Abstract
Background
There is growing evidence supporting a role for TRPA1 receptors in the neurotransmission of peripheral mechanical stimulation. In order to enhance understanding of TRPA1 contributions to mechanotransmission, we examined the effects a selective TRPA1 receptor antagonist, A-967079, on spinal neuronal activity following peripheral mechanical stimulation in uninjured, CFA-inflamed, and osteoarthritc (OA) rats.
Results
Systemic injection of A-967079 (30 μmol/kg, i.v.) decreased the responses of wide dynamic range (WDR), and nociceptive specific (NS) neurons following noxious pinch stimulation of the ipsilateral hind paw in uninjured and CFA-inflamed rats. Similarly, A-967079 reduced the responses of WDR neurons to high-intensity mechanical stimulation (300 g von Frey hair) of the knee joint in both OA and OA-sham rats. WDR neuronal responses to low-intensity mechanical stimulation (10 g von Frey hair) were also reduced by A-967079 administration to CFA-inflamed rats, but no effect was observed in uninjured rats. Additionally, the spontaneous activity of WDR neurons was decreased after A-967079 injection in CFA-inflamed rats but was unaltered in uninjured, OA, and OA-sham animals.
Conclusions
Blockade of TRPA1 receptors disrupts transmission of high-intensity mechanical stimulation to the spinal cord in both uninjured and injured rats indicating that TRPA1 receptors have an important role in noxious mechanosensation in both normal and pathological conditions. TRPA1 receptors also contribute to the transmission of low-intensity mechanical stimulation, and to the modulation of spontaneous WDR firing, but only after an inflammatory injury.
Background
The transient receptor potential (TRP)A1 receptor is part of a larger family of TRP ion channels that includes TRPV, TRPM, TRPC, TRPN, TRPP, and TRPML [1-3]. TRPA1 is expressed in small and medium-sized peptidergic primary afferent sensory neurons that also express TRPV1 [4-8]. A recent report has also demonstrated localization of TRPA1 to a subclass of large diameter primary afferent sensory neurons and epidermal kerantinocytes [8]. TRPA1 is activated by a variety of chemical irritants that include allyl isothiocyanate (mustard oil), allicin, gingerol, cinnamaldehyde, eugenol, and methysalicylate [5,6,9,10]. Local application of these TRPA1 agonists induces spontaneous pain in humans [11], as well as nocifensive behaviors and hypersensitivity to peripheral stimulation in animals [10,12].
There is now growing evidence demonstrating an important role for TRPA1 receptors in mechanotransmission [13-15]. TRPA1 ablated mice (TRPA1-/-) were less sensitive to low-intensity mechanical stimuli compared to wild-type mice, and responses to high-intensity mechanical stimulation were appreciably impaired [13]. Using the skin-nerve preparation, it was found that TRPA1 receptors in normal skin are necessary for mechanotransmission in several type of primary afferent fibers including slowly adapting C-fibers (all ranges of intensity), Aδ-fiber mechanonociceptors (only high-intensity), and slowly adapting Aβ-fibers (all ranges of intensity) [8]. Additionally, application of the TRPA1 antagonist, HC-030031 attenuated C-fiber responses to high-intensity mechanical stimulation of normal skin [16]. After an inflammatory injury, there is an increase in TRPA1 expression as well as a decrease in mechanical thresholds of TRPA1-containing primary afferent neurons [17,18]. Furthermore, mechanical sensitivity of inflamed animals was significantly decreased by systemic (HC-030031) or intraplantar (AP18) administration of TRPA1 receptor antagonists [19,20].
In order to further delineate the role of TRPA1 receptors in normal and pathological mechanotransmission, we administered a selective TRPA1 receptor antagonist, A-967079 (Figure 1), to examine its effects on spinal nociceptive neurons in uninjured, inflamed, and osteoarthritic (OA) rats. Systemic delivery of A-967079 has been shown to decrease mechanical sensitivity in several rat models of pathological nociception [21]. We also examined the actions of A-967079 on the spontaneous firing of spinal wide dynamic range (WDR) neurons in each of the animal models examined. Spontaneous activity of WDR neurons is heightened following a chronic injury and is an indication of central sensitization and possibly non-evoked or ongoing pain [22-27].
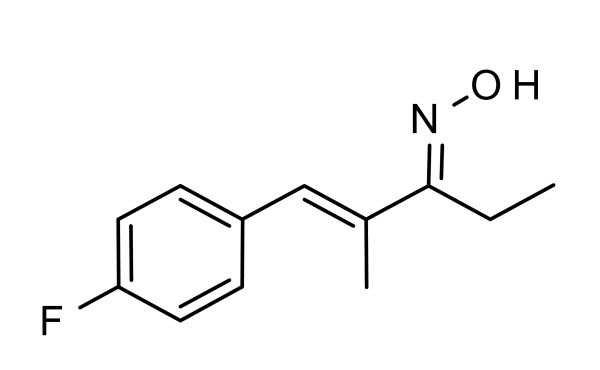
Figure 1.
Structure of A-967079.
Methods
All animal handling and experimental protocols were approved by Abbott's Institutional Animal Care and Use Committee (IACUC), and were conducted in accordance with the ethical principles for pain-related animal research of the American Pain Society. Male Sprague-Dawley rats (Charles River, MA, 250-400 g) were used for all experiments and were housed in a temperature controlled room with a 12/12-hr day/night cycle. Food and water were available ad libitum.
Compound
A-967079 was synthesized at Abbott Laboratories (Abbott Park, IL). A-967079 is a TRPA1 receptor antagonist with IC50's of 67 nM and 289 nM at human and rat TRPA1 receptors, respectively, and has good penetration into the CNS [21]. A-967079 (10 μM) is weak (ED50 > 5 μM) or not active at 89 different G-protein-coupled receptors, enzymes, transporters, and ion channels including other TRP channels (CEREP, Poitiers, France; and unpublished data), and thus is selective for TRPA1 receptors.
Animal models
Uninjured, inflamed, osteoarthritic and sham osteoarthritic animals were used for the experiments. Osteoarthritis was induced 21 days prior to electrophysiological testing by injecting 3 mg Monoiodoacetate (MIA, 50 μl) i.a. through the infrapatella ligament of the right knee. Animals were anesthetized with 5% isoflurane followed by 2.5% maintenance during the MIA injection procedure. MIA was dissolved in 0.9% sterile saline and administered using a 29-gauge needle. Sham OA animals received an i.a injection of saline 21 days prior to testing. Chronic inflammation was induced by injecting complete Freund's adjuvant (CFA, 50%, 150 μl) into the plantar surface of the rat's right hindpaw 48 hours prior to the electrophysiology experiments.
Electrophysiological protocol
On the day of neuronal recording, all animals were initially anesthetized with pentobarbital (50 mg/kg, i.p.). Catheters were placed into the left and right external jugular veins. A laminectomy was performed to remove vertebral segments T12-L3 for uninjured and CFA rats, and T9-L2 for OA and OA-sham rats to record from spinal neurons that receive input from either the right paw or knee, respectively. All animals were then secured in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA) supported by clamps attached to the vertebral processes on either side of the exposure site. The exposed area of the spinal cord was first enveloped by agar and then filled with mineral oil. A stable plane of anesthesia was maintained throughout the experiment by a continuous infusion of propofol at a rate of 8-12 mg/kg/hr (i.v.). Body temperature was kept at approximately 37°C by placing the animals on a circulating water blanket.
Platinum-iridium microelectrodes (Frederick Haer, Brunswick, ME) were used to record extracellular activity of spinal dorsal horn neurons. The activity of WDR neurons was recorded from all groups of rats. WDR neurons were defined as those neurons that respond in a graded manner to both low- and high-intensity stimulation. The firing of nociceptive specific (NS) neurons was also recorded from CFA and uninjured rats. NS neurons were defined as those neurons that respond only to high-intensity (noxious) stimulation. Spike waveforms were monitored on an oscilloscope throughout the experiment, digitized (32 points), and then stored for off-line analysis (SciWorks, Datawave Technologies, Longmont, CO) to ensure that the unit under study was unambiguously discriminated throughout the experiment.
Each neuron was characterized to confirm WDR or NS response patterns. For CFA and uninjured animals, the neuronal receptive field (RF) on the ipsilateral hind paw was (in order) gently tapped, brushed, given a noxious pinch with forceps, and stimulated with a 10-g von Frey hair for 2-3 sec. In OA and OA-sham rats, neurons were characterized by responses to manual gentle rubbing, and a 300-g von Frey applied to the knee for 2-3 sec. Only neurons that specifically responded to knee joint stimulation, without responding to stimulation of the surrounding skin/tissue, were kept for recordings in OA and OA-sham rats.
After characterization, three baseline responses, separated by 5 min each, to specific stimulation (see below) of the neuronal RF were recorded. Spontaneous and evoked neuronal activity was then measured 5, 15, 25, and 35 min after systemic injection of A-967079 (30 μmol/kg, i.v.) or vehicle (polyethylene glycol). The intravenous injection of A-967079 or vehicle was completed over a 6-7 min period. The i.v. dose of A-967079 was selected based on extrapolated plasma levels that were effective in behavioral studies (data not shown). Except for 2 experiments in which two easily distinguished neurons were simultaneously recorded on one electrode, only one neuron was studied per rat. Since only one vehicle was used in these experiments, vehicle data was combined when a clear "no effect' was observed in at least 2-3 animals from a particular subset of groups (e.g. OA and sham OA).
Only one form of test stimulation was given in each specific experiment. In uninjured and CFA-inflamed rats, WDR responses to either low-intensity (10 g von Frey hair for 15 s) or high-intensity (noxious pinch with 22 mm mini bulldog clamp for 10 s) mechanical stimulation of the RF on the right hindpaw were recorded. Responses of NS neurons to noxious pinch (22 mm mini bulldog clamp for 10 s) of the RF were also recorded in uninjured and CFA-inflamed rats. Finally, in OA and OA-sham rats, a 300 g von Frey hair test stimulus was applied to the right knee joint for 10 s to evoke WDR activity.
Data analysis
At the onset of each experiment, spontaneous neuronal firing was counted for 5 min to determine baseline levels (prior to characterization). Post-drug spontaneous firing was measured in the 5 min leading up to each stimulus at 5, 15, 25 and 35 min after injection. Evoked activity was measured by counting the spikes during the time of stimulus presentation. Thus, the total number of spikes was counted for 10 s during pinch and 300 g von Frey stimulation, and for 15 s during the 10 g von Frey stimulation. A mean of the three pre-drug evoked stimuli was calculated to represent baseline evoked activity. For each neuron, the post-drug spontaneous and evoked activity was calculated as a percent of the respective baseline levels.
Statistical analysis was performed on baseline levels of neuronal spontaneous activity for uninjured, CFA, OA and OA-sham rats by conducting a one-way ANOVA followed by a Fisher's LSD. Baseline levels of evoked firing for a particular stimulus were compared by using t-tests. For comparisons to baseline firing levels, statistical significance of post-drug activity was established by using a repeated-measures ANOVA followed by a Fisher's least significant difference (LSD) test. A two-way ANOVA followed by a Fisher's LSD was used for comparison of drug and vehicle groups. Post-hoc tests were only performed if the relevant parameter was significant in the ANOVA. All data are presented as mean ± SEM, and a difference was considered significant if it reached a p-value of less than 0.05.
Results
Baseline neuronal activity
Discharge activity was recorded from 63 WDR and 17 NS neurons from the deep dorsal horn with a mean depth of 704.7 ± 23.8 μm, and 814.7 ± 45.9 μm, respectively, from the surface of the spinal cord. Rates of spontaneous and evoked firing for WDR neurons under baseline (pre-drug) conditions in uninjured, CFA-inflamed, OA and OA-sham rats are shown in Table 1. NS neuronal activity was also examined in uninjured and CFA-inflamed rats. The mean rate of spontaneous firing for NS neurons in uninjured and CFA-inflamed rats was 0.03 ± 0.02 and 0.18 ± 0.06 spikes/sec, respectively. However, no spontaneous firing was observed in 6 of 9 NS neurons recorded from uninjured rats and in 1 of 8 neurons recorded from CFA-inflamed rats. Thus, due to the very low levels of spontaneous firing of NS neurons, this measure was not used for subsequent analysis. The mean number of baseline discharges from NS neurons following noxious pinch stimulation in uninjured and CFA rats was, 15.64 ± 5.02 and 16.42 ± 2.58 spikes/sec, respectively.
Table 1.
Baseline discharge rates of WDR neurons
|
Spontaneous (spikes/sec) |
10-g von Frey (spikes/sec) |
Noxious pinch (spikes/sec) |
300-g von Frey (spikes/sec) |
|
| Uninjured | 1.73 ± 0.38 | 10.87 ± 4.37 | 52.80 ± 11.34 | |
| CFA-inflamed | 4.43 ± 0.79++ | 19.99 ± 3.66* | 44.19 ± 9.87 | |
| OA | 3.93 ± 0.54+ | 27.39 ± 3.83 | ||
| OA-sham | 2.11 ± 0.81 | 18.04 ± 2.45 | ||
significant vs. uninjured group +p < 0.05, and ++p < 0.01 (Fisher's LSD), *p < 0.05 (t-test).
Effects of A-967079 on WDR and NS neuronal activity in uninjured and CFA-inflamed rats
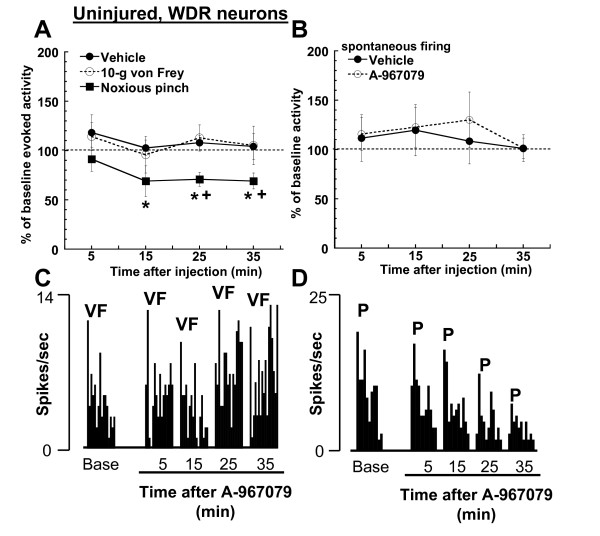
Systemic administration of A-967079 (30 μmol/kg, i.v.) reduced the responses of WDR neurons to high-intensity (pinch), but not low-intensity (10 g von Frey hair) stimulation of the RF in uninjured rats (Figure 2A, C, D). The reduction in WDR responses to pinch was significant compared to both baseline levels (p = 0.02, repeated-measures ANOVA), and the vehicle group (p = 0.0002, two way ANOVA). A-967079 decreased the evoked pinch responses of WDR neurons by approximately 30% from baseline levels. This effect was observed 15 min after injection and remained at this level for the duration of recording period (35 min post-injection). Spontaneous firing of WDR neurons was not altered by the injection of A-967079 to uninjured rats (Figure 2B).
Figure 2.
In uninjured rats, (A) systemic administration of A-967079 (30 μmol/kg, i.v.) reduced WDR responses to noxious pinch (28 mm mini bulldog clamp for 10 sec), but not to 10-g von Frey stimulation (n = 5-7 per group) of the RF on the hind paw. (B) Spontaneous firing of WDR neurons was unaltered by A-967079 injection to uninjured rats (n = 7-12 per group). Representative ratemeters showing the responses of a single WDR neuron to; (C) 10-g von Frey hair stimulation (15 sec), and (D) a noxious pinch (28 mm mini bulldog clamp for 10 sec) both before and after injection of A-967079 (30 μmol/kg, i.v.) to uninjured rats. VF = 10-g von Frey hair stimulation, P = noxious pinch (mini bulldog clamp). Since vehicle did not alter WDR firing in animals receiving either a von Frey (n = 5) or pinch stimulus (n = 2), this data was combined for comparison. *p < 0.05, vs. baseline firing; +p < 0.05 vs. vehicle-treated group (Fisher's LSD).
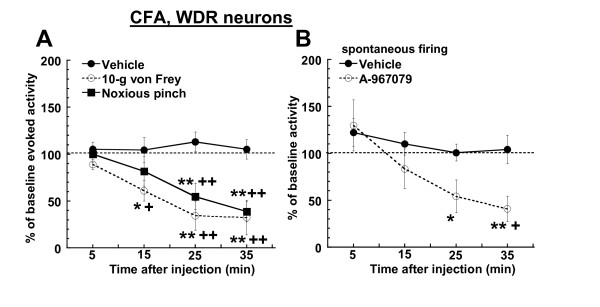
Similar to its actions in uninjured rats, administration of A-967079 (30 μmol/kg, i.v.) to CFA-inflamed rats significantly reduced WDR neuronal responses to noxious pinch stimulation (Figures 3A and 4A) compared to baseline firing (p = 0.0013, repeated-measures ANOVA) and the vehicle group (p = 0.0001, two-way ANOVA). The maximum observed effect (61.1 ± 10.97% decrease from baseline levels) on pinch-evoked activity in inflamed rats occurred 35 min after injection. In contrast to uninjured rats, injection of A-967079 to CFA-inflamed rats also significantly (p = 0.0004, and p = 0.0001 for the repeated-measures and two-way ANOVA's, respectively) reduced responses of WDR neurons to 10-g von Frey hair stimulation (Figures 3A and 4B). The maximal observed decrease in von Frey-evoked activity was 67.69 ± 18.39% from baseline levels (35 min post-injection), and was thus comparable to the effects of A-967079 on pinch-evoked activity in inflamed rats.
Figure 3.
In CFA-inflamed rats, systemic administration of A-967079 (30 μmol/kg, i.v.) reduced (A) WDR responses to noxious pinch (28 mm mini bulldog clamp for 10 sec), and 10-g von Frey stimulation (n = 6 per group) of the RF on the hind paw. (B) Injection of A-967079 (30 μmol/kg, i.v.) also decreased the spontaneous firing of WDR neurons in CFA-inflamed rats (n = 6-12 per group). Since vehicle did not alter WDR firing in animals that received either a von Frey (n = 4) or pinch stimulus (n = 2), this data was combined for comparison. *p < 0.05, **p < 0.01 vs. baseline firing; ++p < 0.01 vs. vehicle-treated group (Fisher's LSD).
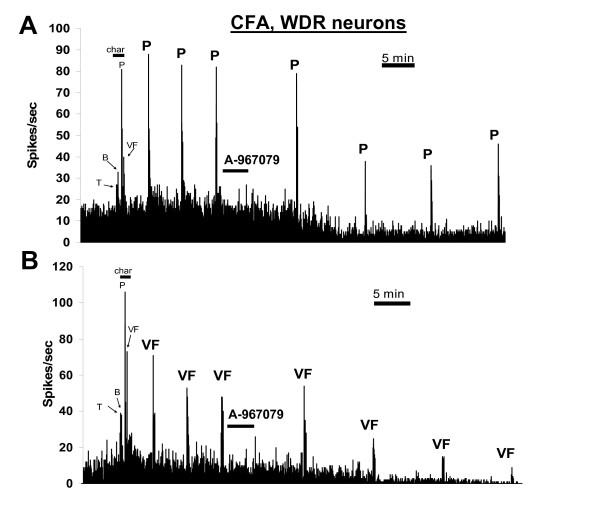
Figure 4.
Effects of A-967079 (30 μmol/kg, i.v.) on single WDR neurons recorded from CFA-inflamed rats. Both evoked and spontaneous firing was attenuated by injection of A-967079. VF = 10-g von Frey hair stimulation, char = characterization period, T = non-noxious tap, B = non-noxious brush, P = noxious pinch (mini bulldog clamp). The neuronal RF on the hind paw was stimulated.
Also in contrast to the effects in uninjured rats, systemic injection of A-967079 significantly attenuated the spontaneous firing of WDR neurons in CFA-inflamed rats (Figures 3B and 4A, B) compared to both baseline levels (p = 0.001, repeated-measures ANOVA) and the vehicle group (p = 0.04, two-way ANOVA). This effect of A-967079 on spontaneous firing was detected by 25 min after injection, and reached a maximally observed reduction (59.27 ± 13.65% from baseline levels) 35 min post-injection.
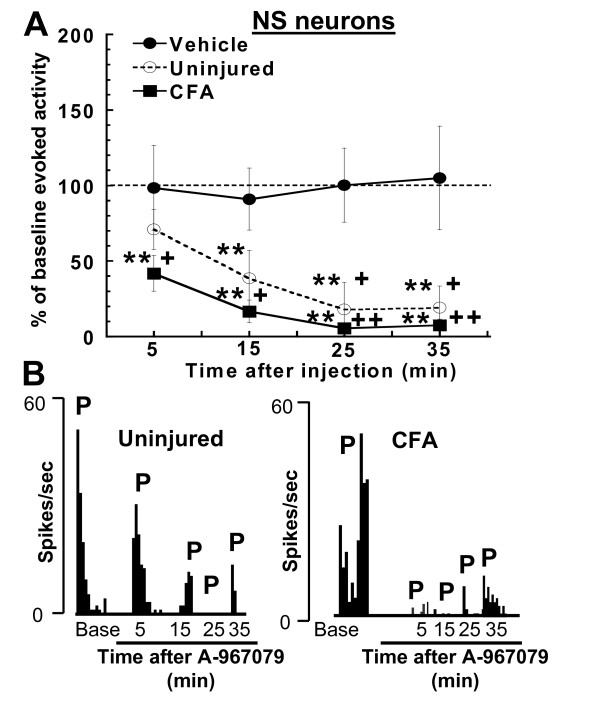
NS neuronal activity in both uninjured and CFA-inflamed rats was substantially decreased by A-967079 (30 μmol/kg, i.v.) (Figure 5) compared to baseline levels (p = 0.0001 for both groups, repeated-measures ANOVA) as well as the vehicle group (p = 0.0004 and p = 0.0001, two-way ANOVA for uninjured and CFA rats, respectively). The pinch-evoked firing of NS neurons was significantly (p < 0.05, Fisher's LSD) reduced 5 min after injection of A-967079, and was nearly eliminated by 25 min post-injection in both groups of rats. Responses of NS neurons to noxious pinch stimulation were maximally decreased by 82.09 ± 17.55%, and 92.49 ± 3.79% from baseline levels in uninjured and inflamed rats, respectively.
Figure 5.
(A) Systemic administration of A-967079 (30 μmol/kg, i.v.) reduced NS responses to a noxious pinch (28 mm mini bulldog clamp for 10 sec) in both uninjured and CFA-inflamed rats (n = 5-6 per group). (B) Representative ratemeters showing the responses of a single NS neuron to noxious pinch (10 sec) stimulation of the hind paw in uninjured (left), and CFA-inflamed (right) rats before and after injection of A-967079 (30 μmol/kg, i.v.). P = 10 sec pinch (mini bulldog clamp) stimulation. Since vehicle did not alter NS firing in CFA (n = 3) or uninjured animals (n = 2), this data was combined for comparison **p < 0.01 vs. baseline firing; +p < 0.05, ++p < 0.01 vs. vehicle-treated group (Fisher's LSD).
Effects of A-967079 on WDR neuronal activity in OA and sham OA rats
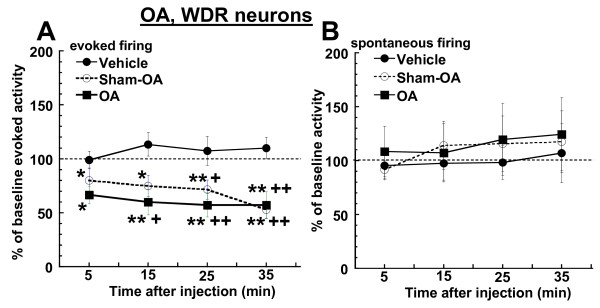
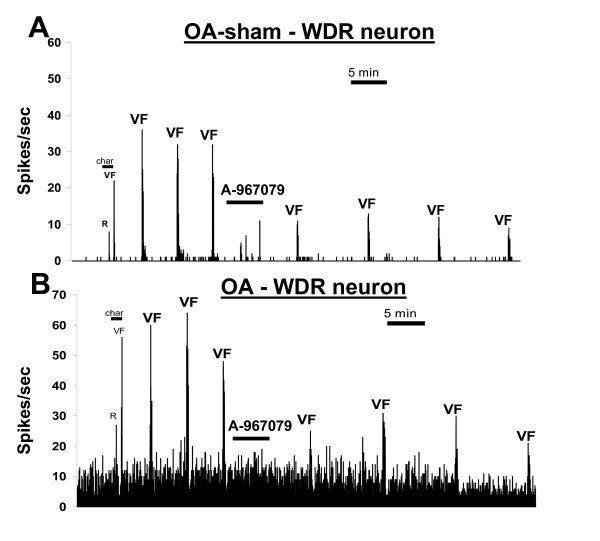
The firing of WDR neurons was also examined in MIA-induced OA and sham OA rats. The testing parameters in these two groups of rats were limited to high-intensity stimulation due to the difficulty and questionable reliability of stimulating the knee joint through its surrounding tissue with a low-intensity stimulus. Responses of WDR neurons to a 300-g von Frey were significantly reduced from baseline levels of firing (p = 0.015 and p = 0.0009, repeated measure ANOVA, respectively) after injection of A-967079 (30 μmol/kg, i.v.) to both OA and sham OA rats (Figures 6A and 7). This evoked response was also significantly reduced compared to the vehicle group (p = 0.0001 for both groups, two-way ANOVA). The effects of A-967079 in these models were similar, as evoked firing of WDR neurons from OA rats was maximally reduced by 42.92 ± 12.3% from baseline levels, while the same stimulation was maximally decreased by 47.35 ± 8.06% in OA-sham rats at 35 min post-injection. Spontaneous firing of WDR neurons was not altered by A-967079 in either OA or OA-sham rats (Figures 6B and 7).
Figure 6.
In OA and OA-sham rats, systemic administration of A-967079 (30 μmol/kg, i.v.) reduced (A) the responses of WDR neurons high-intensity von Frey hair stimulation (300 g for 10 s) of the knee joint, but did not alter the (B) spontaneous firing of WDR neurons (n = 7-8 per group). Since vehicle did not alter WDR firing in OA (n = 5) or OA-sham rats (n = 2), this data was combined for comparison. *p < 0.05, **p < 0.01 vs. baseline firing; +p < 0.05, ++p < 0.01 vs. vehicle-treated group (Fisher's LSD).
Figure 7.
Effects of A-967079 (30 μmol/kg, i.v.) on single WDR neurons recorded from either (A) an OA-sham or (B) an OA rat. In both cases, A-967079 reduced the evoked responses of WDR neurons following high-intensity stimulation (300 g von Frey for 10 s), but did not change the spontaneous activity. R = gentle manual rubbing of the knee joint, VF = 300-g von Frey hair stimulation of the knee, char = characterization period.
Discussion
Systemic administration of the selective TRPA1 receptor antagonist, A-967079, reduced the responses of spinal WDR and NS neurons to high-intensity mechanical stimulus in both uninjured rats and in rats with OA or an inflammatory injury. These in vivo effects add to the growing evidence confirming that TRPA1 receptors have an important role in the transmission of noxious mechanical signals to the spinal cord under both normal and pathological conditions [8,13,16,19,20]. In contrast, the transmission of low-intensity mechanical stimulation was impeded by A-967079 only following an inflammatory injury, and was not altered in uninjured animals. Clearly, the contributions of TRPA1 to mechanosensation shift to include lower intensity stimulation following the inflammatory insult, and this data appears to be consistent with other reports demonstrating an enhanced role for TRPA1 during inflammation [17-20,28].
The neuronal responses to high-intensity peripheral stimulation were attenuated by injection of A-967079 in each of the animal models examined (uninjured, CFA-inflamed, OA, and OA-sham). The intensity of the two stimuli, a clamp-inflicted pinch and 300-g von Frey hair, would certainly be considered in the noxious range. It is likely that A-967079 acted on a similar set of pathways into the spinal cord to modulate the high-intensity stimulation across the different animal models. Furthermore, since both NS and WDR neuronal activity was modulated by A-967079, these pathways likely influence both of these classes of nociceptive neurons. Since A-967079 was administered systemically we cannot determine its exact site(s) of action, but expression of TRPA1 has been localized to different sized primary afferent sensory neurons as well as epidermal kerantinocytes [4-8]. In studies using the skin-nerve preparation, it was reported that application of a TRPA1 antagonist (HC-030031) did not alter the responses of C- and A-fibers to low-intensity mechanical stimulation of normal skin [16]. However, HC-030031 reduced the responsiveness of C-fibers once the stimulus force was increased into the high-intensity range, but Aδ-fiber activity was still unaffected. Thus, TRPA1 receptors on C nociceptors, particularly the slow-adapting subtype [13], appear to play a significant role in high-intensity mechanotransmission. Input to the spinal cord from these TRPA1-containing C-fibers was likely impeded by A-967079 administration in each of the animal models examined, and highlights the importance of this subclass of afferent neurons in transferring noxious mechanical stimulation from the periphery to the spinal cord in both normal and pathological states. It is interesting to note that while WDR neuronal responses to high-intensity stimuli were significantly attenuated by injection of A-967079, the responses of NS neurons to the same simulation were almost completely eliminated. WDR neuronal activity is influenced by a diversity of afferent fibre types, including non-TRPA1-containing fibers. Thus, it is not surprising that the responses of WDR neurons to high-intensity mechanical stimulation were not totally blocked by the injection of A-967079. When combined with the localization data, the almost complete suppression of NS neuronal firing after administration of A-967079 suggests that the mechanical responses of this neuronal class are greatly influenced by input from a specific set of C-fibers that contain TRPA1 receptors.
The transmission of low-intensity mechanical stimulation to WDR neurons was also reduced by A-967079 administration, but only in the CFA-inflamed rats. This additional action of A-967079 in CFA-injected rats indicates that TRPA1 has a role in mechanical hypersensitivity during inflammation. Consistent with this data, injection of TRPA1 receptor antagonists, HC-030031 and AP18, reduced behavioral mechanical hypersensitivity in CFA-inflamed animals [19,20]. The mechanical thresholds of TRPA1-containing primary afferent neurons have been shown to shift lower following CFA-induced inflammation [18], and may be the means through which A-967079 attenuated WDR responses to low-intensity stimulation. There are several factors that may contribute to the lowering of mechanical thresholds for TRPA1-containing neurons after CFA injection, including TRPA1 upregulation in the dorsal root ganglion [17,18], and direct activation or indirect sensitization of the receptor by bradykinin, prostaglandins, and other endogenous agents released during inflammation [8,10,28-32]. We did not examine the effects of A-967079 on low-intensity stimulation in OA rats due to the difficulty in reproducibly stimulating the knee joint through surrounding skin/tissue with a low force stimulus. Nonetheless, antagonism of TRPA1 receptors reduces mechanical allodynia in neuropathic rats [19], demonstrating that TPRPA1 receptors may have a role in pathological mechanotransmission in a model other than inflammatory injury.
A-967079 also reduced the "heightened" spontaneous firing of WDR neurons in CFA-inflamed rats. As we and others have observed previously, the spontaneous firing of WDR neurons is elevated following CFA-induced inflammation [23,24,33-35], and likely reflects injury-related sensitization of this class of spinal neurons [22-27]. Given that WDR neurons are an integral component of the ascending nociceptive pathway, and that higher levels of WDR firing generally code for increased somatosensory intensity, the observation that WDR neurons in inflamed rats spontaneously discharge at rates higher than normal may indicate that there is unevoked or "nagging" discomfort in the animal. Unevoked pain is observed in the majority of patients with chronic pain, and is a primary reason for seeking medical care [36,37]. Suzuki and Dickenson [27] have reported that heightened spontaneous discharges of WDR neurons are either reduced, or unaltered by drugs that are effective or ineffective, respectively, in treating unevoked pain in humans. Since accurate assessment of unevoked pain in animals is difficult [38], examining the ongoing firing of WDR neurons in animal models of pathological pain may provide some insight into this undesirable condition. The current data would suggest that antagonism of TRPA1 receptors may be an effective means to alleviate unevoked pain or discomfort following an inflammatory injury.
Spontaneous firing of WDR neurons was also elevated in OA rats, but unlike in CFA-inflamed rats, this increase in ongoing discharges was not altered by injection of A-967079. One difference between these models was the site of neuronal recording since input from the knee (OA) and hind paw (CFA) arrive at different sections of the lumbar spinal cord. However, both of these sections are likely influenced by fibers containing TRPA1 receptors since injection of A-967079 decreased evoked neuronal firing in both OA and CFA rats. Albeit speculative, the differential effect of A-967079 on spontaneous firing in CFA and OA rats may be more related to the degree of inflammation on the days of neuronal recording. In the MIA-induced model of OA, inflammation of the knee region has mostly dissipated by day 7 after the MIA insult [39]. Thus, inflammation would not factor into the neuronal activity recorded on day 21 in OA rats. In contrast, the release of inflammatory mediators in the CFA assay may be an important component to the augmented contributions of TRPA1 in this model [28,30,31]. These mediators probably not only factored into the effects of A-967079 on low-intensity mechanical stimulation, but also on the antagonist-related reduction of heightened spontaneous firing.
Conclusions
The TRPA1 receptor has a significant role in the transmission of high-intensity mechanical stimulation to different classes of spinal nociceptive neurons in uninjured, inflamed and osteoarthritic animals. Additionally, there is an enhanced role for TRPA1 receptors following an inflammatory injury that includes modulation of low-intensity mechanical input to the spinal cord, and reducing elevated levels of spontaneous WDR neuronal firing. Thus, blockade of TRPA1 receptors is likely to be an effective means to alleviate pain associated with intense mechanical stimulation in both normal and pathological conditions, as well as reducing mechanical allodynia and "nagging" or unevoked pain after an inflammatory injury.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SM and KLC carried out, analyzed, and designed all aspects of the electrophysiological studies. SM, KLC, and PRK were involved in constructing the original concept of the study. SM wrote the manuscript while KLC, RJP, MEC, and PRK participated in critically reviewing the manuscript. RJP, MEC, and SD were involved in the design and synthesis of A-967079. All authors have read and approved the final manuscript
Contributor Information
Steve McGaraughty, Email: Steve.P.McGaraughty@abbott.com.
Katharine L Chu, Email: Katharine.Chu@abbott.com.
Richard J Perner, Email: Richard.J.Perner@abbott.com.
Stan DiDomenico, Email: Stanley.Didomenico@abbott.com.
Michael E Kort, Email: Michael.E.Kort@abbott.com.
Philip R Kym, Email: Philip.R.Kym@abbott.com.
Acknowledgements
All work is funded by Abbott Laboratories.
References
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch. 2005;451:1–10. doi: 10.1007/s00424-005-1462-y. [DOI] [PubMed] [Google Scholar]
- Minke B. TRP channels and Ca2+ signaling. Cell Calcium. 2006;40:261–275. doi: 10.1016/j.ceca.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/S0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, García-Añoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–40561. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/S0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Namer B, Seifert F, Handwerker HO, Maihöfner C. TRPA1 and TRPM8 activation in humans: effects of cinnamaldehyde and menthol. Neuroreport. 2005;16:955–999. doi: 10.1097/00001756-200506210-00015. [DOI] [PubMed] [Google Scholar]
- Andrade EL, Luiz AP, Ferreira J, Calixto JB. Pronociceptive response elicited by TRPA1 receptor activation in mice. Neuroscience. 2008;152:511–520. doi: 10.1016/j.neuroscience.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, Schafer WR. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Chen J, Faltynek CR, Moreland RB, Neelands TR. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur J Neurosci. 2008;27:605–611. doi: 10.1111/j.1460-9568.2008.06030.x. [DOI] [PubMed] [Google Scholar]
- Kerstein PC, del Camino D, Moran MM, Stucky CL. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol Pain. 2009;5:19. doi: 10.1186/1744-8069-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham JP, Kelly S, Donaldson LF. Inflammation reduces mechanical thresholds in a population of transient receptor potential channel A1-expressing nociceptors in the rat. Eur J Neurosci. 2008;27:3151–3160. doi: 10.1111/j.1460-9568.2008.06256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, Henze DA, Kane SA, Urban MO. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, Jegla T, Patapoutian A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin D, Mikusa J, Baker S, Zhong C, Simler G, Zhang X, Bianchi B, DiDomenico S, Perner RJ, Reilly R, Kort M, Jacobson P, Honore P, Chen J, Joshi S, Kym P. A-967079, a potent and selective TRPA1 antagonist, is efficacious in rat preclinical pain models. Society for Neuroscience Abstracts. 2009. 761.3.
- Chapman V, Suzuki R, Dickenson AH. Electrophysiological characterization of spinal neuronal response properties in anaesthetized rats after ligation of spinal nerves L5-L6. J Physiol. 1998;507:881–894. doi: 10.1111/j.1469-7793.1998.881bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu KL, Faltynek CR, Jarvis MF, McGaraughty S. Increased WDR spontaneous activity and receptive field size in rats following a neuropathic or inflammatory injury: implications for mechanical sensitivity. Neurosci Lett. 2004;372:123–126. doi: 10.1016/j.neulet.2004.09.025. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Brown BS, Zhu CZ, Zhong C, Joshi SK, Honore P, Faltynek CR, Jarvis MF. Contributions of central and peripheral TRPV1 receptors to mechanically evoked and spontaneous firing of spinal neurons in inflamed rats. J Neurophysiol. 2008;6:3158–3166. doi: 10.1152/jn.90768.2008. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Scanio MJ, Kort ME, Faltynek CR, Jarvis MF. A selective Nav1.8 sodium channel blocker, A-803467, attenuates spinal neuronal activity in neuropathic rats. J Pharmacol Exp Ther. 2008;324:1204–1211. doi: 10.1124/jpet.107.134148. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Dart MJ, Yao BB, Meyer MD. A CB(2) receptor agonist, A-836339, modulates wide dynamic range neuronal activity in neuropathic rats: contributions of spinal and peripheral CB(2) receptors. Neuroscience. 2009;158:1652–1661. doi: 10.1016/j.neuroscience.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Dickenson AH. Differential pharmacological modulation of the spontaneous stimulus-independent activity in the rat spinal cord following peripheral nerve injury. Exp Neurol. 2006;198:72–80. doi: 10.1016/j.expneurol.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1987. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley SM, Hughes PA, Page AJ, Kwan KY, Martin CM, O'Donnell TA, Cooper NJ, Harrington AM, Adam B, Liebregts T, Holtmann G, Corey DP, Rychkov GY, Blackshaw LA. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology. 2009;137:2084–2095. doi: 10.1053/j.gastro.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Orengo L, Dhaka A, Heuermann RJ, Young TJ, Montana MC, Cavanaugh EJ, Kim D, Story GM. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol Pain. 2008;4:30. doi: 10.1186/1744-8069-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- Materazzi S, Nassini R, Andrè E, Campi B, Amadesi S, Trevisani M, Bunnett NW, Patacchini R, Geppetti P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Natl Acad Sci USA. 2008;105:12045–12050. doi: 10.1073/pnas.0802354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YB, Ling QD, Ruda MA, Kenshalo DR. Electrophysiological changes in adult rat dorsal horn neurons after neonatal peripheral inflammation. J Neurophysiol. 2003;90:73–80. doi: 10.1152/jn.01019.2002. [DOI] [PubMed] [Google Scholar]
- Kitagawa J, Kanda K, Sugiura M, Tsuboi Y, Ogawa A, Shimizu K, Koyama N, Kamo H, Watanabe T, Ren K, Iwata K. Effect of chronic inflammation on dorsal horn nociceptive neurons in aged rats. J Neurophysiol. 2005;93:3594–3604. doi: 10.1152/jn.01075.2004. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Faltynek CR, Jarvis MF. Systemic and site-specific effects of A-425619, a selective TRPV1 receptor antagonist, on wide dynamic range neurons in CFA-treated and uninjured rats. J Neurophysiol. 2006;95:18–25. doi: 10.1152/jn.00560.2005. [DOI] [PubMed] [Google Scholar]
- Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. J Pain. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Birklein F, Riedl B, Sieweke N, Weber M, Neundörfer B. Neurological findings in complex regional pain syndromes--analysis of 145 cases. Acta Neurol Scand. 2000;101:262–269. doi: 10.1034/j.1600-0404.2000.101004262x./. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004;112:12–15. doi: 10.1016/j.pain.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Bove SE, Calcaterra SL, Brooker RM, Huber CM, Guzman RE, Juneau PL, Schrier DJ, Kilgore KS. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage. 2003;11:821–830. doi: 10.1016/S1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]