Abstract
Background
The 2007 update to the American Heart Association (AHA) Guidelines for Cardiovascular Disease Prevention in Women recommend a simplified approach to risk stratification. We assigned Women's Health Initiative (WHI) participants to risk categories as described in the guideline, and evaluated clinical event rates within and between strata.
Methods and Results
The WHI enrolled 161,808 women aged 50-79 years, and followed them prospectively for 7.8 years (mean). Applying the 2007 AHA guideline categories, 11% of women were high risk, 72% at-risk and 4% optimal risk; 13% of women did not fall into any category, that is, lacked risk factors but did not adhere to a healthy lifestyle (moderate intensity exercise for 30 minute most days and <7% of calories from saturated fat). Among high risk, at-risk and optimal risk women, rates of myocardial infarction (MI)/coronary death were 12.5, 3.1 and 1.1% /10-years (p for trend <0.0001); the event rate was 1.3% among women who could not be categorized. We observed a graded relationship between risk category and cardiovascular event rates for white, black, Hispanic and Asian women, although event rates differed among ethnic groups (p for interaction =0.002). The AHA guideline predicted coronary events with accuracy similar to current Framingham risk categories (Area under receiver operating characteristic curve [AUC] for Framingham risk 0.665, for AHA risk 0.664; p=0.94), but less well than proposed Framingham 10-year risk categories of <5%, 5-20%, >20% (AUC for Framingham risk 0.724, for AHA risk 0.664; p<0.0001).
Conclusions
Risk stratification as proposed in the 2007 AHA guideline is simple, accessible to patients and providers, and identifies cardiovascular risk with accuracy similar to that of the current Framingham algorithm.
Keywords: women, prevention, risk factors
Global risk stratification in the National Cholesterol Education Program Adult Treatment Panel III1,2 is underpinned by the Framingham algorithm,3 which has a number of limitations4-6 and is underutilized by practitioners.7,8 The 2007 update to the American Heart Association (AHA) Evidence-Based Guidelines for Cardiovascular Disease Prevention in Women9 recommended a new risk stratification scheme, categorizing women as high risk, at-risk or optimal risk (Table 1). The guideline was endorsed by professional organizations representing primary care and specialty providers, women's health advocacy groups, the Centers for Disease Control and Prevention, and the National Heart, Lung and Blood Institute, but the predictive accuracy of this approach to risk stratification has not been confirmed.
Table 1.
Risk Categories in 2007 AHA Cardiovascular Prevention Guidelines for Women
| Risk category | Risk characteristic |
|---|---|
| High risk | Established cardiovascular disease |
| Diabetes mellitus | |
| End-stage or chronic renal disease | |
| 10-year Framingham global risk >20% | |
| At risk | ≥ 1 major risk factor of cardiovascular disease including: |
| Cigarette smoking | |
| Poor diet | |
| Physical inactivity | |
| Obesity, especially central adiposity | |
| Family history of premature cardiovascular disease (<55y in male relative or <65y in female relative) | |
| Hypertension | |
| Dyslipidemia | |
| Evidence of subclinical vascular disease | |
| Metabolic syndrome | |
| Poor exercise capacity on treadmill test and/or abnormal heart rate recovery after stopping exercise | |
| Optimal risk | Framingham global risk <10% and a healthy lifestyle with no risk factors |
The Women's Health Initiative (WHI) is a large, diverse cohort followed prospectively for 7.8 years for cardiovascular events. To evaluate the new risk stratification scheme, we categorized WHI participants as described in the 2007 AHA guideline, and assessed their cardiovascular event rates.
Methods
Study population
The WHI includes 161,808 postmenopausal women, aged 50 to 79 years at baseline, enrolled at 40 clinical sites from 1993-8 into 4 randomized trials and an observational study.10 Recruitment and baseline data collection have been previously reported.11-14 In brief, eligible women had anticipated longevity of at least 3 years and were likely to be residing in the same geographic area for at least 3 years. Women joining the dietary modification trial consumed an average American diet and had no history of breast or colon cancer12; those joining the hormone trials had no history of breast cancer or other cancers within the preceding 10 years.13 Calcium/vitamin D trial participants were a subset of women in the diet and hormone trials.10 The protocol and consent forms were approved by institutional review boards of the participating institutions; all trial participants provided written informed consent.
Variables
Women reporting prior MI or coronary revascularization were considered to have established coronary disease and those with prior stroke to have established cerebrovascular disease. Women reporting carotid or peripheral arterial revascularization were considered to have established peripheral arterial disease. Diabetes mellitus requiring dietary or pharmacologic therapy (excluding gestational diabetes), high cholesterol requiring drug treatment, family history of coronary disease (male first degree relative with MI before age 55 or female relative before age 65) and cigarette smoking were assessed by self-report at baseline. Hypertension included both measured high blood pressure and self-reported high blood pressure requiring pills. If a woman had missing data for a given risk characteristic, she would be classified on the basis of other reported factors; in their absence, she would be considered to have no risk factors. Lipid profiles and fasting glucose levels were not available for most women, so for this analysis, body mass index ≥30 kg/m2 with waist circumference >35″ was used as a surrogate for the metabolic syndrome.15,16
The AHA guideline includes several criteria that are not explicitly defined. For this analysis, poor exercise capacity was identified by self-report of being limited “a lot” in climbing multiple flights of stairs or walking several blocks. Total physical activity was assessed by questions on a frequency and duration scale for walking and other types of activity.17 We defined physical inactivity as the lowest quintile, <1.25 MET-hours/week. For the optimal risk category, we defined the physical activity component of a healthy lifestyle as ≥10 MET-hours/week, which approximates 30 minutes of walking, 6 days per week, consistent with the physical activity component of healthy lifestyle as described in the 2007 AHA guideline.
We assessed dietary nutrient consumption by food frequency questionnaire.18 The AHA guideline considers women consuming a “poor diet” as at-risk for cardiovascular disease without an explicit definition. For this analysis, we defined poor diet as consuming >10% of calories from saturated fat. For a healthy diet, the guideline recommends a diet rich in fruits and vegetables, whole-grain, high-fiber foods, fish at least twice weekly, saturated fat <10% of energy, and if possible <7%, cholesterol <300 mg/day, alcohol <1 drink/day, sodium <2.3 g/day and trans fat <1% of energy. In view of 1) the limited dietary assessment likely to be available to practitioners using this guideline, and 2) the fact that very low saturated fat consumption (<6.1% of calories) was required to demonstrate cardiovascular event reduction in the dietary modification trial,19 we elected to use the AHA guideline saturated fat criterion (<7% of total calories) as shorthand for a healthy diet. In prior analyses, WHI participants adhering to saturated fat restrictions generally met recommendations for fruit and vegetable and dietary cholesterol consumption.20
Framingham algorithm
The Framingham algorithm at baseline was calculated in the random subsample of women with fasting lipid profiles: 1% of observational study, 8.6% of hormone trial and 4.3% of dietary modification trial participants, oversampled for minority women. High sensitivity C-reactive protein was not measured in these samples. The Framingham algorithm assigns points for each of the following factors: age, total and high density lipoprotein cholesterol levels, systolic blood pressure, treatment for hypertension and current cigarette smoking.1 Each calculated point total is assigned a corresponding 10-year risk for MI/CHD death. The high risk category includes those with 10-year risk >20%, prior MI, stroke coronary revascularization procedure or diabetes mellitus.
Outcomes ascertainment
Participants reported emergency room visits, overnight hospital stays and outpatient coronary revascularization procedures at least annually. Medical records for all deaths, overnight hospitalizations and outpatient coronary revascularization procedures were scrutinized for potential outcomes of interest. Centrally-trained physician adjudicators classified outcomes on the basis of medical record review. MI was categorized using an algorithm which included symptoms, electrocardiographic findings and cardiac enzymes.21 Stroke required rapid onset of a persistent neurologic deficit not due to trauma, tumor, infection or other cause.22
Statistical analysis
Differences in baseline characteristics across risk strata were evaluated with ANOVA F-tests for continuous covariates and Chi-square tests for categorical covariates. Annualized event rates were compared across subgroups using likelihood ratio testing from generalized linear models with a Poisson distribution and log link function. Hazard ratios, 95% confidence intervals and associated p-values were from Cox proportional hazards models. Areas under receiver operating characteristic curves (AUCs) were computed using logistic regression. Curves were compared using nonparametric methods for correlated AUCs.23 Analyses were performed using the SAS System for Windows v9 (SAS Institute, Cary, NC) and R v2.7.2 (The R Foundation for Statistical Computing). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
The cohort included 161,808 women aged 50 to 79 years at study entry, with follow up of 7.8 ± 1.6 years (mean ± standard deviation). High risk, at-risk and optimal risk women constituted 11, 72 and 4% of the cohort, respectively. A considerable proportion of women (13%) did not fall into any category, that is, they lacked risk factors, but did not adhere to a healthy lifestyle. The uncategorized women consumed 7-10% of calories in the form of saturated fat and performed 1.25 to <10 MET-hours/week of physical activity, thus meeting neither our definition of “poor diet” (>10% of calories from saturated fat) nor physical inactivity (<1.25 MET-hours/week), either of which would have placed them in the at-risk category. Neither did they meet our definition of healthy lifestyle (not smoking, <7% of calories from saturated fat and ≥10 MET-hours/week of physical activity), which would have qualified them for the optimal risk category.
The number and percentage of women with each risk characteristic are shown in descending order of frequency within each risk stratum (Table 2). Among women in the high risk category, self-reported diabetes mellitus was the most common qualifying characteristic (6%), followed by prevalent coronary heart disease (CHD)(3%). Among women in the at-risk category, “poor diet”, defined in this analysis as >10% of calories from saturated fat, was the most common characteristic (43%), followed by hypertension (26%) and obesity (25%).
Table 2.
Number (%) of women meeting each risk criterion, assessed at baseline, and 10-year event rate
| N (% of cohort) | MI/CHD death | MI/CHD death/stroke | |||
|---|---|---|---|---|---|
| High risk | N | (%/10y) | N | (%/10y) | |
| Diabetes mellitus | 9618 (5.9) | 871 | (12.7) | 1273 | (18.9) |
| Prior MI or coronary revascularization | 5060 (3.2) | 705 | (20.3) | 943 | (27.8) |
| Established peripheral arterial disease | 3270 (2.0) | 313 | (13.6) | 458 | (20.2) |
| Prior stroke | 2165 (1.3) | 187 | (12.3) | 363 | (24.8) |
| Aortic aneurysm | 301 (0.2) | 40 | (19.5) | 57 | (28.7) |
| At-risk | |||||
| Poor diet | 69627 (43.0) | 1605 | (3.0%) | 2864 | (5.4%) |
| Hypertension | 41926 (25.9) | 1414 | (4.5%) | 2521 | (8.1%) |
| BMI ≥30 kg/m2 | 40071 (24.8) | 1020 | (3.3%) | 1754 | (5.7%) |
| BMI ≥30 kg/m2 with waist >35″ | 35115 (21.7) | 936 | (3.5%) | 1600 | (6.0%) |
| Physical inactivity | 25572 (15.8) | 659 | (3.4%) | 1167 | (6.1%) |
| Family history of premature MI | 23905 (14.8) | 692 | (3.8%) | 1180 | (6.5%) |
| Dyslipidemia | 16949 (10.5) | 515 | (4.1%) | 854 | (6.8%) |
| Poor exercise capacity | 15557 (9.6) | 624 | (5.4%) | 1079 | (9.5%) |
| Current smoker | 9683 (6.0) | 334 | (4.5%) | 558 | (7.7%) |
| Optimal risk | 6784 (4.2) | 59 | (1.1%) | 119 | (2.2%) |
| Not in any category | 20820 (12.9) | 217 | (1.3%) | 441 | (2.6%) |
Women were assigned to the highest risk category for which they qualfied. A woman with diabetes and prior stroke would be counted in both rows. MI, myocardial infarction; BMI, body mass index
Among high risk women, the rate of MI/CHD death ranged from 12.3 to 20.3%/10-years. Women with at-risk characteristics had coronary event rates ranging from 3.0 to 5.4%/10-years, whereas optimal risk women had the lowest coronary event rate, 1.1%/10-years. The event rate among women who could not be categorized was similar to that of optimal risk women (1.3%/10-years). Clinical event rates for individual risk characteristics did not overlap between categories.
Selected baseline characteristics are shown by risk category (Table 3). Age and ethnicity differed between risk categories (p<0.0001 for each); women in lower risk categories were younger and more likely to be white or Asian.
Table 3.
Baseline characteristics by AHA risk category
| High risk | At-risk | Optimal risk | Not in any category | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | mean (SD) or % |
N | mean (SD) or % |
N | mean (SD) or % |
N | mean (SD) or % |
p value | |
| Age at screening, years | 17578 | 65.5 (7.1) | 116626 | 63.1 (7.2) | 6784 | 62.0 (7.2) | 20820 | 62.2 (7.2) | <.0001 |
| 50-59 | 3772 | 21.5 | 39013 | 33.5 | 2669 | 39.3 | 8104 | 38.9 | |
| 60-69 | 8216 | 46.7 | 52517 | 45.0 | 2945 | 43.4 | 8908 | 42.8 | |
| 70-79 | 5590 | 31.8 | 25096 | 21.5 | 1170 | 17.2 | 3808 | 18.3 | |
| Body mass index, kg/m2 | 17409 | 30.5 (6.7) | 115630 | 28.5 (6.0) | 6713 | 23.7 (2.8) | 20611 | 24.4 (2.8) | <.0001 |
| <25 | 3635 | 20.9 | 36157 | 31.3 | 4536 | 67.6 | 12003 | 58.2 | |
| 25 - <30 | 5481 | 31.5 | 39402 | 34.1 | 2177 | 32.4 | 8608 | 41.8 | |
| ≥30 | 8293 | 47.6 | 40071 | 34.7 | 0 | 0.0 | 0 | 0.0 | |
| Waist circumference, inches | 17514 | 93.9 (15.2) | 116205 | 87.5 (13.6) | 6764 | 76.2 (8.3) | 20744 | 78.1 (8.6) | <.0001 |
| ≤35 | 6806 | 38.9 | 66987 | 57.6 | 6280 | 92.8 | 18513 | 89.2 | |
| >35 | 10708 | 61.1 | 49218 | 42.4 | 484 | 7.2 | 2231 | 10.8 | |
| Physical activity quintiles | 16890 | 10.0 (12.3) | 111848 | 11.2 (13.0) | 6784 | 26.8 (15.9) | 18818 | 16.6 (14.4) | <.0001 |
| <1.25 (MET h/wk) | 4147 | 24.6 | 25572 | 22.9 | 0 | 0.0 | 0 | 0.0 | |
| 1.25 - <5.5 | 4011 | 23.7 | 23688 | 21.2 | 0 | 0.0 | 4022 | 21.4 | |
| 5.5 - <11.6 | 3376 | 20.0 | 21952 | 19.6 | 542 | 8.0 | 4887 | 26.0 | |
| 11.63 - <21.0 | 2804 | 16.6 | 20612 | 18.4 | 2492 | 36.7 | 4483 | 23.8 | |
| ≥21.0 | 2552 | 15.1 | 20024 | 17.9 | 3750 | 55.3 | 5426 | 28.8 | |
| Ethnicity | <.0001 | ||||||||
| White | 12696 | 72.2 | 96547 | 82.8 | 6024 | 88.8 | 18266 | 87.7 | |
| Black | 3016 | 17.2 | 10594 | 9.1 | 222 | 3.3 | 795 | 3.8 | |
| Hispanic | 919 | 5.2 | 4705 | 4.0 | 181 | 2.7 | 707 | 3.4 | |
| American Indian | 171 | 1.0 | 480 | 0.4 | 15 | 0.2 | 49 | 0.2 | |
| Asian / Pacific Islander | 479 | 2.7 | 2707 | 2.3 | 262 | 3.9 | 744 | 3.6 | |
| Unknown | 297 | 1.7 | 1593 | 1.4 | 80 | 1.2 | 259 | 1.2 | |
| % of calories from saturated fat | 16763 | 10.5 (3.4) | 113741 | 10.8 (3.3) | 6784 | 5.6 (1.1) | 19771 | 8.1 (1.4) | <.0001 |
| <7 | 2513 | 15.0 | 14868 | 13.1 | 0 | 0.0 | 3121 | 15.8 | |
| 7 – 10 | 5199 | 31.0 | 29246 | 25.7 | 0 | 0.0 | 16650 | 84.2 | |
| >10 | 9051 | 54.0 | 69627 | 61.2 | 0 | 0.0 | 0 | 0.0 | |
p value is from ANOVA for continuous variables (age, body mass index, waist circumference, physical activity, calories from saturated fat) or a chi-square test for categorical variables.
The number of cardiovascular events and annualized event rate are shown by risk category (Table 4). Among women in the high risk category, the rate of MI/CHD death was 12.5%/10-years, and of MI/CHD death/stroke was 19.0%/10-years. In contrast, these events occurred at about 1/10th that frequency among optimal risk women, while at-risk women had intermediate event rates (p for trend <0.0001 across risk strata). Women who did not fall into any risk category had event rates similar to the optimal risk group.
Table 4.
Number and percent (per 10 years) of women with cardiovascular events by AHA risk category
| High risk | At-risk | Optimal risk | Not in any category | |||||
|---|---|---|---|---|---|---|---|---|
| N (%/10y) | HR (95% CI) | N (%/10y) | HR (95% CI) | N (%/10y) | HR | N (%/10y) | HR (95% CI) | |
| MI | 1141 (9.1) | 10.58 (7.90-14.16) | 2224 (2.5) | 2.86 (2.14-3.81) | 47 (0.9) | 1.00 | 178 (1.1) | 1.21 (0.88-1.67) |
| CHD death | 631 (4.9) | 19.46 (11.46-33.05) | 747 (0.8) | 3.20 (1.89-5.44) | 14 (0.3) | 1.00 | 52 (0.3) | 1.16 (0.64-2.09) |
| Stroke | 936 (7.4) | 6.44 (4.99-8.31) | 2366 (2.6) | 2.26(1.76-2.90) | 63 (1.2) | 1.00 | 235 (1.4) | 1.19 (0.90-1.57) |
| Hospitalized angina | 1902 (15.7) | 12.19 (9.59-15.50) | 3011 (3.4) | 2.62 (2.06-3.33) | 69 (1.3) | 1.00 | 267 (1.6) | 1.24 (0.95-1.62) |
| MI/CHD death | 1568 (12.5) | 11.63 (8.97-15.09) | 2749 (3.1) | 2.81 (2.17-3.64) | 59 (1.1) | 1.00 | 217 (1.3) | 1.17 (0.88-1.56) |
| MI/CHD death/stroke | 2342 (19.0) | 8.76 (7.29-10.54) | 4922 (5.5) | 2.51 (2.09-3.01) | 119 (2.2) | 1.00 | 441 (2.6) | 1.18 (0.96-1.45) |
| MI/CHD death/angina | 3204 (27.2) | 11.76 (9.83-14.06) | 5575 (6.3) | 2.70 (2.26-3.23) | 125(2.3) | 1.00 | 473 (2.8) | 1.21 (0.99-1.47) |
| MI/CHD death/stroke/ angina | 3868 (33.4) | 9.78 (8.44-11.33) | 7642 (8.7) | 2.52 (2.17-2.91) | 185 (3.5) | 1.00 | 688 (4.1) | 1.19 (1.01-1.40) |
MI, myocardial infarction; CHD, coronary heart disease; HR, hazard ratio; CI, confidence interval
HR (95% CI) are from Cox proportional hazards analyses, where the ‘Optimal’ group is the referent for risk category. P-value for each individual or composite event was <0.0001. This was the case whether “Not in any category” women were omitted, combined with “Optimal” or with “At-risk” women or included as a separate category.
Cardiovascular event rates were examined among women from different ethnic groups by risk category (Table 5). The numbers of events in the low risk categories are small for ethnic minority women. A graded increase in event rate across risk groups was consistently observed in all ethnic groups, although the absolute event rates differed between groups (p for interaction =0.002). For each risk category, event rates were higher among white and black women compared with Hispanic or Asian women.
Table 5.
Number of cardiovascular events and 10-year event rate by ethnicity and AHA risk category
| High risk | At-risk | Optimal risk | Not in any category |
|||||
|---|---|---|---|---|---|---|---|---|
| N (%/10-years) | ||||||||
| White | 3011 | (36.0%) | 6498 | (8.9%) | 172 | (3.6%) | 637 | (4.4%) |
| Black | 581 | (29.1%) | 694 | (8.9%) | 7 | (4.0%) | 17 | (2.7%) |
| Hispanic | 115 | (18.9%) | 185 | (5.5%) | 3 | (2.2%) | 15 | (2.8%) |
| Asian | 71 | (22.3%) | 121 | (6.1%) | 2 | (1.0%) | 12 | (2.1%) |
Cardiovascular events included myocardial infarction/coronary death/stroke/hospitalized angina P-value from a likelihood ratio test examining the interaction of ethnicity with risk category = 0.002; result was the same when “Not in any category” women were combined with “Optimal” or with “at-risk” women or when “Not in any category” women were excluded.
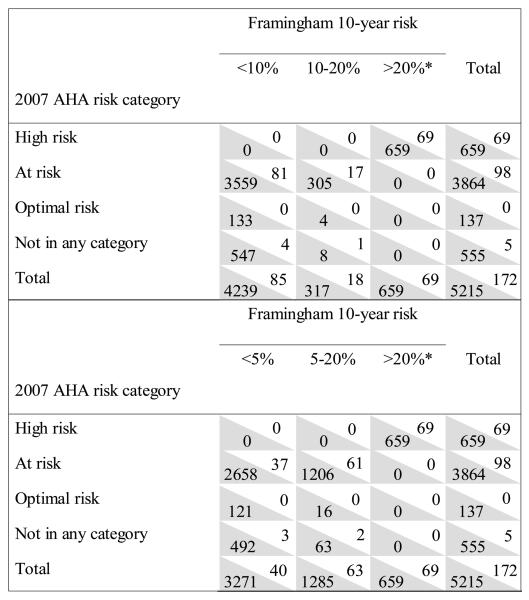
In a random subsample of women with measured lipids at baseline, the numbers of women with and without MI/CHD death are shown by risk category for the 2007 AHA guideline and Framingham algorithm (Figure 1). Mean calculated Framingham 10-year risk was 2% for the entire subsample, with 80%, 6% and 14% in the low, intermediate and high risk categories, respectively (including assignment to the high risk group on the basis of clinical characteristics). Among women in the AHA at-risk category, Framingham 10-year risk was <10% for 3640 (92%) and 10-20% for 322 (8%). AUC for prediction of CHD events was no different for the two approaches to risk stratification (p=0.94). We also considered Framingham categories of <5%, 5-20% and >20% (Figure 1). Among women in the AHA at-risk category, Framingham risk was <5% for 3695 (68%) and 5-20% for 1267 (32%). These modified Framingham categories, more accurately predicted CHD events; AUC was 0.724 for the Framingham algorithm compared with 0.664 for the AHA guideline (p<0.0001).
Figure 1.
Numbers of women in 2007 AHA guideline and Framingham risk categories with and without myocardial infarction/CHD death. Each cell includes the number of MI/CHD death cases (white background) and noncases (shaded background). Analyses include only the subsample with measured lipids. Upper panel shows Framingham risk categories of <10, 10-20 and >20%. For Framingham 10-year risk, AUC=0.665; for AHA risk, AUC=0.664 (p=0.94 vs Framingham). Lower panel shows Framingham risk categories of <5, 5-20 and >20%. For Framingham 10-year risk, AUC=0.724; for AHA risk, AUC=0.664 (p <0.0001 vs Framingham). *CHD or equivalent, including Framingham risk score >20%.
Discussion
We classified a large, diverse cohort into optimal, at-risk and high risk categories as proposed in the 2007 update of the Evidence-Based Guidelines for Cardiovascular Disease Prevention in Women. Most women were at-risk, but a significant proportion (13%) could not be categorized. Incident cardiovascular events increased across risk strata (p for trend <0.0001); this relationship was apparent among white, black, Asian and Hispanic women, although absolute event rates varied among ethnic groups (p for interaction = 0.002). Event rates did not overlap for individual characteristics defining at-risk vs high risk women. The 2007 AHA guideline and Framingham 10-year risk categories (<10%, 10-20%, >20%) predicted coronary events with similar accuracy. In contrast, modified Framingham 10-year risk categories of <5%, 5-20% and >20%, predicted coronary events more accurately than the 2007 AHA guideline (AUC 0.724 vs 0.664, p<0.0001).
Strengths of this analysis include the large, diverse population, careful prospective collection and adjudication of cardiovascular events, and richness of the baseline dataset including dietary and physical activity variables. Limitations include the lack of available data on renal function, the fact that lipoproteins were only measured in a random subsample of participants and the potential inaccuracy of self-reported medical conditions such as diabetes mellitus or hypercholesterolemia. High sensitivity C-reactive protein was not measured in the random sample, precluding assessment of the Reynolds risk score.
Age is not a specified criterion for risk categorization in the 2007 AHA guideline. The prevalence of several high risk and at-risk criteria such as diabetes, hypertension and dyslipidemia increase with age,12-14 thereby incorporating, at least in part, the cardiovascular risk associated with aging. Further, this approach focuses on long term effects of risk factors, potentially avoiding the false reassurance which a low 10-year Framingham risk may provide to younger individuals with clinically important risk characteristics.24,25
The 2007 AHA guideline for women recommends a diet rich in vegetables and fruits, whole-grain, high-fiber foods, oily fish at least twice weekly, limiting alcohol, salt <2.3 g/day, saturated fat <7% of calories, trans fats <1% of calories and dietary cholesterol <300 mg/day, an assessment well beyond the scope of most practitioners' office visits. Applying just the salt, beverage, saturated and trans fat and dietary cholesterol criteria to the WHI, fewer than 1% of women fell into the optimal risk category and 16% could not be categorized, that is, had no risk criteria, but did not adhere to a healthy lifestyle. For this analysis, we chose saturated fat <7% of calories as shorthand for a healthy diet 1) to reflect a feasible degree of dietary assessment in community practice, 2) with knowledge that WHI participants adhering to the saturated fat restriction generally are consuming at least 5 daily servings of fruits/vegetables and <300 mg/day of cholesterol,18 and that 3) cardiovascular event reduction in the dietary modification trial was only observed at very low levels of saturated fat consumption (<6.1% of calories).20
A shortcoming of the 2007 AHA guideline in this analysis was its inability to classify all women. Certainly the 13% of women who remained uncategorized could be addressed by changing our definition of “optimal lifestyle,” which included consuming <7% of calories from saturated fat and performing physical activity equivalent to 30 minutes of walking, 6 days per week. On the other hand, the no man's land, or no woman's land, between poor diet/physical inactivity and optimal lifestyle warrants scrutiny. In our analysis this encompasses women consuming between 7 and 10% of calories from saturated fat and/or with physical activity levels between 1.25 and 10 MET-hrs per week. In fact, the event rates among the uncategorized women were similar to the optimal risk women, from which limited cardiovascular benefit from prudent lifestyle could be inferred. One approach to resolving the problem of uncategorizable women would be to combine them with the optimal risk group, as their event rates are similar. This could lead to the inference that lifestyle doesn't matter. Alternatives would be to combine them with the at risk group, or to eliminate the gap between definitions of optimal lifestyle and poor diet/sedentary lifestyle.
Risk stratification permits providers and payors to efficiently direct attention and resources toward patients likely to benefit from initiation or intensification of preventive therapies. An at-risk label may also focus patients' attention on adherence to lifestyle or other interventions. For risk stratification to be effective, it must be 1) used and understood by providers, 2) comprehensible to patients, and 3) valid across a spectrum of individuals.
Incorporation of ATP III into clinical practice has lagged behind provider awareness of the guideline.26 Doubtless many factors contribute to the gap between what clinicians do and what scientific evidence supports; one potential obstacle to risk stratification using ATP III is its complexity. The 2007 AHA prevention guideline for women requires no mathematical calculation, but does require knowledge of a list of qualifying risk characteristics. The idea that specific characteristics or behaviors increase an individual's likelihood of having a cardiovascular event may be more accessible to both providers and patients than concepts of global risk and extrapolation from population to individual risk.
Another advantage of the AHA guideline is that a qualifying risk characteristic directly pinpoints the target for intervention. Broadly recommended healthful behaviors such as physical activity, attainment and maintenance of ideal body weight, avoidance of cigarette smoking, and consumption of a prudent diet1,27 are directly addressed in laymen's terms, so that a woman told she was at-risk due to physical inactivity could undertake appropriate remedial action.
If validity is the property of a measurement method to measure what it is intended to measure,28 then the 2007 AHA risk stratification scheme is valid. Our findings support its predictive accuracy among older women from a variety of ethnic groups. Accuracy among younger women or among men remains to be demonstrated.
The ultimate measure of a guideline is whether it improves clinical decision-making, thereby enhancing efficiency and reducing clinical events. Global risk assessment appears to improve risk factor management only modestly in practice.29 The impact of the risk stratification approach proposed in the 2007 AHA prevention guideline for women will need to be demonstrated in the field.
“What is known”
-The 2007 American Heart Association guideline for cardiovascular disease prevention in women recommended categorizing women as high risk, at- risk or optimal risk on the basis of prevalent medical conditions, conventional coronary risk factors, diet and physical activity.
“What this article adds”
- In the Women's Health Initiative, a large, diverse cohort of postmenopausal women, prediction of cardiovascular risk by the AHA guideline did not differ from Adult Treatment Panel III modified Framingham categories of <10%, 10-20% and >20%, but the AHA guideline was less accurate than Framingham categories of <5%, 5-20% and >20 (p<0.0001).
- Whether the AHA guideline will be used by practitioners, and whether its use would improve clinical decision-making and patient outcomes remains to be demonstrated.
Acknowledgments
Funding source
The Women's Health Initiative program is funded by the National Heart, Lung and Blood Institute, NIH, United States Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221. The sponsor participated in design and management of the Women's Health Initiative and reviewed the manuscript, but had no role in data analysis or interpretation, preparation or approval of the manuscript.
Footnotes
The WHI investigators are listed at www.whiscience.org/collaborators/investigators.php
Clinical Trial Registration Information ClinicalTrials.gov NCT00000611
Disclosures
Dr. Hsia is employed by and owns stock in AstraZeneca.
References
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ, National Heart, Lung, and Blood Institute. American College of Cardiology Foundation. American Heart Association Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PWF, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 4.D'Agostino RB, Grundy S, Sullivan LM, Wilson P, CHD Risk Prediction Group Validation of the Framingham Coronary Heart Disease Prediction Scores. Results of a Multiple Ethnic Groups Investigation. JAMA. 2001;286:180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D'Agostino RB, Levy D. Framingham risk score and prediction of lifetime risk for coronary heart disease. Am J Cardiol. 2004;94:20–24. doi: 10.1016/j.amjcard.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Cook N. Should Age and Time Be Eliminated From Cardiovascular Risk Prediction Models? Rationale for the Creation of a New National Risk Detection Program. Circulation. 2005;111:657–658. doi: 10.1161/01.CIR.0000154544.90488.52. [DOI] [PubMed] [Google Scholar]
- 7.Sibley C, Blumenthal RS, Bairey Merz CN, Mosca L. Limitations of Current Cardiovascular Disease Risk Assessment Strategies in Women. J Women's Health. 2006;15:54–56. doi: 10.1089/jwh.2006.15.54. [DOI] [PubMed] [Google Scholar]
- 8.Tsang JLY, Mendelsohn A, Tan MKK, Hackam DG, Leiter LA, Fitchett D, Lin PJ, Grima E, Langer A, Goodman SG, Vascular Protection Registry and Guidelines Oriented Approach to Lipid Lowering Registry Investigators Discordance Between Physicians' Estimation of Patient Cardiovascular Risk and Use of Evidence-Based Medical Therapy. Am J Cardiol. 2008;102:1142–5. doi: 10.1016/j.amjcard.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 9.Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, Ganiats TG, Gomes AS, Gornik HL, Gracia C, Gulati M, Haan CK, Judelson DR, Keenan N, Kelepouris E, Michos ED, Newby LK, Oparil S, Ouyang P, Oz MC, Petitti D, Pinn VW, Redberg RF, Scott R, Sherif K, Smith SC, Jr, Sopko G, Steinhorn RH, Stone NJ, Taubert KA, Todd BA, Urbina E, Wenger NK, Expert Panel/Writing Group. American Heart Association. American Academy of Family Physicians. American College of Obstetricians and Gynecologists. American College of Cardiology Foundation. Society of Thoracic Surgeons. American Medical Women's Association. Centers for Disease Control and Prevention. Office of Research on Women's Health. Association of Black Cardiologists. American College of Physicians. World Heart Federation. National Heart, Lung, and Blood Institute. American College of Nurse Practitioners Evidence-based Guidelines for Cardiovascular Disease Prevention in Women: 2007 Update. Circulation. 2007;115:1481–1501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 10.The Women's Health Initiative Study Group Design of the Women's Health Initiative Clinical Trial and Observational Study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 11.Hays J, Hunt JR, Hubbell A, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women's Health Initiative Recruitment Methods and Results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 12.Ritenbaugh C, Patterson RE, Chlebowski RT, et al. The Women's Health Initiative Dietary Modification Trial: Overview and Baseline Characteristics of Participants. Ann Epidemiol. 2003;13:S87–S97. doi: 10.1016/s1047-2797(03)00044-9. [DOI] [PubMed] [Google Scholar]
- 13.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women's Health Initiative Postmenopausal Hormone Trials: Overview and Baseline Characteristics of Participants. Ann Epidemiol. 2003;13:S78–S86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 14.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: Baseline Characteristics of Participants and Reliability of Baseline Measures. Ann Epidemiol. 2003;13:S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson G, Hedberg P, Jonason T, Lönnberg I, Tenerz A, Forberg R, Ohrvik J. Waist circumference alone predicts insulin resistance as good as the metabolic syndrome in elderly women. Eur J Intern Med. 2008;19:520–6. doi: 10.1016/j.ejim.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Howard BV, Criqui MH, Curb JD, Rodabough R, Safford MM, Santoro N, Wilson AC, Wylie-Rosett J. Risk Factor Clustering in the Insulin Resistance Syndrome and its Relationship to Cardiovascular Disease in Postmenopausal White, Black, Hispanic, and Asian/Pacific Islander Women. Metabolism. 2003;52:362–371. doi: 10.1053/meta.2003.50057. [DOI] [PubMed] [Google Scholar]
- 17.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 18.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 19.Hsia J, Rodabough R, Rosal MC, Cochrane B, Howard BV, Snetselaar L, Frishman WH, Stefanick ML. Compliance with National Cholesterol Education Program Dietary and Lifestyle Guidelines Among Older Women with Self-reported Hypercholesterolemia: The Women's Health Initiative. Am J Med. 2002;113:384–392. doi: 10.1016/s0002-9343(02)01218-4. [DOI] [PubMed] [Google Scholar]
- 20.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SA, Black HR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low-Fat Dietary Pattern and Risk of Cardiovascular Disease. JAMA. 2006;295:655–66. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 21.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M, Daugherty S, WHI Morbidity and Mortality Committee Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 22.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J, WHI Investigators Effects of Conjugated Equine Estrogen on Stroke in the Women's Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 24.Ridker PM, Cook N. Should Age and Time Be Eliminated From Cardiovascular Risk Prediction Models? Rationale for the Creation of a New National Risk Detection Program. Circulation. 2005;111:657–658. doi: 10.1161/01.CIR.0000154544.90488.52. [DOI] [PubMed] [Google Scholar]
- 25.Cavanaugh-Hussey MW, Berry JD, Lloyd-Jones DM. Who exceeds ATP-III risk thresholds? Systematic examination of the effect of varying age and risk factors levels in the ATP-III risk assessment tool. Prev Med. 2008;47:619–23. doi: 10.1016/j.ypmed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christian AH, Mills T, Simpson SL, Mosca L. Quality of Cardiovascular Disease Preventive Care and Physician/Practice Characteristics. J Gen Intern Med. 2006;21:231–237. doi: 10.1111/j.1525-1497.2006.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services. US Department of Agriculture . Dietary Guidelines for Americans, 2005. 6th ed. US Government Printing Office; Washington, DC: Jan, 2005. [Google Scholar]
- 28.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453–473. doi: 10.1002/(sici)1097-0258(20000229)19:4<453::aid-sim350>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Sheridan SL, Crespo E. Does the routine use of global coronary heart disease risk scores translate into clinical benefits or harms? A systematic review of the literature. BMC Health Serv Res. 2008;8:60. doi: 10.1186/1472-6963-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]