Abstract
Objective
To assess the safety and efficacy of rapamycin (RAPA) in the treatment of diffuse systemic sclerosis (SSc)
Methods
Eighteen patients with ≤5 years of diffuse SSc were randomized to receive RAPA or methotrexate (MTX) in a single-blind, 48-week study. Abnormalities in clinical and laboratory parameters were compared between the two treatment groups. The potential efficacy of the study drugs was evaluated by comparing the baseline and 48-week assessments, including the modified Rodnan Skin score (mRSS) and the Health Assessment Questionnaire Disability Index.
Results
Baseline patient characteristics were similar in both groups (N = 9 in each). One patient who never took the study drug in the RAPA group was excluded from analysis. Three patients in each group withdrew from the study: two were treatment-related (severe hypertriglyceridemia associated with RAPA; pancytopenia associated with MTX) and four were SSc-related. Hypertriglyceridemia was the most notable side-effect associated with RAPA, but it was generally well-tolerated and treatable. The incidence and severity of other adverse drug reactions were comparable between two groups. Within each group, the mRSS score showed a significant improvement from baseline (P < 0.05). In the RAPA group, the patient global assessment showed a significant improvement from baseline while forced vital capacity declined from baseline. The disease activity scores at 48 weeks and their changes from baseline were not significantly different between two groups.
Conclusion
RAPA has a reasonable safety profile in a select group of scleroderma patients. Larger trials are needed to assess its efficacy in early diffuse SSc.
Introduction
Systemic sclerosis (SSc) is a multi-system, autoimmune disorder with an unknown etiology and a highly variable disease progression. While skin thickening is the most visible manifestation of SSc, fibrotic damage can also occur in the musculoskeletal system and in the visceral organs, including the heart, kidneys, lungs, and gastrointestinal tract. The pathogenesis of SSc is complex, but three key processes are hypothesized as being the most important: excessive collagen synthesis and deposition in various organs; small vessel vasculopathy; and activation of the immune system (1, 2). The interplay of these processes likely contributes to the progression of SSc. Treatment of SSc is usually organ-specific and mostly guided by experience from case series and a few randomized, controlled trials (RCTs) (3, 4).
Since an activated immune system is a key process underlying SSc, several immunomodulatory agents have been studied as potential treatments for SSc. One such agent is MTX (5-7). Two RCTs have demonstrated either trends toward (5), or actual significance for (6), improvement in skin thickening and in global assessment, favoring MTX.
Cyclosporine A (CsA), another immunomodulatory agent, may improve skin thickening by suppressing T-cell activation via inhibition of the calcineurin signaling pathway, which then leads to a decrease in gene transcription for pro-inflammatory cytokines such as interleukin-2 (IL-2). CsA was used to treat 10 patients with diffuse SSc in an open labeled study with historical controls (8). Although a clinically significant reduction in skin thickening was seen in 60% of the patients treated with CsA, significant renal dysfunction and/or new-onset hypertension occurred in 8 of these 10 patients. Because renal impairment is a frequent organ complication observed in SSc itself, any treatment that increases the likelihood of renal disease decreases the merit in its use.
Since RAPA blocks the response of T cells to cytokines including IL-2 (9), we hypothesized that RAPA therapy might lead to similar clinical improvements as were seen with CsA while being less toxic. Unlike CsA, RAPA may have additional immunomodulatory effects on fibrogenesis via its role in other cellular pathways. RAPA binds to the intracellular receptor, FK506 binding proteins (FKBP12), to form a complex that inhibits mammalian Target of RAPA (mTOR). This complex is a multifunctional protein with a key role in the regulation of cell growth, proliferation, and differentiation (10). Blockade of mTOR by RAPA inhibits cytokine-stimulated signal transductions for cellular proliferation (11) and also reduces production of cytokines (10). The synthesis of collagen type I in human fibroblasts is regulated by mTOR (12), and mTOR inhibition has been shown to decrease extracellular matrix deposition in an animal study (13). Thus, RAPA may have a more specific effect on the fibrogenesis of SSc than CsA or MTX.
In the present report, we describe a 48-week, single-blind, randomized, phase I study of RAPA versus MTX in the treatment of diffuse SSc. MTX was used as a comparator for several reasons: it has been compared to placebo, with results suggesting more improvement in skin thickening and global assessment in the MTX arm compared to placebo (5, 6); it is often used as a treatment for SSc; it has a well-described toxicity profile; it is well-tolerated by the majority of patients with SSc; and it is recommended by EULAR/EUSTAR as treatment for early diffuse SSc (3). Our primary aim was to evaluate and compare the major toxicities associated with RAPA use in diffuse SSc to those of MTX. Toxicity data for RAPA comes primarily from organ transplantation studies (14). In its limited use in rheumatologic diseases, RAPA demonstrated a reasonable safety profile and treatment response (15, 16). Although the toxicities associated with RAPA may be different from those of MTX, we hypothesized that the major toxicities associated with RAPA would not be greater than those of MTX and therefore should be well-tolerated by patients with SSc. Our secondary aim was to examine the potential efficacy of RAPA by monitoring several clinical parameters commonly used in SSc clinical trials to measure disease activity.
Patients and Methods
Patients
All enrolled patients met the 1980 preliminary classification criteria for SSc as defined by the American Rheumatism Association (17). All patients were required to have diffuse cutaneous scleroderma (with skin thickening proximal as well as distal to the elbows and knees, often involving the chest and abdomen, with or without facial involvement), a disease duration of ≤5 years from the first sign or symptom typical of SSc other than Raynaud's phenomenon, be ≥18 and ≤70 years old, and be using reliable contraception (preferably including a barrier method) if female and of childbearing potential. The study protocol was approved by the institutional review board at the University of California, Los Angeles. All patients provided fully-informed, written consent to participate in this study.
Patients were excluded from the study if they had any of the following: 1) SSc-like diseases such as eosinophilic fasciitis or those following environmental exposure; 2) pregnancy; 3) breastfeeding; 4) leukocyte count <4.0 × 109/l, platelet count <100 × 109/l, serum creatinine ≥2 mg/dl, elevated liver function tests (LFTs) greater than twice the upper limits of normal (ULN); 5) forced vital capacity (FVC) <45% or diffusing capacity (DLco) <40% of their predicted values; 6) chronic illnesses that might cause serious debilitation including intractable malabsorption; 7) poorly-controlled hypertension or congestive heart failure; 8) liver disease; 9) malignancy; 10) alcohol use of greater than 2 standard sized drinks per week; 11) previous treatment with MTX or RAPA for >30 days; 12) concomitant use of any sulfhydryl containing angiotensin-converting enzyme (ACE) inhibitor such as captopril (other ACE-inhibitors were permissible); 13) prednisone use >10 mg/day or equivalent (lower dosage was allowed if it had been stable for at least 30 days); or 14) participation in another clinical research study within the last 30 days. Patients were required to discontinue other immunosuppressive or antimetabolite agents, or agents with putative disease-modifying potential, for at least one month prior to initiating the study drug.
Treatment
Enrolled patients who satisfied the inclusion and exclusion criteria were randomized to receive either RAPA or MTX by a predetermined computer-generated randomization schedule developed by the UCLA Department of Statistics. The initial dose of oral RAPA was 6 mg once daily. RAPA serum trough levels were assessed 2 weeks after any change in RAPA dose and every 8 weeks thereafter. RAPA dose and levels have a linear relationship (online supplement Figure 1), which facilitated achievement of target doses. The dose was adjusted to achieve and maintain a serum level between 5-15 ng/ml, which is the level typically used in the immunosuppressive management of organ transplant recipients (18). All RAPA blood levels were obtained through Quest Laboratories ®.
The initial dose of oral MTX was 10 mg weekly; the weekly dose was increased by 2.5 mg every 4 weeks to the target dose of 20 mg/wk as tolerated. Oral folic acid was administered at a daily dose of 1 mg.
An unblinded investigator not involved in the direct care of the patients served as a medication control officer (MCO). The MCO was responsible for initiating therapy with RAPA and MTX, increasing the dose per protocol, monitoring the serial laboratory profile including the RAPA serum trough levels, and adjusting the dosage of the study medications as needed to minimize toxicity while maintaining therapeutic levels and doses of the study drugs. In some cases, the study medication was discontinued if adverse events occurred and restarted at a lower dose per protocol when patients recovered from these events.
Baseline measurements and serial clinical monitoring
At baseline and at the 48-week visits, each patient had the following assessments: a complete history and physical exam, comprehensive metabolic panel (CMP), creatine phosphokinase (CPK), complete blood count (CBC), fasting lipid panel (FLP), urinalysis, urine pregnancy test if patient was female of child-bearing potential, chest radiograph, 12-lead electrocardiogram (EKG), and pulmonary function tests (PFTs). Urinalysis and urine pregnancy tests were also collected at 24 weeks. Serial CMP and CBC were obtained every 4 weeks to monitor toxicities associated with the study drugs. Because RAPA is associated with hypercholesterolemia and hypertriglyceridemia, a FLP was performed every 2 months, or more frequently if the results were abnormal and required intervention with cholesterol or triglyceride-lowering medications.
Physical examination at baseline, 24 weeks, and 48 weeks included measurements of modified Rodnan Skin Score (mRSS), palpation of tendon friction rubs, and patient's joint tenderness and swelling counts (8 joints). Patient and physician global assessments via visual analogue scale (0-100 mm VAS) and Health Assessment Questionnaire Disability Index (HAQ-DI) were also collected at baseline, 24 weeks, and 48 weeks. The physician global assessment was performed by the clinician on a 10-cm line who was blind to the patient global assessment. Patients returned for follow-up every 2 months. During these visits, the clinician performed limited physical examinations including vital signs and mRSS, and asked open-ended questions regarding potential adverse events. Patients were seen at every visit by the same clinician who was blind to the patient's treatment allocation.
Outcome measurements
The primary goal of this study was to compare the toxicities of RAPA and MTX in the treatment of diffuse SSc over 48 weeks. Laboratory results and blood pressure values from every 2-monthly visits were considered in the analysis. The incidences of abnormal laboratory values were compared between two treatment groups and distributed into grades of severity using published guidelines (19-21). If a patient had multiple abnormally-elevated laboratory values during the course of the study, only the most abnormal one observed during the study was included and assigned to the corresponding grade. While this analysis is non-statistical and does not indicate when the abnormalities occurred, it provides a general view of the possible adverse effects that may be associated with the study drugs and allows for a comparison of some of the laboratory abnormalities between the two groups.
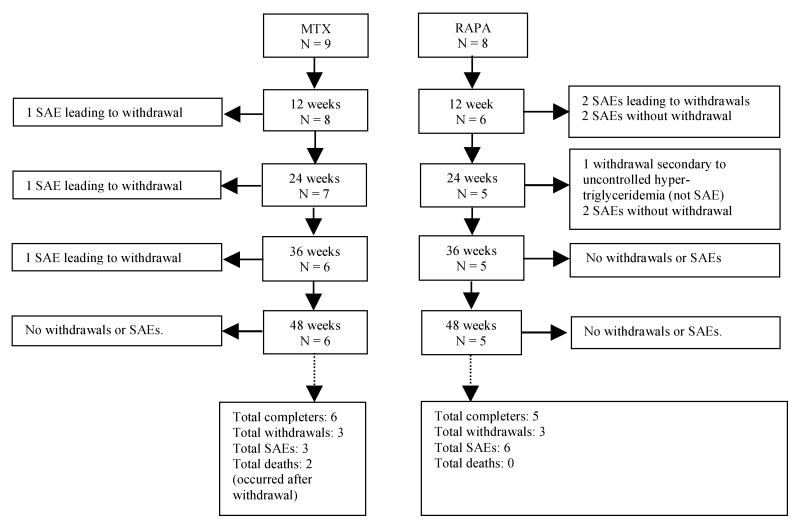
Serious adverse events (SAEs) (22) occurring during the study and withdrawals from the study are outlined in a patient flow diagram (Figure 1). At the completion of the study, an independent blinded reviewer determined whether the SAEs in this study were related to the study drug or were part of the SSc disease progression.
Figure 1.
Patient withdrawals and serious adverse events (SAEs) during the 48-week study. All SAEs were determined by the independent reviewer to be related to complications of SSc, with the exception of the first SAE in the MTX group (pancytopenia) which was determined to be treatment-related.
The secondary goal of this study was to examine the potential efficacy of RAPA compared to MTX in the treatment of SSc. We used several clinical assessments commonly used in SSc clinical trials to measure disease activity, including mRSS, patient and physician global assessments, HAQ-DI, FVC and DLco (as percent of predicted), joint tenderness and swelling count, and presence of tendon friction rubs. The disease activity scores at 48 weeks and their changes from baseline were compared within and between the two treatment groups in patients who completed the study. Using the definitions demonstrated in published SSc trials for the minimum clinically important differences (MCID) (mRSS improvement of ≥5.3 or HAQ-DI improvement of ≥0.14) (23), we determined which patients met these MCIDs at 48 weeks by calculating the change scores from baseline in patients who completed the study.
Statistical Analysis
Results are expressed as means ± standard deviation (SD), unless otherwise specified. For continuous data, student's t-test was used to compare differences between groups and paired t-test was used to compare changes within groups. For analysis requiring non-parametric methods, Wilcoxon rank sum test and Wilcoxon signed rank test were used in comparisons between groups and within groups, respectively. Categorical data were analyzed using Fisher's exact test or Chi-square test. P-values ≤ 0.05 were considered significant. Since the analyses were exploratory, no adjustment was made for multiple comparisons. A completer analysis was used to examine the disease activity scores at 48 weeks and their changes from baseline (Tables 4 and 5). The data from all the study participants were considered in the analysis of baseline characteristics and toxicity profile of the study drugs (Tables 1 and 3). Statistical calculations were performed using SAS software (SAS Institute, Cary, NC).
Table 4.
Measurements of SSc disease activity at 48 weeks and change scores at 48 weeks in patients who completed the study.
| MTX (n = 6) | RAPA (n = 5) | |||||
|---|---|---|---|---|---|---|
| 48 weeks (n) | Change from baseline (n) | P* | 48 weeks | Change from baseline | P | |
| mRSS, 0-51 | 15.5 ± 8.0 (6) | -6.8 ± 6.1 (6) | 0.041 | 18.4 ± 10.9 (5) | -5.6 ± 3.9 (5) | 0.033 |
| HAQ-DI score, 0-3 | 0.95 ± 0.47 (5) | -0.03 ± 0.69 (5) | 0.940 | 1.70 ± 0.85 (5) | 0.17 ± 0.81 (5) | 0.656 |
| Patient global assessment VAS, 0-100 | 39.2 ±36.6 (5) | -11.0 ± 31.7 (5) | 0.481 | 49.0 ± 20.9 (4) | -11.5 ± 6.2 (4) | 0.035 |
| Physician global assessment VAS, 0-100 | 21.3 ± 10.8 (6) | -5.5 ± 11.2 (6) | 0.283 | 32.2 ± 19.2 (5) | -8.4 ± 7.5 (5) | 0.066 |
| Tender joint count, 0-8 | 2.8 ± 3.7 (6) | -0.3 ± 2.5 (6) | 0.756 | 2.6 ± 3.3 (5) | 0.0 ± 2.1 (5) | 1.000 |
| Tendon friction rub, 0-5 | 0 ± 0 (6) | -0.2 ± 0.4 (6) | 0.496 | 0.2 ± 0.5 (5) | -0.4 ± 0.9 (5) | 0.614 |
| FVC, % predicted | 93.3 ± 6.8 (5) | 1.2 ± 14.1 (6) | 0.859 | 87.8 ± 9.6 (4) | -10.5 ± 6.6 (4) | 0.050 |
| DLco, % predicted | 75.7 ± 16.3 (5) | -5.2 ± 13.2 (6) | 0.429 | 64.2 ± 19.6 (4) | -12.7 ± 10.0 (3) | 0.160 |
P values listed are within-group comparisons for change scores from baseline to 48 weeks. Between-group comparisons did not show significant differences in any change scores from baseline to 48 weeks (P all > 0.05, not listed).
Negative numbers denotes improvement, except for FVC and DLco.
() = number of subjects for which scores were available.
Table 5.
Number of patients in each group meeting the MCIDs for mRSS and HAQ-DI.
| MTX (n = 6) | RAPA (n = 5) | |
|---|---|---|
| mRSS ≥5.3 | 4 | 3 |
| HAQ-DI ≥0.14 | 1 | 2 |
None of the patients met the MCIDs for both scores except for one patient in the RAPA group.
() = number of subjects who completed the study.
Table 1.
Demographic and baseline characteristics of study participants. Data presented as mean ± SD.
| MTX (n = 9) |
RAPA* (n = 8) |
P | |
|---|---|---|---|
| Demographics | |||
| Age, years | 51.6 ± 15.4 | 52.8 ± 9.8 | 0.85 |
| Sex, F/M (percentage females) | 7/2 (77.8) | 5/3 (62.5) | 0.62 |
| Ethnicity | |||
| Caucasian | 6 | 5 | – |
| Hispanic | 1 | 3 | – |
| African American | 1 | 0 | – |
| Other | 1 | 0 | – |
| History and physical examination | |||
| Duration of SSc, years | 1.44 ± 0.97 | 1.64 ± 1.63 | 0.85 |
| HAQ-DI score, 0-3 | 1.19 ± 0.70 | 1.61 ± 0.54 | 0.18 |
| Patient global assessment VAS, 0-100 | 58.9 ± 29.0 | 58.9 ± 25.1 | 1.00 |
| Physician global assessment VAS, 0-100 | 33.4 ± 13.0 | 46.9 ± 15.2 | 0.08 |
| mRSS, 0-51 | 24.6 ± 7.7 | 28.1 ± 8.8 | 0.39 |
| Tender joint count, 0-8 | 3.4 ± 3.2 | 3.1 ± 2.9 | 0.84 |
| Tendon friction rub, 0-5 | 0.11 ± 0.33 | 0.50 ± 0.76 | 0.19 |
| Systolic blood pressure, mmHg | 122.9 ± 21.4 | 113.3 ± 8.2 | 0.60 |
| Diastolic blood pressure, mmHg | 68.9 ± 6.1 | 66.7 ± 10.8 | 0.65 |
| Weight, kg | 68.4 ± 6.5 | 68.9 ± 9.0 | 0.92 |
| Laboratory variables | |||
| FVC, % predicted | 85.2 ± 17.0 | 92.1 ± 12.1 | 0.37 |
| DLco, % predicted | 74 ± 22 | 77 ± 12 | 0.76 |
| White blood cell count, ×1,000/mm3 | 8.67 ± 2.85 | 8.29 ± 2.43 | 0.66 |
| Hemoglobin, gm/dl | 11.74 ± 1.34 | 12.04 ± 1.04 | 0.63 |
| Platelets, ×1,000/mm3 | 372.17 ± 91.64 | 320.20 ± 112.75 | 0.42 |
| Total creatinine kinase, u/l | 82.50 ± 44.04 | 132.29 ± 42.44 | 0.04 |
| Serum creatinine, mg/dl | 0.88 ± 0.30 | 0.74 ± 0.22 | 0.30 |
| AST, u/l | 16.17 ± 5.46 | 22.80 ± 7.05 | 0.11 |
| ALT, u/l | 11.50 ± 3.51 | 15.60 ± 2.88 | 0.07 |
| Alkaline phosphatase, u/l | 66.17 ± 13.53 | 54.20 ± 19.88 | 0.27 |
| Total bilirubin, mg/dl | 0.40 ± 0.21 | 0.28 ± 0.08 | 0.26 |
| Serum albumin, gm/dl | 3.75 ± 0.40 | 3.64 ± 0.35 | 0.64 |
| Lipid profile | |||
| Total cholesterol, mg/dl | 180.0 ± 38.2 | 190.0 ± 39.8 | 0.64 |
| Triglycerides, mg/dl | 217.3 ± 129.6 | 241.0 ± 152.4 | 0.48 |
| LDL, mg/dl | 88.0 ± 33.8 | 100.0 ± 56.6 | 0.75 |
| HDL, mg/dl | 46.4 ± 19.4 | 43.0 ± 21.2 | 0.85 |
1 patient randomized to the RAPA group never took the study drug and was excluded from all the analyses.
Table 3.
Number of patients in each treatment group who developed abnormal values* (AEs) while taking the study drug, distributed by laboratory parameters and blood pressure measurements.
| MTX (n = 9) | RAPA (n = 8) | |
|---|---|---|
| Hemoglobin (decrease from baseline) † | ||
| 1.0 – 1.4 | 4 | 0 |
| 1.5- 2.0 | 0 | 4 |
| 2.1 – 2.9 | 1 | 0 |
| ≥ 3 | 2 € | 3 |
| White Blood Cell † | ||
| 3.0 – 3.9 | 1 € | 0 |
| < 3.0 | 0 | 0 |
| Platelets † | ||
| 75 – 140 | 0 | 2 |
| 50 – 74.9 | 0 | 0 |
| 20 – 49.9 | 1 € | 0 |
| < 20 | 0 | 0 |
| Creatinine † | ||
| 1.1 – 1.2 ULN | 0 | 0 |
| 1.3 – 1.8 ULN | 1 | 1 |
| > 1.9 ULN | 0 | 0 |
| Systolic Blood Pressure ‡ | ||
| ≥ 120 – 139 | 2 | 2 |
| ≥ 140 – 159 | 2 | 2 |
| ≥ 160 | 1 | 1 |
| Diastolic Blood Pressure ‡ | ||
| ≥ 80 – 89 | 4 | 5 |
| ≥ 90 – 99 | 0 | 0 |
| ≥ 100 | 0 | 1 |
| Cholesterol § | ||
| 200 – 239 | 4 | 2 |
| ≥ 240 | 1 | 4 |
| Triglyceride § | ||
| 150 – 199 | 1 | 1 |
| 200 – 499 | 6 | 5 |
| ≥ 500 | 0 | 2 |
| AST † | ||
| 1.2 – 1.5 ULN | 1 | 0 |
| 1.6 – 3.0 ULN | 0 | 1 |
| > 3.0 ULN | 0 | 0 |
| ALT † | ||
| 1.2 – 1.5 ULN | 0 | 0 |
| 1.6 – 3.0 ULN | 0 | 1 |
| > 3.0 ULN | 0 | 0 |
| Alkaline phosphatase † | ||
| 1.1 – 1.5 ULN | 1 | 0 |
| 1.6 – 3.0 ULN | 0 | 0 |
| 3.1 – 5.0 ULN | 0 | 0 |
| > 5 ULN | 0 | 0 |
Each parameter was divided into categories of severity based on published guidelines. Adjacent categories are combined if their frequencies are zero.
If a patient had several abnormal clinical values during the study course, only the most severe value is counted.
Based on the Rheumatology Common Toxicity Criteria (19).
Based on Seventh Report of the Joint National Committee Guidelines (21).
Based on the Adult Treatment Panel III (ATP III) guidelines (20).
Patient #9 accounted for one instance of leukopenia, anemia, and thrombocytopenia.
Results
Patient characteristics at baseline
Eighteen patients with ≤5 years of diffuse SSc were enrolled in the study and randomized to receive either RAPA or MTX. Each group consisted of nine patients initially, but one patient randomized to the RAPA group chose to withdraw from the study prior to taking RAPA and was excluded from all the analyses. The patient demographics, baseline clinical and laboratory characteristics, and baseline assessments of disease activity are shown in Table 1. There were no statistical differences between the two, except for a statistically significant difference in CPK (means ± SD: 82.5 ± 44.0 for MTX and 132.3 ± 42.4 for RAPA, P < 0.045; both means were within normal limits) that was not clinically significant. While some of the parameters that measure disease activity were higher in the RAPA group (e.g. physician global assessment, HAQ-DI, mRSS), suggesting a more severe disease in this group at baseline, these differences compared to the MTX group were not statistically significant. Other assessments of disease activity at baseline were similar between the two groups (Table 1).
Adverse events (AE)
Figure 1 summarizes the patient flow, withdrawals, and SAEs in both treatment groups at 12-week intervals during the study period. Three patients in each treatment group withdrew before completing the study. There were a total of 3 SAEs (one each in three patients) in the MTX group and 6 SAEs (one each in 2 patients and 2 each in 2 patients) in the RAPA group (Table 2). Most of these case were determined to be SSc-related. There were two deaths in the MTX group that occurred within 6 months of withdrawal and no deaths in the RAPA group. These deaths were deemed to be the result of rapid progression of SSc.
Table 2.
Serious adverse events (SAEs) occurring during the study in patients treated with MTX or RAPA. All SAEs listed here resulted in hospitalizations.
| Patient # | Month of SAE | First SAE | Second SAE | Comments |
|---|---|---|---|---|
| MTX | ||||
| 9 * | 2nd | Pancytopenia† SRC |
— | Developed pancytopenia after withdrawal; resolved after discontinuation of MTX SRC controlled with ACEI |
| 13 * ‡ | 6th | Respiratory failure Pericardial effusion |
— | Hospitalization complicated by infection Rehospitalized multiple times after withdrawal from the study Death at 3 months after withdrawal |
| 2 * ‡ | 8th | Nausea, vomiting | — | Death at 4 ½ months after withdrawal |
| RAPA | ||||
| 8 * | 1st | GIB; then withdrew | — | One month after GIB, was hospitalized for SRC |
| 14 * | 1st | CHF Severe GERD |
— | Hypertriglyceridemia (455 mg/dl) while on RAPA |
| 1 | 1st | Septic joint | — | |
| 5th | — | Malnutrition | ||
| 5 | 3rd | Onset of SRC Nausea, vomiting |
— | SRC controlled with ACEI GI symptoms controlled with metoclopramide |
| 4th | — | SRC | ||
Withdrawals from study.
Treatment-related SAE per independent reviewer.
Death after withdrawal from study, secondary to failure to thrive.
SRC = SSc renal crisis; ACEI = angiotensin-converting enzyme inhibitor; GIB = gastrointestinal bleed; CHF = congestive heart failure; GERD = gastroesophageal reflux disease.
Abnormalities in various laboratory parameters and blood pressure are presented in Table 3 for all the patients who participated in the study. Seven cases of a decline in hemoglobin from baseline were observed in each treatment group. Two of the cases in the MTX group involved ≥3 gm/dl decrease in hemoglobin: one was due to MTX-induced pancytopenia and the second occurred in a patient who became seriously ill with respiratory failure and pericardial effusion (the etiology of his hemoglobin drop was unknown). Three of the cases in the RAPA group involved ≥3 gm/dl decrease in hemoglobin: one case each resulted from severe gastrointestinal bleeding (watermelon stomach), from SRC, and from an undetermined cause.
No case of leukopenia was seen in the RAPA group. One patient in the MTX group developed scleroderma renal crisis (SRC) and leukopenia (3.7 × 1,000/mm3) (patient 9 in Table 2). He withdrew from the study one week later when he wad admitted to the hospital with pancytopenia. An independent reviewer determined that his pancytopenia was attributable to MTX, likely due to accumulation of MTX from worsening renal function. No other cases of thrombocytopenia were observed in the MTX group during the study. Mild degrees of thrombocytopenia (138 × 1,000/mm3 and 116 × 1,000/mm3) were seen in two patients in the RAPA group. The latter case occurred during SRC.
One case of elevated Cr occurred in each treatment group (both due to SRC). Neither patient required dialysis. Both treatment groups had incidences of hypertension that were similarly distributed across the categories of severity. There was one incidence of systolic pressure ≥160 mmHg in each group, both occurring during SRC.
At baseline, 47% of all the patients had elevated serum cholesterol of ≥200 mg/dl (four in each group). During the course of the study, patients in the RAPA group had a mean increase in total cholesterol of 69.0 ± 68.8 mg/dl which was a 34% increase over baseline. The total cholesterol of most patients in the MTX group remained the same or decreased slightly during the course of the study. Only one patient in the MTX group had a total cholesterol greater ≥240 mg/dl during the study.
At baseline, 35% of all the patients had elevated serum triglyceride of ≥200 mg/dl (three in each group). All the patients in the RAPA group had hypertriglyceridemia at one point during the study, with an increase in serum triglyceride from baseline of <100 mg/dl in 4 patients and >2000 mg/dl in 2 patients. Several patients in the MTX group had moderately high triglyceride levels, but none was >490 mg/dl. Most of the increases from baseline were <40 mg/dl in the MTX group, in contrast to the much larger increases observed in the RAPA group.
Three patients in the RAPA group and one patient in the MTX group carried a diagnosis of dysplipidemia that was being treated with a statin or fenofibrate. During the study, two of these patients in the RAPA group required changes to their pre-existing therapy (their triglyceride levels were 150s mg/dl at baseline controlled with therapy, which increased to maximum of 3264 mg/dl in one patient and 238 mg/dl in the other patient while on RAPA). One patient in the RAPA group required statin initiation, but her triglyceride level increased up to 2205 mg/dl despite adding a fenofibrate to the statin. The patient subsequently withdrew from the study secondary to intractable hypertriglyceridemia. Since the total cholesterol and triglyceride levels in the MTX group remained relatively stable during the study, no one in this group was initiated on a statin or fenofibrate and no changes were made to prior statin therapy.
No significant hepatoxicity was seen in either treatment group in the present study. There was one mild case of elevated AST (<1.5 ULN) and elevated alkaline phosphatase (<1.5 ULN) in the MTX group, both of which resolved without any intervention or discontinuation of MTX. In the RAPA group, there was one case of elevated AST and ALT (both approximately 1.7 ULN) which occurred at about the time the patient was hospitalized for congestive heart failure with dilated cardiomyopathy, severe GERD, and elevated CPK at 1447 u/l.
Results of serial clinical monitoring over 48 weeks
Table 4 shows the data on the secondary outcomes for patients who completed the 48-week study. Comparisons were made between the two treatment groups on several parameters that measure disease activity in SSc. Similar to the baseline comparison, the scores for mRSS, physician global assessment, and HAQ-DI remained higher in the RAPA group at 48 weeks compared to the MTX group, but these differences were not statistically significant. MRSS skin scores decreased significantly within both groups at 48 weeks (mean change in mRSS ± SD in MTX group −6.8 ± 6.1, P < 0.045; in RAPA group −5.6 ± 3.9, P < 0.033), without significant differences in the changes between groups. In the RAPA group, there was a significant decrease in patient global score at 48 weeks (mean change ± SD: −11.5 ± 6.2, P < 0.035) and in FVC (percent predicted, -10.6 ± 6.6, P = 0.05). No other statistically significant changes in scores were found within or between the RAPA and MTX groups.
Patients meeting MCID
We determined which patients met the definition of MCIDs for mRSS and/or HAQ-DI at 48 weeks. Table 5 shows that a similar proportion of patients in both the MTX and RAPA groups achieved the MCID for mRSS (4 of 6 [67%] patients in MTX group; 3 of 5 [60%] patients in RAPA group) and for HAQ-DI (1 of 5 [20%] patients in MTX group; 2 of 5 [40%] patients in RAPA group; one patient in the MTX group who completed the study did not fill out the HAQ-DI at 48 weeks).
Discussion
The immunomodulary effects of RAPA on fibrogenesis and T-cells make RAPA a potential novel therapy for patients with SSc. RAPA is approved by the FDA in renal transplant patients for the prevention of organ rejection, but studies on its safety and use in rheumatologic diseases have been sparse (15, 16). This study was a phase I, single-blind, randomized parallel trial of RAPA versus MTX with the primary aim of assessing the safety profile of RAPA treatment in SSc patients and the secondary aim of examining the potential efficacy of RAPA as a therapy for SSc.
One of the most common toxicities associated with the administration of RAPA is hypertriglyceridemia (18). In the present study, seven of eight patients in the RAPA group experienced moderate to high levels of hypertriglyceridemia at one point during the study. Hypertriglyceridemia was treatable in most of these patients, with only one withdrawal due to uncontrolled hypertriglyceridemia. Hypertriglyceridemia in the MTX group was less severe and the increases from baseline levels were much smaller compared to those on treatment with RAPA. A sizable proportion of patients in both groups had elevated cholesterol and triglyceride levels at baseline as well as prior diagnosis of hypertension. It is possible that having an unfavorable cardiovascular risk profile at baseline may increase the risk for developing dyslipidemia (or worsening of pre-existing dyslipidemia) when treated with RAPA.
Bone marrow suppression is an adverse effect that can be seen with MTX and RAPA. There were two cases of mild thrombocytopenia in the RAPA group. One patient in the MTX group withdrew from the study after being admitted to the hospital with pancytopenia, which was felt to be due to MTX in setting of SRC. Clinically significant hepatoxicity was not observed in either group in this study.
MTX and RAPA rarely increase blood pressure. The incidence and severity of hypertension and renal insufficiency in this study were not higher in the RAPA group compared with the MTX group. Four patients in the RAPA group and six patients in the MTX group had diagnosis of hypertension prior to the study. Thus, the higher incidences of hypertension observed in both groups are not surprising. Two patients in this study had systolic hypertension ≥160 mmHg and were hospitalized for SRC. These same two patients accounted for all the cases of renal insufficiency. In summary, the occurrences of hypertension and renal insufficiency in this study were likely SSc-related instead of drug toxicities.
A similar proportion of patients in each group withdrew from the study. The majority of patients who withdrew had evidence of SSc-progression, with the exception of one case of pancytopenia secondary to MTX described above and one due to severe, intractable hypertriglyceridemia. The high withdrawal rate was due to several enrolled patients who became seriously ill after entry and a number of patients who experienced adverse events serious enough that they no longer wished to continue in the study. In the present study, 35% of the patients withdrew within the 48 weeks of the study; in comparison, 27% who withdrew in the first 12 months of the D-penicillamine trial in early diffuse SSc (24).
Both groups had similar baseline characteristics (Table 1). A significant within-group improvement in the mRSS was noted in both groups, while a significant within-group improvement in the physician global assessment and a significant decline in FVC were also noted in the RAPA group (Table 2). The decline in FVC was the result of a considerable fall of FVC in two of the RAPA patients that may be related to progressive restrictive lung. The number of patients meeting the definition of MCIDs for mRSS and/or HAQ-DI at 48 weeks was similar in both groups. The improvement in skin score with MTX therapy in this study is consistent with results in prior larger studies (5, 6). However, since a placebo arm was not included in this study, these changes in scores may have occurred naturally and not necessarily be due to the study drugs.
Our study has several limitations: small sample size, single-blinded design, lack of placebo group, and high withdrawal rate early in the study. Thus, statements about the frequency of adverse events and efficacy of either MTX or RAPA are limited based on this study. Although having a placebo group would have strengthened our study, MTX was a reasonable choice as a comparator for several reasons as described in our introduction. While the study was not double-blinded, the introduction of an MCO to manage the toxicity of the study drug allowed the evaluating clinician to be blind to patient's treatment allocation. We felt this was reasonable given that it was a phase I trial and the budget was limited.
In conclusion, studies to investigate new drugs are essential in debilitating conditions such as SSc where treatment options are very limited. An earlier trial showed that CsA had a positive effect on skin scores, but the use of CsA was limited by its side-effect of nephrotoxicity and hypertension (8). Although RAPA was not associated with an increased incidence of these side-effects, its most common toxicity was hypertriglyceridemia which was generally tolerable and treatable in most patients. Strategies to better control dyslipidemia secondary to RAPA may improve the apparent efficacy/toxicity ratio. Our study was not designed to address efficacy and cannot support the use of RAPA in current clinical management of SSc. However, our results from this phase I trial indicate that RAPA has a reasonable safety profile in a select group of scleroderma patients and may be worthy of further evaluation in a randomized, controlled trial with a larger number of participants to further examine its safety and efficacy in the treatment of SSc.
Supplementary Material
Online supplement
Although the dose of RAPA has been shown to have a linear relationship to blood levels in the transplant literature, it has not been well documented in the rheumatologic literature (SLE, or now SSc). During this study we drew trough RAPA serum levels 2 weeks following any change in the RAPA dosing as well as every 8 weeks during the study. The initial serum levels to a standard 6 mg daily dose ranged widely from 7 ng/dl to 28 ng/ml. We then titrated the RAPA dose up or down as dictated by the serum level. Generally one change in dose was all that was required to produce a therapeutic blood level of RAPA. Further changes were made based on therapeutic RAPA serum levels.
Figure 1. The trough serum level of RAPA (Baseline) are shown at 2 weeks after starting the initial dose of 6 mg/day. The trough serum levels of RAPA shown at 3 and 6 months after starting RAPA demonstrate that target levels of 5-15 ng/ml can be reached easily.
Acknowledgments
We acknowledge the contribution of Harold E. Paulus, MD, who served as the independent, blind reviewer who determined whether a Serious Adverse Event was related to study drugs or was part of the SSc disease progression. We also acknowledge Wyeth Pharmaceuticals for supplying Rapamycin for this study.
Supported by a grant from the Oxnard Foundation. Dr. Khanna was supported by a National Institutes of Health Award (NIAMS K23 AR053858-01A1) and the Scleroderma Foundation (New Investigator Award).
References
- 1.Charles C, Clements P, Furst DE. Systemic sclerosis: hypothesis-driven treatment strategies. Lancet. 2006;367(9523):1683–91. doi: 10.1016/S0140-6736(06)68737-0. [DOI] [PubMed] [Google Scholar]
- 2.Denton C. Therapeutic targets in systemic sclerosis. Arthritis Res Ther. 2007;9 2:S6. doi: 10.1186/ar2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowal-Bielecka O, Landewe R, Avouac J, et al. EULAR/EUSTAR recommendations for the treatment of systemic sclerosis (SSc) [abstract] Ann Rheum Dis. 2007;66 2:213. [Google Scholar]
- 4.Henness S, Wigley F. Current drug therapy for scleroderma and secondary Raynaud's phenomenon: evidence-based review. Curr Opin Rheumatol. 2007;19(6):611–618. doi: 10.1097/BOR.0b013e3282f13137. [DOI] [PubMed] [Google Scholar]
- 5.Van den Hoogen F, Boerbooms A, Swaak A, Rasker J, van Lier H, van de Putte L. Comparison of methotrexate with placebo in the treatment of systemic sclerosis: a 24 week randomized double-blind trial, followed by a 24 week observational trial. Br J Rheumatol. 1996;35(4):364–72. doi: 10.1093/rheumatology/35.4.364. [DOI] [PubMed] [Google Scholar]
- 6.Pope J, Bellamy N, Seibold J, Baron M, Ellman M, Carette S, et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheum. 2001;44(6):1351–8. doi: 10.1002/1529-0131(200106)44:6<1351::AID-ART227>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Krishna Sumanth M, Sharma V, Khaitan B, Kapoor A, Tejasvi T. Evaluation of oral methotrexate in the treatment of systemic sclerosis. Int J Dermatol. 2007;46(2):218–23. doi: 10.1111/j.1365-4632.2007.02887.x. [DOI] [PubMed] [Google Scholar]
- 8.Clements P, Lachenbruch P, Sterz M, Danovitch G, Hawkins R, Ippoliti A, et al. Cyclosporine in systemic sclerosis. Results of a forty-eight-week open safety study in ten patients. Arthritis Rheum. 1993;36(1):75–83. doi: 10.1002/art.1780360113. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration. Rapamune. 2003 April; URL: http://www.fda.gov/medwatch/SAFETY/2003/03Oct_PI/Rapamune_PI.pdf.
- 10.Young D, Nickerson-Nutter C. mTOR--beyond transplantation. Curr Opin Pharmacol. 2005;5(4):418–23. doi: 10.1016/j.coph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Kahan BD, Camardo JS. Rapamycin: clinical results and future opportunities. Transplantation. 2001;72(7):1181–93. doi: 10.1097/00007890-200110150-00001. [DOI] [PubMed] [Google Scholar]
- 12.Shegogue D, Trojanowska M. Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway. J Biol Chem. 2004;279(22):23166–75. doi: 10.1074/jbc.M401238200. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Wu J, Frizell E, Liu SL, Bashey R, Rubin R, et al. Rapamycin inhibits hepatic stellate cell proliferation in vitro and limits fibrogenesis in an in vivo model of liver fibrosis. Gastroenterology. 1999;117(5):1198–204. doi: 10.1016/s0016-5085(99)70406-3. [DOI] [PubMed] [Google Scholar]
- 14.Wyeth-Ayerst Research. Rapamune (sirolimus) maintenance regimen: summary for presentation to the subcommittee of the antiviral drugs advisory committee on immunosuppressive drugs. 2002 Jan; URL: http://www.fda.gov/ohrms/dockets/ac/02/briefing/3832b1_01_Wyreth-Ayerst.pdf.
- 15.Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54(9):2983–8. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadiminti U, Arbiser JL. Rapamycin (sirolimus) as a steroid-sparing agent in dermatomyositis. J Am Acad Dermatol. 2005;52(2 Suppl 1):17–9. doi: 10.1016/j.jaad.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 17.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 18.Danovitch GM. Handbook of kidney transplantation. 4th. Philadelphia: Lppincott Williams & Wilkins; 2005. [Google Scholar]
- 19.Woodworth T, Furst DE, Alten R, Bingham C, Yocum D, Sloan V, et al. Standardizing assessment and reporting of adverse effects in rheumatology clinical trials II: the Rheumatology Common Toxicity Criteria v.2.0. J Rheumatol. 2007;34(6):1401–14. [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 21.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. The FDA Safety Information and Adverse Event Reporting Program. 2004 Jan; URL: http://69.20.19.211/medwatch/report/DESK/advevnt.htm.
- 23.Khanna D, Furst DE, Hays RD, Park GS, Wong WK, Seibold JR, et al. Minimally important difference in diffuse systemic sclerosis: results from the D-penicillamine study. Ann Rheum Dis. 2006;65(10):1325–9. doi: 10.1136/ard.2005.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clements PJ, Furst DE, Wong WK, Mayes M, White B, Wigley F, et al. High-dose versus low-dose D-penicillamine in early diffuse systemic sclerosis: analysis of a two-year, double-blind, randomized, controlled clinical trial. Arthritis Rheum. 1999;42(6):1194–203. doi: 10.1002/1529-0131(199906)42:6<1194::AID-ANR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online supplement
Although the dose of RAPA has been shown to have a linear relationship to blood levels in the transplant literature, it has not been well documented in the rheumatologic literature (SLE, or now SSc). During this study we drew trough RAPA serum levels 2 weeks following any change in the RAPA dosing as well as every 8 weeks during the study. The initial serum levels to a standard 6 mg daily dose ranged widely from 7 ng/dl to 28 ng/ml. We then titrated the RAPA dose up or down as dictated by the serum level. Generally one change in dose was all that was required to produce a therapeutic blood level of RAPA. Further changes were made based on therapeutic RAPA serum levels.
Figure 1. The trough serum level of RAPA (Baseline) are shown at 2 weeks after starting the initial dose of 6 mg/day. The trough serum levels of RAPA shown at 3 and 6 months after starting RAPA demonstrate that target levels of 5-15 ng/ml can be reached easily.