RVLM (Bic2) 70–90 minutes after Bic1, and responses were not different from Bic1. Values are means ± SE; bpm, beats per min.
Experimental Protocol: Effects of Pregnancy and AT1 Receptor Blockade on Responses to Bilateral GABAA Receptor Blockade in the RVLM of SAD Rats
Animals
Pregnant rats (n = 6, 348 ± 8 g) weighed more than nonpregnant rats (n = 9, 238 ± 7 g) and had 13 ± 1.0 fetuses with an average weight of 4.6 ± 0 g.
Effects of SAD in nonpregnant and pregnant rats
MAP was lower and HR was higher in pregnant compared with nonpregnant rats both before and after SAD (Table 1). Ten to fifteen minutes after SAD, MAP and HR were elevated above pre-SAD values in both groups. RSNA was also elevated above pre-SAD values (pregnant = 156 ± 13; nonpregnant = 191 ± 20% control), and the increases were not significantly different between groups. Arterial baroreflex responses to intravenous phenylephrine were tested before and 10–15 min after SAD surgery. Mean data for changes in MAP, RSNA, and HR are shown in Table 2. Phenylephrine significantly increased MAP in both nonpregnant and pregnant rats before and after SAD. Prior to SAD, pressor responses to phenylephrine were greater in nonpregnant compared with pregnant rats. Following SAD, the relationship between nonpregnant and pregnant groups was reversed so that pressor responses to phenylephrine were greater in pregnant compared with nonpregnant rats. Maximum MAP with phenylephrine was higher in nonpregnant (178 ± 4 mmHg) compared with pregnant rats (155 ± 6 mmHg), which could have limited the increase in MAP in nonpregnant rats. Before SAD, baroreflex-mediated decreases in RSNA were significant and not different between groups, while baroreflex-mediated decreases in HR were attenuated in pregnant compared with nonpregnant rats. SAD attenuated arterial baroreflex decreases in RSNA by 92 and 94% in nonpregnant and pregnant rats, respectively. Baroreflex bradycardia was not significant in pregnant rats either before or after SAD and was not significant after SAD in nonpregnant rats (Table 2). Following SAD, MAP and HR slowly decreased to stable levels over the next hour. Immediately prior to the experimental protocol (90–120 min), HR was not different from pre-SAD values in either pregnant or nonpregnant rats. MAP decreased in both nonpregnant and pregnant rats, but remained greater than pre-SAD values in nonpregnant rats (Table 1). RSNA was not recorded continuously, and the value for integrated nerve activity at the beginning of an experimental protocol after all surgical preparation was completed was defined as 100%.
Functional identification of the RVLM
Following surgical exposure of the brain stem, the RVLM was identified bilaterally based on excitatory responses to microinjected l-glutamate. Responses were not different between the left and right RVLM, and these values were averaged to obtain one value for the response to RVLM microinjection of l-glutamate for each animal. Table 3 contains group means for responses to l-glutamate in the experimental protocol. l-Glutamate increased MAP and RSNA similarly in nonpregnant and pregnant groups. Although the modest increase in HR due to RVLM l-glutamate was significant in nonpregnant but not pregnant rats, changes in HR were not different between groups (Table 3).
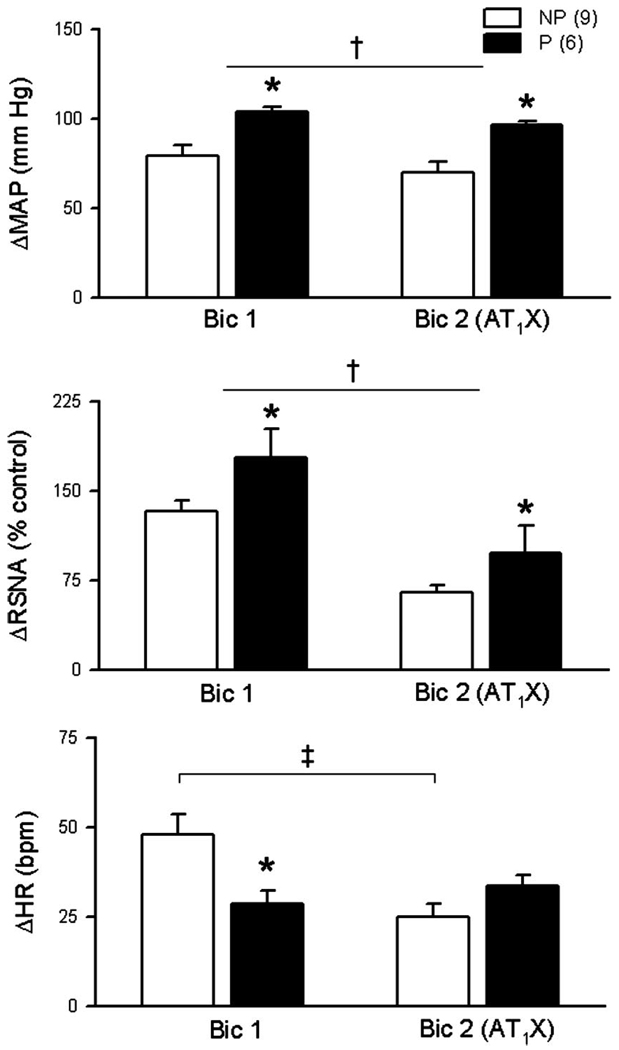
Effects of pregnancy and AT1 receptor blockade on responses to bilateral injection of bicuculline in the RVLM
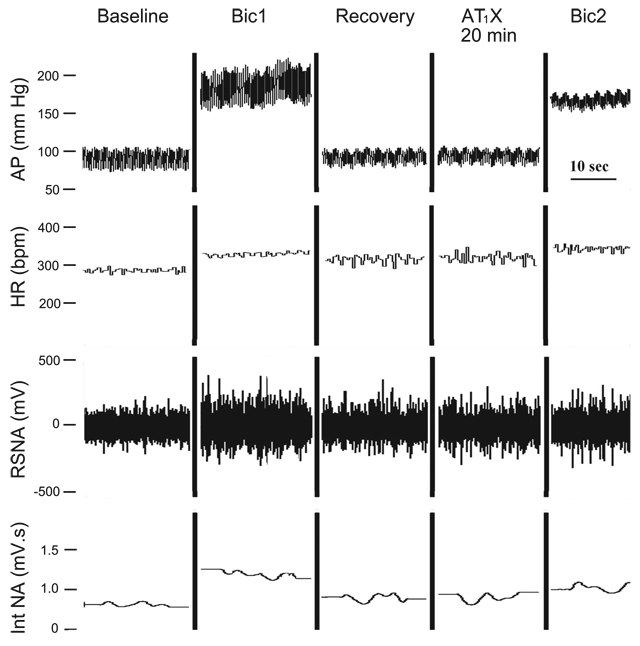
Responses to bilateral bicuculline before and after AT1 receptor blockade in one nonpregnant rat are shown in Fig. 2. Mean data for the nonpregnant and pregnant groups are shown in Fig. 3. Pressor and sympathoexcitatory responses to bilateral blockade of GABAA receptors in the RVLM (Bic1) were greater in pregnant compared with nonpregnant rats. The tachycardic response to bicuculline (Bic1) was less in pregnant animals.
Fig. 2.
Responses to bilateral Bic in the RVLM of 1 NP SAD rat. Two minutes after bilateral microinjection of Bic into the RVLM (Bic1, 4 mM, 100 nl) increases in arterial pressure (AP), heart rate (HR), and RSNA were maximal. Following recovery (~50 min), the angiotensin II type 1 receptor blocker (AT1X) L-158,809 (10 mM, 100 nl), was microinjected bilaterally into the RVLM. Baseline values were stable 20 min after AT1X. In the presence of AT1X, responses to bilateral microinjection of Bic (Bic2) were maximal within 2 min and were attenuated compared with Bic1. Int NA, rectified, integrated, and smoothed RSNA.
Fig. 3.
Baroreflex-independent GABAergic inhibition of RVLM is potentiated in pregnant (P) rats. In SAD rats, bilateral RVLM GABAA receptor blockade (Bic1, 4 mM, 100 nl) increased MAP, RSNA, and HR in both NP and P groups. Pressor and sympathoexcitatory responses were potentiated in P compared with NP rats (main effect of pregnancy). In contrast, the increase in HR following Bic1 was less in P rats. Bilateral blockade of AT1X in the RVLM with L-158,809 (10 mM, 100 nl) attenuated pressor and sympathoexcitatory responses to bilateral Bic (Bic2). However, increases in MAP and RSNA due to blockade of GABAA receptors in the RVLM remained potentiated in P compared with NP rats. AT1X attenuated tachycardic responses in NP rats only. Values are means ± SE. *P different from NP; †main effect of AT1X; ‡Bic1 different from Bic2; P ≤ 0.05.
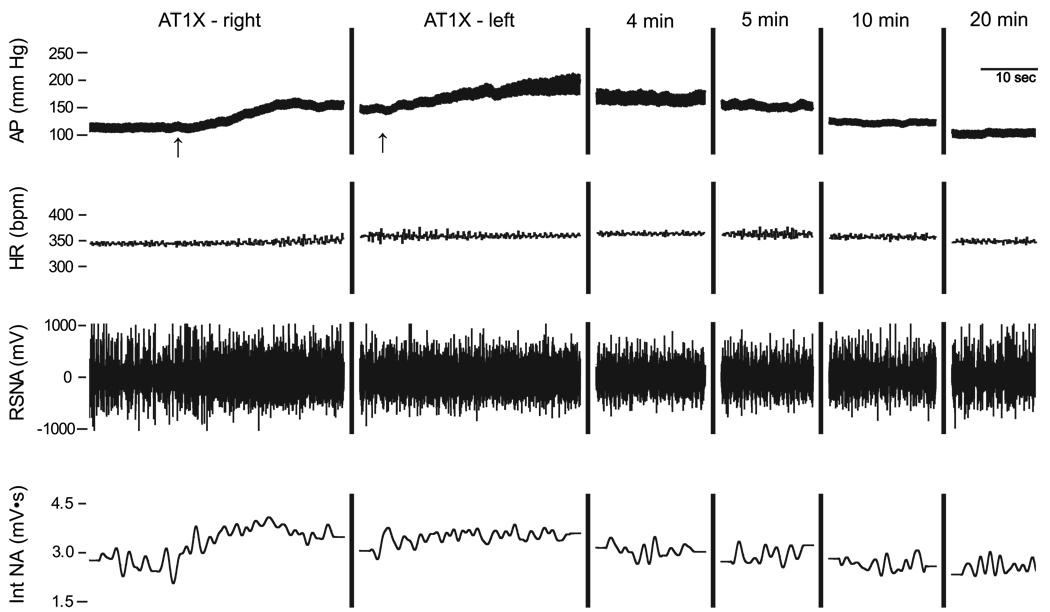
The immediate response to bilateral AT1 receptor blockade in the RVLM was excitatory and peak responses were observed within 1 to 2 min. Figure 4 is an example of an original record from one nonpregnant SAD rat showing increases in arterial pressure, HR, and RSNA following bilateral AT1 receptor blockade (AT1X) and subsequent return to baseline values. Mean peak increases in MAP (nonpregnant = 47 ± 4 mmHg; pregnant = 72 ± 12 mmHg), HR (nonpregnant = 25 ± 2 beats/min; pregnant = 25 ± 6 beats/min), and RSNA (nonpregnant = 38 ± 5% control; pregnant = 54 ± 18% control) were significant in both nonpregnant and pregnant rats. Two-way ANOVA on absolute values of MAP revealed a significant interaction, such that AT1X had a greater initial excitatory effect in pregnant compared with nonpregnant rats. Twenty minutes after bilateral AT1 receptor blockade, all values had returned to levels not different from control. In the presence of L-158,809, increases in MAP and RSNA due to bilateral injection of bicuculline in the RVLM (Bic2) were attenuated in both nonpregnant and pregnant rats. However, in the presence of AT1X, responses to RVLM bicuculline remained augmented in pregnant compared with nonpregnant rats. AT1X attenuated the bicuculline-induced increase in HR only in nonpregnant rats (Fig. 3).
Fig. 4.
Initial response to bilateral AT1X in the RVLM of 1 NP rat. AT1X receptor blocker L-158,809 (10 mM, 100 nl) was microinjected first into the right and then 2.5 min later into the left RVLM (arrows). AP, HR, and RSNA increased to maximal levels within ~2 min. Over the next 20 min, all values returned to control levels.
Histological identification of injection sites
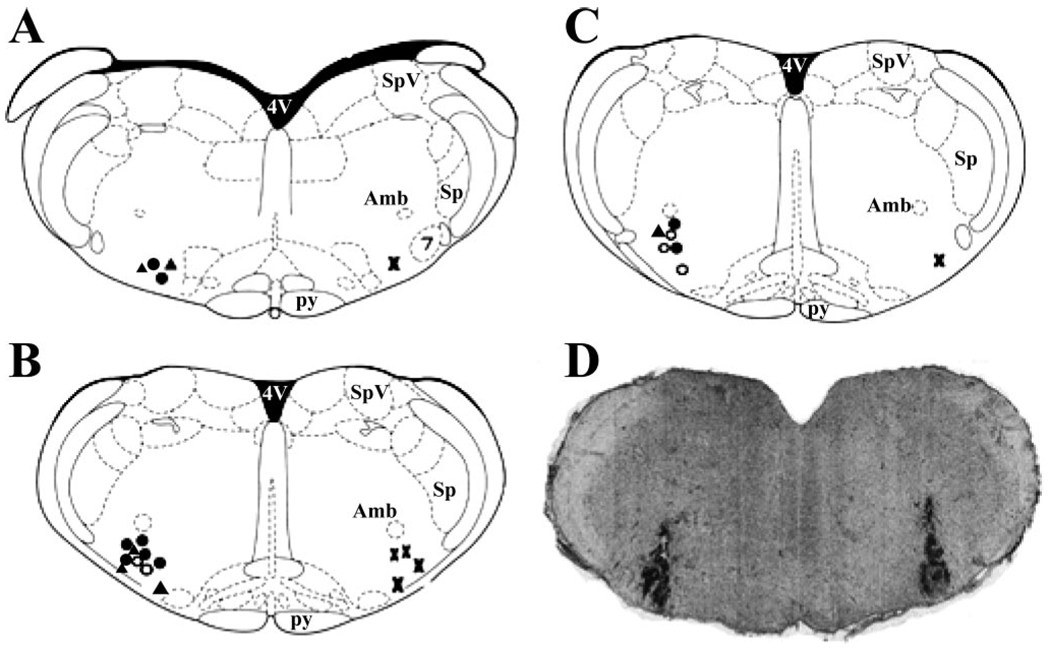
Figure 5 contains estimated locations for the center of microinjection sites in all rats used in these experiments. Bicuculline injections were performed bilaterally, but for clarity the location within the RVLM is marked only on one side of the diagrams. Injection sites for nonpregnant rats are indicated on the left side of the diagrams, and sites for pregnant rats are indicated on the right side. All microinjections were estimated to be within 11.6 to 11.96 mm caudal to bregma and within the ventral-lateral region defined as the RVLM (Fig. 5, A–C). Fig. 5D is an original photomicrograph from one nonpregnant rat, showing bilateral dye spots marking the injection sites.
Fig. 5.
RVLM injection sites. Approximate centers of injection sites are indicated on adapted diagrams from a standard rat atlas (57). A: bregma, −11.6 mm. B: bregma, −11.8 mm. C: bregma, −11.96 mm. Injection sites for NP rats are shown left: closed triangles are for data shown in Table 4, open circles are for data shown in Fig. 1, closed circles are for data shown in Fig. 3. Injection sites for P rats (Fig. 3) are shown right (X). D: coronal section of brain stem from 1 NP rat showing bilateral Chicago Sky Blue dye spots in the region of the RVLM.
DISCUSSION
Previously our laboratory demonstrated that arterial baroreflex sympathoexcitation due to baroreceptor unloading is attenuated in near-term pregnant compared with nonpregnant rats (43, 51). In response to a hypotensive challenge, fos expression is less in pregnant compared with nonpregnant rats, suggesting that neuronal activation of the RVLM during baroreceptor unloading is suppressed in pregnant rats (17). These observations could be explained by either decreased excitation or increased inhibition of the RVLM when baroreceptor input is minimal. A major finding of the present study is that the influence of baseline baroreflex-independent GABAA inhibition of the RVLM appears to be increased in pregnant rats. When this inhibition was removed (bilateral injection of bicuculline in the RVLM of SAD rats), sympathoexcitatory responses were greater in pregnant compared with nonpregnant rats. Thus, rather than a deficit in baseline excitatory drive to the RVLM, a greater influence of arterial pressure independent GABAergic inhibition of the RVLM is implicated in pregnancy. Prior blockade of AT1 receptors within the RVLM attenuated excitatory responses to disinhibition of the RVLM in both groups. However, ANG II mechanisms alone cannot account for differences between nonpregnant and pregnant rats. Another interesting and unexpected observation in the present experiments is that the initial response to bilateral blockade of AT1 receptors in the RVLM of SAD rats was transient sympathoexcitation, which was greater in pregnant animals.
Responses to Unilateral Activation of the RVLM
Pressor and sympathoexcitatory responses to unilateral activation of the RVLM with l-glutamate were not different between pregnant and nonpregnant rats. If, as we hypothesized, the RVLM was under greater baseline inhibition in pregnant animals, we might have expected decreased responses to an excitatory stimulus. The dose of l-glutamate used to activate the RVLM was relatively high (10 mM, 30 nl), and it is likely that near-maximum responses were achieved, which could have masked subtle differences between nonpregnant and pregnant rats. However, pilot experiments in baroreceptor-intact rats, revealed that the dose-response relationship for increased MAP and RSNA due to microinjection of l-glutamate into the RVLM (30 nl, 1–100 mM) was not attenuated in pregnant compared with nonpregnant rats (41). In addition, in the present experiments, excitatory responses to bilateral GABAA receptor blockade in the RVLM of pregnant rats were substantial and greater than those in nonpregnant rats, suggesting that tonic endogenous sympathoexcitatory drive to the RVLM is well preserved in pregnancy.
GABAA receptor blockade in the RVLM: baroreflex-independent inhibition
The present experiments demonstrated that in the absence of arterial baroreflex afferent input, pressor and sympathoexcitatory responses to GABAA receptor blockade in the RVLM were greater in pregnant compared with nonpregnant rats. Thus, the influence of pressure-independent GABAergic inhibition of the RVLM appears to be augmented by pregnancy. In these experiments in SAD rats, the vagi, and therefore afferent inputs from cardiopulmonary receptors, were intact. Therefore one possible explanation for differences between pregnant and nonpregnant rats could be differences in cardiopulmonary reflex inhibition. However, sympathoinhibition through cardiopulmonary reflexes is attenuated rather than enhanced in pregnancy. Cardiopulmonary reflex-mediated diuresis, natriuresis, and inhibition of RSNA are all blunted in pregnant rats (34, 38, 56). In addition, pregnancy reduces afferent information from cardiopulmonary receptors by increasing the stimulus threshold for low-frequency units and selectively inactivating high-frequency discharging receptors (33, 64). Since pregnancy is associated with decreased afferent input from cardiopulmonary receptors and blunted cardiopulmonary reflex responses, differences in cardiopulmonary inhibitory input could not account for our observation of augmented GABAergic inhibition of the RVLM in pregnant rats.
The apparent potentiation of baroreflex-independent GABAA inhibition of the RVLM in pregnancy could be due to an actual increase in GABAergic inputs, increased release of GABA from nerve terminals within the RVLM, or enhanced postsynaptic responsiveness to GABA in the RVLM. Potential sources of arterial baroreflex-independent inhibitory input to the RVLM include the caudal midline medulla (58), paraventricular nucleus (PVN) of the hypothalamus (71), the CVLM (15, 16, 18, 60), and GABA interneurons within the RVLM (19). The caudal midline medulla does not appear to be tonically active (58), and most studies suggest that sympathoinhibition from the PVN is related to decreased excitatory input from the PVN to RVLM and involves GABA within the PVN rather than the RVLM (29). Therefore any increase in GABAergic input to the RVLM would likely involve regions other than the PVN.
The CVLM is tonically active and is required for baroreflex-dependent inhibition of RVLM sympathoexcitatory neurons and also accounts for a substantial baroreflex-independent GABAergic input to the RVLM (19, 29, 60). In addition, GABAergic interneurons within the RVLM have been reported to provide tonic inhibition of sympathetic outflow (19). The physiological stimulus for activating baroreflex-independent inhibitory pathways is not known. However, it is possible that pregnancy could be associated with an increase in baroreflex-independent GABAergic input onto RVLM presympathetic neurons, and this could originate from sources within or outside of the RVLM. Future studies are needed to determine whether this is the case and to determine which brain regions might be involved.
Another possibility that could explain our results would be increased sensitivity of RVLM GABAA receptors to endogenously released GABA in pregnant compared with nonpregnant rats. Ovarian hormones and their metabolites are known to influence CNS GABAergic mechanisms. The major metabolite of progesterone, 3α-hydroxy-dihydroprogesterone (3α-OH-DHP), belongs to a class of endogenous compounds termed neurosteroids or neuroactive steroids, which mediate rapid nongenomic membrane effects. 3α-OH-DHP binds to a unique site on the GABAA receptor complex and is a potent endogenous positive modulator of GABAA receptor function (4). The ovarian hormones estrogen and progesterone, which are elevated in near-term pregnant rats (27), participate in brain region-specific regulation of GABAA receptor subunit composition, regulation of enzymes involved in neurosteroid metabolism, and phosphorylation of synaptic GABAA receptors (4, 52, 59). These genomic and posttranslational effects of ovarian hormones can modulate the efficacy of neurosteroid interaction with GABAA receptors in a brain region and cell type specific manner (4).
Importantly, both plasma and CNS concentrations of 3α-OH-DHP are elevated during pregnancy (13, 25, 26, 59). Reports differ on how gestational day is defined in rats, which has created confusion in interpretation of the literature regarding neurosteroid levels in pregnancy, and this issue warrants clarification. For example, our laboratory defines the day following mating as day 1 of pregnancy. Concas et al. (13) define the day following mating as day 0 of pregnancy. These investigators measured cerebrocortical concentrations of 3α-OH-DHP at several time points during gestation and reported the highest brain concentrations of 3α-OH-DHP on day 19 (our day 20) and levels not different from control at the next time point measured, day 21 (our day 22), which is immediately prior to delivery. Similar to our laboratory, Frye and colleagues (25, 26) define the day following mating as day 1 of pregnancy and reported increased brain concentrations of 3α-OH-DHP both on day 18 (26) and day 21 (25), the day the present experiments were performed. Therefore, available data indicate that brain levels of 3α-OH-DHP are elevated on day 21 of pregnancy, as defined by our laboratory.
Because CNS concentrations of 3α-OH-DHP appear to be elevated, positive modulation of GABAA receptor function is a possible mechanism for augmented GABAergic inhibition in the RVLM of near-term pregnant rats. Previously our laboratory demonstrated that depressor and sympathoinhibitory responses due to activation of GABAA receptors in the RVLM of nonpregnant female rats are potentiated when preceded by RVLM microinjection of 3α-OH-DHP (32). In the presence of increased circulating levels of 3α-OH-DHP, inhibition of spinally projecting RVLM neurons in response to a pressor stimulus is augmented in female nonpregnant rats (44). Acute administration of 3α-OH-DHP, either intravenously (32, 51) or microinjected into the RVLM (31), but not into the NTS or CVLM (23) of nonpregnant female rats, mimics the effects of pregnancy on arterial baroreflex control of efferent sympathetic nerve activity. Thus, similar to the effects of pregnancy, arterial baroreflex-mediated increases in sympathetic nerve activity are reduced and baroreflex-mediated inhibition of neurons in the RVLM is increased by 3α-OH-DHP.
It is important to consider that GABAA receptors in the RVLM mediate both baroreflex-dependent and baroreflex-independent sympathoinhibition (19, 29, 60). Therefore, if elevated 3α-OH-DHP in pregnant rats positively modulates postsynaptic GABAA receptors in the RVLM, whether baroreflex-independent or due to increased arterial baroreflex input, potentiated responses to endogenously released GABA would be expected. We previously demonstrated potentiated inhibition of RSNA due to increased arterial baroreceptor input during elevated arterial pressure in pregnant compared with nonpregnant rats (43, 51). Prior to SAD in the present experiments, although pressor responses to intravenous phenylephrine were significantly smaller in pregnant rats, reflex decreases in RSNA were similar to nonpregnant rats. Although indirect, this observation is consistent with previous observations of potentiated sympathoinhibition when arterial blood pressure is increased in pregnant rats (43, 51).
On the other hand, baroreflex sympathoexcitation is mediated by withdrawal of baseline baroreflex GABAergic tone in the RVLM. If all else was equal between pregnant and nonpregnant rats, we might predict potentiated sympathoexcitatory responses upon removal of baseline baroreflex input during hypotension in pregnant rats. However, responses to baroreceptor unloading are attenuated in pregnant animals (7, 14, 43, 51, 55). The exact nature of the interaction between baroreflex-dependent and baroreflex-independent influences on sympathetic outflow is not known. It is possible, and even likely, that the effects of these two types of GABAergic sympathoinhibition are not simply additive. Electrophysiological recording experiments indicate that there are individual RVLM-projecting GABAergic neurons within the CVLM that can be driven by both baroreflex-dependent and baroreflex-independent inputs (60). Likewise, in the RVLM, an individual presympathetic neuron can receive both baroreflex-dependent and baroreflex-independent GABAergic input (60, 65). The connectivity is such that baroreflex-dependent and baroreflex-independent RVLM GABAergic pathways could interact so that their combined effects are occlusive. In other words, one input could compensate for the absence of the other in certain situations. Combined interpretation of results from previous studies (42, 51) and the present data are consistent with occlusive summation of GABAergic inputs on presympathetic neurons in the RVLM. In baroreflex-intact pregnant rats, if GABAA receptors are sensitized to endogenous GABA within the RVLM, during a hypotensive stimulus pressure-independent inhibition of the RVLM may be sufficient to maintain inhibition of presympathetic neurons that receive both inputs, despite withdrawal of baroreflex inhibition. Such an interaction could explain reduced sympathoexcitatory responses during arterial baroreceptor unloading in pregnant animals (7, 14, 51, 55). Similarly, in SAD rats, potentiation of responses to endogenous GABA (baroreflex independent) in pregnant rats could compensate for the loss of baroreflex-dependent GABAergic inhibition in the RVLM. Subsequent sympathoexcitatory responses to removal of baroreflex-independent GABAergic inhibition would then be greater in pregnant compared with nonpregnant animals (present results).
Although the present experiments suggest that the influence of baroreflex-independent GABAergic sympathoinhibition is potentiated in pregnancy, and results are consistent with sensitization of GABA receptors in the RVLM, other mechanisms could certainly contribute. For example, a recent study by Daubert et al. (20) proposed that impaired baroreflex control of HR in near-term pregnant rabbits is related to insulin resistance and decreased cerebrospinal fluid concentrations of insulin. Whether this applies to control of efferent sympathetic nerve activity or might involve GABAergic mechanisms remains to be tested. However, it is intriguing to consider that a rat model of metabolic syndrome that exhibits insulin resistance (69) is characterized by increased expression of the GABA synthesizing enzyme GAD 65 in the RVLM (10).
Effects of RVLM AT1 receptor blockade in the RVLM
AT1 receptors have been described on catecholamine-containing, glutamate and GABA neurons within the RVLM (36). In addition, presynaptic AT1 receptors have been identified and proposed to modulate both glutamate and GABA release (12, 22, 36, 45, 46). Thus, AT1 receptor activation has the potential of being either excitatory or inhibitory within the RVLM, and the overall effect of endogenous ANG II in the RVLM is likely dependent on (and possibly modulates) the balance between excitatory and inhibitory inputs to presympathetic neurons (36, 63).
Endogenous ANG II, or ANG II peptides, appear to contribute to tonic activity of RVLM sympathoexcitatory neurons under certain physiological conditions (1a, 68). In regard to pregnancy, there is indirect evidence that sympathoexcitatory effects of angiotensin may be augmented (9, 14, 35, 55). In addition, an interaction between GABA and ANG II has been demonstrated within the RVLM (63, 68) and in other brain regions associated with cardiovascular function (1a, 19, 45). Results of the present experiments indicate that prior blockade of AT1 receptors in the RVLM attenuated bicuculline-induced increases in MAP and RSNA to a similar degree in pregnant and nonpregnant rats. Reduced responses to bicuculline after AT1 receptor blockade in both groups could be due to elimination of an excitatory effect of ANG II which is unmasked by GABAA receptor blockade (68). Alternately, AT1 receptor blockade could attenuate responses to bicuculline by blocking an effect of ANG II to enhance GABA release within the RVLM (22, 45). Regardless of the mechanism for effects of AT1 receptor blockade. pressor and sympathoexcitatory responses to bicuculline remained augmented in pregnant compared with nonpregnant rats in the absence of AT1 receptor activation. Therefore, differences in ANG II actions within the RVLM alone cannot account for differences between pregnant and nonpregnant rats.
An interesting observation in these experiments is that, although 20 min after L-158,809 MAP, RSNA, and HR were no different from control values, the initial effects of bilateral AT1 receptor blockade in the RVLM of SAD rats were excitatory. Previous studies in male rats have reported excitatory responses to RVLM AT1 receptor antagonists (2, 24), although other studies have reported no baseline effects of AT1 receptor blockade in the RVLM of normal rats (1a, 63).
In conscious rats, microinjection into the RVLM of losartan or L-158,809 (the AT1 receptor antagonist used in the present experiments) increased MAP (24). In urethane-anesthetized hypoxic, but not normoxic rats, microinjection of the AT1 receptor antagonists, candesartan or L-158,809, into the RVLM increased MAP and RSNA, suggesting that endogenous ANG II was providing baseline inhibition of the RVLM. This excitatory effect of AT1 receptor blockade was reversed to sympathoinhibition in the presence of bicuculline in the RVLM (63). The relationship whereby excitatory effects of endogenous ANG II are unmasked by prior blockade of GABAA receptors in the RVLM has also been demonstrated in normal anesthetized rats (68). Regarding potential mechanisms, since AT1 receptors have been identified on GABA neurons within the RVLM (36), endogenous ANG II could be activating sympathoinhibitory GABAergic interneurons. Another possible explanation could be ANG II mediated potentiation of GABA release in the RVLM, similar to mechanisms proposed for inhibitory actions of ANG II in the PVN of the hypothalamus (22, 45).
In the present experiments, the initial pressor response to blockade of AT1 receptors in the RVLM was greater in pregnant compared with nonpregnant rats. Considering that excitatory responses to GABAA receptor blockade were also greater in pregnant rats, involvement of a GABAergic mechanism in the apparent inhibitory effects of ANG II seems likely. Although responses to exogenous ANG II were blocked, MAP, RSNA, and HR stabilized to values not different from control after the initial excitation due to AT1 receptor blockade. This return to control levels most likely represents the overall balance between excitatory and inhibitory effects of ANG II in the RVLM. Similar to studies discussed above, in the absence of GABAergic influence, the predominant effect of ANG II was likely sympathoexcitatory in both nonpregnant and pregnant rats.
Perspectives and Significance
These experiments in SAD rats suggest that independent of arterial baroreflexes, the influence of GABAergic sympathoinhibition in the RVLM was greater in near-term pregnant compared with nonpregnant rats. Endogenous ANG II likely contributed to the excitatory response following disinhibition of the RVLM in both groups, but ANG II mechanisms alone cannot account for the effects of pregnancy. Although multiple mechanisms probably contribute to CNS adaptations during pregnancy, one possible mechanism consistent with present results is positive modulation of GABAA receptors in the RVLM of pregnant rats by the neurosteroid metabolite of progesterone, 3α-OH-DHP, which is increased in pregnancy. Functionally, potentiation of GABAergic influences at the level of the RVLM could serve as a protective mechanism during pregnancy to limit abrupt increases in sympathetic nerve activity in response to a variety of stimuli.
ACKNOWLEDGMENTS
The authors thank Glenn Phaup, Kayla Terry, and Korryn Shogie for expert technical assistance in performing these experiments.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1-36245 (to C. M. Heesch) and University of Missouri Research Council Grant (to L. Kvochina).
REFERENCES
- 1.American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol. 2002;283:R281–R283. doi: 10.1152/ajpregu.00279.2002. [DOI] [PubMed] [Google Scholar]; 1a Averill DB, Diz DI. Angiotensin peptides and baroflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2000;51:119–128. doi: 10.1016/s0361-9230(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 2.Averill DB, Tsuchihashi T, Khosla MC, Ferrario CM. Losartan, non-peptide angiotensin II-type 1 (AT1) receptor antagonist, attenuates pressor and sympathoexcitatory responses evoked by angiotensin II and l-glutamate in rostral ventrolateral medulla. Brain Res. 1994;665:245–252. doi: 10.1016/0006-8993(94)91344-7. [DOI] [PubMed] [Google Scholar]
- 3.Barron WM, Mujais SK, Zinaman M, Bravo EL, Lindheimer MD. Plasma catecholamine responses to physiologic stimuli in normal human pregnancy. Am J Obstet Gynecol. 1986;154:80–84. doi: 10.1016/0002-9378(86)90397-2. [DOI] [PubMed] [Google Scholar]
- 4.Belelli D, Herd MB, Mitchell EA, Peden DR, Vardy AW, Gentet L, Lambert JJ. Neuroactive steroids and inhibitory neurotransmission: mechanisms of action and physiological relevance. Neuroscience. 2006;138:821–829. doi: 10.1016/j.neuroscience.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Brooks VL, Kane CM, Van Winkle DM. Altered heart rate baroreflex during pregnancy: role of sympathetic and parasympathetic nervous systems. Am J Physiol Regul Integr Comp Physiol. 1997;273:R960–R966. doi: 10.1152/ajpregu.1997.273.3.R960. [DOI] [PubMed] [Google Scholar]
- 6.Brooks VL, Kane CM, Welch LS. Regional conductance changes during hemorrhage in pregnant and nonpregnant conscious rabbits. Am J Physiol Regul Integr Comp Physiol. 1999;277:R675–R681. doi: 10.1152/ajpregu.1999.277.3.R675. [DOI] [PubMed] [Google Scholar]
- 7.Brooks VL, Quesnell RR, Cumbee SR, Bishop VS. Pregnancy attenuates activity of the baroreceptor reflex. Clin Exp Pharmacol Physiol. 1995;22:152–156. doi: 10.1111/j.1440-1681.1995.tb01972.x. [DOI] [PubMed] [Google Scholar]
- 8.Brooks VL, Quesnell RR, Kane CM, Keil LC. Hemodynamic and hormonal responses to hemorrhage in conscious rabbits at mid- and late gestation. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1082–R1090. doi: 10.1152/ajpregu.1998.275.4.R1082. [DOI] [PubMed] [Google Scholar]
- 9.Brooks VL, Welch LS, Kane CM. Role of angiotensin II in altered baroreflex function of conscious rabbits during late pregnancy. Am J Obstet Gynecol. 2001;184:476–482. doi: 10.1067/mob.2001.109593. [DOI] [PubMed] [Google Scholar]
- 10.Buck BJ, Kerman IA, Burghardt PR, Koch LG, Britton SL, Akil H, Watson SJ. Upregulation of GAD65 mRNA in the medulla of the rat model of metabolic syndrome. Neurosci Lett. 2007;419:178–183. doi: 10.1016/j.neulet.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- 12.Chan SHH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JYH. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–780. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- 13.Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RE, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crandall ME, Heesch CM. Baroreflex control of sympathetic outflow in pregnant rats: effects of captopril. Am J Physiol Regul Integr Comp Physiol. 1990;258:R1417–R1423. doi: 10.1152/ajpregu.1990.258.6.R1417. [DOI] [PubMed] [Google Scholar]
- 15.Cravo SL, Morrison SF. The caudal ventrolateral medulla is a source of tonic sympathoinhibition. Brain Res. 1993;621:133–136. doi: 10.1016/0006-8993(93)90308-a. [DOI] [PubMed] [Google Scholar]
- 16.Cravo SL, Morrison SF, Reis DJ. Differentiation of two cardiovascular regions within caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 1991;261:R985–R994. doi: 10.1152/ajpregu.1991.261.4.R985. [DOI] [PubMed] [Google Scholar]
- 17.Curtis KS, Cunningham JT, Heesch CM. Fos expression in brain stem nuclei of pregnant rats after hydralazine-induced hypotension. Am J Physiol Regul Integr Comp Physiol. 1999;277:R532–R540. doi: 10.1152/ajpregu.1999.277.2.R532. [DOI] [PubMed] [Google Scholar]
- 18.Dampney RAL, Blessing WW, Tan E. Origin of tonic GABAergic inputs to vasopressor neurons in the subretrofacial nucleus of the rabbit. J Auton Nerv Syst. 1988;24:227–239. doi: 10.1016/0165-1838(88)90123-3. [DOI] [PubMed] [Google Scholar]
- 19.Dampney RAL, Horiuchi J, Tagawa T, Fontes MAP, Potts PD, Polson JW. Medullary and supramedullar mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand. 2003;177:209–218. doi: 10.1046/j.1365-201X.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- 20.Daubert DL, Chung MY, Brroks VL. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2188–R2195. doi: 10.1152/ajpregu.00614.2006. [DOI] [PubMed] [Google Scholar]
- 21.DeSwiet M. Hypertension in Pregnancy. New York: Elsevier Science; 1988. [Google Scholar]
- 22.Ferguson AV, Latchford KJ. Local circuitry regulates the excitability of rat neurohypophysial neurones. Exp Physiol. 2000;85:153S–161S. doi: 10.1111/j.1469-445x.2000.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 23.Foley CM, Bruno SB, Kvochina L, Heesch CM. Alterations in baroreflex function by hindbrain microinjection of allopregnanolone in female rats (Abstract) FASEB J. 2005;19:A618. [Google Scholar]
- 24.Fontes MAP, Martins-Pinge MC, Naves V, Campagnole-Santos MJ, Lopes OU, Khosla MC, Santos RAS. Cardiovascular effects produced by microinjection of angiotensins and angiotensin antagonists into the ventrolateral medulla of freely moving rats. Brain Res. 1997;750:305–310. doi: 10.1016/s0006-8993(96)01476-x. [DOI] [PubMed] [Google Scholar]
- 25.Frye CA, Bayon LE. Seizure activity is increased in endocrine states characterized by decline in endogenous levels of the neurosteroid 3α,5α-THP. Neuroendocrinology. 1998;68:272–280. doi: 10.1159/000054375. [DOI] [PubMed] [Google Scholar]
- 26.Frye CA, Walf AA. Hippocampal 3α,5α-THP may alter depressive behavior of pregnant and lactating rats. Pharmacol Biochem Behav. 2004;78:531–540. doi: 10.1016/j.pbb.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Garland HO, Atherton JC, Baylis C, Morgan MRA, Milne CM. Hormone profiles for progesterone, oestradiol, prolactin, plasma renin activity, aldosterone and corticosterone during pregnancy and pseudopregnancy in two strains of rat: correlation with renal studies. J Endocrinol. 1987;113:435–444. doi: 10.1677/joe.0.1130435. [DOI] [PubMed] [Google Scholar]
- 28.Greenwood JP, Scott EM, Stoker JB, Walker JJ, Mary DASG. Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation. 2001;104:2200–2204. doi: 10.1161/hc4301.098253. [DOI] [PubMed] [Google Scholar]
- 29.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 30.Heesch CM, Laiprasert JD, Kvochina L. RVLM glycine receptors mediate GABAA and GABAB independent sympathoinhibition from CVLM in rats. Brain Res. 2006;1125:46–59. doi: 10.1016/j.brainres.2006.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heesch CM, Laiprasert JD, Rogers RC, Ghosh S. Effects of 3α-hydroxy dihydroprogesterone (3α-OH-DHP) in the rostral ventrolateral medulla (RVLM) of female rats (Abstract) Soc Neurosci Abstr. 1997;23:153. [Google Scholar]
- 32.Heesch CM, Rogers RC. Effects of pregnancy and progesterone metabolites on regulation of sympathetic outflow. Clin Exp Pharmacol Physiol. 1995;22:136–142. doi: 10.1111/j.1440-1681.1995.tb01970.x. [DOI] [PubMed] [Google Scholar]
- 33.Hines T. Baroreceptor afferent discharge in the pregnant rat. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1433–R1440. doi: 10.1152/ajpregu.2000.278.6.R1433. [DOI] [PubMed] [Google Scholar]
- 34.Hines T, Mifflin SW. Gestational effects on volume-sensitive cardiopulmonary receptor reflexes in the rat. Am J Physiol Regul Integr Comp Physiol. 1995;268:R736–R743. doi: 10.1152/ajpregu.1995.268.3.R736. [DOI] [PubMed] [Google Scholar]
- 35.Hines T, Porter J. Role for central angiotensin II in control of blood pressure during pregnancy. Am J Physiol Regul Integr Comp Physiol. 1989;257:R1457–R1461. doi: 10.1152/ajpregu.1989.257.6.R1457. [DOI] [PubMed] [Google Scholar]
- 36.Hu L, Zhu D, Yu Z, Wang JQ, Sun ZJ, Yao T. Expression of angiotensin II type 1 (AT1) receptor in the rostral ventrolateral medulla in rats. J Appl Physiol. 2002;92:2153–2161. doi: 10.1152/japplphysiol.00261.2001. [DOI] [PubMed] [Google Scholar]
- 37.Ito S, Komatsu K, Tsukamoto K, Sved AF. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension. 2000:413–417. doi: 10.1161/01.hyp.35.1.413. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman S, Deng Y. Renal response to atrial stretch during pregnancy in conscious rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R902–R906. doi: 10.1152/ajpregu.1993.265.4.R902. [DOI] [PubMed] [Google Scholar]
- 39.Kiely JM, Gordon FJ. Non-NMDA receptors in the rostral ventrolateral medulla mediate somatosympathetic pressor responses. J Auton Nerv Syst. 1993;43:231–240. doi: 10.1016/0165-1838(93)90329-s. [DOI] [PubMed] [Google Scholar]
- 40.Krieger EM. Neurogenic hypertension in the rat. Circ Res. 1964;15:511–521. doi: 10.1161/01.res.15.6.511. [DOI] [PubMed] [Google Scholar]
- 41.Kvochina L, Heesch CM. Excitatory responses in rostral ventrolateral medulla (RVLM) of virgin and pregnant rats (abstract) FASEB J. 2002;16:A500. [Google Scholar]
- 42.Kvochina L, Heesch CM. Increased tonic inhibitory influences on rostral ventrolateral medulla in term pregnant compared with virgin rats (Abstract) FASEB Journal. 2003;17:A24. [Google Scholar]
- 43.Laiprasert JD, Hamlin R, Heesch CM. Afferent baroreceptor discharge in pregnant rats. Am J Physiol Heart Circ Physiol. 2001;281:H2456–H2462. doi: 10.1152/ajpheart.2001.281.6.H2456. [DOI] [PubMed] [Google Scholar]
- 44.Laiprasert JD, Rogers RC, Heesch CM. Neurosteroid modulation of arterial baroreflex-sensitive neurons in rat rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 1998;274:R903–R911. doi: 10.1152/ajpregu.1998.274.4.R903. [DOI] [PubMed] [Google Scholar]
- 45.Latchford KJ, Ferguson AV. Angiotensin II activates a nitric-oxide-driven inhibitory feedback in the rat paraventricular nucleus. J Neurophysiol. 2003;89:1238–1244. doi: 10.1152/jn.00914.2002. [DOI] [PubMed] [Google Scholar]
- 46.Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–5049. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindheimer MD, Katz AI. Renal physiology and disease in pregnancy. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. New York: Raven; 1992. pp. 3371–3432. [Google Scholar]
- 48.Lipski J, Kanjhan R, Kruszewska B, Rong W. Properties of presympathetic neurones in the rostral ventrolateral medulla in the rat: an intracellular study “in vivo.”. J Physiol. 1996;490:729–744. doi: 10.1113/jphysiol.1996.sp021181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucini D, Strappazzon P, Vecchia LD, Maggioni C, Pagani M. Cardiac autonomic adjustments to normal human pregnancy: insight from spectral analysis of R-R interval and systolic arterial pressure variability. J Hypertens. 1999;17:1899–1904. doi: 10.1097/00004872-199917121-00019. [DOI] [PubMed] [Google Scholar]
- 50.Lumbers ER, Yu ZY. A method for determining baroreflex-mediated sympathetic and parasympathetic control of the heart in pregnant and non-pregnant sheep. J Physiol. 1999;515:555–566. doi: 10.1111/j.1469-7793.1999.555ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masilamani SME, Heesch CM. Effects of pregnancy and progesterone metabolites on baroreflex control of sympathetic outflow and heart rate in conscious rats. Am J Physiol Regul Integr Comp Physiol. 1997;272:R924–R934. doi: 10.1152/ajpregu.1997.272.3.R924. [DOI] [PubMed] [Google Scholar]
- 52.Mody I. Aspects of the homeostatic plasticity of GABAA receptor-mediated inhibition. J Physiol. 2005;562:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreira TS, Sato MA, Takakura ACT, Menani JV, Colombari E. Role of pressor mechanisms from the NTS and CVLM in control of arterial pressure. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1416–R1425. doi: 10.1152/ajpregu.00053.2005. [DOI] [PubMed] [Google Scholar]
- 54.O’Hagan KP, Casey SM. Arterial baroreflex during pregnancy and renal sympathetic nerve activity during parturition in rabbits. Am J Physiol Heart Circ Physiol. 1998;274:H1635–H1642. doi: 10.1152/ajpheart.1998.274.5.H1635. [DOI] [PubMed] [Google Scholar]
- 55.O’Hagan K, Skogg KA, Stevenson J. AT1 receptor block does not affect arterial baroreflex during pregnancy in rabbits. Am J Physiol Heart Circ Physiol. 2001;280:H1996–H2005. doi: 10.1152/ajpheart.2001.280.5.H1996. [DOI] [PubMed] [Google Scholar]
- 56.Patel KP, Zhang PL. Role of renal nerves in renal responses to acute volume expansion during pregnancy in rats. Proc Soc Exp Biol Med. 1993;203 doi: 10.3181/00379727-203-43585. [DOI] [PubMed] [Google Scholar]
- 57.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. compact 3rd edition. New York: Academic; 1997. p. 3. [Google Scholar]
- 58.Potas JR, Dampney RAL. Sympathoinhibitory pathway from caudal midline medulla to RVLM is independent of baroreceptor reflex pathway. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1071–R1078. doi: 10.1152/ajpregu.00559.2002. [DOI] [PubMed] [Google Scholar]
- 59.Russell JA, Brunton PJ. Neuroactive steroids attenuate oxytocin stress responses in late pregnancy. Neuroscience. 2006;138:879–889. doi: 10.1016/j.neuroscience.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol. 2002;29:514–521. doi: 10.1046/j.1440-1681.2002.03665.x. [DOI] [PubMed] [Google Scholar]
- 61.Schreihofer AM, Ito S, Sved AF. Brain stem control of arterial pressure in chronic arterial baroreceptor-denervated rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1746–R1755. doi: 10.1152/ajpregu.00307.2005. [DOI] [PubMed] [Google Scholar]
- 62.Schreihofer AM, Sved AF. Use of sinoaortic denervation to study the role of baroreceptors in cardiovascular regulation. Am J Physiol Regul Integr Comp Physiol. 1994;266:R1705–R1710. doi: 10.1152/ajpregu.1994.266.5.R1705. [DOI] [PubMed] [Google Scholar]
- 63.Sheriff MJ, Fontes MAP, Killinger S, Horiuchi J, Dampney RAL. Blockade of AT1 receptors in the rostral ventrolateral medulla increases sympathetic activity under hypoxic conditions. Am J Physiol Regul Integr Comp Physiol. 2006;290:R733–R740. doi: 10.1152/ajpregu.00410.2005. [DOI] [PubMed] [Google Scholar]
- 64.Storey E, Kaufman S. Effect of pregnancy and 5α-pregnan-3α-ol-20-one on atrial receptor afferent discharge in rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1427–R1433. doi: 10.1152/ajpregu.00693.2003. [DOI] [PubMed] [Google Scholar]
- 65.Sun MK, Guyenet PG. GABA-mediated baroreceptor inhibition of reticulospinal neurons. Am J Physiol Regul Integr Comp Physiol. 1985;249:R672–R680. doi: 10.1152/ajpregu.1985.249.6.R672. [DOI] [PubMed] [Google Scholar]
- 66.Sved AF, Ito S, Madden CJ, Stocker SD, Yajima Y. Excitatory inputs to the RVLM in the context of the baroreceptor reflex. Ann NY Acad Sci. 2002;940:247–258. doi: 10.1111/j.1749-6632.2001.tb03681.x. [DOI] [PubMed] [Google Scholar]
- 67.Sved AF, Ito S, Yajima Y. Role of excitatory amino acid inputs to the rostral ventrolateral medulla in cardiovascular regulation. Clin Exp Pharmacol Physiol. 2002;29:503–506. doi: 10.1046/j.1440-1681.2002.03663.x. [DOI] [PubMed] [Google Scholar]
- 68.Tagawa T, Fontes MAP, Potts PD, Allen AM, Dampney RA. The physiological role of AT1 receptors in the ventrolateral medulla. Braz J Med Biol Res. 2000;33:643–652. doi: 10.1590/s0100-879x2000000600005. [DOI] [PubMed] [Google Scholar]
- 69.Wisloff U, Najjar SM, Oyvind E, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei KLSJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 70.Yang SN, Lippoldt A, Jansson A, Phillips MI, Ganten D, Fuxe K. Localization of angiotensin II AT1 receptor-like immunoreactivity in catecholaminergic neurons of the rat medulla oblongata. Neuroscience. 1997;81:503–515. doi: 10.1016/s0306-4522(97)00057-2. [DOI] [PubMed] [Google Scholar]
- 71.Yang Z, Coote JH. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurones in the rostral ventrolateral medulla of the rat. J Physiol. 1998;513:521–530. doi: 10.1111/j.1469-7793.1998.521bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]