Abstract
Background
Ulipristal (CDB-2914; UPA) is a progesterone receptor modulator with contraceptive potential. To test its effects when delivered by an intrauterine system (IUS), we prepared control and UPA-filled IUS and evaluated their effects in rhesus macaques.
Study Design
Short lengths of Silastic tubing either empty (n=3), or containing UPA (n=5), were inserted into the uteri of 8 ovariectomized macaques. Animals were cycled by sequential treatment with estradiol and progesterone. After 3.5 cycles, the uterus was removed.
Results
During treatment, animals with an empty IUS menstruated for a mean total of 11.66 ± 0.88 days while UPA-IUS treated animals bled for only 1 ± 0.45 days. Indices of endometrial proliferation were significantly reduced by UPA-IUS treatment. The UPA exposed endometria were atrophied with some glandular cysts while the blank controls displayed a proliferative morphology without cysts. Androgen receptors were more intensely stained in the glands of the UPA-IUS treated endometria than in the blank-IUS treated controls.
Conclusions
In rhesus macaques, a UPA-IUS induced endometrial atrophy and amenorrhea. The work provides proof of principle that an IUS can deliver effective intrauterine concentrations of Ulipristal.
Keywords: CDB-2914, Ulipristal, intrauterine, rhesus macaque, endometrium
1.3. Introduction
An estrogen-free, bleed-free hormonal contraceptive method would be valuable to women from both medical and cultural viewpoints. The use of a progesterone receptor modulator (PRM) as a contraceptive is attractive because such molecules, including those that antagonize P action, can suppress ovulation, induce endometrial atrophy, and result in amenorrhea, a condition that is perceived favorably in many cultural settings [1,2]. PRMs are a new class of PR ligands that exert clinically relevant, tissue-selective, P agonist, antagonist, partial, or mixed agonist/antagonist effects on various P target tissues [3-5]. PRMs either antagonize or modulate P action by binding to PR and completely or partially inhibiting P-dependent gene expression. CDB-2914, also called VA-2914, and now named Ulipristal acetate (UPA) derives from 19-norprogesterone (17 alpha-acetoxy-11-[4-N,N-dimethylaminophenyl]-19-norpregna-4,9-diene-3,20-dione) and is an antagonistic PRM [6] currently undergoing clinical investigation.
UPA, like mifepristone, binds PR with high affinity and antagonizes P action. In bioassays UPA did not exhibit progestational activity in the estradiol-primed immature female rabbit at doses that exhibited anti-progestational activity. A dose-dependent inhibition of ovulation was shown in the rat model. Neither UPA nor mifepristone exhibited glucocorticoid activity as determined by thymus involution in rats; mifepristone was twice as potent as UPA in antagonizing glucocorticoid action [7]
UPA has potential clinical applications for regular and emergency contraception, the treatment of fibroids and endometriosis, cervical ripening for induction of labor, and the treatment of breast cancer and gliomas [8,9]. In preliminary studies of the contraceptive effects of UPA delivered orally or vaginally, ovulation was suppressed and amenorrhea induced in a large percentage of the treated women [unpublished report, Population Council, 2005]. However, the ability of UPA to suppress endometrial growth and bleeding when delivered by an intrauterine system (IUS) has not been determined. Although copper-based intrauterine devices are highly effective contraceptives, patient compliance is reduced because of heavy menstrual bleeding, breakthrough bleeding and cramping [10]. Levonorgestrel-filled IUS have fewer side effects than copper-IUS but breakthrough bleeding in the early months reduces patient compliance [11]. In previous work we showed that IUS delivery of the antiprogestin, ZK 230 211 (Schering AG), could suppress endometrial development in the rhesus macaque model [12]. Because PRMs vary in their effectiveness when delivered from intrauterine devices [4] and because the doses necessary to suppress the endometrium and induce amenorrhea are unknown, and finally because UPA is a multi potential PRM [13], we explored the effects of a UPA-IUS on the endometrium in rhesus macaques.
2. Methods
2.1 IUS preparation
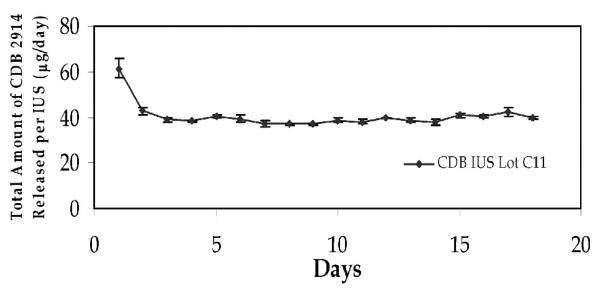
A mixture of silicone elastomer (R-2602; Nu-Sil Silicone Technology, Toms River, NJ) containing 20% micronized UPA was prepared and injected into a small inert silicone tube. Upon polymerization of silicone matrix, the two ends of the tube were sealed with medical silicone adhesive to form tubes of 10 or 15 mm length. The in vitro release rate of UPA from these IUS was measured by suspending the tubes in a glass vial containing distilled water with constant shaking in a 37° C water bath. Distilled water in the vial was changed daily. An aliquot of a daily sample of distilled water was analyzed for UPA with HPLC (Phenomenex C-18 column, Torrance, California) using 80% acetonitrile and 20% water as mobile phase. The delivery rate of UPA from the tubes was 20 mcg per day (10 mm length) and 40 mcg per day (15 mm length) (see Fig. 1).
Fig. 1.
In vitro release rate. Analysis of a 40 mcg/day CDB-IUS showed that after an initial surge to around 60 mcg/day the release rate stablilized at ~40 mcg/day.
2.2 Animals
Eight adult rhesus macaques were ovariectomized by the surgical staff of the Oregon National Primate Research Center (ONPRC) in compliance with the Animal Welfare Act and under the supervision of the ONPRC/OHSU Institutional Animal Care and Use Committee. The monkeys were treated with estradiol (E2 ) and P Silastic capsules implanted subcutaneously (s.c.) for 3 months to create artificial menstrual cycles [14]. Briefly, a Silastic capsule 3 cm in length (0.34 cm inner diameter; 0.64 cm outer diameter; Dow Corning; Midland, MI, USA) filled with crystalline E2 was first inserted s.c. to induce an artificial proliferative phase. After 14 days of E2 priming, a Silastic capsule 6 cm in length (0.34 cm inner diameter; 0.64 cm outer diameter) containing crystalline P was inserted s.c. for an additional 14 days to stimulate an artificial secretory phase. Such implants typically produce serum levels of E2 around 80.66 ± 23 pg/ml and serum levels of P around 4.42 ± 1.6 ng/ml [14].
The P implant was removed on Day 28 to complete each cycle and induce menstruation, and the E2 implant remained in place to renew endometrial proliferation. Vaginal swabs were done daily to assess bleeding. After 3 cycles to establish pretreatment bleeding patterns, menstruation was induced in each animal by removing the P implant, and the IUS were inserted after the last day of bleeding.
2.2.1. IUS insertions
Empty (blank) silicone tubes (15 mm length) were prepared as controls and were inserted into the uteri of 3 animals. The UPA-filled capsules were inserted into the uteri of 5 animals. Three animals received the 20 mcg/day and 2 received the 40 mcg/day dose. However, no significant differences were noted in the effects of the two doses and the results were combined for this report. To place the IUS, the uterine cavity was opened by hysterotomy and the IUS were inserted and held in place with a suture through the myometrium.
2.2.2.Treatment phase
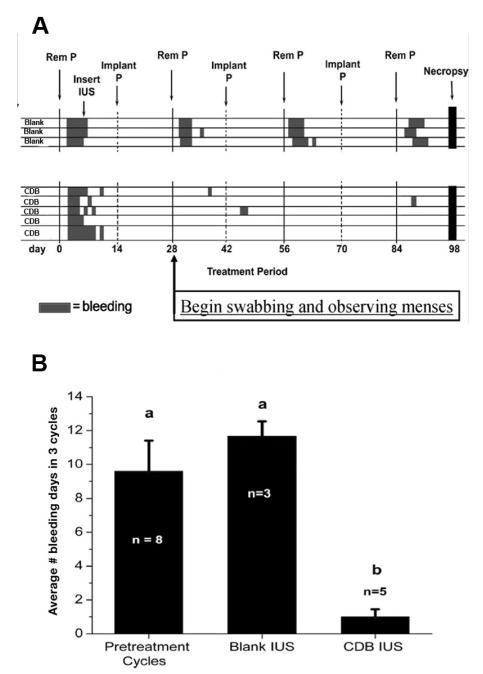
During the treatment phase, the s.c. P implant was sequentially inserted and removed from each animal every 14 days, with the E2 implant remaining in place, to induce 3.5 cycles. The macaques were hysterectomized on Day 14 of the 4th artificial proliferative phase. After separation from the cervix and oviducts, the uterus was cut into four quarters along the longitudinal axis. The endometrium from two of the quarters (half the endometrium) was dissected free of myometrium, weighed on a Denver Instruments SI-64 balance (Arvada, CO) and frozen immediately for further analysis. The endometrial weight so obtained was multiplied by 2 to provide an estimate of the total endometrial weight for each animal. The other two quarters were used for immunocytochemistry (ICC) and morphological studies. Several cross-sections (~2 mm thick) were cut freehand from the lumen to the myometrial border with a razor blade under stereomicrocope magnification. These slices were processed further to assess histological development, steroid receptor immunocytochemistry and markers of proliferation. Steroid receptors included estrogen, progestin and androgen receptors (ER, PR, AR ), and the proliferation markers were Ki-67 and Phospho-H3 as described below. The overall experimental design is illustrated in Figure 2.
Fig. 2.
Bleeding patterns.
A). Each row represents the bleeding pattern of a single animal. Three pretreatment cycles were measured before the IUS were inserted. During the treatment phase, all the Blank-IUS treated animals bled for ~3-4 days each time P was withdrawn. In the CDB-IUS treated ones, however, only 3 of the 5 animals bled. In these animals the duration of bleeding was around one day, was not always associated with P withdrawal, and was not correlated with dose.
B) The bar graphs indicate the mean number of total bleeding days for the pretreatment, Blank-IUS and CDB-IUS treated cycles.
2.3.1. Histology and immunocytochemistry (ICC)
Samples for histology were fixed in a mixture of 2% glutaraldehyde and 3% paraformaldehyde, embedded in glycol methacrylate (GMA), sectioned, and stained with hematoxylin. For ICC, fresh tissue samples were microwave-stabilized [15] for 7 s and embedded in Tissue Tek II OCT (Miles Inc., Elkhart, IN, USA) and frozen in liquid propane. Cryostat sections (7 μm) were thaw-mounted on Superfrost Plus (Fisher Scientific, Pittsburgh, PA, USA) slides, placed on ice and microwave irradiated for 2 s. The microwave-treated sections were fixed in 0.2% picric acid and 2% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature, immersed in 85% ethanol + 1.5% polyvinylpyrollidone (PVP) at 4°C, rinsed in PBS, immersed in 0.37% glycine in PBS + PVP, and then immersed in 0.1% gelatin in PBS + PVP at 4°C. To inhibit endogenous peroxidase activity, the sections were incubated with glucose oxidase (1 unit/mL), NaAzide (1 mM) and glucose (10 mM) in PBS for 45 min. Sections were incubated with blocking serum for 20 min and then with the primary monoclonal anti-ER (1D-5; detects ER alpha, Biogenex, San Ramon, CA, USA), anti-PR (JZB-39; courtesy of Geoffrey Greene, University of Chicago, detects PR-A and PR-B), anti-AR (F39.4; Biogenex, San Ramon CA), anti-Ki-67 antigen (Dako Corp., Carpinteria, CA, USA); Phospho H3 was detected with a rabbit polyclonal antibody (Upstate Biotechnology, Lake Placid NY), and all were incubated overnight. Omission of the first antibody and incubation of tissue sections with irrelevant and/or isotype-matched mouse IgG were used as method controls. In each case, primary antibody was detected with either biotinylated anti-mouse IgG (for 1D-5, anti Ki-67, F39.4), anti-rat IgG (for JZB-39) or anti-rabbit IgG (for Phospho H3) secondary antibody. Final visualization was achieved using the ABC kit (Vector Laboratories, Burlingame, CA), 0.025% 3,3′diaminobenzidine/4HCl (Dojindos DAB; Wako Chemicals, Richmond, VA) in Tris buffer and 0.03% H2O2 (Fisher Scientific, Pittsburgh, PA), and 0.026% osmium tetroxide (Electron Microscopy Sciences, Hatfield, PA). Sections were then postfixed with 2% paraformaldehyde and lightly counterstained with hematoxylin to facilitate identification of cellular elements.
2.3.1. Quantitation of KI-67 and Phospho H3 positive cells
The specimens stained with either the Ki-67 or the Phospho H3 antibody were of sufficient homogeneity, high contrast and low background to allow computer-assisted thresholding and subsequent computer-assisted counting. Digital images were captured with an Optronics DEI-750 CCD camera (Optronics, Goleta CA) and analyzed with Image Pro-plus (Media Cybernetics Inc., Silver Springs, MD). Minor adjustments to contrast and sharpness were made with Adobe Photoshop (Adobe Systems Incorporated, San Jose, CA) before printing. The digital images were captured at ~25X original magnification (2.5X objective). After importing into Image Pro-plus, the endometrial glands were traced and defined as regions of interest (ROI), their area was measured, and the number of Phospho-H3 or Ki-67 positive cells per unit gland area was determined and expressed as positive cells per 10,000 μm2. These and all other numeric data (e.g., wet weight, number of bleeding days) were analyzed by one-way ANOVA followed by Fishers LSD [16].
3. Results
3.1. Number of bleeding days
During the course of the 3 treatment cycles, animals bearing the blank IUS bled for a mean total of 11.66 ± 0.88 days, which was not significantly different from the total number of days animals bled during the pretreatment cycles, while the UPA-IUS treated animals bled for a mean total of only 1 ± 0.45 days (P<0.05; Fig. 2B). In the blank-IUS controls, the bleeding occurred within 1-2 days of P withdrawal in all the animals and persisted for 3-4 days, typical of normal menstruation. In the UPA-IUS animals, the bleeding was not always associated with P withdrawal and only occurred in 3 of the 5 animals (Fig. 2A). In these 3 animals, 1 was treated with a high dose UPA-IUS and 2 were treated with the low dose UPA-IUS. There was no clear correlation between dose and days of bleeding in these 3 animals.
3.2. Endometrial wet weights
The blank-IUS treated animals had mean (±SE) endometrial wet weights of 409 ± 112 mg while the UPA-IUS treated ones had significantly reduced mean weights of 188 ± 30 mg (p<0.05).
3.3. Histology
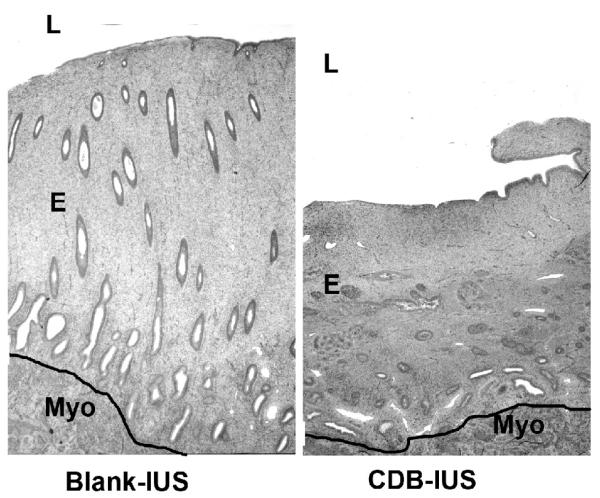
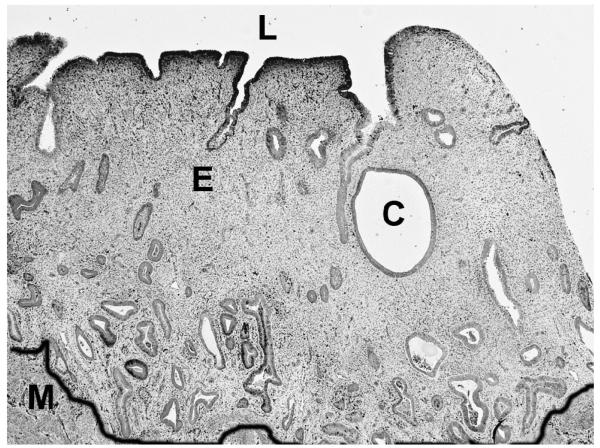
After 3.5 cycles, the endometria treated with a blank-IUS were in a proliferative state marked by typically straight tubular glands in a normal stroma. The UPA–IUS treated endometria were much thinner than the controls, and consisted of a moderately compacted stroma with glands that were generally small and tubular (Fig. 3) but in some animals were large and dilated, resembling cysts (Fig. 4). The epithelium lining the cystic, dilated glands varied from cuboidal to columnar and showed minimal evidence of proliferative activity (Fig. 5). Both the blank-IUS and the UPA–IUS treated endometria contained a layer of neutrophils and other leucocytes concentrated below the luminal epithelium and scattered thinly throughout the endometrium. An increase in subluminal leucocytes is a characteristic response of the endometrium to a sterile foreign body in the lumen.
Fig. 3.
Blank vs CDB-IUS on endometrial histology. Treatment with the CDB-IUS induced overall endometrial atrophy. L= lumen, E= endometrium, Myo =myometrium. The black line was drawn to demarcate the endometrial-myometrial border. Original magnification=25×.
Fig. 4.
Cystic dilation of endometrial glands. L=lumen, E= endometrium, M= myometrium, C= cystic, dilated gland. The black line was drawn to demarcate the endometrial-myometrial border. CDB-IUS induced some cystic dilation of glands. Original magnification=40×.
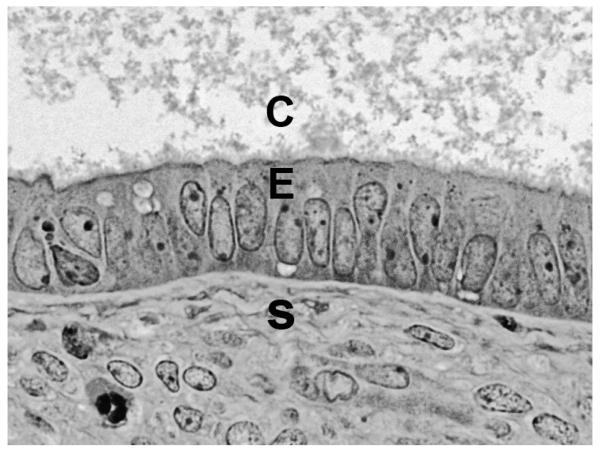
Fig. 5.
Epithelium lining the dilated cyst. The epithelium of the cyst shown in Figure 4 shows minimal evidence of proliferation. C= cyst lumen, E= lining epithelium, S = stroma. Original magnification=640×.
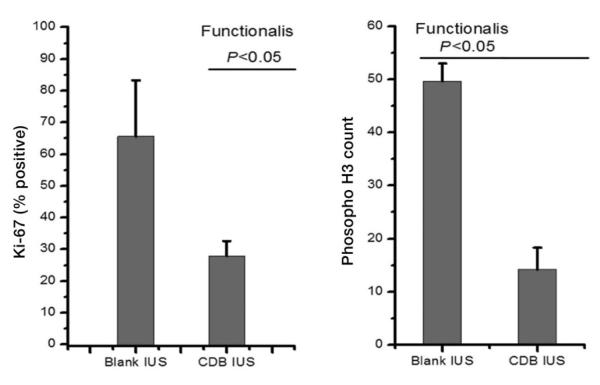
3.4. Proliferation markers
The number of Ki-67 and Phospho-H3 positive gland cells were significantly lower in the UPA-IUS treated than the blank-IUS treated endometria (p<0.05; Fig. 6).
Fig. 6.
Effects on proliferative indices. Counts of Ki-67 and Phospho-H3 in the glands of the functionalis zone showed that the CDB-IUS significantly suppressed proliferation indices.
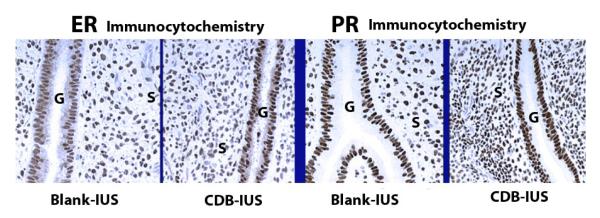
3.5. Steroid receptor immunocytochemistry
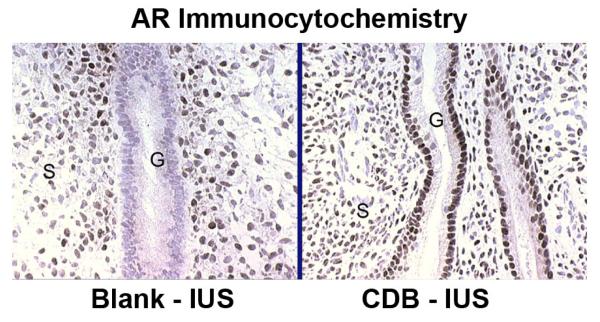
The cellular distribution and staining intensity of ER and PR did not differ between the blank and the UPA-IUS treated animals (Fig. 7). However, AR, which was nondetectable in the glands of the blank-IUS treated endometria, was strongly upregulated in the glands of the UPA-IUS treated endometria. This upregulatory effect was specific to glandular AR as stromal AR staining was clearly evident after both treatments (Fig. 8).
Fig. 7.
ER and PR staining. There were no clear differences between the effects of the Blank and CDB-IUS treatments. G = gland, S= Stroma. Brown-black nuclei are positive. Original magnification=340×.
Fig. 8.
AR staining. CDB-IUS treatment induced a marked upregulation of AR expression in the glands. G = gland, S= Stroma. Brown-black nuclei are positive. Original magnification=340×.
4. Discussion
Compared to the blank-IUS, the UPA-IUS induced significant reductions in endometrial wet weights, indices of proliferation, and days of bleeding. Suppression of menstrual bleeding in the UPA-IUS treated animals was likely due to blockade of progestational development. For example, during the 2 weeks the s.c. P implant was in place, the effects of circulating P would be blocked by the high local concentrations of UPA in the endometrium, so that progestational development of the endometrium would be suppressed. When the s.c. P implant was removed, the endometrium would not be developed as a secretory endometrium and normal menstruation would not occur. In contrast, endometria of animals treated with the blank IUS would undergo relatively normal progestational development during P treatment and would respond to P withdrawal by menstruating normally. A similar cessation of menstruation was observed in sixteen female cynomolgus monkeys treated with oral administration of UPA in doses of 1.5 and 25 mg/kg/day [unpublished report, MPI Research, Mattawan, Michigan 2001].
Menstrual blockade is also consistent with the action of systemically administered asoprisnil, a mixed agonist-antagonist PRM which induced amenorrhea in non-human primates and women [17] and with the suppressive effects of a ZK 230 211-IUS on menstruation in the macaque [12] . The minor bleeding that occurred in the UPA-IUS treated animals may have been due to ongoing atrophic processes in the endometrium.
The reduced endometrial wet weight may be attributed to two mechanisms: blockade of P action, as discussed above, and suppression of E2-dependent proliferation [1,2]. When the samples were taken, the tissue had been exposed to ~70 pg/mL E2/day in the absence of P for 2 weeks. In the animals treated with the blank-IUS, this level of E2 drove normal levels of proliferation indicated by typically high Ki-67 and Phospho-H3 expression. However, in the UPA-treated animals, Ki-67 and Phospho-H3 indices were significantly reduced, and this lowered level of proliferation likely contributed to the reduction in wet weight seen with UPA treatment. There was no evidence for an unopposed action of E2 that typically would follow blockade or absence of P action. Rather, UPA itself opposed the effects of E2 on proliferation. A blockade of E2 action on cell division by antagonistic PRMs has been referred to previously as a non-competitive anti-estrogenic effect [18] or an endometrial antiproliferative effect [1].
The increase in AR detected here and in other studies of antiprogestins is a hallmark of antiprogestins that have antiproliferative effects [12,19-21]. A working hypothesis is that androgens, which are known to suppress estrogen effects on proliferation, bind to the elevated AR, activate it and subsequently mediate the suppressive effects of antiprogestins on E2-dependent proliferation [22]. This hypothesis is consistent with the effects of flutamide, an antiandrogen, which blocked the endometrial antiproliferative effects of a different antagonistic PRM [23]. However, the detailed mechanism underlying the endometrial antiproliferative effect remains to be elucidated.
Unlike AR, the staining intensity of ER and PR were essentially identical in the blank-IUS and the UPA-IUS treated endometria. Because the tissue was sampled after 2 weeks of E2 treatment in the absence of P, it was evident that the UPA-IUS did not prevent E2 from maintaining ER and PR levels. Consequently, as has been noted in reports of other antiprogestins [18,22], UPA inhibits E2-dependent proliferation at a post-receptor step, presumably within the signaling cascades that regulate the cell cycle.
The most notable histological changes were the overall decrease in endometrial thickness, the moderate stromal compaction and the development of glandular cysts. These effects have been observed with other antiprogestins and PRMs [20,24]. Cystic dilatation of the endometrial glands was noted previously in a longer-term study of female cynomolgus monkeys treated systemically with oral UPA [unpublished report, MPI Research, Mattawan, Michigan, 2001]. In women, different endometrial changes have been reported with different PRMs administered orally and according to the duration of the treatment. Cystic dilatation of the glands with inactive epithelium have been reported [25,26] sometimes associated with endometrial thickening, a finding in contrast with our present findings in the monkeys. In most cases, however, the endometrium was inactive with low cell proliferation in keeping with the findings in this study. However, longer term studies are warranted to assess the effect of the different PRMs on the endometrium, and direct administration of a very low dose of PRM within the uterus may induce a different response as compared with high doses given orally. Not all PRMs, including the classic, mifepristone, can be easily delivered by an IUS [4]. The effect of IUS-delivery of ZK 230 211, a selective progestin antagonist, on the human endometrium has been studied [27]. In that work, ZK 230 211 failed to induce endometrial suppression and did not upregulate the AR. The study was of short duration and longer term effects have not been studied [27].
In summary, local administration of Ulipristal blocked the systemic effects of both P and E2 on the endometrium and led to amenorrhea in the rhesus macaque. Clinically, a UPA-IUS could provide estrogen-free and bleed-free contraception and may offer control of heavy menstrual bleeding.The current work provides proof of principle to support development of a Ulipristal-IUS for women. If the results are confirmed in women, such a development would provide a new and important contraceptive method with the potential of additional medical benefits.
Acknowledgments
We thank Kunie Mah for her expert work in immunocytochemistry, Andrea Lawson for help with computerized morphometrics, and the morphology core of the Eunice Kennedy Shriver NICHD/NIH Cooperative Agreement U54 (HD 18185) for histology support. This work was further supported by NIH grants RR00163 and HD 43209 as well as the Population Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note: In the figures we use the abbreviation “CDB” as shorthand for Ulipristal (CDB-2914).
References
- [1].Brenner RM, Slayden OD. Progesterone receptor antagonists and the endometrial antiproliferative effect. Semin Reprod Med. 2005;23:74–81. doi: 10.1055/s-2005-864035. [DOI] [PubMed] [Google Scholar]
- [2].Chwalisz K, Brenner RM, Fuhrmann U, Hess-Stumpp H, Elger W. Antiproliferative effects of progesterone antagonists and progesterone receptor modulators (PRMs) on the endometrium. Steroids. 2000;65:741–51. doi: 10.1016/s0039-128x(00)00190-2. [DOI] [PubMed] [Google Scholar]
- [3].Chwalisz K, Perez MC, Demanno D, Winkel C, Schubert G, Elger W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr Rev. 2005;26:423–38. doi: 10.1210/er.2005-0001. [DOI] [PubMed] [Google Scholar]
- [4].Goodman AL, Hodgen GD. Progesterone Receptor Antagonists. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive Endocrinology, Surgery, and Technology. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 548–58. Chapter 27. [Google Scholar]
- [5].Elger W, Bartley J, Schneider B, Kaufmann G, Schubert G, Chwalisz K. Endocrine pharmacological characterization of antiprogestins and progesterone receptor modulators (PRMs) with respect to PR-agonistic and -antagonistic activity. Steroids. 2000;65:713–23. doi: 10.1016/s0039-128x(00)00178-1. [DOI] [PubMed] [Google Scholar]
- [6].Attardi BJ, Burgenson J, Hild SA, Reel JR, Blye RP. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: in vitro comparison to mifepristone and CDB-2914. Mol Cell Endocrinol. 2002;188:111–23. doi: 10.1016/s0303-7207(01)00743-2. [DOI] [PubMed] [Google Scholar]
- [7].Hild SA, Reel JR, Hoffman LH, Blye RP. CDB-2914: anti-progestational/anti-glucocorticoid profile and post-coital anti-fertility activity in rats and rabbits. Hum Reprod. 2000;15:822–29. doi: 10.1093/humrep/15.4.822. [DOI] [PubMed] [Google Scholar]
- [8].Blithe DL, Nieman LK, Blye RP, Stratton P, Passaro M. Development of the selective progesterone receptor modulator CDB-2914 for clinical indications. Steroids. 2003;68:1013–17. doi: 10.1016/s0039-128x(03)00118-1. [DOI] [PubMed] [Google Scholar]
- [9].Gainer E, Ulmann A. Pharmacologic properties of CDB (VA)-2914. Steroids. 2003;68:1005–11. doi: 10.1016/s0039-128x(03)00130-2. [DOI] [PubMed] [Google Scholar]
- [10].Ortiz ME, Croxatto HB, Bardin CW. Mechanisms of action of intrauterine devices. Obstet Gynecol Survey. 1996;51(Suppl):S42–S51. doi: 10.1097/00006254-199612000-00014. [DOI] [PubMed] [Google Scholar]
- [11].McGavigan CJ, Cameron IT. The Mirena levonorgestrel system. Drugs Today (Barc ) 2003;39:973–84. doi: 10.1358/dot.2003.39.12.799415. [DOI] [PubMed] [Google Scholar]
- [12].Nayak NR, Slayden OD, Chwalisz K, Lehtinen M, Brenner RM. Antiprogestin-releasing intrauterine devices: A novel approach to endometrial contraception. Contraception. 2007;75:S104–S111. doi: 10.1016/j.contraception.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chabbert-Buffet N, Pintiaux-Kairis A, Bouchard P. Effects of the progesterone receptor modulator VA2914 in a continuous low dose on the hypothalamic-pituitary-ovarian axis and endometrium in normal women: a prospective, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2007;92:3582–89. doi: 10.1210/jc.2006-2816. [DOI] [PubMed] [Google Scholar]
- [14].Slayden OD, Chwalisz K, Brenner RM. Reversible suppression of menstruation with progesterone antagonists in rhesus macaques. Hum Reprod. 2001;16:1562–74. doi: 10.1093/humrep/16.8.1562. [DOI] [PubMed] [Google Scholar]
- [15].Slayden OD, Koji T, Brenner RM. Microwave stabilization enhances immunocytochemical detection of estrogen receptor in frozen sections of macaque oviduct. Endocrinology. 1995;136:4012–21. doi: 10.1210/endo.136.9.7649110. [DOI] [PubMed] [Google Scholar]
- [16].Petersen RG. Design and Analysis of Experiments. 66 edition Marcel Dekker, Inc.; New York: 1985. [Google Scholar]
- [17].Chwalisz K, Garg R, Brenner R, Slayden O, Winkel C, Elger W. Role of nonhuman primate models in the discovery and clinical development of selective progesterone receptor modulators (SPRMs) Reprod Biol Endocrinol. 2006;4(Suppl 1):S8. doi: 10.1186/1477-7827-4-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wolf JP, Hsiu JG, Anderson TL, Ulmann A, Baulieu EE, Hodgen GD. Noncompetitive antiestrogenic effect of RU 486 in blocking the estrogen- stimulated luteinizing hormone surge and the proliferative action of estradiol on endometrium in castrate monkeys. Fertil Steril. 1989;52:1055–60. doi: 10.1016/s0015-0282(16)53174-4. [DOI] [PubMed] [Google Scholar]
- [19].Slayden OD, Nayak NR, Burton KA, et al. Progesterone antagonists increase androgen receptor expression in the rhesus macaque and human endometrium. J Clin Endocrinol Metab. 2001;86:2668–79. doi: 10.1210/jcem.86.6.7606. [DOI] [PubMed] [Google Scholar]
- [20].Narvekar N, Cameron S, Critchley H, LIn S, Cheng L, Baird D. Low-dose mifepristone inhibits endometrial proliferation and up-regulates androgen receptor. J Clin Endocrinol Metab. 2004;89:2491–97. doi: 10.1210/jc.2003-031945. [DOI] [PubMed] [Google Scholar]
- [21].Brenner RM, Slayden OD, Nayak NR, Baird DT, Critchley HO. A role for the androgen receptor in the endometrial antiproliferative effects of progesterone antagonists. Steroids. 2003;68:1033–39. doi: 10.1016/s0039-128x(03)00120-x. [DOI] [PubMed] [Google Scholar]
- [22].Brenner RM, Slayden OD, Critchley HO. Anti-proliferative effects of progesterone antagonists in the primate endometrium: a potential role for the androgen receptor. Reproduction. 2002;124:167–72. doi: 10.1530/rep.0.1240167. [DOI] [PubMed] [Google Scholar]
- [23].Slayden OD, Brenner RM. Flutamide counteracts the antiproliferative effects of antiprogestins in the primate endometrium. J Clin Endocrinol Metab. 2003;88:946–49. doi: 10.1210/jc.2002-021763. [DOI] [PubMed] [Google Scholar]
- [24].Baird DT, Brown A, Critchley HO, Williams AR, Lin S, Cheng L. Effect of long-term treatment with low-dose mifepristone on the endometrium. Hum Reprod. 2003;18:61–8. doi: 10.1093/humrep/deg022. [DOI] [PubMed] [Google Scholar]
- [25].Williams AR, Critchley HO, Osei JI, et al. The effects of the selective progesterone receptor modulator asoprisnil on the morphology of uterine tissues after 3 months treatment in patients with symptomatic uterine leiomyomata. Hum Reprod. 2007;22:1696–704. doi: 10.1093/humrep/dem026. [DOI] [PubMed] [Google Scholar]
- [26].Mutter GL, Bergeron C, Deligdisch L, et al. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod Pathol. 2008;21:591–8. doi: 10.1038/modpathol.2008.19. [DOI] [PubMed] [Google Scholar]
- [27].Heikinheimo O, Vani S, Carpen O, et al. Intrauterine release of progesterone antagonist ZK230211 is feasible and results in novel endometrial effects: a pilot study. Hum Reprod. 2007;22:2515–22. doi: 10.1093/humrep/dem235. [DOI] [PubMed] [Google Scholar]