Abstract
Background
We compare the genotype distribution for the serotonin transporter polymorphism (5-HTTLPR) in a sample of older Taiwanese adults with samples of various racial and ethnic groups collected in other studies. We also explore interactions among sex, stressors, and 5-HTTLPR genotype on depressive symptoms in our sample.
Methods
Using a nationally-representative sample of 984 Taiwanese aged 53 and older, we model depressive symptoms as a function of 5-HTTLPR genotype and two classes of stressors: lifetime trauma and recent major life events. We test two- and three-way interactions among stressors, 5 HTTLPR, and sex.
Results
This sample exhibits higher frequency of S/S and lower frequency of L/L genotype than Western samples, but the distribution is comparable to those in East Asian populations. Nearly 9% carry an allele (XL) that has rarely been reported in the literature. Although the gene-environment (GxE) interaction with recent major life events is not significant, our results suggest that trauma has a worse effect on depressive symptoms for those with S/S or S/L genotype than for those who do not carry the S allele (p<0.05). We find no evidence that this GxE interaction varies by sex.
Conclusions
Previous studies of this GxE interaction have been inconclusive, perhaps because interactions between genotype and stressful events are more prominent under extreme stressors. Our findings underscore the need to move beyond a bi-allelic parameterization of the 5-HTTLPR polymorphism and raise questions about why East Asian populations exhibit low rates of depression despite a high frequency of the S allele.
Keywords: SLC6A4 protein, depressive disorder, life change events, stressful events, Taiwan
INTRODUCTION
Serotonin (5-HT), a key central nervous system neurotransmitter, is involved in regulating a broad range of psychological traits, behaviors, and physical functions including mood, sleep, appetite, and sexual activity. The serotonin transporter protein (5-HTT), which terminates the action of serotonin by facilitating its reuptake from the synapse, appears to be part of the pathway leading to psychiatric disorders and has been a target of widely used pharmacological treatments. It is thus not surprising that the gene associated with serotonin transport (SLC6A4) has been the focus of extensive research. More than a decade ago, a polymorphism in the promoter region of the gene encoding 5-HTT, referred to as 5-HTTLPR, was identified by Heils et al. [1]: a 44bp deletion/insertion generated two alleles of 5-HTTLPR, with the 14-repeat short (S) variant having less transcriptional activity and lower serotonin uptake than the 16-repeat long (L) variant. Researchers speculated that the differential transcriptional activity caused by this polymorphism would influence complex traits and diseases, including affective disorders [1, 2].
Subsequent studies suggest a nuanced relationship between the 5-HTTLPR polymorphism and psychiatric disorders. First, although most studies have considered the 5-HTTLPR polymorphism to be functionally bi-allelic, researchers have identified additional alleles. Gelernter et al. [3] identified two uncommon alleles that are longer than the L variant: 1) an extra-long allele (XL) was found in both an African American and a Japanese sample; and 2) a single instance of an allele of length between the L and XL alleles (VL) was also observed in the Japanese sample. In the same year, Delbruck and colleagues [4] identified a “novel allelic variant” (XL) among persons of African origin but not among Caucasians or East Asians. One study in Japan reported two XL variants–with 18 and 20 repeats [5]–whereas another identified alleles with 19 and 20 repeats in a Japanese sample [6]. With more detailed genotyping of 5-HTTLPR among Japanese and Caucasian subjects, Nakamura et al. [7] identified four S alleles and six L alleles as well a few other variants (15-, 19-, 20- and 22-repeats) found only in the Japanese sample. Thus, a dichotomous classification of 5-HTTLPR is likely to obscure potential differences in allele effects.
A second more recent body of research has focused on whether the effects of 5-HTTLPR on psychiatric disorders are moderated by exposure to stressors. A widely-cited study found no direct association between 5-HTTLPR and depression, yet revealed that, among those exposed to major life stressors, the short allele was related to depressive symptoms in an additive fashion: levels of depressive symptoms were lowest among persons with L/L genotype, intermediate among S/L, and highest among those with S/S genotype [8]. They found a similar pattern among adults exposed to child maltreatment. Subsequent attempts to replicate these findings yielded mixed results. Two recent meta-analyses concluded that there is no evidence of such a gene-environment (GxE) interaction [9, 10], but most of the studies evaluated focused on major life stressors rather than more severe trauma.
Furthermore, among studies that examined the GxE interaction, the pattern of that interaction varied. Some studies using measures of major life events replicated the additive effect of the S allele [11–13], but others reported a recessive model (S/S vs. S/L or L/L) [14–18] and many found no GxE interaction [19–24]. Among studies investigating the GxE interaction with trauma, one study replicated the additive effect of the S allele among girls (but not boys) exposed to traumatic family conflicts [12], whereas another reported the reverse relationship (number of L alleles associated with higher risk) among those who experienced traumatic events [25]. Studies focusing on child abuse have reported a recessive effect [26–28], a dominant model [29], or no GxE interaction [28, 29].
A third source of complexity arises from studies hypothesizing that demographic characteristics (e.g., sex, race and ethnicity) are likely to moderate this GxE interaction [12, 30]. Under stressful circumstances, susceptibility to depression among individuals carrying the S allele was significant only for women in several studies [11, 12, 30]. Three other studies reported no sex difference in the GxE interaction [16, 18, 24]. Although the distribution of 5-HTTLPR genotype varies substantially across racial and ethnic groups [3, 4, 7, 16, 30–32], we know of only two studies that explicitly tested an interaction among race, stressors, and 5-HTTLPR in a model of depression. These studies found no difference in the GxE interaction between blacks and whites [30] or between Asians and non-Asians [16].
This study uses data from a large national sample in Taiwan to examine these issues. By comparing the 5-HTTLPR genotype distribution in our study with other samples from different racial and ethnic groups, we underscore the large variation across populations. Our sample displays a much higher frequency of XL alleles than most previous studies, challenging the contention that the XL allele is rare. We examine GxE interactions with two types of stressors—recent major life events and lifetime trauma—on depressive symptoms among older adults, a segment of the population that has rarely been the focus of such studies [17]. We also explore whether the GxE interaction varies by sex. The estimates provide insight into the phenotype associated with the XL allele.
MATERIALS AND METHODS
Data
The 2006 wave of the Social Environment and Biomarkers of Aging Study (SEBAS) comprised a nationally representative sample of Taiwanese aged 53 and older. Written informed consent was obtained for participation in both the in-home interview and hospital visit, which entailed a medical examination. The research was approved by human subjects committees in Taiwan and at Georgetown University and Princeton University. In-home interviews were completed with 1,284 respondents, including 757 participants aged 60 and over who participated in the medical examination in the 2000 SEBAS (89.5% response rate) as well as 527 respondents from a younger cohort aged 53 to 60 (80.2% response rate).
Among those interviewed, 80.7% (n=1,036) also participated in the medical exam in the 2006 study; 0.2% (n=3) died before the exam, 2.5% (n=32) were not eligible because of a health condition, and 16.6% (n=213) declined. Both the youngest (aged 53–59) and the oldest (80+) respondents were less likely to participate in the medical exam than those aged 60–79. Participation was also lower among less educated respondents and those with ADL limitations compared with their respective counterparts. Participants did not differ significantly from non-participants in terms of self-reported health status.
As part of the medical exam, hospital personnel drew venous blood, which was subsequently analyzed at Union Clinical Laboratories (UCL) in Taipei. Additional details about the SEBAS study are provided elsewhere [33, 34].
Measures
Depressive symptoms are measured by a 10-item subset of the 20-item Center for Epidemiologic Studies Depression scale (CES-D). The CES-D, which focuses on depressive symptoms experienced in the most recent week, was developed for use in epidemiological studies of general populations rather than for clinical diagnosis of depression [35]. The CES-D score in this analysis uses standard coding based on the number and severity of symptoms (range 0 to 30; high = more symptoms; Cronbach’s alpha = 0.83). The CES-D has demonstrated reliability in older populations [36]. Using clinical diagnosis of depression as the criterion, a 10-item form of the instrument showed good predictive ability among elderly Chinese [37].
We include two measures of stressors—lifetime exposure to trauma and recent major life events–based on two batteries of questions in the home interview, one pertaining to traumatic events that respondents may have experienced at any time in the past and a second pertaining to major life events during the most recent 12 months. The specific stressors are described in Table 2. Because preliminary analyses indicated a threshold effect on depressive symptoms at a level of two types of trauma (with insufficient sample size to determine the effects of three or more traumas), we coded trauma as a dichotomous variable (0–1 versus 2+). We coded major life events in a similar way: 0–1 versus 2+; fewer than 2% of respondents reported more than two such events.
Table 2.
Mean (SD) or Percent Distribution for Variables Included in the Model by 5-HTTLPR Genotype
| 5-HTTLPR genotype |
||||||
|---|---|---|---|---|---|---|
| Variable | Total | S/S | S/L | S/XL | L/L | L/XL |
| Age (53–97)1 | 65.9 (9.9) | 65.7 (10.0) | 66.7 (10.0) | 65.6 (8.4) | 63.9 (9.2) | 65.8 (10.2) |
| Female (%) | 46.2% | 46.1% | 45.5% | 60.7% | 34.9% | 62.5% |
| Respondent’s years of schooling (0–17)1 | 7.0 (4.8) | 6.9 (4.7) | 7.1 (4.9) | 6.1 (5.4) | 7.7 (4.9) | 7.1 (4.4) |
| Types of trauma (out of 7) experienced during lifetime2 | ||||||
| Zero | 69.8% | 68.6% | 69.2% | 65.6% | 79.5% | 79.2% |
| One | 20.7% | 20.9% | 20.4% | 29.5% | 16.9% | 12.5% |
| Two or more | 9.4% | 10.5% | 10.4% | 4.9% | 3.6% | 8.3% |
| Number of major life events (out of 5) in past 12 months3 | ||||||
| Zero | 59.0% | 59.0% | 57.8% | 55.7% | 62.6% | 75.0% |
| One | 29.6% | 29.6% | 30.0% | 34.4% | 25.3% | 25.0% |
| Two or more | 11.4% | 11.4% | 12.3% | 9.8% | 12.1% | 0.0% |
| Center for Epidemiologic Studies Depression scale (CES-D) (0–27)1 | 4.7 (5.5) | 4.7 (5.3) | 4.9 (5.7) | 5.6 (6.0) | 4.1 (5.7) | 2.9 (3.5)4 |
| Number of respondents | 984 | 449 | 367 | 61 | 83 | 24 |
| Percent of respondents | 100.0% | 45.6% | 37.3% | 6.2% | 8.4% | 2.4% |
For continuous variables, the observed range is given in parentheses.
Respondents were asked whether they had ever experienced seven types of trauma that involved threat of serious injury or death: natural disaster; human-made disaster; serious accident; residence in mainland China during the Japanese invasion; physical abuse; family member, romantic partner or very close friend died because of an accident, homicide, or suicide; and any other situation in which they or someone else was in danger of death or serious physical injury. Exposure to trauma does not vary significantly by 5-HTTLPR genotype (Χ2=10.5, df=2, two-tailed p~ 0.23).
Respondents were asked whether they experienced five major life events: death of spouse; death of another close family member; death of a close friend; major deterioration in the health of a family member; and loss or damage to personal property. Exposure to major life events does not vary significantly by 5-HTTLPR genotype (Χ2=5.76, df=8, two-tailed p~ 0.67).
Mean CES-D score differs significantly (p<0.05) from those of S/S (t=2.4, Satterthwaite’s df=28.9, p~0.03), S/L (t=2.6, df=31.5, p~0.02), and S/XL (t=2.6, df=69.7, p~0.01) genotypes (based on two-tailed t tests).
To determine 5-HTTLPR genotype, DNA was extracted from venous blood using the technique described in Gustincich et al. [38] and then amplified with polymerase chain reaction (PCR). The forward primer used was 5′-GGC GTT GCC GCT CTG AAT GCC A-3′, while the reverse primer was 5′-GAG GGA CTG AGC TGG ACA ACC AC-3′. PCR was performed in a total volume of 20.4μL containing 2.0 μL dNTP mix(2.5mM), 2.0 μL Taq buffer, 0.4 μL of the Taq polymerase Enzyme(5 Units), 12.0 μL DEPC H2O, 2.0 μL of the primer mix and 2.0 μL of the genomic DNA (approximately 150 ng). Cycling conditions consisted of 1) a 10 min denaturation at 95°C, 2) 45 cycles of 30 sec denaturation at 95°C, 3) 30 sec annealing at 65°C, 4) 60 sec extension at 75°C, and 5) a final cycle of 75°C for 5 min. The PCR products were separated by electrophoresis in a 2% agarose gel prepared with ethidium bromide. Three allele variants of the gene polymorphism were identified based on the PCR fragment sizes: short (S; 486bp, 14 repeats), long (L; 529bp, 16 repeats), or extra-long (XL; 612 or 654bp, 20 or 22 repeats). Subjects were classified into five genotypes: S/S, S/L, L/L, S/XL, and L/XL (no respondents were homozygous for the XL allele). The genotype frequencies are in Hardy-Weinberg equilibrium (χ2=2.59, df=3, p~0.46).
Analytic Strategy
Genotype information for 5-HTTLPR is available for 1,019 of the 1,036 medical exam participants. The sample used for model estimation (n=984) excludes another 35 participants who are missing the CES-D or traumatic events.
Using linear regression, we model depressive symptoms as a function of 5-HTTLPR and stressors controlling for age, sex and respondent’s education. We include a random effect for township to adjust for the clustered sample design. The few persons with L/XL genotype (n=24) are combined with L/L genotype following previous practice [26, 27]. Models 1 and 2 test the GxE interaction for trauma and major life events, respectively. Model 3 includes both GxE interactions. Subsequently, we re-estimate these models including two- and three-way interactions among stressful exposure, 5-HTTLPR, and sex. We calculate predicted CES-D scores by genotype and stressful experience using the coefficients from Model 3 and mean values for age, percent female and education. All analyses were performed using Stata, Version 10.1 [39].
RESULTS
Distributions of 5-HTTLPR genotype across populations
Table 1 compares the 5-HTTLPR distributions for our Taiwan sample with other samples from diverse populations. These studies emerged from a PubMed search for distributions of the 5-HTTLPR polymorphism for specified racial or ethnic groups. For studies that reported distributions for control and patient samples, we present results for the controls. In contrast to the Taiwan study, which is based on a national sample, the other studies comprise various sampling strategies (e.g., convenience, clinical, and community samples). Sample sizes range from fewer than 100 to more than 1000 participants in the Taiwan sample and two Caucasian samples.
Table 1.
Distribution by 5-HTTLPR Genotype in Selected Studies1
| Genotypes (%) |
Alleles (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Sample | Ages | S/S | S/L | L/L | S/XL | L/XL | S | L | VL & XL2 |
| Current | Nationally-representative (n=1019), Taiwan | 53+ | 45 | 37 | 8 | 6 | 2 | 67 | 28 | 4 |
| Caucasian | ||||||||||
| Bellivier et al. [53] | Blood donors at the Pitié-Salpétrière Hospital3 (n=99), France | 35+ | 12 | 59 | 29 | NR | NR | 41 | 59 | NR |
| Brummett et al. [30] | Convenience sample including caregivers and controls (n=203), North Carolina, U.S. | mean=58.3, SD=14.7 | 24 | 44 | 32 | NR | NR | 46 | 54 | NR |
| Caspi et al. [8] | Representative birth cohort (n=1037), New Zealand | From birth | 17 | 51 | 31 | NR | NR | 43 | 57 | NR |
| Chipman et al. [19] | Community-based (n=2095), Canberra and Queanbeyan, Australia | 20–24 | 21 | 46 | 33 | NR | NR | 44 | 56 | NR |
| Chorbov et al. [25] | European-American twins from Missouri birth records (n=238) some of which had been diagnosed with major depressive disorder and others not | 18–23 | 14 | 51 | 35 | NR | NR | 40 | 60 | NR |
| Delbruck et al. [4] | North American Caucasians of Western European descent (n=62) from Philadelphia | NR | NR | NR | NR | NR | NR | 44 | 56 | NR |
| Delbruck et al. [4] | Western European Caucasians from Berlin, Germany (n=216) | NR | NR | NR | NR | NR | NR | 39 | 61 | NR |
| Delbruck et al. [4] | Western European Caucasians from Alsace, France (n=73) | NR | NR | NR | NR | NR | NR | 46 | 54 | NR |
| Gelernter et al. [3] | Unrelated adults living in Connecticut (n=104) | NR | NR | NR | NR | NR | NR | 40 | 60 | NR |
| Hu et al. [32] | Persons with (n=177) and without (n=120) psychiatric disorders, group 1, U.S. | NR | 16 | 41 | 43 | NR | NR | 37 | 63 | NR |
| Hu et al. [32] | Persons with (n=154) and without (n=132) psychiatric disorders, group 2, U.S. | NR | 12 | 45 | 42 | NR | NR | 35 | 65 | NR |
| Hu et al. [32] | Persons with (n=480) and without (n=291) psychiatric disorders, Finland | NR | 15 | 52 | 34 | NR | NR | 40 | 60 | NR |
| Jacobs et al. [14] | Female twins (n=356 pairs), Belgium | 18–46 | 22 | 47 | 31 | NR | NR | 46 | 55 | NR |
| Nakamura et al. [7] | Unrelated Caucasians from genomic DNAs of CEPH families (n=76) | NR | 20 | 49 | 31 | 0 | 0 | 45 | 55 | 0 |
| Otte et al. [54] | Patients with coronary disease (n=557), San Francisco and Palo Alto, U.S. | mean=68, SD=11 | 17 | 52 | 31 | NR | NR | 43 | 57 | NR |
| Rees et al. [55] | Unrelated blood donors3 (n=118), South Wales, U.K. | mean=44, SD=11 | 20 | 50 | 30 | NR | NR | 45 | 55 | NR |
| Scheid et al. [22] | Pregnant women (n=568) recruited from prenatal clinics in five Michigan communities | 81% aged 20–34 | 17 | 52 | 31 | NR | NR | 43 | 57 | NR |
| Williams et al. [56] | Volunteers (n=70) in good physical and medical health | NR | 20 | 40 | 40 | NR | NR | 40 | 60 | NR |
| All Caucasian samples (weighted mean) | 19 | 48 | 33 | 0 | 0 | 43 | 57 | 0 | ||
| African | ||||||||||
| Delbruck et al. [4] | Samples from Congo (n=32) | NR | NR | NR | NR | NR | NR | 9 | 84 | 6 |
| Delbruck et al. [4] | Samples from Gabon (n=12) | NR | NR | NR | NR | NR | NR | 21 | 79 | 0 |
| African-American | ||||||||||
| Brummett et al. [30] | Convenience sample including caregivers and controls (n=203), North Carolina, U.S. | mean=58.3, SD=14.7 | 17 | 39 | 45 | NR | NR | 36 | 64 | NR |
| Delbruck et al. [4] | African Americans (n=186) from Philadelphia | NR | NR | NR | NR | NR | 29 | 69 | 2 | |
| Gelernter et al. [3] | Unrelated adults living in Connecticut (n=51) | NR | NR | NR | NR | NR | NR | 25 | 70 | 6 |
| Hu et al. [32] | Persons with drug addictions (n=414) and with no psychiatric diagnosis (n=210), U.S. | NR | 7 | 37 | 56 | NR | NR | 26 | 75 | NR |
| Williams et al. [56] | Volunteers (n=85) in good physical and medical health | NR | 11 | 35 | 54 | NR | NR | 28 | 72 | NR |
| All African/African-American samples (weighted mean) | 9 | 37 | 53 | 0 | 0 | 27 | 72 | 1 | ||
| Chinese | ||||||||||
| Li et al. [57] | Healthy volunteers who had a normal check-up3 (n=96), Guangzhou, China | 18+ | 57 | 35 | 7 | NR | NR | 75 | 25 | NR |
| Shen et al. [58] | Healthy blood donors3 (n=628), Shanghai, China | mean=33.3, SD=10.1 | 49 | 39 | 6 | 4 | 2 | 71 | 26 | 3 |
| You et al. [59] | Healthy blood donors and hospital staff3 (n=90), Hunan Province, China | mean=30.2, SD=11.1 | 49 | 44 | 7 | NR | NR | 71 | 29 | NR |
| All Chinese samples (weighted mean) | 50 | 39 | 6 | 3 | 2 | 72 | 26 | 2 | ||
| Japanese | ||||||||||
| Delbruck et al. [4] | (n=14) | NR | NR | NR | NR | NR | NR | 71 | 29 | 0 |
| Gelernter et al. [3] | Samples (n=48) collected in Japan | NR | NR | NR | NR | NR | NR | 80 | 17 | 3 |
| Kunugi et al. [6] | Healthy volunteers from students and hospital staff3 (n=212), Japan | mean=32, SD=13 | 62 | 30 | 5 | 2 | 0 | 79 | 20 | 1 |
| Nakamura et al. [7] | Selected from population of unrelated Japanese in Ehima (n=131), Japan | NR | 634 | 31 | 1 | 3 | 2 | 81c | 17 | 2 |
| Narita et al. [5] | Healthy infants3 (n=115), Dokkyo University Koshigaya Hospital, Japan | < 6 months | 74 | 23 | 2 | 1 | 0 | 86 | 13 | 0.4 |
| Ohara et al. [60] | Healthy volunteers from medical staff and students3 (n=92), Hamamatsu, Japan | mean=35.2, SD=14.5 | 59 | 35 | 7 | NR | NR | 76 | 24 | NR |
| All Japanese samples (weighted mean) | 64 | 30 | 4 | 2 | 0.4 | 80 | 19 | 1 | ||
| Korean | ||||||||||
| Han et al. [61] | Unrelated males (n=158) with no history of psychiatric disorders from psychiatric department staff at two hospitals and medical students, South Korea | NR | 61 | 34 | 5 | NR | NR | 78 | 22 | NR |
| Kim et al. [17] | Community-based (n=732), Kwangju, South Korea | 65+ | 53 | 34 | 13 | NR | NR | 70 | 30 | NR |
| All Korean samples (weighted mean) | 54 | 34 | 12 | 0 | 0 | 71 | 29 | 0 | ||
| Native American | ||||||||||
| Hu et al. [32] | Community-based sample of Plains Native Americans with (n=335) and without (n=121) psychiatric diagnoses | NR | 42 | 48 | 10 | NR | NR | 66 | 34 | NR |
| Hu et al. [32] | Community-based sample of Southwest Native Americans with (n=359) and without (n=205) psychiatric diagnoses | NR | 42 | 44 | 14 | NR | NR | 64 | 36 | NR |
| All Native American samples (weighted mean) | 42 | 46 | 12 | 0 | 0 | 65 | 35 | 0 | ||
These studies emerged from a PubMed search for distributions of the 5-HTTLPR polymorphism; the search was restricted to studies that specified racial or ethnic groups of participants (and provided separate estimates if participants represented more than one group).
We group VL and XL alleles because some researchers do not distinguish among alleles longer than the L allele.
The study reported the distributions for both patient and control samples; we present results only for the latter.
Includes two people (1.5% of the sample) with a 15-repeat allele.
NR = Not reported; CEPH = Center d’Etude du Polymorphisme Humain.
The comparisons reveal striking differences in the distributions of 5-HTTLPR by race and ethnic group. Almost half of the SEBAS respondents (45%) are classified as homozygous for the S allele, a lower prevalence than those observed in the other East Asian studies (49–74%) and similar to Native American samples (42%), but much higher than in Caucasian samples from various countries (12–24%) and African Americans (7–17%). Conversely, the proportion with the L/L genotype in the SEBAS sample (8%) is substantially lower than in Caucasians (29–43%) and African Americans (45–56%), but is generally comparable to Native American (10–14%) and other East Asian samples (1–13%).
Notably, the majority of studies in Table 1– including all studies based on Caucasian samples – found only the S and L alleles. The few studies that found alleles longer than L are from East Asian (Chinese or Japanese), African or African-American populations. Four of the six Japanese samples found individuals carrying an XL allele. In the SEBAS sample, almost 9% (95% C.I. 7.1–10.6%) carries an XL allele, a prevalence greater than those in other East Asian samples; only two studies report a higher frequency, and these are based on very small samples of Africans [4] and African-Americans [3].
Effects of 5-HTTLPR and Stressors on the CES-D Score
Table 2 provides a description of the sample used in the statistical models. Nine percent experienced two or more traumatic events in their lifetime, and 11% experienced at least two major life events during the past year. Variations in the distribution of stressors by genotype are not statistically significant. The average CES-D score by genotype is generally consistent with the presumed differences in transcriptional efficiency of the alleles. The mean for L/XL (2.9) is significantly lower (p<0.05) than those for S/S (4.7), S/L (4.9) and S/XL (5.6), but does not differ significantly from that of L/L (4.1).
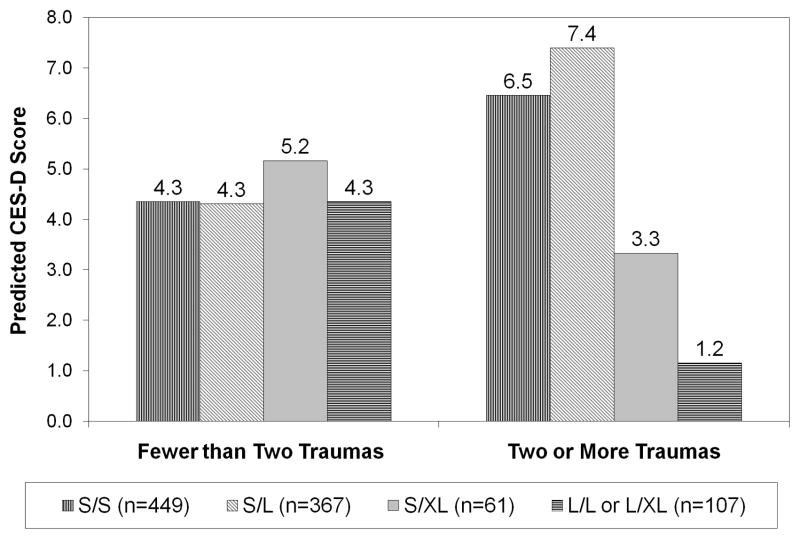
As expected, the estimated coefficients from models of the CES-D score indicate sizeable main effects for trauma and major life events, but the main effects for 5-HTTLPR are not significant (Table 3). The GxE interactions are significant for trauma (p<0.05 based on a Wald joint test) but not major life events. As depicted in Figure 1, there is little genotypic variation in predicted depressive symptom scores among persons who experienced one or no traumatic event. However, among those exposed to at least two traumas, predicted CES-D scores are higher for individuals with the S/S and S/L genotypes than other genotypes. The differences between persons with S/S or S/L genotype and those with L/L or L/XL genotype are significant (p< 0.01) and large—about one standard deviation in the score (5.5 points). The significance of the GxE interaction with trauma but not with major life events suggests that the interaction may be more prominent with exposure to extreme stressors.
Table 3.
Estimated Coefficients from Linear Regression Models1 Predicting CES-D in 2006 (n=984)
| Variable | (1) | (2) | (3) |
|---|---|---|---|
| Age | 0.081** | 0.081*** | 0.081*** |
| Female | 1.199** | 1.150** | 1.228*** |
| Respondent’s years of schooling | −0.153** | −0.170*** | −0.158*** |
| 5-HTTLPR genotype | |||
| S/S (ref group) | |||
| S/L | −0.012 | −0.014 | −0.041 |
| S/XL | 0.656 | 0.513 | 0.818 |
| L/L or L/XL | −0.235 | −0.335 | −0.000 |
| 2+ Traumas (lifetime) | 2.165** | -- | 2.108** |
| 2+ Traumas x S/L | 1.383 | -- | 0.983 |
| 2+ Traumas x S/XL | −4.0222 | -- | −3.945 |
| 2+ Traumas x (L/L or L/XL) | −5.385*3 | -- | −5.301*4 |
| Joint test for interaction terms (df=3) | Χ2=9.3, p~0.025 | -- | Χ2=8.0, p~0.047 |
| 2+ Life Events (past yr) | -- | 1.897* | 1.881* |
| 2+ Life Events x S/L | -- | 1.076 | 0.539 |
| 2+ Life Events x S/XL | -- | −1.423 | −1.508 |
| 2+ Life Events x (L/L or L/XL) | -- | −2.240 | −2.022 |
| Joint test for interaction terms (df=3) | -- | Χ2=3.7, p~0.294 | Χ2=2.3, p~0.521 |
| Constant | −0.235 | −0.113 | −0.454 |
p<0.05,
p<0.01, two-tailed tests.
Models include a random effect for township of residence to adjust for the clustered sampling design.
The difference between this coefficient and the coefficient for 2+ Traumas x S/L is marginally significant (Wald test: Χ2=2.9, df=1, p~0.09).
This coefficient differs significantly from the coefficient for 2+ Traumas x S/L (Wald test: Χ2=7.2, df=1, p~0.01).
This coefficient differs significantly from the coefficient for 2+ Traumas x S/L (Wald test: Χ2=6.2, df=1, p~0.01).
Figure 1. Predicted CES-D Score by Trauma Exposure and 5-HTTLPR Genotype.
Note: Predicted scores are calculated using the coefficients from the Model 3 (Table 3); assigning values for age, percent female, and education equal to the sample mean; and setting major life events to zero.
The effect of trauma on the CES-D score is greater for the S/L than the S/XL genotype; the difference between these coefficients is marginally significant in Model 1 (p~0.09) but not Model 3. With only 24 respondents with L/XL genotype, we do not have sufficient statistical power to distinguish between L/L and L/XL. However, we estimated additional models (not shown) similar to those in Table 3, but excluding persons with L/XL genotype. The magnitude of the coefficient for the interaction between trauma and L/L remains similar but owing to the loss of statistical power is reduced to marginal significance (p~0.09).
In the models including interaction terms with sex (not shown), the set of two- or three-way interactions with sex are not jointly significant. These findings may be driven by limited statistical power: in the three-way tabulations, several cells contain fewer than five respondents.
DISCUSSION
This study provides the most comprehensive review to date of ethnic variation in the 5-HTTLPR polymorphism. Although variation in the frequencies of the S and L alleles were identified in earlier work, some studies have erroneously assumed that the 5-HTTLPR polymorphism is bi-allelic. Our review demonstrates that extra-long alleles occur in several samples of persons of African origin and East Asians, but they have not been reported in any Caucasian group.
Still, we know relatively little about the XL allele. In light of the non-randomness and geographic restriction of most of the samples in Table 1, it is impossible to ascertain the prevalence of XL alleles in any large-scale population but Taiwan. Our data, derived from a nationally representative sample, show that the XL allele is not rare in Taiwan.
Recent studies demonstrate that 5-HTTLPR is more highly polymorphic than previously believed and that a reassessment of 5-HTTLPR as functionally tri-allelic is premature for at least two reasons. First, despite our reference to an XL allele, several studies, including our own, find that XL comprises variants with different numbers of repeats, at minimum 18, 19, 20 and 22 repeats. Second, SNP and other variations have been identified within the S and L alleles. Nakamura et al. [7] found several novel variants consisting of different combinations of the 14- and 16-repeats. Hu et al. [32] failed to detect most of Nakamura’s novel variants in an ethnically diverse sample but demonstrated a large difference in transcriptional efficiency resulting from a single base substitution in an L allele, with one of the L variants having a similar degree of 5-HTT expression as the S allele. Wendland et al. [40] demonstrated that this SNP (rs25531) occurs in the S allele—albeit very rarely—as well as the L allele. Such findings raise the question of whether these variants are at least as important functionally as differences in the tandem repeat structure.
The absence of a significant GxE interaction with recent major life events in our analysis is consistent with recent meta-analyses [9, 10], which are based primarily on samples of Caucasians. Nonetheless, we do find evidence of a GxE interaction with exposure to trauma, which has been less frequently studied than stressful life events. Consistent with Aguilera et al. [29], our results suggest that trauma has a greater effect on depressive symptoms for those with S/S or S/L genotypes than for those with L/L genotype, suggesting dominance of the S allele. However, other studies of trauma have found different patterns including additive and recessive effects of the S allele as well as a reversed relationship (L allele associated with greater risk); a few found no GxE interaction. Unlike some previous studies [11, 12, 30], we found no evidence that the GxE interaction varies by sex. Still, we are cautious about drawing firm conclusions in light of Munafò and colleagues’ contention [10] that most published studies are underpowered to examine these interactions1 and that variation in the pattern of the interaction across studies weakens researchers’ claims to have replicated Caspi’s original study.
Our study raises an important question that has received little attention in the literature: Is the high prevalence of the S allele in East Asian populations accompanied by high rates of depression or depressive symptoms? Because of enormous variation in how depression is measured across studies, and the selection biases inherent in relying on clinical data, this question is difficult to address. Nevertheless, two reviews based on a large number of comparable community-based studies suggest generally lower rates of depression in East Asian populations [41, 42], despite their presumed unfavorable allele distribution for 5-HTTLPR.
These paradoxical findings are consistent with several hypotheses. One is that East Asians fail to acknowledge symptoms of mental illness because of social stigma and potential “loss of face” [42–47] or because the stoicism associated with Chinese culture encourages individuals to tolerate emotional difficulties rather than seek help [48, 49]. A second related hypothesis is that East Asians have a greater tendency toward somatization of psychiatric symptoms, in part because they may not view psychiatric symptoms as signs of illness [48–50]. A third contention is that the strong family and social networks in East Asian societies buffer individuals from the potentially negative impacts of stressful events [16, 48, 49, 51]. An extension of this argument posits a coevolution of 5-HTTLPR and collectivist cultures–East Asia, for example–in which values of social harmony and support act as buffers to reduce stress and resultant affective disorders among genetically susceptible populations [52]. A final hypothesis is that these differences arise from ethnic variation in the phenotypes associated with the 5-HTTLPR polymorphism.
One objective of our study was to assess phenotypic variation associated with the XL allele. Given that the L allele is believed to result in more efficient serotonin function than the S allele, are individuals who possess the XL allele especially resilient to depression? We are not aware of any other study that has addressed this question. Our analysis suggests that the XL allele confers similar or perhaps even greater resilience than the L allele. However, the sampling variability of these estimates coupled with our inability to distinguish the effects of L/XL from L/L genotype, or to analyze variants of the S, L and XL alleles, prevents us from reaching a firmer conclusion.
This study has several advantages over related analyses, most notably the use of a national sample and identification of a relatively large number of individuals carrying the XL allele (N=89). Nevertheless, we have limited statistical power to examine some of the interactions of interest or to consider each of the observed genotypes. Although we found no evidence of gene-environment correlation (rGE) for this specific genotype-exposure combination, it remains possible that exposure to trauma is influenced by unmeasured genetic differences and that part of our estimated GxE effect could be the result of GxG interactions.
Our analysis raises several important issues that require further research. One question, which we are beginning to explore, is how the transcriptional efficiency of the XL allele compares with that of the S and L alleles. Additional laboratory research is needed to identify the functional importance of other alleles of the 5-HTTLPR polymorphism. In addition, our findings suggest that Risch and colleagues’ [9] failure to find evidence of an interaction between 5-HTTLPR and stressful life events on depression may be premature. Community-based studies based on large and diverse samples, with detailed genotyping of 5-HTTLPR variants, are necessary to identify more precisely the nature of the association between the serotonin transporter genotype and depression, as well as potential variability by the severity of stressful experience and ethnicity. It seems virtually certain that the links between 5-HTTLPR and depression are more complex than previously thought.
Acknowledgments
This work was supported by the Demography and Epidemiology Unit of the Behavioral and Social Research Program of the National Institute of Aging [grant numbers R01AG16790, R01AG16661] and the Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant number R24HD047879].
We are grateful to Dr. Min-Long Lai and Ms. Susana Ong at Union Clinical Laboratory in Taipei for their assistance with the laboratory assays. We also thank Dr. Daniel Notterman at Princeton University and Dr. Avshalom Caspi at Duke University for their helpful comments and advice.
Footnotes
Contributor Information
Noreen Goldman, Office of Population Research, Princeton University
Dana A. Glei, Center for Population and Health, Georgetown University.
Yu-Hsuan Lin, Population and Health Research Center, Bureau of Health Promotion, Department of Health, Taiwan
Maxine Weinstein, Center for Population and Health, Georgetown University
References
- 1.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 2.Collier DA, Stöber G, Li T, et al. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- 3.Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- 4.Delbruck SJ, Wendel B, Grunewald I, et al. A novel allelic variant of the human serotonin transporter gene regulatory polymorphism. Cytogenet Cell Genet. 1997;79:214–220. doi: 10.1159/000134726. [DOI] [PubMed] [Google Scholar]
- 5.Narita N, Narita M, Takashima S, et al. Serotonin transporter gene variation is a risk factor for sudden infant death syndrome in the Japanese population. Pediatrics. 2001;107:690–692. doi: 10.1542/peds.107.4.690. [DOI] [PubMed] [Google Scholar]
- 6.Kunugi H, Hattori M, Kato T, et al. Serotonin transporter gene polymorphisms: ethnic difference and possible association with bipolar affective disorder. Mol Psychiatry. 1997;2:457–462. doi: 10.1038/sj.mp.4000334. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura M, Ueno S, Sano A, et al. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5:32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- 8.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 9.Risch N, Herrell R, Lehner T, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munafò MR, Durrant C, Lewis G, et al. Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Eley TC, Sugden K, Corsico A, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- 12.Sjöberg RL, Nilsson KW, Nordquist N, et al. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. Int J Neuropsychopharmacol. 2006;9:443–449. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm K, Mitchell PB, Niven H, et al. Life events, first depression onset and the serotonin transporter gene. Br J Psychiatry. 2006;188:210–215. doi: 10.1192/bjp.bp.105.009522. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs N, Kenis G, Peeters F, et al. Stress-related negative affectivity and genetically altered serotonin transporter function: evidence of synergism in shaping risk of depression. Arch Gen Psychiatry. 2006;63:989–996. doi: 10.1001/archpsyc.63.9.989. [DOI] [PubMed] [Google Scholar]
- 15.Kendler KS, Kuhn JW, Vittum J, et al. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- 16.Taylor SE, Way BM, Welch WT, et al. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Kim JM, Stewart R, Kim SW, et al. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biol Psychiatry. 2007;62:423–428. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Cervilla JA, Rivera M, Molina E, et al. The 5-HTTLPR s/s genotype at the serotonin transporter gene (SLC6A4) increases the risk for depression in a large cohort of primary care attendees: the PREDICT-gene study. Am J Med Genet B Neuropsychiatr Genet. 2006;141:912–917. doi: 10.1002/ajmg.b.30455. [DOI] [PubMed] [Google Scholar]
- 19.Chipman P, Jorm AF, Prior M, et al. No interaction between the serotonin transporter polymorphism (5-HTTLPR) and childhood adversity or recent stressful life events on symptoms of depression: results from two community surveys. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:561–565. doi: 10.1002/ajmg.b.30480. [DOI] [PubMed] [Google Scholar]
- 20.Surtees PG, Wainwright NW, Willis-Owen SA, et al. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry. 2006;59:224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Gillespie NA, Whitfield JB, Williams B, et al. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35:101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- 22.Scheid JM, Holzman CB, Jones N, et al. Depressive symptoms in mid-pregnancy, lifetime stressors and the 5-HTTLPR genotype. Genes Brain Behav. 2007;6:453–464. doi: 10.1111/j.1601-183X.2006.00272.x. [DOI] [PubMed] [Google Scholar]
- 23.Power T, Stewart R, Ancelin ML, et al. 5-HTTLPR genotype, stressful life events and late-life depression: No evidence of interaction in a French population. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Laucht M, Treutlein J, Blomeyer D, et al. Interaction between the 5-HTTLPR serotonin transporter polymorphism and environmental adversity for mood and anxiety psychopathology: evidence from a high-risk community sample of young adults. Int J Neuropsychopharmacol. 2009;12:737–747. doi: 10.1017/S1461145708009875. [DOI] [PubMed] [Google Scholar]
- 25.Chorbov VM, Lobos EA, Todorov AA, et al. Relationship of 5-HTTLPR genotypes and depression risk in the presence of trauma in a female twin sample. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:830–833. doi: 10.1002/ajmg.b.30534. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Zalsman G, Huang YY, Oquendo MA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry. 2006;163:1588–1593. doi: 10.1176/ajp.2006.163.9.1588. [DOI] [PubMed] [Google Scholar]
- 29.Aguilera M, Arias B, Wichers M, et al. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychol Med. 2009:1–8. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- 30.Brummett BH, Boyle SH, Siegler IC, et al. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR) Behav Genet. 2008;38:34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelernter J, Cubells JF, Kidd JR, et al. Population studies of polymorphisms of the serotonin transporter protein gene. Am J Med Genet. 1999;88:61–66. [PubMed] [Google Scholar]
- 32.Hu XZ, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glei DA, Goldman N. Dehydroepiandrosterone sulfate (DHEAS) and risk for mortality among older Taiwanese. Ann Epidemiol. 2006;16:510–515. doi: 10.1016/j.annepidem.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Chang M, Glei D, Goldman N, Weinstein M. The Taiwan biomarker project. In: Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial surveys. Washington, D.C.: The National Academies Press; 2007. pp. 3-1-3–16. Committee on advances in collecting and utilizing biological indicators and genetic information in social science surveys, Committee on Population, Division of Behavioral and Social Sciences and Education. [Google Scholar]
- 35.Radloff LS. The CES-D Scale: A Self-report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:149–166. [Google Scholar]
- 36.Hertzog C, Van Alstine J, Usala PD, et al. Measurement properties of the Center for Epidemiological Studies Depression Scale (CES-D) in older populations. Psychol Assess. 1990;2:64–72. [Google Scholar]
- 37.Cheng ST, Chan AC. The Center for Epidemiologic Studies Depression Scale in older Chinese: thresholds for long and short forms. Int J Geriatr Psychiatry. 2005;20:465–470. doi: 10.1002/gps.1314. [DOI] [PubMed] [Google Scholar]
- 38.Gustincich S, Manfioletti G, Del Sal G, et al. A fast method for high-quality genomic DNA extraction from whole human blood. BioTechniques. 1991;11:298–300. 302. [PubMed] [Google Scholar]
- 39.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 40.Wendland JR, Martin BJ, Kruse MR, et al. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 41.Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307–311. doi: 10.1192/bjp.174.4.307. [DOI] [PubMed] [Google Scholar]
- 42.Weissman MM, Bland RC, Canino GJ, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- 43.Compton WM, 3rd, Helzer JE, Hwu HG, et al. New methods in cross-cultural psychiatry: psychiatric illness in Taiwan and the United States. Am J Psychiatry. 1991;148:1697–1704. doi: 10.1176/ajp.148.12.1697. [DOI] [PubMed] [Google Scholar]
- 44.Lin TY. Psychiatry and Chinese culture. West J Med. 1983;139:862–867. [PMC free article] [PubMed] [Google Scholar]
- 45.Shen YC, Zhang MY, Huang YQ, et al. Twelve-month prevalence, severity, and unmet need for treatment of mental disorders in metropolitan China. Psychol Med. 2006;36:257–267. doi: 10.1017/S0033291705006367. [DOI] [PubMed] [Google Scholar]
- 46.Georg Hsu LK, Wan YM, Chang H, et al. Stigma of depression is more severe in Chinese Americans than Caucasian Americans. Psychiatry. 2008;71:210–218. doi: 10.1521/psyc.2008.71.3.210. [DOI] [PubMed] [Google Scholar]
- 47.Griffiths KM, Nakane Y, Christensen H, et al. Stigma in response to mental disorders: a comparison of Australia and Japan. BMC Psychiatry. 2006;6:21. doi: 10.1186/1471-244X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker G, Gladstone G, Chee KT. Depression in the planet’s largest ethnic group: the Chinese. Am J Psychiatry. 2001;158:857–864. doi: 10.1176/appi.ajp.158.6.857. [DOI] [PubMed] [Google Scholar]
- 49.Xu JM. Some issues in the diagnosis of depression in China. Can J Psychiatry. 1987;32:368–370. [PubMed] [Google Scholar]
- 50.Kleinman A. Culture and depression. N Engl J Med. 2004;351:951–953. doi: 10.1056/NEJMp048078. [DOI] [PubMed] [Google Scholar]
- 51.Chen CN, Wong J, Lee N, et al. The Shatin community mental health survey in Hong Kong. II. Major findings. Arch Gen Psychiatry. 1993;50:125–133. doi: 10.1001/archpsyc.1993.01820140051005. [DOI] [PubMed] [Google Scholar]
- 52.Chiao JY, Blizinsky KD. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene. Proc Biol Sci. 2009 doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bellivier F, Henry C, Szoke A, et al. Serotonin transporter gene polymorphisms in patients with unipolar or bipolar depression. Neurosci Lett. 1998;255:143–146. doi: 10.1016/s0304-3940(98)00677-6. [DOI] [PubMed] [Google Scholar]
- 54.Otte C, McCaffery J, Ali S, et al. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: the Heart and Soul Study. Am J Psychiatry. 2007;164:1379–1384. doi: 10.1176/appi.ajp.2007.06101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rees M, Norton N, Jones I, et al. Association studies of bipolar disorder at the human serotonin transporter gene (hSERT; 5HTT) Mol Psychiatry. 1997;2:398–402. doi: 10.1038/sj.mp.4000256. [DOI] [PubMed] [Google Scholar]
- 56.Williams RB, Marchuk DA, Gadde KM, et al. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Nie Y, Xie J, et al. The association of serotonin transporter genetic polymorphisms and irritable bowel syndrome and its influence on tegaserod treatment in Chinese patients. Dig Dis Sci. 2007;52:2942–2949. doi: 10.1007/s10620-006-9679-y. [DOI] [PubMed] [Google Scholar]
- 58.Shen Y, Li H, Gu N, et al. Relationship between suicidal behavior of psychotic inpatients and serotonin transporter gene in Han Chinese. Neurosci Lett. 2004;372:94–98. doi: 10.1016/j.neulet.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 59.You JS, Hu SY, Chen B, et al. Serotonin transporter and tryptophan hydroxylase gene polymorphisms in Chinese patients with generalized anxiety disorder. Psychiatr Genet. 2005;15:7–11. doi: 10.1097/00041444-200503000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Ohara K, Nagai M, Tsukamoto T, et al. Functional polymorphism in the serotonin transporter promoter at the SLC6A4 locus and mood disorders. Biol Psychiatry. 1998;44:550–554. doi: 10.1016/s0006-3223(98)00112-7. [DOI] [PubMed] [Google Scholar]
- 61.Han DH, Park DB, Na C, et al. Association of aggressive behavior in Korean male schizophrenic patients with polymorphisms in the serotonin transporter promoter and catecholamine-O-methyltransferase genes. Psychiatry Res. 2004;129:29–37. doi: 10.1016/j.psychres.2004.06.013. [DOI] [PubMed] [Google Scholar]