SUMMARY
In many experimental models and clinical examples, defects in the differentiation of the second heart field (SHF) and heart outflow tract septation defects are combined, although the mechanistic basis for this relationship has been unclear. We found that as the initial SHF population incorporates into the outflow tract, it is replenished from the surrounding progenitor territory. In retinoic acid (RA) receptor mutant mice, this latter process fails, and the outflow tract is shortened and misaligned as a result. As an additional consequence, the outflow tract is misspecified along its proximal-distal axis, which results in ectopic expression of TGFβ2 and ectopic mesenchymal transformation of the endocardium. Reduction of TGFβ2 gene dosage in the RA receptor deficient background restores septation but does not rescue alignment defects, indicating that excess TGFβ causes septation defects. This may be a common pathogenic pathway when second heart field and septation defects are coupled.
INTRODUCTION
The tissue of the first heart field will ultimately become the myocardium and endocardium of the left ventricle and of portions of both atrial chambers. The second heart field (SHF) is a progenitor population of splanchnic and pharyngeal mesoderm that is located dorsal to the pericardial cavity. SHF cells are added progressively to both ends of the heart tube (Buckingham et al., 2005; Horsthuis et al., 2009), in mouse during the E8.0–10.5 period, fully constituting the right ventricle and outflow tract (OFT) and contributing to portions of both atrial chambers at the inflow region of the heart.
The OFT initially exits the heart solely from the right ventricle. Through the continuing recruitment of tissue from the second heart field, the OFT lengthens and repositions by E10.5–11.0 to overlie the interventricular septum. Around E11.0–11.5, the OFT becomes septated (divided) by the expansion and fusion of cushions positioned on opposite sides of its inner wall to form the ascending aorta and pulmonary trunk. Lengthening and repositioning of the OFT in the period prior to septation is necessary for the ascending aorta, once formed, to connect to the left ventricle. Consequently, when development of the SHF is compromised, alignment defects such as double outlet right ventricle (DORV; the aorta and pulmonary trunk both exit from the right ventricle) or overriding aorta (the aorta straddles the interventricular septum) occur. Problems in SHF development can in addition compromise the septation process, resulting in a persisting single outflow vessel. This phenotype is often called persistent truncus arteriosus but in many cases should be described as common arterial trunk (CAT) (Kirby, 2008). The mechanistic relationship between the SHF and the septation process has been unclear.
Retinoic acid (RA) is a vitamin A derivative that is widely used in development as a signaling molecule. Mouse embryos lacking the major RA synthetic enzyme Raldh2 show a profound disruption of the SHF starting as early as E7.5 (Ryckebusch et al., 2008; Sirbu et al., 2008). This indicates an early role for RA in delimiting the domain of mesoderm that is competent to become the SHF. In this study, we show that RA signaling also has a later and distinct role in the further recruitment of splanchnic mesoderm to a second heart field fate. Our results suggest a specific effect on the subdomain known as the secondary heart field, which normally contributes the distal myocardium of the outflow tract and the mesodermal portion of smooth muscle of the great vessels (Buckingham et al., 2005; Dyer and Kirby, 2009; Choudhary et al., 2009). This secondary heart field deficiency in RA receptor null embryos results in a shortened outflow tract and thereby in alignment defects. As a related consequence, the tissue of the shortened outflow tract is misspecified along its proximal-distal axis at the time when septation is initiated. We show that the CAT septation defect is a consequence of outflow tract axial misspecification, and one that results specifically from altered TGFβ signaling.
RESULTS
Temporal requirement for RA and RA receptor activity
RA signals are received by a heterodimer of one RAR and one RXR, which are members of the nuclear receptor family. Embryos lacking the α1 isoform of the RARα gene plus all isoforms of the RARβ gene (RARα1/RARβ) have common arterial trunk (CAT) with 100% penetrance (Lee et al., 1997). Similarly, embryos lacking RARα1 and all isoforms of the RXRα gene (RARα1/RXRα) have CAT with high although not full penetrance. The outflow tract in both mutant backgrounds is shorter and right-sided, which is suggestive of a SHF deficiency.
To address the temporal requirement for RA receptor function, we made use of the Tg(CAGG-Cre/Esr1) transgenic line, in which a tamoxifen-dependent version of Cre recombinase is ubiquitously expressed. In CAGG-Cre-expressing conditional RXRα embryos, RXRα protein is mostly eliminated by 24 hr following a single injection of tamoxifen (Fig. S1A), consistent with previous applications of CAGG-Cre to other conditional gene targets (Hayashi and McMahon, 2002; Xu et al., 2005). We crossed the CAGG-Cre transgene into a background of germline RARα1 deficiency combined with a conditional RXRα allele. In control embryos that lacked the Cre gene or were wildtype for the RXRα gene, or in embryos that were not treated with tamoxifen, there was no incidence of OFT defects. In tamoxifen-treated Cre/RARα1/RXRα embryos, a range of phenotypes was recovered (Table 1A). Disruption of RA signaling at any time prior to E9.5 (i.e., treatment at E8.5 or earlier) resulted in embryos with a right-sided common arterial trunk, and in additional embryos with alignment defects but with normal septation. The range of phenotypes might reflect variability in the extent of RXRα gene recombination as well as the variability in phenotype seen in germline RARα1/RXRα mutants Importantly, the presence of functional RA receptors prior to E9.0 was not sufficient to support normal outflow tract development if the receptors were eliminated thereafter. Disruption of RA signaling after E10.5 did not cause any outflow tract phenotype. These observations plus those described below bracket the critical time when these RA receptors function in OFT development to the period approximately from E9.0–E10.5.
Table 1.
Outflow tract phenotypes in RA receptor mutant embryos.
| A. Tamoxifen treatment of CAGG-Cre,RARα1−/−, RXRαflox/flox embryos | ||||||||||
| Injection time |
Mutants | Litters | CAT | DORV | Overriding Aorta |
Normal | ||||
| E6.75–7.0 | 14 | 5 | 7 | 50% | 3 | 21% | 1 | 7% | 3 | 21% |
| E7.5–8.0 | 17 | 7 | 5 | 29% | 2 | 21% | 2 | 12% | 8 | 47% |
| E8.5 | 10 | 5 | 2 | 20% | 2 | 20% | 2 | 20% | 4 | 40% |
| E9.0–9.5 | 17 | 8 | 0 | 0% | 7 | 41% | 4 | 21% | 6 | 35% |
| E10.0–10.5 | 6 | 4 | 0 | 0% | 0 | 0% | 0 | 0% | 6 | 100% |
| B. Tissue specific inactivation in Cre,RARα1−/−, RXRαflox/flox embryos | ||||||||||
| Cre line | Mutants | Litters | CAT | DORV | Overriding Aorta |
Normal | ||||
| Mesp1Cre | 18 | 11 | 9 | 50% | 3 | 17% | 3 | 17% | 3 | 17% |
| Myf5Cre | 8 | 2 | 0 | 0% | 0 | 0% | 0 | 0% | 8 | 100% |
| Mef2cCre | 10 | 8 | 0 | 0% | 0 | 0% | 0 | 0% | 10 | 100% |
| Tie2Cre | 7 | 3 | 0 | 0% | 0 | 0% | 0 | 0% | 7 | 100% |
| C. Rescue of septation in RARα1/RARβ mutants by reduction of TGFβ2 gene dosage | ||||||||||
| Genotype | Mutants | Litters | CAT | DORV | Overriding Aorta |
Normal | ||||
|
RARα1−/− RARβ−/− |
* | * | * | 100% | 0 | 0% | 0 | 0% | 0 | 0% |
|
RARα1−/− RARβ−/− TGFβ2−/+ |
9 | 7 | 4 | 44% | 5 | 55% | 0 | 0% | 0 | 0% |
A. CAGG-Cre,RARα1−/−, RXRαflox/flox embryos were isolated following a single i.p. tamoxifen injection at the indicated times, and analyzed for cardiovascular defects. B. Phenotypes were scored in RARα1−/−, RXRαflox/flox embryos carrying the indicated Cre genes. C. Phenotypes were scored in double receptor mutant embryos with or without heterozygosity of Tgfb2.
The complete penetrance of CAT in RARα1/RARβ embryos is based on some embryos from the current study plus many from earlier analyses, totaling over 100 embryos in all.
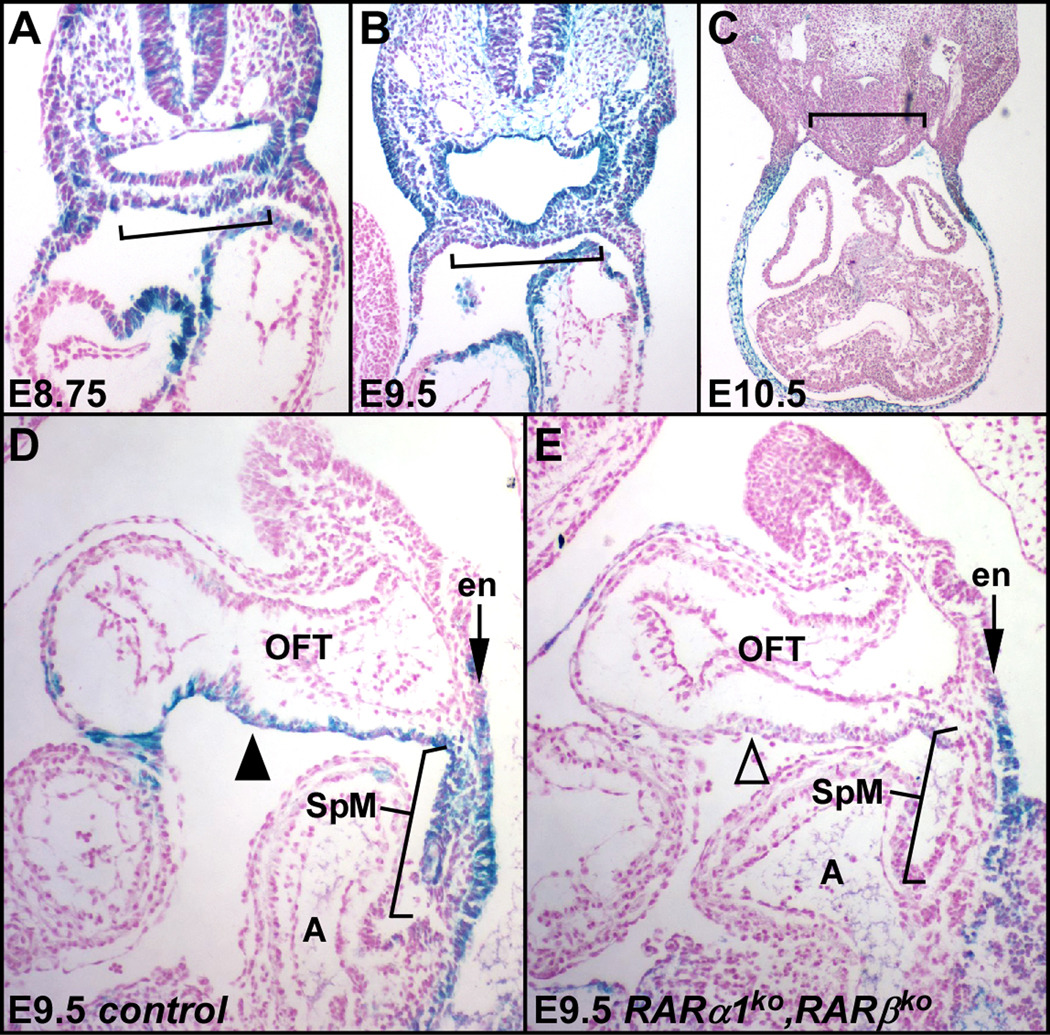
The RARE-lacZ transgene contains a multimerized RA response element (RARE) coupled to a heterologous basal promoter, and acts as an in vivo sensor for the presence of RA and RA receptors. Xgal staining of normal RARE-lacZ transgenic embryos (Fig. 1A-C) revealed that endogenous levels of RA sufficient to activate the transgene are present widely in E8.75 and E9.5 embryos, including the splanchnic mesoderm where the second heart field is located. Staining in E10.5 and E11.5 embryos was noticeably absent from the splanchnic mesoderm (Fig. 1C and data not shown). As described previously (Moss et al., 1998) and in our results (data not shown), there was only limited RARE-lacZ staining in the outflow tract itself at E10.5, and no expression at E11.5, although significant reexpression occurs at E12.5. This pattern is consistent with a role for retinoic acid in SHF development during the period prior to E10.5, although not directly with the process of OFT septation, which occurs at E11.0–11.5.
Fig. 1.
Visualization of endogenous RA response. A–C. Xgal stained sections of normal RARE-lacZ transgenic embryos are shown at E8.75, E9.5, and E10.5 in transverse section. D–E. Sagittal sections of control (D) and RARα1/RARβ mutant (E) littermate embryos at E9.5. Brackets indicate the splanchnic mesoderm (SpM). A, atria; en, endoderm; OFT, outflow tract; V, ventricle; arrowheads, OFT myocardium.
Spatial requirement for RA receptor activity
We previously showed that RA receptor function in the neural crest cell lineage was not required for normal outflow tract development (Jiang et al., 2002). To define the tissue specificity of RA signaling, we crossed the RARE-lacZ transgene into the RARα1/RARβ mutant background. In E9.5 mutant embryos, staining was compromised selectively in the splanchnic mesoderm (Fig. 1D–E). In contrast, endodermal response to RA (Fig. 1D–E), and Hox and Cyp26 gene expression (Fig. S2A) were unchanged, similarly, most aspects of pharyngeal development are normal in RARα1/RARβ mutants (Lee et al., 1997). These observations indicate that the RARα1/RARβ mutant background selectively interferes with mesodermal RA signaling.
We made use of conditional mutagenesis to further define the specificity of RA signaling. The recombination pattern achieved by each line when crossed with the conditional reporter allele R26R in a wildtype background is shown in Fig. S1B. Each Cre allele was crossed with the conventional RARα1 mutation and the conditional RXRα allele, and E14 embryos were evaluated for heart and outflow tract morphology. The early-acting and mesoderm-specific Mesp1Cre allele caused a high frequency of outflow tract septation and alignment defects (Table 1B). These results confirm a mesodermal requirement for RA signaling in OFT development. Mef2c(AHF)Cre (hereafter referred to as Mef2cCre) drives recombination specifically in the second heart field, Tie2Cre is specific for endothelium and endocardium, and Myf5Cre is expressed in paraxial but not splanchnic mesoderm. There was no cardiovascular phenotype when any of these three Cre lines were crossed with the RARα1/RXRα alleles (Table 1B). Both Mef2cCre and Tie2Cre are sufficiently active to cause OFT defects when crossed to other conditional genes (Gu et al., 2003; McCulley et al., 2008), so normal OFT development in our study cannot likely be attributed to ineffective recombination efficiency. The conclusion from this analysis is that RA receptor function occurs within the recombination domain of Mesp1Cre, but occurs earlier in the differentiation sequence or outside of the spatial domain of the tissues defined by the other Cre lines. In fact, as described below, RA signaling is required to activate Mef2c expression within the progenitors of the second heart field.
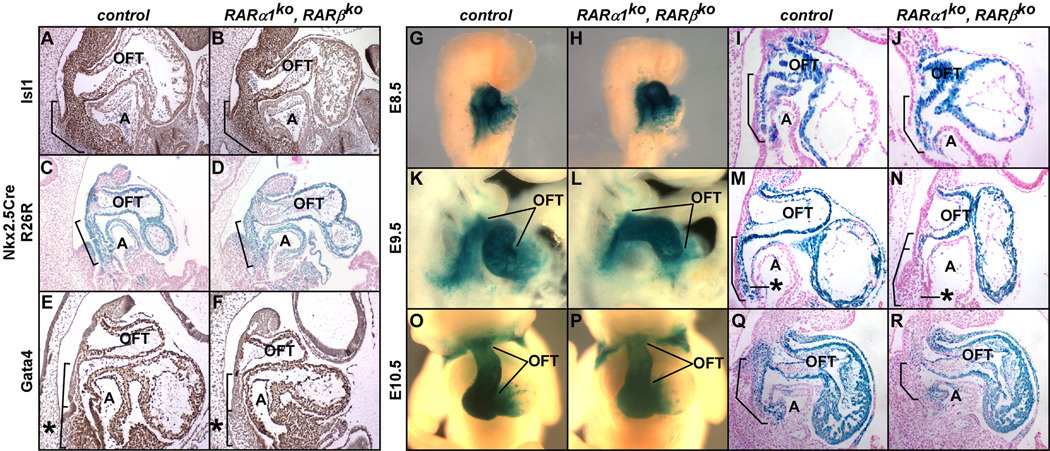
Retinoic acid controls renewal of the second heart field
Second heart field progenitors express Islet-1 (Isl1) and Nkx2.5. Based on these markers (Fig. 2A–D, Fig. S2B) and Fgf8 expression (Fig. S2C), the allocation and distribution of the SHF progenitor population was normal in RARα1/RARβ mutants. Mef2c is regulated by Isl1 and Nkx2.5 (Dodou et al., 2004; Tanaka et al., 1999), and is induced in committed albeit still multipotent SHF cells that will contribute to the ends of the outflow and inflow tracts (Verzi et al., 2005)(Fig. 2G–R). Mef2cCre/R26R is a validated surrogate for Mef2c gene expression during the period and in the tissues relevant for this study (Verzi et al., 2005). In normal embryos through the E8.5–10.5 interval, the Mef2cCre/R26R-positive SHF extends fully between the outflow and inflow tracts (brackets in Fig. 2I,M,Q). A comparable pattern was seen at E8.5 in RARα1/RARβ mutants (Fig. 2J) as in normal controls, and the length of the OFT in E8.5 mutants was normal as well (Fig. 2G–H), indicating the normal initial differentiation of the SHF. However, the Mef2c+ SHF population was severely diminished in E9.5 RARα1/RARβ mutants (Fig. 2N; see also Fig. S2C), and at E10.5, there was no Mef2cCre/R26R staining of the splanchnic mesoderm in mutant embryos (Fig. 2R). Proliferation and apoptosis were both normal in the mutant splanchnic mesoderm at E8.5 and E9.5 (Fig. S2D). Because Mef2c-expressing cells migrate to the heart tube, we infer that the absence of Mef2c expression in the splanchnic mesoderm in mutant embryos starting at E9.5 represents a failure to replace early Mef2c+ cells that move into the outflow tract. The deficiency in the Mef2c+ SHF explains the shortened and ultimately right-sided outflow tract seen in RAR mutants. This model also explains why Mef2cCre did not cause OFT defects in conditional RARα1/RXRα mutants (Table 1B): RA signaling is needed in order to activate Mef2cCre in the domain of the SHF that will be added to the outflow tract at E9.0–10.5, but once Mef2c is activated, there is no longer a continuing need for RA signaling (at least through RARα1 and RXRα) to maintain the committed phenotype of these cells. Indeed, the Mef2cCre/R26R staining pattern was unchanged in conditional Mef2cCre/RARα1/RXRα/R26R embryos (Fig. S2E).
Fig. 2.
Markers of the SHF. Littermate pairs were analyzed for expression of Isl1 (E10.5; A,B), Nkx2.5Cre/R26R (E9.75; C,D), GATA4 (E9.5; E,F), and Mef2cCre/R26R (G–R). Asterisks, caudal portion of the SHF. Related images are in Fig. S2.
GATA factors are known to regulate Mef2c expression (Dodou et al., 2004), and Gata4 has been previously implicated as a retinoic acid responsive gene (Arceci et al., 1993; Ghatpande et al., 2000). Interestingly, GATA4 expression in RARα1/RARβ mutants was compromised in the same domain of the SHF that was lacking Mef2c activity (compare Fig. 2E–F, M–N).
A recent report documented rotation of the outflow tract as SHF cells are added to the arterial pole, and suggested that failure of rotation may cause alignment and septation defects (Bajolle et al., 2006; Bajolle et al., 2008). Using Sema3c as a marker, we did not observe a rotation defect in RAR mutant embryos (Fig. S2F). We also previously described normal rotation of neural crest cell mesenchyme in the OFT of mutant embryos (Jiang et al., 2002). It therefore appears that interruption of accretion of tissue to the outflow tract in RAR mutants does not interfere with the rotation of tissue that has been added previously.
Deficient SHF recruitment results in improper OFT specification
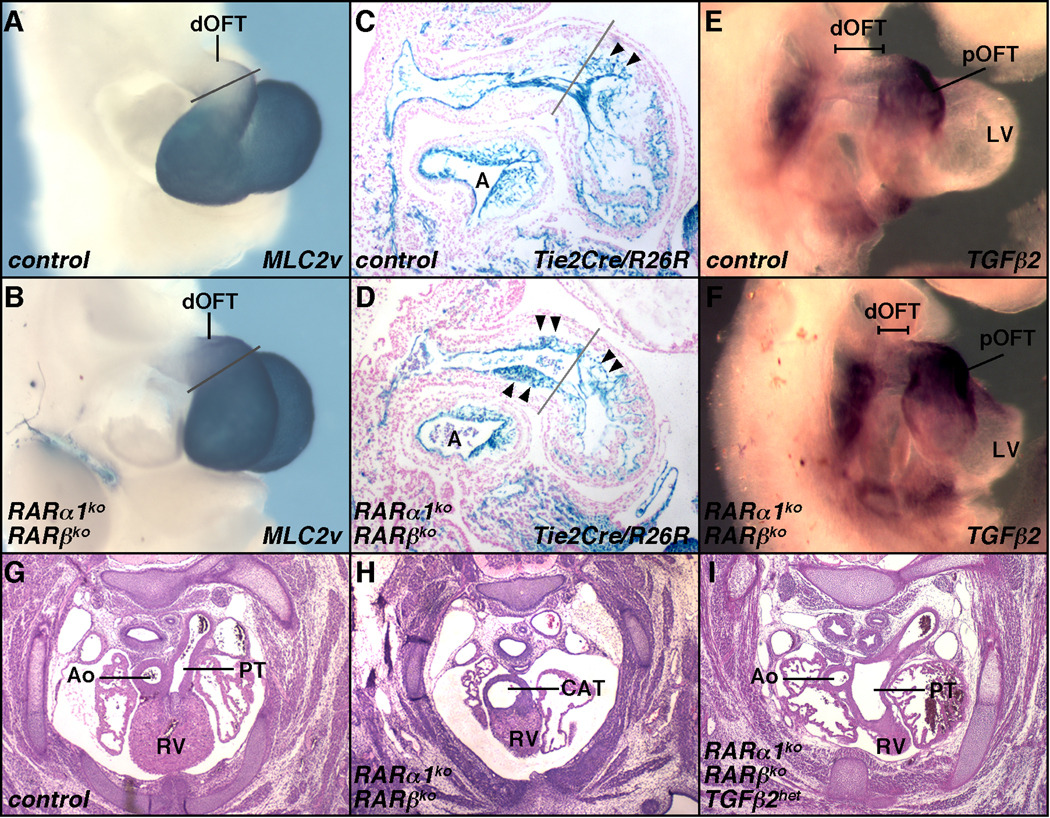
We observed no difference between control and mutant embryos in expression of several markers (αSA, MF20, SM22, SMA) that are expressed throughout the outflow tract at E9.5–10.5 (Fig. S3A–H). However, we did note that the distal OFT displayed inappropriate proximal character, as defined by the myocardial marker myosin light chain 2v (MLC2v) (Fig. 3A–B). We presume that absence of Mef2c+ splanchnic mesodermal cells in mutants (Fig. 2N,R) arrests the recruitment of second heart field cells to the outflow tract, and thereby causes previously committed cells to be retained more distally in the OFT.
Fig. 3.
OFT axial misspecification and elevated TGFβ cause CAT. A–B. Ectopic expression of the proximal marker MLC2v in the distal OFT of RAR mutant embryos at E10.5. C–D. Endocardial mesenchyme (arrowheads) in the distal OFT of mutant embryos at E10.5, visualized by Tie2Cre/R26R. Gray lines in A–D are positioned at the 90° bend between the proximal and distal segments of the OFT. E–F. Elevated Tgfb2 expression in the distal OFT in mutants at E9.75. G–I. Rescue of septation defects in E14.5 RAR mutants by reduced Tgfb2 gene dosage: a normal control embryo (G), a RARα1/RARβ mutant with CAT (H), and a RARα1/RARβ mutant also heterozygous for Tgfb2 (I). In rescued embryos, OFT septation occurs but both outflow vessels originate from the right ventricle (the RV source of the ascending aorta is not seen in this panel). Ao, ascending aorta; PT, pulmonary trunk. See also Fig. S3.
In the proximal and distal OFT, cushions form by infiltration of mesenchymal cells between the endocardium and myocardium. In normal embryos, neural crest-derived mesenchymal cells migrate throughout the cushions, whereas mesodermal cells derived by endocardial mesenchymal transformation (EMT) are located only in the proximal cushions. We previously showed that the number, migration, and differentiation of neural crest cells was normal in RARα1/RARβ mutant embryos (Jiang et al., 2002). However, Tie2Cre/R26R revealed endocardium-derived mesenchymal cells in the distal segment of the outflow tract (Fig. 3C–D) where such cells are not normally found, and corresponding to the territory where MLC2v was ectopically expressed. Thus, in mutant embryos, tissue that should normally become proximal OFT is retained in the distal OFT, and the process of endocardial EMT that normally occurs in the proximal OFT also extends into the distal OFT.
Altered TGFβ signaling accounts for septation defects but not alignment defects in RAR mutants
TGFβ2 was a likely candidate to explain the ectopic endocardium-derived mesenchyme in RAR mutants: TGFβ promotes endocardial EMT in vitro (Eisenberg and Markwald, 1995), Tgfb2 is expressed by the myocardium in regions of EMT, Tgfb2 null mutants are defective in outflow tract development (Sanford et al., 1997), and TGFβ expression is inversely associated with RA signaling in other contexts (Kubalak et al., 2002; Chen et al., 2007). Indeed, we found that Tgfb2 expression was elevated in RAR mutants in the distal segment of the outflow tract (Fig. 3E–F, Fig. S3I).
To address whether elevated TGFβ was related to any of the OFT phenotypes, we combined RARα1/RARβ deficiency with heterozygosity of TGFβ2. CAT is a 100% penetrant phenotype in RARα1/RARβ mutants, and in all such embryos, the single outflow tract originates from the right ventricle (i.e., the OFT is shortened and therefore misaligned). However, by reducing Tgfb2 gene dosage, normal septation was restored in half of the embryos (Table 1C, Fig. 3G–I). Importantly, second heart field defects (e.g., absence of Mef2cCre/R26R staining in the splanchnic mesoderm and ectopic expression of MLC2v in the distal outflow tract; Fig. S3J-K) were unchanged by reduced Tgfb2 gene dosage. Consequently, in all cases where septation was rescued, the ascending aorta always originated from the right ventricle, and DORV was the resultant phenotype (Table 1C, Fig. 3I).
DISCUSSION
Our results demonstrate that the outflow tract phenotype of RAR mutants - a combination of a deficiency in outflow tract lengthening and a failure in septation - results from two independent processes that can be distinguished temporally, genetically, and molecularly. The underlying requirement for RA signaling in the E9.0–10.5 period is to promote the recruitment and commitment of Isl1+, Nkx2.5+ progenitors to a Mef2c+ fate, in order to replace previously specified Mef2c+ cells as they migrate into the outflow tract and terminally differentiate. As a direct consequence of the disruption of this process in RAR mutants, the outflow tract is shortened and ultimately right-sided. For lack of accretion of additional tissue to the end of the outflow tract, the tissue in the distal OFT retains a proximal identity that is evidenced by expression of the proximal genes MLC2v and Tgfb2, and by ectopic mesenchymal transformation of the endocardium. Misexpression of Tgfb2 is causative specifically for compromised septation.
Our study visualizes the dynamic nature of SHF and OFT development. Isl1+,Nkx2.5+ progenitors continually generate Mef2c+ cells, probably starting as early as E7.5 but clearly continuing through E10.5. Mef2c expression does not characterize a self-renewing progenitor population, since despite a relatively high proliferation rate it does not renew itself as development proceeds, at least to a level sufficient for the needs of outflow tract morphogenesis. RA signaling through RARα1/RARβ and RARα1/RXRα is specifically associated with the later (E9.0–10.5) differentiation of the Mef2c+ population.
The secondary heart field has been defined in chick embryos as the splanchnic mesodermal subdomain of the SHF between the outflow and inflow tracts that is restricted in fate to conotruncal myocardium and smooth muscle of the outflow vessels, but does not contribute to the right ventricle or atria (Buckingham et al., 2005; Dyer and Kirby, 2009). In RARα1/RARβ mutants, the SHF contribution to the right ventricle and to the atrial chambers was normal, based on morphological criteria and as visualized using Mef2cCre/R26R. The selective defect in what we suggest to be the secondary heart field was apparent by the absence of Mef2cCre/R26R staining in the splanchnic mesoderm of E9.5 and E10.5 mutant embryos. A recent report described the expression of transgenes in subdomains of the mouse SHF that are added relatively late to the outflow tract (Bajolle et al., 2008), which may correspond to the same territory as is missing in our RAR mutants. Our studies therefore lend support to the distinct identity of the secondary heart field in mammalian embryos, and demonstrate the importance of RA signaling for its derivation from splanchnic mesodermal progenitors.
Our observations indicate that GATA4, a known regulator of Mef2c expression (Dodou et al., 2004), is not expressed in RARα1/RARβ mutants in the same territory that is deficient in Mef2c expression (Fig. 2E–F,M–N). Indeed, there are several similarities between the RAR-deficient and GATA-deficient phenotypes. Combined Gata4/Gata6 double knockout did not impact the initial appearance of the Isl1+ and Nkx2.5+ splanchnic mesoderm at E8.5 (Zhao et al., 2008), just as in RARα1/RARβ mutants (Fig. 2A–D, Fig. S2B). Germline Gata4 null mutants die too early to evaluate their ultimate outflow tract phenotype, but Gata4 hypomorphs have DORV (Crispino et al., 2001; Pu et al., 2004), a clear indication that GATA4 is required in SHF development. Similarly, combined heterozygosity of Gata4 and Gata6 together results in a single outflow vessel (CAT) (Xin et al., 2006), which is a completely penetrant phenotype in RARα1/RARβ mutants. These observations are consistent with a model of Gata4 as a downstream target of RA action that converges with Isl1 and Nkx2.5 to regulate Mef2c expression and thereby to regulate differentiation of the secondary heart field.
In the absence of differentiation of additional progenitors to a Mef2c+ fate, the cells normally fated to be the proximal OFT remain as the distal segment of what will ultimately be a shortened and right-sided outflow tract. These cells express the proximal markers MLC2v and Tgfb2. Our results therefore imply that specification of outflow tract axial patterning occurs in the splanchnic mesoderm, and not after the second heart field cells have become incorporated into the outflow tract and begun differentiation. A previous study reached similar conclusions based on the expression of marker transgenes (Bajolle et al., 2008).
Our results show that ectopic expression of TGFβ in the outflow tract specifically accounts for septation defects, at least in RARα1/RARβ mutants. Although we suggest that ectopic endocardial EMT contributes to septation failure, it remains possible that the effects of TGFβ on neural crest cells in the outflow tract (Choudhary et al., 2006) are also relevant. The relative importance and role of endocardium-derived and neural crest-derived mesenchyme in the overall process of outflow tract septation remains to be clarified.
EXPERIMENTAL PROCEDURES
Detailed experimental procedures are provided as supplemental information. Standard procedures were used for histology, Xgal staining, whole mount and section in situ hybridization, immunohistochemistry, and Western blotting. For tamoxifen-induced gene knockout, pregnant females were treated with a single i.p. dose of 75 mg/kg tamoxifen; for phenotype analysis, embryos were isolated at E14.5 and analyzed histologically, and for analysis of RXRα protein, females were treated at E8.5 and whole embryos were isolated at defined times thereafter and individually homogenized prior to Western analysis. All mouse lines used in this study have been previously described (see supplemental information).
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the many investigators who shared mouse lines and other reagents that were used in this study. This investigation was conducted in a facility constructed with support from an NIH Research Facilities Improvement Program grant. This study was supported by NIH grant HL078891 to H.M.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajolle F, Zaffran S, Kelly RG, Hadchouel J, Bonnet D, Brown NA, Buckingham ME. Rotation of the myocardial wall of the outflow tract is implicated in the normal positioning of the great arteries. Circ Res. 2006;98:421–428. doi: 10.1161/01.RES.0000202800.85341.6e. [DOI] [PubMed] [Google Scholar]
- Bajolle F, Zaffran S, Meilhac SM, Dandonneau M, Chang T, Kelly RG, Buckingham ME. Myocardium at the base of the aorta and pulmonary trunk is prefigured in the outflow tract of the heart and in subdomains of the second heart field. Dev Biol. 2008;313:25–34. doi: 10.1016/j.ydbio.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lu J, Cardoso WV. Inhibition of Tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- Choudhary B, Ito Y, Makita T, Sasaki T, Chai Y, Sucov HM. Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFbeta receptor (Tgfbr2) mutant mice. Dev Biol. 2006;289:420–429. doi: 10.1016/j.ydbio.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Choudhary B, Zhou J, Li P, Thomas S, Kaartinen V, Sucov HM. Absence of TGFbeta signaling in embryonic vascular smooth muscle leads to reduced lysyl oxidase expression, impaired elastogenesis, and aneurysm. Genesis. 2009;47:115–121. doi: 10.1002/dvg.20466. [DOI] [PubMed] [Google Scholar]
- Crispino JD, Lodish MB, Thurberg BL, Litovsky SH, Collins T, Molkentin JD, Orkin SH. Proper coronary vascular development and heart morphogenesis depend on interaction of GATA-4 with FOG cofactors. Genes Dev. 2001;15:839–844. doi: 10.1101/gad.875201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131:3931–3942. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- Dyer LA, Kirby ML. The role of secondary heart field in cardiac development. Dev Biol. 2009;336:137–144. doi: 10.1016/j.ydbio.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- Ghatpande S, Ghatpande A, Zile M, Evans T. Anterior endoderm is sufficient to rescue foregut apoptosis and heart tube morphogenesis in an embryo lacking retinoic acid. Dev Biol. 2000;219:59–70. doi: 10.1006/dbio.1999.9601. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Horsthuis T, Christoffels VM, Anderson RH, Moorman AF. Can recent insights into cardiac development improve our understanding of congenitally malformed hearts? Clin Anat. 2009;22:4–20. doi: 10.1002/ca.20723. [DOI] [PubMed] [Google Scholar]
- Jiang X, Choudhary B, Merki E, Chien KR, Maxson RE, Sucov HM. Normal fate and altered function of the cardiac neural crest cell lineage in retinoic acid receptor mutant embryos. Mech Dev. 2002;117:115–122. doi: 10.1016/s0925-4773(02)00206-x. [DOI] [PubMed] [Google Scholar]
- Kirby ML. Pulmonary atresia or persistent truncus arteriosus: is it important to make the distinction and how do we do it? Circ Res. 2008;103:337–339. doi: 10.1161/CIRCRESAHA.108.174862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalak SW, Hutson DR, Scott KK, Shannon RA. Elevated transforming growth factor beta2 enhances apoptosis and contributes to abnormal outflow tract and aortic sac development in retinoic X receptor alpha knockout embryos. Development. 2002;129:733–746. doi: 10.1242/dev.129.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RY, Luo J, Evans RM, Giguere V, Sucov HM. Compartment-selective sensitivity of cardiovascular morphogenesis to combinations of retinoic acid receptor gene mutations. Circ Res. 1997;80:757–764. doi: 10.1161/01.res.80.6.757. [DOI] [PubMed] [Google Scholar]
- McCulley DJ, Kang JO, Martin JF, Black BL. BMP4 is required in the anterior heart field and its derivatives for endocardial cushion remodeling, outflow tract septation, and semilunar valve development. Dev Dyn. 2008;237:3200–3209. doi: 10.1002/dvdy.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss JB, Xavier-Neto J, Shapiro MD, Nayeem SM, McCaffery P, Drager UC, Rosenthal N. Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Dev Biol. 1998;199:55–71. doi: 10.1006/dbio.1998.8911. [DOI] [PubMed] [Google Scholar]
- Pu WT, Ishiwata T, Juraszek AL, Ma Q, Izumo S. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol. 2004;275:235–244. doi: 10.1016/j.ydbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Ryckebusch L, Wang Z, Bertrand N, Lin SC, Chi X, Schwartz R, Zaffran S, Niederreither K. Retinoic acid deficiency alters second heart field formation. Proc Natl Acad Sci USA. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFb2 knockout mice have multiple developmental defects that are nonoverlapping with other TGFb knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Zhao X, Duester G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev Dyn. 2008;237:1627–1635. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–1280. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Xin M, Davis CA, Molkentin JD, Lien CL, Duncan SA, Richardson JA, Olson EN. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci USA. 2006;103:11189–11194. doi: 10.1073/pnas.0604604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Cerrato F, Baldini A. Timed mutation and cell-fate mapping reveal reiterated roles of Tbx1 during embryogenesis, and a crucial function during segmentation of the pharyngeal system via regulation of endoderm expansion. Development. 2005;132:4387–4395. doi: 10.1242/dev.02018. [DOI] [PubMed] [Google Scholar]
- Zhao R, Watt AJ, Battle MA, Li J, Bondow BJ, Duncan SA. Loss of both GATA4 and GATA6 blocks cardiac myocyte differentiation and results in acardia in mice. Dev Biol. 2008;317:614–619. doi: 10.1016/j.ydbio.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.