Abstract
Background
A central problem in posttraumatic stress disorder (PTSD) is a reduced capacity to suppress fear under safe conditions. Previously, we have shown that combat-related PTSD patients have impaired inhibition of fear-potentiated startle. Given the high comorbidity between PTSD and depression, our goal was to see whether this impairment is specific to PTSD, or a nonspecific symptom associated with both disorders.
Methods
Fear-potentiated startle (FPS) was assessed in 106 trauma-exposed individuals divided into four groups: a) No diagnosis control, b) PTSD only, c) major depression (MDD) only, and d) comorbid PTSD and MDD. We used a novel conditional discrimination procedure, in which one set of shapes (the danger signal) was paired with aversive airblasts to the throat, and different shapes (the safety signal) were presented without airblasts. The paradigm also included fear inhibition transfer test.
Results
Subjects with comorbid MDD and PTSD had higher FPS to the safety signal and to the transfer test compared to controls and MDD only subjects. In contrast to the control and MDD groups, the PTSD and comorbid PTSD and MDD groups did not show fear inhibition to safety cues.
Conclusions
These results suggest that impaired fear inhibition may be a specific biomarker of PTSD symptoms.
Keywords: PTSD, Depression, Hyper-arousal Symptoms, Physiology, Fear-Potentiated Startle
Introduction
Posttraumatic stress disorder (PTSD) and major depressive disorder (MDD) can develop independently, but are frequently comorbid in individuals who experience traumatic events. The National Comorbidity Survey of 1995 (1) estimated that 48% of individuals who met criteria for PTSD also met criteria for MDD. A more recent examination of the onset of these mental illnesses indicated that a prior diagnosis of PTSD increased the odds of developing MDD by a factor of 5.7 in one study (2), and a factor of 11.7 in another study (3). One reason for the high rates of comorbidity of these disorders is the large number of symptoms that are observed in both diagnoses. According to the DSM-IV-TR (4), PTSD is characterized by three major symptom clusters following an event that elicited fear, helplessness, or horror. The first symptom category, referred to as cluster B, covers symptoms of re-experiencing the event, such as intrusive thoughts, nightmares, and flashbacks induced by reminders of the event. Cluster C symptoms include avoidance of stimuli associated with the trauma, while cluster D incorporates symptoms of increased arousal. The latter two symptom clusters include restricted range of affect, emotional detachment, loss of interest, difficulties sleeping, and impaired concentration that are also cardinal symptoms of MDD. Furthermore, suicidality is frequently seen in both disorders. This overlap in clinical presentation of the two disorders has lead some researchers to argue for a distinct depressive subtype of PTSD, rather than the presence of two separate disorders (5). Given the complexity of clinical symptoms, biomarkers of specific symptoms could provide a useful tool to discriminate between these disorders. Additionally, understanding physiological differences that may underlie symptomatology is critical for elucidating differential neural circuitry in stress-related psychopathology.
One of the core problems in PTSD is the reduced ability to suppress fear under safe conditions. Neuroimaging studies have found that responses to fearful stimuli can be so powerful that the inputs from the prefrontal cortex are not able to inhibit amygdala activity (6–13). Differential activation of these brain areas or impaired connectivity between them may account for some of the symptoms of PTSD; especially those in the hyper-arousal symptom cluster. Conceptualizing PTSD as a disorder of fear conditioning (14–17) can lead to the use of fear inhibition experiments to identify vulnerable and resilient individuals and can be used for testing treatments that bolster resilience.
A recent meta-analysis of 15 studies using fear conditioning found that patients with anxiety disorders showed greater levels of fear responses compared to healthy controls (18). Several studies have focused specifically on fear-potentiated startle, defined as the relative increase in the startle response elicited in the presence of a conditioned stimulus (CS) paired with an aversive unconditioned stimulus (US) (19–22). Early studies with Vietnam and Gulf War veterans found that enhanced fear-potentiated startle was observed only in an aversive context (20, 21). One study (21) found equivalent levels of fear potentiation to the danger signal in the PTSD and control groups, but the PTSD subjects also had potentiated startle responses to the safety cue, whereas the controls did not. This finding suggested that PTSD is associated with impaired inhibition of fear.
Our laboratories have recently developed a startle paradigm, based on an animal model (23), that measures baseline startle response as well as fear-potentiated startle and the inhibition of fear-potentiated startle. We have used this paradigm to measure responses to danger and safety cues using electromyogram (EMG) recordings of the eyeblink muscle contraction to an acoustic startle probe delivered in the presence and absence of the CS (19, 24). Using this paradigm, we found that PTSD subjects with higher current symptoms showed impairment in transferring inhibition of fear to a test stimulus that paired the danger and safety cues(25). The current study used fear potentiation of the startle response to investigate fear inhibition differentially in PTSD and MDD. We hypothesized that deficits in fear inhibition would be specific to patients with PTSD.
Methods
Study Subjects
One hundred and six subjects were included in the study. Participants were recruited as part of a larger study investigating the genetic and environmental factors that contribute to PTSD in a primarily African-American, low socioeconomic, inner city population (see (26, 27) for full description of larger study sample). Exclusion criteria for participation in the study included active psychosis, pregnancy, hearing impairment and major medical illnesses as assessed by health and physical examinations which were conducted by medical professionals. Medication use was not an exclusion criterion for the study. All subjects were screened for auditory impairment using an audiometer (Grason-Stadler, Model GS1710). The subjects were required to detect tones at 30 dB(A)SPL at frequencies ranging from 250 to 4000 Hz. Prior to their participation, all participants provided written informed consents that were approved by the Emory University Institutional Review Board.
Clinical Assessment
The Structured Clinical Interview for DSM IV (SCID, (28) was administered to all subjects. In addition to the diagnostic interview, all participants completed the modified PTSD Symptom Scale for PTSD and the Beck Depression Inventory for depression assessment. The Traumatic Events Inventory was used to assess lifetime trauma exposure.
Modified PTSD Symptom Scale
The modified PTSD Symptom Scale (PSS) is a psychometrically valid 17-item self-report scale assessing PTSD symptomatology over the two weeks prior to rating (29–31). The PSS interview has been validated with the widely used measure of PTSD – the Clinician Administered PTSD Scale (CAPS; (30, 32). The categorical definition of PTSD was determined based on DSM-IV A-E criterion responses to the PSS questionnaire (A, presence of trauma; B, presence of at least 1 intrusive symptom; C, presence of at least 3 avoidance / numbing symptoms; and D, presence of at least 2 hyperarousal symptoms; and E, present for at least 1 month).
The Beck Depression Inventory (BDI) was administered to measure depressive symptoms. This interview was conducted at least 1-week prior to the conditioning session. The BDI consists of a 21-item questionnaire (33). Each of the items measures the presence and severity of depressive symptoms that are rated on a scale from 0 to 3. A categorical definition of MDD was determined by a cutoff score of 15 on the BDI. A current diagnosis of MDD was also confirmed by the SCID.
Childhood Trauma Questionnaire (CTQ)
The CTQ is a self-report inventory assessing childhood physical, sexual and emotional abuse. Studies have established the internal consistency, stability over time, and criterion validity of both the original 70-item CTQ and the current brief version (34, 35). The CTQ yields a total score and subscale scores for each of the types of child abuse. The CTQ total score was used as a covariate in the analyses.
Traumatic Events Inventory (TEI)
The traumatic events inventory (31) assesses lifetime history of trauma exposure and is a measure of both child abuse and non-child abuse trauma. The TEI assesses past experience and frequency of 13 separate types of traumatic events as well as feelings of terror, horror, and helplessness with such events.
Startle Response Measurements
The startle response data were acquired using Biopac MP150 for Windows (Biopac Systems, Inc., Aero Camino, CA) and stored on the hard drive of a Windows XP laptop. All data were sampled at 1000 Hz and amplified with a gain of 5000 using the EMG module of the Biopac system. The acquired data were filtered, rectified, and smoothed using MindWare software (MindWare Technologies, Ltd., Gahanna, OH) and exported for statistical analyses. The EMG signal was filtered with low- and high- frequency cutoffs at 28 and 500 Hz, respectively. The maximum amplitude of the eyeblink muscle contraction 20 – 200 ms after presentation of the startle probe was used as a measure of the acoustic startle response.
As previously described (19, 24) the eyeblink component of the acoustic startle response was measured by EMG recordings of the right orbicularis oculi muscle with two 5-mm Ag/AgCl electrodes filled with electrolyte gel. One electrode was positioned 1cm below the pupil of the right eye and the other was 1cm below the lateral canthus. We used disposable electrodes from Biopac (EL504) pre-coated with electrolyte gel. Impedance levels were less than 6 kilo-ohms for each participant as measured by a Checktrode impedance meter (1089 MKIII, UFI, Morro Bay, CA). A background white noise of 70-dB (A) SPL was presented continuously throughout the session; startle probe delivery was superimposed on the background noise. The startle probe was a 108-dB (A) SPL, 40ms burst of broadband noise with near instantaneous rise time, delivered binaurally through headphones (Maico, TDH-39-P).
Experimental Design
Fear-potentiated startle (FPS) and inhibition of FPS was assessed using a conditional discrimination paradigm termed AX+/BX− (19). Each session consisted of a startle habituation phase followed by three blocks of conditioning that occurred without any breaks. The conditioning phase was seamlessly followed by a testing block for fear inhibition. Each conditioned stimulus (CS) was a compound of two shapes presented on a computer monitor. The AX+ compound served as the reinforced stimulus (CS+), and the BX− compound served as the non-reinforced stimulus (CS−). The AX+ and BX− cues consisted of a set of 2 blue, black or purple shapes (star, triangle or square) presented centrally on a monitor (with counterbalanced shape assignment across the CSs). For any given pair, the cues differed on both color and shape. Each compound CS had one novel cue (A or B) and one common cue ‘X’ (see Figure 1A and 1B). The fear inhibition test stimulus was a compound of the previously conditioned A and B cues (Figure 1C) that was used to determine transfer of inhibition (by B) to the fear response to A (19, 23). For each compound stimulus, the shapes were presented simultaneously and in one of two pseudorandom sequences. The aversive stimulus (US) was a 250-ms airblast with an intensity of 140 psi directed to the larynx. The air blast was emitted by a compressed air tank attached to the polyethylene tubing and controlled by a solenoid switch. This US has been used in our studies previously (19, 36) and produces robust fear-potentiated startle.
Figure 1.
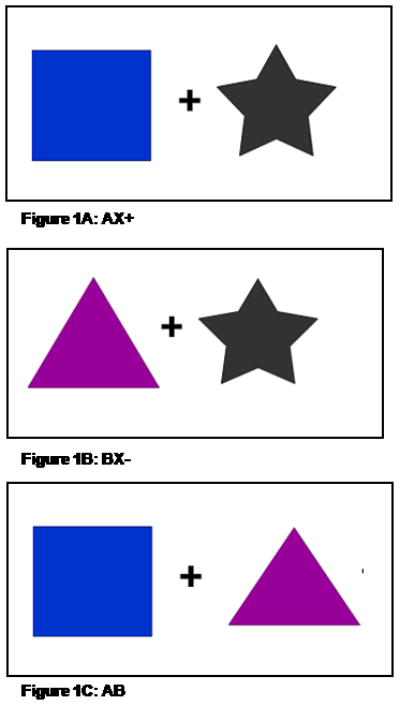
Examples of the CSs presented on a computer monitor during conditioning.
A. AX+, the reinforced stimulus; B. BX−, the non-reinforced stimulus; and C. AB, inhibition test stimulus. Shape and color assignment was counterbalanced across subjects.
The habituation phase consisted of six startle probes presented alone (noise-alone trials, NA). Immediately following habituation, participants underwent the conditioning phase, which consisted of three blocks, each of which included four trials of each CS type and four noise-alone trials for a total of 12 trials per block. A block of three AB trials was presented after the conditioning phase. All AX+ trials were reinforced with the US, while the BX− and AB trials were not reinforced. Both conditioned stimuli were 6 sec in duration. During AX+ trials, the 250-ms airblast co-terminated with the stimulus, and the 40ms startle probe preceded the US (airblast) by 500 ms. The BX− trials terminated immediately after the presentation of the startle probe. The AB trials were designed the same way as the BX− trials. In all phases of the experiment, inter-trial intervals were of randomized duration ranging from 9 to 22 seconds.
Data Analysis
The group variables in the analyses were the diagnostic categories for PTSD and MDD, resulting in 4 groups: a traumatized no diagnosis control, PTSD only, MDD only, and comorbid PTSD and MDD.
Startle reactivity was assessed for the noise alone trials (NA) by averaging the startle response to the probe in the absence of the CSs. Differential conditioning to AX+ and BX−, and fear inhibition to AB was measured by calculating percent potentiation for each CS type, in order to account for individual differences in startle magnitude as well as startle habituation. This value was derived as follows: Percent Startle Potentiation = 100 × (startle magnitude during CS trials − NA startle) / (NA startle), with the NA derived from the same conditioning block as the CS. We then averaged three conditioning blocks for AX+ and BX−. These variables were analyzed using a two-way MANOVA with the between groups factor of PTSD group (2 levels: No PTSD, PTSD) and MDD group (2 levels: No MDD, MDD). The scores on the CTQ (for childhood trauma) were added as covariates in the between-group analyses. In order to assess whether the subjects were learning the discrimination, we used a RM ANOVA comparing trial types (specific contrasts AX+ to BX−, and AX+ to AB) as a within-subjects factor separately for each group. We used multivariate tests because the Sphericity assumption was not met for the repeated measures tests.
In all MANOVAs we used Roy’s Largest Root statistic with an alpha level of 0.05. Significant between-groups differences were followed up by post-hoc Bonferroni tests. All analyses were performed in SPSS 15.0 for Windows (SPSS, Inc). Effect sizes for the MANOVA comparisons are reported as partial η2.
Results
Subject Characteristics
One hundred and six subjects participated in the study; of those, 53 did not meet criteria for either PTSD or MDD; 14 met a diagnosis of PTSD only, 17 had MDD only, and 22 met criteria for both disorders. The subjects’ age ranged from 18–69 years old, 62.1% were female, and 89.1% were African American. Table 1 shows the demographic and clinical information of the subjects across the different groups.
Table 1.
The table displays the demographic and clinical information across the diagnostic groups.
| NO DX | PTSD ONLY | MDD ONLY | PTSD&MDD | ||
|---|---|---|---|---|---|
| n=53 | n=14 | n=17 | n=22 | p | |
| AGE (M, SD) | 45.7 (12.9) | 41.8 (13.6) | 40.4 (12.2) | 46.8 (8.9) | ns |
| SEX (%) | M=41.2%; F=58.8% | M=57.1%; F=42.9% | M=26.7%; F=73.3% | M=28.6%; F=71.4% | ns |
| RACE (%) | AA=94.1%; C=5.9% | AA=100% | AA=80%; C=20% | AA=81%; C=19% | ns |
| CTQ (M, SD) | 37.75 (16.13) | 38.07 (12.27) | 48.24 (15.34)a | 53.56 (25.40)a,b | <0.05 |
| TEI (M, SD) | 2.97 (2.11) | 3.74 (2.29) | 3.58 (1.70) | 5.85 (2.57)a,b,c | <0.0001 |
| PSS (M, SD) | 4.56 (4.65) | 17.98 (8.44)a,c | 7.20 (6.48) | 28.71 (9.62)a,b,c | <0.0001 |
| BDI (M, SD) | 6.87 (5.19) | 8.98 (5.68) | 21.71 (6.36)a,b | 31.36 (11.95)a,b,c | <0.0001 |
Acronyms: CTQ: Childhood Trauma Questionnaire;
TEI: Traumatic Events Inventory (mean indicates number of traumatic events experienced)
BDI: Beck Depression Inventory; PSS; PTSD Symptoms Scale.
different from No Dx;
different from PTSD only;
different from MDD only
Clinical Assessments
As shown in Table 1, all subjects had approximately equivalent levels of childhood trauma. However, the subjects that met current criteria for PTSD had significantly higher PSS scores, F(3,76)=49.00, p<0.001, than traumatized controls (p<0.001) and the MDD group (p=0.01). The comorbid PTSD and MDD subjects had higher PSS scores than all other groups. The MDD group had higher BDI scores, F(3,76)=28.68, p<0.001, than the controls (p<0.001) or the PTSD subjects (p<0.01). The comorbid group was more symptomatic for depression than the controls (p<0.001) and the PTSD subjects (p<0.001).
Although all groups experienced substantial levels of trauma (see Table 2), there were significant differences in both childhood trauma (F(3,96)=4.42, p<0.05) and adult trauma (F(3,97)=8.04, p<0.001) exposure. Subjects with comorbid PTSD and MDD had higher CTQ scores than the no diagnosis (p<0.001) and the PTSD only group (p<0.05) and had experienced more types of trauma than all of the other three groups (all p<0.001). Subjects with MDD only had more childhood trauma than the no diagnosis controls (p<0.05).
Table 2.
The table displays the rates of traumatic events experienced by the subjects in the study sample.
| TRAUMATIC EVENTS INVENTORY | Percent of Sample |
|---|---|
| Natural Disaster | 20.69 |
| Serious Accident or Injury | 55.77 |
| Sudden Life-Threatening Illness | 38.60 |
| Military Combat | 9.26 |
| Close Friend or Family Member Murdered | 3.57 |
| Close Friend or Family Member Committed Suicide | 8.77 |
| Attacked with Weapon | 35.09 |
| Attacked Without Weapon | 34.55 |
| Violence Between Parents or Caregivers | 35.19 |
| Beaten as a Child | 35.71 |
| Sexual Contact Before Age 13 | 21.43 |
| Forced Sexual Contact Between 14 and 17 | 9.09 |
| Forced Sexual Contact After Age 17 | 9.80 |
Fear-Potentiated Startle
Startle magnitude was assessed during noise alone (NA) trials and in the presence of conditioned stimuli (AX+, BX−) and the fear inhibition stimulus (AB). There were no PTSD or MDD group differences in startle magnitude to the noise alone trials without CSs, indicating that baseline startle levels were equivalent in all subjects. However, a two-way MANOVA comparing the PTSD groups X MDD groups on percent startle potentiation to AX+, BX−, and AB showed a significant main effect of PTSD group on AX+ (F(1,97)=6.48, p<0.05, η2=0.07), BX− (F(1,97)=5.66, p<0.05, η2=0.06), and AB (F(1,97)=12.08, p=0.001, η2=0.12), with PTSD patients showing higher FPS to all trial types. There were no significant main effects of MDD group on any trial type, but there was a significant interaction of the two diagnoses on the AB safety transfer (i.e., fear inhibition) trial (F(1,97)=4.83, p<0.05, η2=0.05), with the patients with comorbid PTSD and MDD having the highest FPS to this trial. Figure 2 shows startle potentiation to AX+, BX−, and AB across the four diagnostic groups (No diagnosis, PTSD only, MDD only, and comorbid PTSD and MDD).
Figure 2.
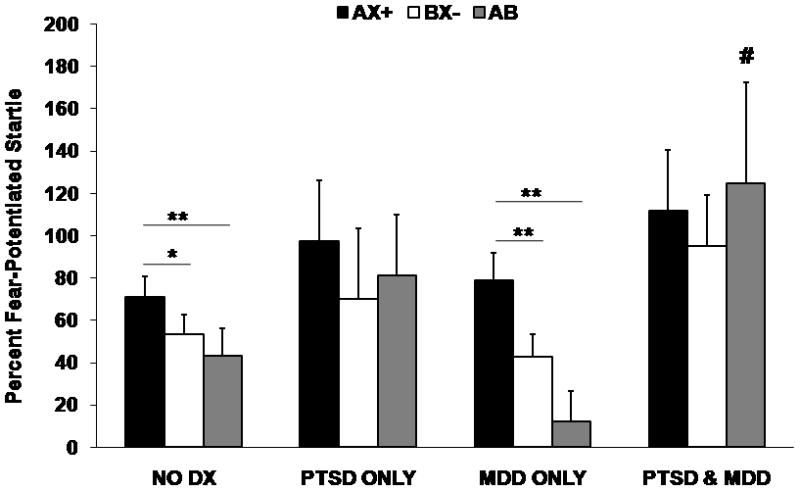
Fear-Potentiated Startle on AX+, BX−, and AB trials across the four diagnostic groups. The Y-axis represents average percent startle potentiation for each trial type. This value was derived as follows: Percent Startle Potentiation = 100 × (startle magnitude during CS trials − NA startle) / (NA startle), with the NA derived from the same conditioning block as the CS.
Significant main effect of PTSD for AX+, BX−, AB trial types; no main effect of MDD. # denotes interaction of PTSD and MDD on AB trial type, p<0.05. * denotes within-subject trial type effect, p<0.05; ** denotes within-subject trial type effect, p<0.01.
RM MANOVA of CS trial type within each diagnostic group showed a significant effect of trial type in the no-diagnosis controls (F(2,51)=5.55, p<0.01) and MDD subjects (F(2,15)=6.63, p<0.01). PTSD subjects and comorbid subjects did not show significant effects. Contrast analyses comparing AX+ to BX− showed that controls and MDD subjects had greater startle potentiation to AX+ than BX− (F(1,52)=7.84, p<0.01, η2=0.13, and F(1,16)=8.03, p=0.01, η2=0.33, respectively). Furthermore, a comparison of AB to AX+ indicated that controls and MDD subjects also had significant transfer of safety on AB trials, F(1,52)=3.99, p=0.05, η2=0.07, and F(1,16)=8.24, p=0.01, η2=0.34, respectively. PTSD subjects and the comorbid group did not show any discrimination between AX+ and BX− (F(1,13)=2.66, ns, and F(1,21)=1.03, ns, respectively) or reduction of fear from AX+ to AB (F(1,13)=0.43, ns, and F(1,21)=0.02, ns, respectively).
In order to examine whether fear inhibition is associated with specific PTSD symptoms, we compared percent startle potentiation on the AB trials between subjects with high and low levels of symptoms on each symptom cluster. We divided subjects into high and low symptom groups by using a median split of the PSS score for intrusive, avoidance, and hyper-arousal symptoms. In order to control for effects of depression, we only analyzed subjects who did not meet criteria for depression. Figure 3 shows startle potentiation on the transfer test (AB trials) between high and low symptom groups for the three symptom clusters. We found AB startle was associated only with hyper-arousal symptoms, in that those with high levels of symptoms had significantly higher startle potentiation on AB (i.e., less fear inhibition), F(1,62)=4.28, p<0.05. The association with hyper-arousal symptoms was not accounted for by startle potentiation to the safety cue (BX− itself, since high and low symptom did not differ on this trial type, F(1,62)=0.70, ns.
Figure 3.
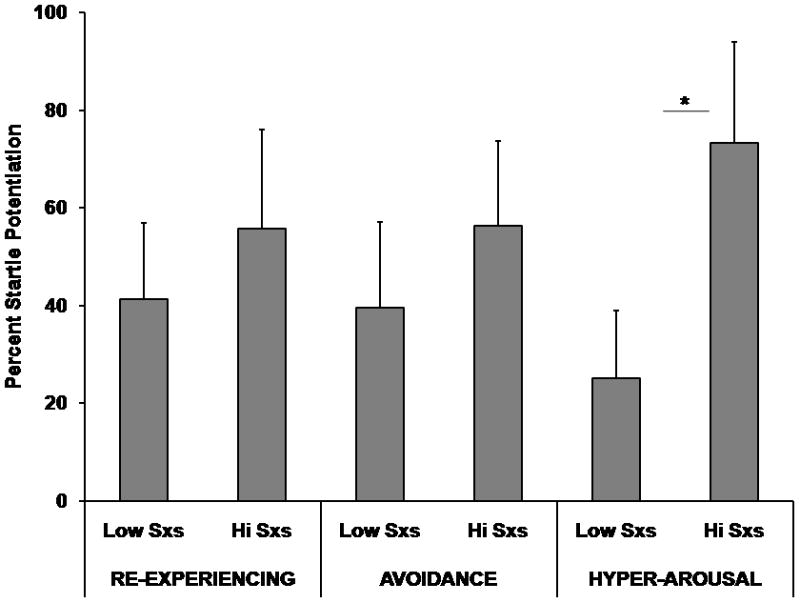
Fear-Potentiated Startle on AB trials across high and low levels of PTSD symptoms on the three symptom clusters. The Y-axis represents average percent startle potentiation. This value was derived as follows: Percent Startle Potentiation = 100 × (startle magnitude during CS trials − NA startle) / (NA startle), with the NA derived from the same conditioning block as the CS. * denotes p<0.05.
Discussion
The objective of this study was to investigate fear inhibition differentially in PTSD and MDD. We found a main effect of PTSD on all fear-potentiated startle trial types, but no main effect of MDD on FPS. There was a significant interaction effect of PTSD and MDD on the safety transfer trial, with patients with comorbid diagnoses have the highest FPS to this trial type. Furthermore, patients with a PTSD diagnosis with or without comorbid depression did not demonstrate discrimination between danger and safety cues. We also found that PTSD patients did not inhibit fear on the safety transfer trials; however, this finding may be secondary to the lack of discrimination. We also found that a reduced capacity for inhibition of FPS and a lack of discrimination between danger and safety cues were specific to PTSD, as the MDD and control subjects did not show these deficits. Finally, we found that FPS to the safety transfer trial was positively correlated with hyper-arousal symptoms of PTSD.
These data are consistent with our previous studies that have found PTSD symptom severity associated with impaired inhibition of fear-potentiated startle (25). However, given the high comorbidity between PTSD and depression, we wanted to see whether this deficit was specific to PTSD. The present study suggests that this may be the case. We found that subjects with PTSD or PTSD comorbid with MDD had reduced fear inhibition. While MDD in addition to PTSD appeared to exacerbate the impairment because this group had the greatest level of startle potentiation to AB, this group also had much higher symptoms of PTSD than the PTSD only group. The fact that the MDD only group did not differ from the no diagnosis control either in the level of potentiation or in their ability to inhibit conditioned fear further suggests that this may be a unique finding in PTSD. Finally, the association between impaired fear inhibition and hyper-arousal symptoms indicates that dysregulated transfer of safety may be a biomarker for such symptoms. Given that we controlled for trauma exposure by including CTQ scores as a covariate in between-group analyses, the biomarker may identify the disorder rather than just trauma-related pathology, or a general vulnerability to develop either PTSD or depression after traumatic experiences (3).
Given that we did not find significant differential conditioning to AX+ and BX−, failure to inhibit on AB trials may be simply secondary to the lack of discrimination on fear-potentiated startle. However, we believe the inability of the PTSD patients in the current study to discriminate BX− from AX+ is another reflection of poor inhibitory control of the startle measure of fear on BX− trials. Heightened responding to BX− suggests over-generalization of fear responses, i.e., inappropriate safety cue processing. A limitation of the current study is the small sample size in the PTSD only and MDD only groups, which did not provide enough power to analyze awareness. However, in another study in this population (Jovanovic et al, in press) as well as other studies in different populations (25) we have found that PTSD patients clearly learn that B is safe, based on an on-line rating of whether each stimulus is “dangerous, safe, or I do not know’, as described previously in healthy controls (24). Thus PTSD patients rate BX− as “safe” and AB as more safe than AX+. Despite this awareness they still fail to inhibit potentiated startle on either BX− or AB test trials. Finally, even though the inhibitory properties of the AB trial are dependent on BX−, since B is the safety cue, one piece of data suggests that AB may be a more robust measure of the reduced capacity for fear inhibition in PTSD: the fact that the effect size for the AB trial is double the effect size of the BX− trial in differentiating PTSD from No PTSD patients.
The results of the present study support an increasing number of investigations that have found deficient safety signal processing in PTSD (8, 18, 25, 37). A small number of prospective studies suggest that impaired suppression of fear during extinction may be a risk factor for PTSD (38, 39). In support of this finding, a recent study found that genetic polymorphisms of the serotonin transporter were linked to fear-potentiated startle (40). On the other hand, our study that examined current symptom severity in Vietnam veterans suggests that fear inhibition may be a state-dependent rather than a trait phenomenon (25); Another possibility is that some aspects of fear inhibition, such as the ability to learn safety cues, may be vulnerability traits (39), while others, such as memory of safety cues, may be acquired as part of the disorder (37).
Impaired safety signal processing in PTSD is consistent with current neurocircuitry models of exaggerated amygdala activity and decreased prefrontal cortex activity in this disorder (7, 10, 11, 41). While no study to date has examined fear-potentiated startle with neuroimaging in PTSD, decades of research on animal models has established a critical role for the amygdala in fear-potentiated startle (22, 42). This paradigm provides an excellent translational tool because it is a well-characterized, objective measure of fear that can be used across mammalian species (22). In addition, fear-potentiated startle is less invasive and less costly than imaging techniques, yet it can serve as an indirect measure of amygdala activity. Recent studies have also found that inhibitory control from the dorsal anterior cingulate cortex is deficient in PTSD (13, 43); this brain area has also been found to affect fear expression in humans (44). Functional neuroimaging studies that have examined connectivity between prefrontal cortex and the amygdala have found impaired inhibition of the amygdala in PTSD (13); however, other studies have reported heightened amygdala activation in PTSD patients, but no reduction in prefrontal inhibition (45). While fear-potentiated startle is a good peripheral measure of fear responses, future studies should incorporate neuroimaging techniques with the paradigm described here in order to acquire direct measures of inhibition of amygdala activity.
Another limitation of the current study is the lack of control over medication status of the subjects. Ideally, subjects would be excluded if they were currently taking psychotropic medication. However, this approach would significantly reduce the sample size of the study, and would likely not reflect a realistic description of the clinical population.
In summary, our findings strongly suggest that inhibition of fear-potentiated startle should be further investigated as a marker of PTSD symptoms. Additionally, these data support the hypothesis that PTSD is associated with reduced responding to safety cues, or over-generalized responding to danger cues. While the current study cannot indicate whether this trait is a vulnerability factor for the disorder or acquired as a consequence of the illness, such phenotypes that serve as intermediate biomarkers of mental disorders are necessary to examine the cascade of effects that lead from trauma to illness.
Acknowledgments
This work was primarily supported by National Institutes of Mental Health (MH071537). The work was also supported by Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01 RR00039), and the Burroughs Wellcome Fund. We thank Allen Graham, BA, Josh Castleberry, BS, Daniel Crain, BS, Abby Powers, BS and Rachel Herschenberg, BS for their assistance with data collection and support.
References
- 1.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Archives of General Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(Suppl 5):4–12. discussion 3–4. [PubMed] [Google Scholar]
- 3.Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: the posttraumatic stress disorder-major depression connection. Biological Psychiatry. 2000;48:902–9. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- 4.APA. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C: American Psychiatric Press; 2000. [Google Scholar]
- 5.Schnurr PP, Friedman MJ. An overview of research findings on the nature of posttraumatic stress disorder. Session: Psychotherapy in Practice. 1997;3:11–25. [Google Scholar]
- 6.Bremner J, Staib L, Kaloupek D, Southwick S, Soufer R, Charney D. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biological psychiatry. 1999;45:806–16. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 8.Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychological Medicine. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53:380–7. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 10.Rauch SL, Shin LM, Phelps EA. Neurocircuitry Models of Posttraumatic Stress Disorder and Extinction: Human Neuroimaging Research-Past, Present, and Future. Biological Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47:769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 12.Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–26. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 13.Lanius RA, Williamson PC, Densmore M, Boksman K, Neufeld RW, Gati JS, Menon RS. The Nature of Traumatic Memories: A 4-T fMRI Functional Connectivity Analysis. Am J Psychiatry. 2004;161:36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- 14.Foa EB, Steketee G, Rothbaum BO. Behavioral/cognitive conceptualizations of post-traumatic stress disorder. Behavioral Therapy. 1989;20:155–76. [Google Scholar]
- 15.Keane TM, Zimering RT, Caddell JM. A behavioral formulation of posttraumatic stress disorder in Vietnam veterans. The Behavior Therapist. 1985;8:9–12. [Google Scholar]
- 16.Falls WA, Davis M. Behavioral and physiological analysis of fear inhibition. In: Friedman MJ, Charney DS, Deutch AY, editors. Neurobiological and clinical consequences of stress: From normal adaptation to PTSD. Philadelphia: Lippincott-Raven Publishers; 1995. pp. 177–202. [Google Scholar]
- 17.Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Sciences. 2003;1008:112–21. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- 18.Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research & Therapy. 2005;43:1391–424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan EJ. Fear Potentiation and Fear Inhibition in a Human Fear-Potentiated Startle Paradigm. Biological Psychiatry. 2005;57:1559–64. doi: 10.1016/j.biopsych.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Grillon C, Morgan CA, 3rd, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biological Psychiatry. 1998;44:1027–36. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- 21.Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–42. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- 22.Davis M. Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and the fear-potentiated startle effect. Pharmacology and Therapeutics. 1990;47:147–65. doi: 10.1016/0163-7258(90)90084-f. [DOI] [PubMed] [Google Scholar]
- 23.Myers KM, Davis M. AX+, BX− discrimination learning in the fear-potentiated startle paradigm: possible relevance to inhibitory fear learning in extinction. Learn Mem. 2004;11:464–75. doi: 10.1101/lm.74704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jovanovic T, Norrholm SD, Keyes M, Fiallos A, Jovanovic S, Myers KM, Davis M, Duncan EJ. Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behav Neurosci. 2006;120:995–1004. doi: 10.1037/0735-7044.120.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos A, Myers KM, Davis M, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009 doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 Polymorphisms and Childhood Abuse With Risk of Posttraumatic Stress Disorder Symptoms in Adults. JAMA. 2008;299:1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Archives of General Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSMIV-Patient Edition (SCID-P) Washington, D.C: American Psychiatric Press; 1995. [Google Scholar]
- 29.Falsetti S, Resnick H, Resick P, Kilpatrick D. The Modified PTSD Symptom Scale: A brief self-report measure of posttraumatic stress disorder. The Behavior Therapist. 1993;16:161–2. [Google Scholar]
- 30.Foa EB, Tolin DF. Comparison of the PTSD symptom scale-interview version and the clinician-administered PTSD scale. Journal of Traumatic Stress. 2000;13:181–91. doi: 10.1023/A:1007781909213. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic Stress Disorder Among African Americans in an Inner City Mental Health Clinic. Psychiatr Serv. 2005;56:212–5. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- 32.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. Journal of Traumatic Stress. 1993;6:459–73. [Google Scholar]
- 33.Beck AT, Ward CH, Mendelsohn M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein DP, Fink L. Childhood Trauma Questionnaire A retrospective self-report manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- 35.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27:169–90. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 36.Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning & Memory. 2006;13:681–5. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. Journal of Psychiatric Research. 2008;42:515–20. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. Prospective Prediction of Posttraumatic Stress Disorder Symptoms Using Fear Potentiated Auditory Startle Responses. Biological Psychiatry. 2009;65:235–40. doi: 10.1016/j.biopsych.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosomatic Medicine. 2006;68:307–11. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- 40.Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Öhman A. Genetic gating of human fear learning and extinction: Possible implications for gene-environment interaction in anxiety disorder. Psychological Science. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 41.Liberzon I, Sripada CS. Progress in Brain Research. Vol. 167. Elsevier; 2007. The functional neuroanatomy of PTSD: a critical review; pp. 151–69. [DOI] [PubMed] [Google Scholar]
- 42.Davis M. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci. 1992;13:35–41. doi: 10.1016/0165-6147(92)90014-w. [DOI] [PubMed] [Google Scholar]
- 43.Shin LM, Bush G, Whalen PJ, Handwerger K, Cannistraro PA, Wright CI, Martis B, Macklin ML, Lasko NB, Orr SP, Pitman RK, Rauch SL. Dorsal anterior cingulate function in posttraumatic stress disorder. Journal of Traumatic Stress. 2007;20:701–12. doi: 10.1002/jts.20231. [DOI] [PubMed] [Google Scholar]
- 44.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry. 2007;62:1191–4. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 45.Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biological Psychiatry. 2004;55:263–72. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]


