Abstract
The influx of Ca2+ ions through voltage-dependent calcium (CaV) channels links electrical signals to physiological responses in all excitable cells. Not surprisingly, blocking CaV channel activity is a powerful method to regulate the function of excitable cells, and this is exploited for both physiological and therapeutic benefit. Nevertheless, the full potential for CaV channel inhibition is not being realized by currently available small molecule blockers or second messenger modulators due to limitations in targeting them either to defined groups of cells in an organism or to distinct sub-cellular regions within a single cell. Here, we review early efforts to engineer protein molecule blockers of CaV channels to fill this crucial niche. This technology would greatly expand the toolbox available to physiologists studying the biology of excitable cells at the cellular and systems level.
Electrical signals, or action potentials, generated by ionic fluxes through ion channel proteins residing in the plasma membranes of cells, constitute one of the most prevalent and important cell signaling mechanisms in biology. Electrical signals co-ordinate the activity of the millions of cells required to generate the heartbeat; underlie the orchestrated firing of neurons that enable sight, speech, movement, and formation of memories; and regulate the release of hormones that control glucose homeostasis, growth, and development. Although the spectrum of biological responses dependent on electrical signals is impressively diverse, they all utilize a similar signal transduction paradigm: membrane depolarization leads to the opening of voltage-dependent Ca2+ (CaV) channels, permitting a Ca2+ influx that triggers the appropriate cell biological response. The central role CaV channels play in transducing electrical signals into biological responses position them as attractive targets for potentially regulating a wide range of physiological processes. Indeed, modulation of CaV channels by a variety of second messenger pathways and small molecules is widely exploited as a means to regulate physiology and as a therapy for various diseases. In this review, we will discuss nascent efforts to engineer protein molecules for custom inhibition of CaV channels. As a prelude to in-depth discussion of this topic, we will first briefly review the structure-function of CaV channels, traditional CaV channel blockers, and the rationale for developing such novel protein inhibitors of CaV channels.
Structure-function of CaV channels
CaV channels are divided into two main families depending on their threshold for activation. There are three types of low-voltage-activated (CaV,LVA), or T-type, Ca2+ channels (CaV3.1 – CaV3.3) encoded by distinct genes (CACNA1G, CACNA1H, and CACNA1I), each with multiple splice variants (93). Functionally, CaV,LVA channels activate at a relatively negative threshold of around −60 mV, have a small conductance, and rapidly inactivate (111). CaV,LVA channels are found in many excitable cell types including sinoatrial node cells, smooth muscle, and neurons, where they contribute to pacemaking (91). CaV,LVA channels will not be discussed any further in this review.
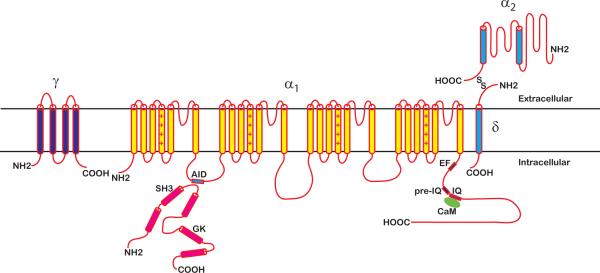
High-voltage-activated calcium (CaV,HVA) channels currently include seven members (CaV1.1–CaV1.4, CaV2.1–CaV2.3) encoded by distinct genes each with multiple splice variants (19). CaV,HVA channels typically have an activation threshold of around −30 mV, the exception being CaV1.3 which activates at a threshold of around −50 mV (75, 123). Structurally, CaV,HVA channels are hetero-multimeric proteins comprised of a main α1 subunit assembled with auxiliary β and α2δ subunits, calmodulin, and sometimes a γ subunit (Figure 1).
Figure 1. Multi-subunit structure of CaV1–2 channels.
CaV1–2 channels are comprised of a main pore-forming α1 subunit together with accessory proteins that include β and α2δ subunits, and calmodulin. Some channel complexes also include a γ subunit.
α1 subunits
CaV,HVA channel α1 subunits are the pore-forming proteins and define the identity of the channel complex. All CaV,HVA channel α1 subunits have a similar architecture, comprised of four homologous domains (I–IV), each with six membrane spanning segments (S1–S6). The four domains are connected by intracellular loops of varying lengths, along with cytosolic N- and C-termini. The S4 segment of each domain contains positively charged residues that are an integral part of the voltage sensor. The S5–S6 pore loops from each domain collaborate to form the selectivity filter, and the S6 segments line the channel pore (19).
β subunits
There are four auxiliary CaVβ subunits (β1–β4) encoded by different genes, each with multiple splice variants (17, 18, 31, 92, 98, 101, 110). At the primary sequence level the different CaVβs display two conserved domains separated by an alternatively spliced linker region, and variable N- and C-termini. Crystal structures revealed the conserved core of CaVβs contain src homology 3 (SH3) and guanylate kinase-like (GK) motifs that interact intramolecularly (23, 88, 117). This functional signature suggests a kinship to the membrane-associated guanylate kinase (MAGUK) super-family of scaffold proteins, which all contain an SH3-GK module, and organize intracellular signaling pathways by co-localizing diverse proteins (2, 48). Functionally, CaVβs: are necessary for trafficking pore-forming α1 subunits to the plasma membrane; produce depolarizing shifts in the voltage-dependence of channel activation; elevate single-channel open probability (Po); and impart characteristic inactivation properties to CaV,HVA channels (24, 29, 63, 70, 86, 92, 105). CaVβs bind with high affinity to α1 subunits using an `α binding pocket' (ABP) formed by non-contiguous residues in the GK motif, and a conserved 18-residue sequence, the `α interaction domain' (AID), located in the intracellular loop connecting α1-subunit domains I and II (23, 88, 97, 117). Binding of CaVβ to α1 increases the helical propensity of the region from the AID to the end of IS6, suggesting formation of a rigid helix that spans the AID and IS6 (89). This rigid IS6-AID helix is important for the ability of CaVβs to modulate activation and inactivation gating (42, 118).
α2δ subunits
There are currently four α2δ subunit types (α2δ-1 – α2δ-4) encoded by different genes (34, 49, 68, 99). The α2δ subunit is generated in cells as a single gene product which is post-translationally cleaved to generate separate α2 and δ proteins that are held together by disulfide bonds (61). Both the α2 and δ subunits are heavily glycosylated. Topologically, the α2 component is entirely extracellular, while the distal part of the δ peptide spans the plasma membrane. Functionally, α2δ subunits typically increase current amplitude by increasing the surface density of α1 subunits, and also influence channel activation and inactivation gating (40, 104).
Calmodulin
CaV,HVA channels are subject to rich positive and negative feedback regulation by Ca2+ (37). The Ca2+ sensor for the effects of intracellular Ca2+ on CaV,HVA channels is calmodulin (71, 94, 126), which associates in the basal state with an IQ motif in the cytoplasmic C-terminus of CaV,HVA channel α1 subunits (66, 82, 116). There is also evidence that calmodulin binding is important for trafficking CaV,HVA channel α1 subunits (119).
γ subunits
The first γ subunit identified (γ1) was originally isolated as one of the component subunits of CaV,HVA channel purified from skeletal muscle (14). Currently, there are eight members of this protein family, but only a subset has been shown to interact with some CaV,HVA channel α1 subunits (64). Topologically, γ subunits are predicted to have four transmembrane with cytoplasmic N- and C-termini. Functionally, they appear to have inhibitory effects on some CaV,HVA channels (64).
Traditional CaV,HVA channel blockers
Small molecules that block CaV,HVA channels have historically played a critical role in advancing understanding of the different CaV,HVA channel subtypes and their respective biological functions. Furthermore, small molecule CaV,HVA channel blockers are used pharmacologically as an important therapy for various cardiovascular and neurological diseases (69, 114).
CaV1 channels
CaV1.1–CaV1.4 channels, also referred to as L-type channels, are inhibited by three classes of drugs— dihydropyridines, phenylalkylamines, and benzothiazepines— in a state-dependent manner (108). Pharmacological blockade of L-type channels is an important therapy for cardiovascular diseases such as hypertension, angina, and some cardiac arrhythmias (114). The three drug classes inhibit CaV1 channels by binding to partially overlapping residues localized in domains III and IV of the respective pore-forming α1 subunits (53, 57, 95, 102, 108). Drug binding to the CaV1 α1 subunits is believed to couple allosterically to the channel pore and gating machinery (108).
CaV2 channels
Unlike CaV1 channels, there is a dearth of small organic blockers for CaV2.1–CaV2.3 channels. However, CaV2 channel family members are potently blocked by various peptide toxins isolated from predatory marine snails or spider venom (115). Specifically, CaV2.1 (P/Q-type) channels are selectively blocked by ω-agatoxin IVA (80, 120); CaV2.2 channels are inhibited by ω-conotoxin GVIA (3, 96); and CaV2.3 channels repressed by SNX-482 (85, 113). These toxins act by binding the respective pore-forming α1 subunit, and either physically occluding the pore or modifying channel gating. Blockade of CaV2 channels is an effective or potential therapy for an assortment of disorders including neuropathic pain, epilepsy, stroke, and neurodegenerative conditions (114).
Gabapentin
Gabapentin is efficacious in the treatment of neuropathic pain and seizures (106). Though this drug was originally designed as a γ-aminobutyric acid (GABA) derivative, its analgesic action stems from a high-affinity interaction with the α2δ-1 (and α2δ-2) subunit of CaV,HVA channels (13, 41, 51). Under control physiological conditions, gabapentin has only moderate effects on ICa. However, during nerve injury there is a marked up-regulation of α2δ-1 subunits in dorsal root ganglion (DRG) neurons and the spinal dorsal horn (73). Under this condition, gabapentin acutely inhibits ICa in DRG neurons, and this effect may underlie the analgesic effect (74). Chronic exposure to gabapentin suppresses CaV2.1 channel trafficking to the plasma membrane and this may also be a contributing mechanism to the therapeutic effects of the drug (54). Overall, gabapentin provides a nice proof-of-concept that it is possible to design efficacious CaV,HVA channel modulating molecules that target auxiliary subunits rather than the pore-forming α1 subunit (114).
Rationale for engineering proteins to inactivate CaV channels
There are many potential applications of CaV,HVA channel inhibition that cannot be achieved using the traditional CaV,HVA channel blockers described above. The limitations arise because it is difficult to specifically target these inhibitors to either a select group of cells in a tissue or organ, or to CaV,HVA channels localized in spatially distinct regions within a single cell (Figure 2). By contrast, these limitations may be overcome with engineered intracellular proteins that block CaV,HVA channels because these have the capacity to be deployed in defined cell types, and may also be targeted to spatially distinct sub-cellular sites using appropriate addressing motifs. To illustrate the potential niches that can be uniquely filled by engineered protein blockers of CaV,HVA channels, we draw on specific examples from neuroscience and cardiac biology, although the potential applications extend to all excitable cells.
Figure 2. Potential applications for genetically encoded CaV channel inhibitors.
(A) Disruption of specific neural circuits in a living organism. (B) Targeting functionally distinct CaV,HVA channels in localized in different compartments within a single neuron. (C) Focal inhibition CaV1.2 channels in defined region of the heart in living animals. (D) Targeting CaV1.2 channels in a single myocyte with sub-cellular specificity.
Macroscopic neuroscience applications
An important tool for neurophysiologists studying the intricacies of the mammalian brain, or the function of neural circuits in model organisms is the ability to functionally eliminate specific neurons in a living animal and observe the resulting behavioral consequences (Figure 2A) (77). Various tools have been developed to advance this capability, each with its own set of limitations (77). These include: suppressing neuronal excitability by over-expressing potassium (62) or chloride ion channels (72), or a light-gated chloride pump (125); and, inactivating synaptic transmission using small-molecule-mediated cross-linking of synaptic vesicle fusion proteins (65). In principle, blocking CaV2 channels should be highly effective in eliminating neurotransmission because synaptic vesicle fusion is steeply dependent on Ca2+ influx via these channels (transmitter release ∝ ICan, where n = 3 – 5) (12, 30). However, traditional peptide toxin blockers of CaV2 channels cannot be easily targeted to specific neurons in living animals, thus limiting their utility for this purpose. Genetically encoded intracellular blockers of CaV,HVA channels have the advantage that they can be expressed in specified neurons using a number of different approaches that have been developed including utilizing appropriate cis-regulating elements (77).
Microscopic neuroscience applications
Neurons have a highly compartmentalized architecture. A single neuron usually has multiple CaV,HVA channel types that are differentially distributed in different sub-cellular compartments. A single CaV,HVA channel type expressed in a neuron may mediate different biological responses upon Ca2+ influx depending on its sub-cellular localization within the cell (Figure 2B). For example, Ca2+ influx through presynaptic terminus-localized CaV2 channels is the dominant trigger for neurotransmitter release in most neurons (20, 87). However, these same channels regulate neuronal excitability by coupling to Ca2+-activated K+ channels in axons and dendrites (39, 79, 122). Having the capacity to selectively inhibit a particular CaV,HVA channel type located in spatially distinct regions of a neuron would not only advance fundamental understanding of Ca2+ signaling mechanisms in neurons but also permit a more fine-tuned regulation of neuronal activity. While such micro-scale targeting of CaV,HVA channels in single neurons is not possible with the currently available toxin blockers, it may be possible to achieve this objective using engineered protein inhibitors.
Macroscopic cardiac applications
In heart, CaV1.2 channels are critical for excitation-contraction (EC) coupling, membrane excitability, and conduction velocity through the atrio-ventricular (AV) node. Atrial fibrillation (AF) is a prevalent arrhythmia characterized by rapid and un-coordinated activation of the atria due to re-entrant excitation or abnormal impulse formation from ectopic foci (84). A significant portion of the adverse effects associated with AF are due to ventricular tachycardia produced by abnormal activation of the ventricles as a result of the electrical activity in the atria. A treatment for AF is ablation of the AV node to electrically uncouple the atria and ventricles. This treatment is invasive and irreversible, and it has been proposed that inhibiting CaV1.2 channels in the AV node may represent a viable alternative that is reversible (Figure 2C) (32). Protein inhibitors of CaV1.2 channels have the advantage over the small organic blockers because they can be focally expressed, and thus specifically targeted, to the AV node (32, 83).
Microscopic cardiac applications
In single ventricular myocytes most CaV1.2 channels are localized in transverse tubules where they are apposed to nearby clusters of ryanodine receptors (RYR) in the junctional sarcoplasmic reticulum (SR; Figure 2D) (16, 50, 103, 109). This spatial arrangement of CaV1.2 channels and RYRs is critical for the calcium-induced calcium release that underlies cardiac EC coupling (15, 38, 107, 121). However, a portion of ventricular CaV1.2 channels are found localized within caveolae (5). It has been hypothesized that caveolae CaV1.2 channels in heart locally activate Ca2+-sensitive molecules that signal to responses other than contraction, including, potentially, cardiac hypertrophy (52, 81). Testing the function of caveolae-localized CaV1.2 channels in heart requires the selective inactivation of this channel pool in single ventricular myocytes. This requirement is beyond the capabilities of organic CaV1.2 channel blockers, but may be achievable with appropriately targeted protein inhibitors.
Engineering proteins for custom inhibition of CaV,HVA channels
Inducible inhibition of CaV1–2 channels is widely exploited as a mechanism to regulate diverse physiological processes in organisms (37, 67). For example, CaV2 channels are inhibited by many G-protein coupled receptor (GPCR) ligands. In one form of modulation, Gβγ subunits released from heterotrimeric G-proteins upon GPCR activation, bind CaV2 channel α1 subunits and shift them into a reluctant gating mode where they require large depolarizations to open (6, 33, 35, 56, 59). Hallmarks of Gβγ-induced inhibition of CaV2 channels include a slowing of current activation kinetics and relief of inhibition by either large depolarizations or high frequency action potential waveforms (25, 36, 76). In another paradigm, binding of GPCR ligands inhibit ICa by causing the removal of CaV,HVA channels from the cell surface (1, 112). In principle, the myriad physiological mechanisms that exist to inhibit CaV,HVA channels are potential candidate templates for engineering to create novel derivatives for custom applications. In one approach, the engineering is performed at the level of the GPCR, to evolve forms that can be activated by pharmacologically inert ligands (4). In another approach, membrane-tethered ω-conotoxin MVIIA was used to selectively and potently block co-expressed CaV2.2 channels in Xenopus oocytes (58). We will, however, focus the rest of the review on the Rad/Rem/Gem/Kir (RGK) GTPases which have several features that make them particularly well-suited for this purpose.
RGK GTPases
The RGK protein family currently consists of four members; Rem, Rem2, Rad, and Gem (mouse homolog also referred to as Kir). These proteins belong to the Ras superfamily of monomeric GTP binding proteins that function as GTP-regulated switches to regulate a wide variety of essential biological processes in cells (26). Structurally, RGK proteins have several unique features that distinguish them from other Ras GTPases including non-conservative substitutions in the GTP binding domain of residues involved in nucleotide binding and hydrolysis, a long N-terminus extension that is variable within the family, and a relatively conserved C-terminus extension (27, 43, 47, 78, 100). The C-terminus extension of RGK GTPases lack the CAAX prenylation motif found in many Ras-like GTPases (26). Nevertheless, the distal C-terminus extension effectively targets RGK proteins to the plasma membrane utilizing a combination of electrostatic and hydrophobic interactions involving basic and aromatic (or aliphatic) residues with the membrane (55). The C-terminus of RGK GTPases binds calmodulin and 14-3-3 proteins in vitro, and these interactions may regulate the sub-cellular localization of these proteins (8, 9). Functionally, Gem and Rad regulate cytoskeleton remodeling via interactions with Rho kinase (27); siRNA knockdown of Rem2 in neurons inhibits synapse development (90); and loss of Rad in heart leads to heart failure (21).
Crosstalk of RGK GTPases with CaV,HVA channels
A yeast two-hybrid screen using CaVβ3 as bait fished out Gem GTPase as an interaction partner (10). Electrophysiological experiments on recombinant channels reconstituted in Xenopus oocytes revealed that Gem effectively eliminated CaV1.2 and CaV1.3 channel currents (10). Subsequently, the property of dramatically inhibiting CaV,HVA channels was found to extend to all members of the RGK GTPase protein family (45). The mechanism of RGK proteins inhibition of CaV,HVA channels was initially believed to involve disruption of the α1-β subunit interaction (10). However, there is an emerging consensus that this does not occur and that RGK GTPases rather form a ternary complex with α1 and β subunits to inhibit ICa (11, 44). RGK GTPases inhibit ICa by different mechanisms. In some studies, RGK proteins reduce the surface density of CaV,HVA channels (7–10), although this is not universally found (22, 46). A significant portion of inhibition involves channels that remain on the cell surface but are either held in a low open probability mode or else display immobilized voltage sensors.
Engineering RGK proteins for custom applications
RGK proteins are promising candidates for engineering custom CaV,HVA channel blockers given their extreme potency in inhibiting all CaV,HVA channel types. Indeed, over-expression of wild-type RGK GTPases in tissues can be used to achieve a constitutive block of native CaV,HVA channels. In a nice demonstration of this, viral-mediated expression of Gem in adult heart cells ablated native CaV1.2 channel currents, and its focal delivery to the AV node slowed AV nodal conduction and heart rate in an animal model of atrial fibrillation (83). One limitation of using wild-type RGK GTPases is that the inhibition is constitutive, with no facile way for temporal control of channel block. A second disadvantage is that the magnitude of channel block cannot be easily regulated. Whether these capabilities could be engineered into RGK GTPases provided a crucial initial test of the feasibility of exploiting these proteins for custom applications. An important finding that advanced this possibility is that deleting the distal C-terminus of RGK GTPases eliminates their ability to block ICa (22, 45, 124). For both Rem and Rem2, deleting the distal C-terminus results in a redistribution of the GTPase from the plasma membrane to the cytosol. Moreover, constitutively targeting the inactive truncated RGK GTPases to the plasma membrane, using generic membrane-targeting modules, recapitulated their capacity to inhibit ICa (22, 124). This suggested that membrane targeting is essential for the ability of RGK GTPases to inhibit ICa. We took advantage of this feature of RGK GTPases to develop a chemical genetic hybrid approach where Rem derivatives are cytosolic and inactive in the basal state but can be acutely activated to block ICa by induced translocation to the plasma membrane using a small molecule (124). These engineered proteins were termed genetically encoded molecules for inactivating CaV channels (GEMIICCs). The first generation GEMIICC featured the C1 domain from protein kinase Cγ fused to the N-terminus of a C-terminus-truncated Rem, termed Rem265. When expressed in HEK293 cells, C1PKCγ-YFP-Rem265 was predominantly cytosolic. Application of 1 μM phorbol-12,13-dibutyrate (PdBu) resulted in a rapid translocation of C1PKCγ-YFP-Rem265 to the plasma membrane. In cells co-expressing recombinant CaV2.2 channels and C1PKCγ-YFP-Rem265, ICa was high in the basal state reflecting the inability of a cytosol-localized truncated Rem derivative to block CaV,HVA channels. Upon adding PdBu, ICa decreased concomitantly with translocation of C1PKCγ-YFP-Rem265 to the plasma membrane. Moreover, the magnitude of CaV2.2 channel inhibition was easily regulated simply by varying the concentration of PdBu (IC50 = 56 nM). One surprise with the C1PKCγ-YFP-Rem265 GEMIICC was that it showed selectivity in inhibition. Both CaV2.2 and CaV1.2 channels were significantly inhibited by C1PKCγ-YFP-Rem265 in a PdBu-inducible manner. However, CaV2.1 and CaV2.3 channels were unresponsive. This contrasts to wild-type Rem, which effectively inhibits all CaV,HVA channels. The reason for the selectivity of the GEMIICC is unknown, but could reflect different geometric constraints for the distinct channel types. Ultimately, the selectivity of C1PKCγ-YFP-Rem265 may prove fortuitous as it suggests that it may be possible to develop GEMIICCs that are specific for individual CaV,HVA channels.
Many of the potential applications of GEMIICCs may rely on their inducible targeting to spatially distinct plasma membrane sites within a single cell. This level of spatial precision is not possible with the C1PKCγ-YFP-Rem265 GEMIICC. As a prelude to developing GEMIICCs that can be targeted with specificity to defined regions of the plasma membrane, we evaluated the feasibility of using a rapamycin-mediated heterodimerization strategy to facilitate membrane translocation of a Rem265 derivative (28). Rapamycin is an immunosuppressant drug that acts by heterodimerizing two proteins, FKBP and the kinase mTOR. To implement the approach, FKBP was fused to the N-terminus of Rem265, and the rapamycin binding domain of mTOR (FRB) was fused constitutively to the plasma membrane using the membrane-targeting module from Lyn kinase (60) (Figure 3). In the basal state, cells co-expressing YFP-FKBP-Rem265 (YFR) and Lyn-FRB (LDR) displayed YFP fluorescence in the cytosol. Addition of 1 μM rapamycin caused a rapid translocation of YFR to the plasma membrane, with a concomitant inhibition of co-expressed CaV2.2 channels (Figure 3B). The successful implementation of the heterodimerization approach greatly advances the prospect of developing GEMIICCs that target CaV,HVA channels with sub-cellular precision in single excitable cells.
Figure 3. Engineering Rem for acutely inducible inhibition of CaV channels.
(A) Concept for generating inducible inhibition of CaV,HVA channels using a small molecule dimerizer-mediated recruitment of a Rem265 derivative to the plasma membrane. (B) Confocal images showing rapamycin-induced translocation of YFP-FKBP-Rem265 to the plasma membrane in a HEK 293 cell. (C) Whole-cell CaV2.2 currents from a cell co-expressing LDR and YFR before (▲) and after ( ) exposure to 1 μM rapamycin. (D) Time course of rapamycin-mediated inhibition of ICa. (E) Population current density (J) versus voltage (V) plots before (▲) and after (
) exposure to 1 μM rapamycin. (D) Time course of rapamycin-mediated inhibition of ICa. (E) Population current density (J) versus voltage (V) plots before (▲) and after ( ) rapamycin in cells co-expressing LDR and YFR. (Figure adapted from Yang et al., 2007).
) rapamycin in cells co-expressing LDR and YFR. (Figure adapted from Yang et al., 2007).
In summary, we have described recent ongoing efforts to develop technologies that permit spatially restricted and temporally regulated inactivation of CaV channels in vitro and in vivo. It is anticipated that this capability would significantly expand the toolkit available to physiologists to probe and manipulate the biology of excitable cells at the cellular and systems level. Potential uses of the technology include: (1) manipulating neuronal excitability in vivo to evaluate the function of specific neural circuits, or as a treatment for neurological disorders such as epilepsy and stroke that are characterized by excessive neural activity and excitotoxicity; (2) selectively inactivating spatially distinct pools of CaV channels within single heart and neuronal cells to discover novel functional paradigms of CaV channel signaling in these cells; and (3) developing inducible animal models of diseases, such as atrial fibrillation, in which a diminished ICa is an important factor.
References
- 1.Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Ferguson SS, Mezghrani A, Dubel SJ, Bourinet E, McRory JE, Zamponi GW. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JM. Cell signalling: MAGUK magic. Current Biol. 1996;6:382–384. doi: 10.1016/s0960-9822(02)00501-8. [DOI] [PubMed] [Google Scholar]
- 3.Aosaki T, Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and omega-conotoxin GVIA. Pflugers Arch. 1989;414:150–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- 4.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for β2-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:7500–7505. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 7.Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Ikeda H, Yamada Y, Seino Y, Hunziker W. Nuclear sequestration of beta-subunits by Rad and Rem is controlled by 14-3-3 and calmodulin and reveals a novel mechanism for Ca2+ channel regulation. J Mol Biol. 2006;355:34–46. doi: 10.1016/j.jmb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Kuwamura N, Yamada Y, Seino Y, Hunziker W. Roles of 14-3-3 and calmodulin binding in subcellular localization and function of the small G-protein Rem2. Biochem J. 2005;390:67–75. doi: 10.1042/BJ20050414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beguin P, Mahalakshmi RN, Nagashima K, Cher DH, Takahashi A, Yamada Y, Seino Y, Hunziker W. 14-3-3 and calmodulin control subcellular distribution of Kir/Gem and its regulation of cell shape and calcium channel activity. J Cell Sci. 2005;118:1923–1934. doi: 10.1242/jcs.02321. [DOI] [PubMed] [Google Scholar]
- 10.Beguin P, Nagashima K, Gonoi T, Shibasaki T, Takahashi K, Kashima Y, Ozaki N, Geering K, Iwanaga T, Seino S. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- 11.Beguin P, Ng YJ, Krause C, Mahalakshmi RN, Ng MY, Hunziker W. RGK small GTP-binding proteins interact with the nucleotide kinase domain of Ca2+-channel beta-subunits via an uncommon effector binding domain. J Biol Chem. 2007;282:11509–11520. doi: 10.1074/jbc.M606423200. [DOI] [PubMed] [Google Scholar]
- 12.Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- 13.Bryans JS, Wustrow DJ. 3-substituted GABA analogs with central nervous system activity: a review. Med Res Rev. 1999;19:149–177. doi: 10.1002/(sici)1098-1128(199903)19:2<149::aid-med3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Campbell KP, Leung AT, Sharp AH. The biochemistry and molecular biology of the dihydropyridine-sensitive calcium channel. Trends Neurosci. 1988;11:425–430. doi: 10.1016/0166-2236(88)90193-2. [DOI] [PubMed] [Google Scholar]
- 15.Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- 16.Carl SL, Felix K, Caswell AH, Brandt NR, Ball WJ, Jr., Vaghy PL, Meissner G, Ferguson DG. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J Cell Biol. 1995;129:672–682. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. Cloning and expression of a neuronal calcium channel beta subunit. J Biol Chem. 1993;268:12359–12366. [PubMed] [Google Scholar]
- 18.Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. Cloning and expression of a third calcium channel beta subunit. J Biol Chem. 1993;268:3450–3455. [PubMed] [Google Scholar]
- 19.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 20.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Chang L, Zhang J, Tseng YH, Xie CQ, Ilany J, Bruning JC, Sun Z, Zhu X, Cui T, Youker KA, Yang Q, Day SM, Kahn CR, Chen YE. Rad GTPase deficiency leads to cardiac hypertrophy. Circulation. 2007;116:2976–2983. doi: 10.1161/CIRCULATIONAHA.107.707257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Puhl HL, 3rd, Niu SL, Mitchell DC, Ikeda SR. Expression of Rem2, an RGK family small GTPase, reduces N-type calcium current without affecting channel surface density. J Neurosci. 2005;25:9762–9772. doi: 10.1523/JNEUROSCI.3111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J. Structural basis of the alpha1-beta subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. doi: 10.1038/nature02641. [DOI] [PubMed] [Google Scholar]
- 24.Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of Ca2+ channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colecraft HM, Patil PG, Yue DT. Differential occurrence of reluctant openings in G-protein-inhibited N- and P/Q-type calcium channels. J Gen Physiol. 2000;115:175–192. doi: 10.1085/jgp.115.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correll RN, Pang C, Niedowicz DM, Finlin BS, Andres DA. The RGK family of GTP-binding proteins: regulators of voltage-dependent calcium channels and cytoskeleton remodeling. Cell Signal. 2008;20:292–300. doi: 10.1016/j.cellsig.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crabtree GR, Schreiber SL. Three-part inventions: intracellular signaling and induced proximity. Trends Biochem Sci. 1996;21:418–422. doi: 10.1016/s0968-0004(96)20027-1. [DOI] [PubMed] [Google Scholar]
- 29.De Waard M, Campbell KP. Subunit regulation of the neuronal alpha 1A Ca2+ channel expressed in Xenopus oocytes. J Physiol. 1995;485:619–634. doi: 10.1113/jphysiol.1995.sp020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dodge FA, Jr., Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dolphin AC. Beta subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 32.Donahue JK, Heldman AW, Fraser H, McDonald AD, Miller JM, Rade JJ, Eschenhagen T, Marban E. Focal modification of electrical conduction in heart by viral gene transfer. Nature Med. 2000;6:1395–1398. doi: 10.1038/82214. [DOI] [PubMed] [Google Scholar]
- 33.Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium ocmponent of sensory neurone action potentials. Nature. 1978;276:837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- 34.Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, et al. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- 35.Elmslie KS. Neurotransmitter modulation of neuronal calcium channels. J Bioenerg Biomembr. 2003;35:477–489. doi: 10.1023/b:jobb.0000008021.55853.18. [DOI] [PubMed] [Google Scholar]
- 36.Elmslie KS, Zhou W, Jones SW. LHRH and GTP-gamma-S modify calcium current activation in bullfrog sympathetic neurons. Neuron. 1990;5:75–80. doi: 10.1016/0896-6273(90)90035-e. [DOI] [PubMed] [Google Scholar]
- 37.Evans RM, Zamponi GW. Presynaptic Ca2+ channels--integration centers for neuronal signaling pathways. Trends Neurosci. 2006;29:617–624. doi: 10.1016/j.tins.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 39.Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Felix R, Gurnett CA, De Waard M, Campbell KP. Dissection of functional domains of the voltage-dependent Ca2+ channel alpha2delta subunit. J Neurosci. 1997;17:6884–6891. doi: 10.1523/JNEUROSCI.17-18-06884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell S, Corradini L, England S, Winks J, Kinloch RA, Hendrich J, Dolphin AC, Webb T, Williams D. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103:17537–17542. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Findeisen F, Minor DL., Jr. Disruption of the IS6-AID linker affects voltage-gated calcium channel inactivation and facilitation. J Gen Physiol. 2009;133:327–343. doi: 10.1085/jgp.200810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finlin BS, Andres DA. Rem is a new member of the Rad- and Gem/Kir Ras-related GTP-binding protein family repressed by lipopolysaccharide stimulation. J Biol Chem. 1997;272:21982–21988. doi: 10.1074/jbc.272.35.21982. [DOI] [PubMed] [Google Scholar]
- 44.Finlin BS, Correll RN, Pang C, Crump SM, Satin J, Andres DA. Analysis of the complex between Ca2+ channel beta-subunit and the Rem GTPase. J Biol Chem. 2006;281:23557–23566. doi: 10.1074/jbc.M604867200. [DOI] [PubMed] [Google Scholar]
- 45.Finlin BS, Crump SM, Satin J, Andres DA. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. Proc Natl Acad Sci U S A. 2003;100:14469–14474. doi: 10.1073/pnas.2437756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finlin BS, Mosley AL, Crump SM, Correll RN, Ozcan S, Satin J, Andres DA. Regulation of L-type Ca2+ channel activity and insulin secretion by the Rem2 GTPase. J Biol Chem. 2005;280:41864–41871. doi: 10.1074/jbc.M414261200. [DOI] [PubMed] [Google Scholar]
- 47.Finlin BS, Shao H, Kadono-Okuda K, Guo N, Andres DA. Rem2, a new member of the Rem/Rad/Gem/Kir family of Ras-related GTPases. Biochem J. 2000;347(Pt 1):223–231. [PMC free article] [PubMed] [Google Scholar]
- 48.Funke L, Dakoji S, Bredt DS. Membrane-associated guanylate kinases regulate adhesion and plasticity at cell junctions. Annu Rev Biochem. 2005;74:219–245. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- 49.Gao B, Sekido Y, Maximov A, Saad M, Forgacs E, Latif F, Wei MH, Lerman M, Lee JH, Perez-Reyes E, Bezprozvanny I, Minna JD. Functional properties of a new voltage-dependent calcium channel alpha(2)delta auxiliary subunit gene (CACNA2D2) J Biol Chem. 2000;275:12237–12242. doi: 10.1074/jbc.275.16.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gathercole DV, Colling DJ, Skepper JN, Takagishi Y, Levi AJ, Severs NJ. Immunogold-labeled L-type calcium channels are clustered in the surface plasma membrane overlying junctional sarcoplasmic reticulum in guinea-pig myocytes-implications for excitation-contraction coupling in cardiac muscle. J Mol Cell Cardiol. 2000;32:1981–1994. doi: 10.1006/jmcc.2000.1230. [DOI] [PubMed] [Google Scholar]
- 51.Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- 52.George MS, Pitt GS. The real estate of cardiac signaling: location, location, location. Proc Natl Acad Sci U S A. 2006;103:7535–7536. doi: 10.1073/pnas.0602389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grabner M, Wang Z, Hering S, Striessnig J, Glossmann H. Transfer of 1,4-dihydropyridine sensitivity from L-type to class A (BI) calcium channels. Neuron. 1996;16:207–218. doi: 10.1016/s0896-6273(00)80037-9. [DOI] [PubMed] [Google Scholar]
- 54.Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 57.Hockerman GH, Johnson BD, Abbott MR, Scheuer T, Catterall WA. Molecular determinants of high affinity phenylalkylamine block of L-type calcium channels in transmembrane segment IIIS6 and the pore region of the alpha1 subunit. J Biol Chem. 1997;272:18759–18765. doi: 10.1074/jbc.272.30.18759. [DOI] [PubMed] [Google Scholar]
- 58.Ibanez-Tallon I, Wen H, Miwa JM, Xing J, Tekinay AB, Ono F, Brehm P, Heintz N. Tethering naturally occurring peptide toxins for cell-autonomous modulation of ion channels and receptors in vivo. Neuron. 2004;43:305–311. doi: 10.1016/j.neuron.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 59.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 60.Inoue T, Heo WD, Grimley JS, Wandless TJ, Meyer T. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat Methods. 2005;2:415–418. doi: 10.1038/nmeth763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP. Structural characterization of the dihydropyridine-sensitive calcium channel alpha 2-subunit and the associated delta peptides. J Biol Chem. 1991;266:3287–3293. [PubMed] [Google Scholar]
- 62.Johns DC, Marx R, Mains RE, O'Rourke B, Marban E. Inducible genetic suppression of neuronal excitability. J Neurosci. 1999;19:1691–1697. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones LP, Wei SK, Yue DT. Mechanism of auxiliary subunit modulation of neuronal alpha1E calcium channels. J Gen Physiol. 1998;112:125–143. doi: 10.1085/jgp.112.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang MG, Campbell KP. Gamma subunit of voltage-activated calcium channels. J Biol Chem. 2003;278:21315–21318. doi: 10.1074/jbc.R300004200. [DOI] [PubMed] [Google Scholar]
- 65.Karpova AY, Tervo DG, Gray NW, Svoboda K. Rapid and reversible chemical inactivation of synaptic transmission in genetically targeted neurons. Neuron. 2005;48:727–735. doi: 10.1016/j.neuron.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 66.Kim EY, Rumpf CH, Fujiwara Y, Cooley ES, Van Petegem F, Minor DL., Jr. Structures of CaV2 Ca2+/CaM-IQ domain complexes reveal binding modes that underlie calcium-dependent inactivation and facilitation. Structure. 2008;16:1455–1467. doi: 10.1016/j.str.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kisilevsky AE, Zamponi GW. Presynaptic calcium channels: structure, regulators, and blockers. Handb Exp Pharmacol. 2008:45–75. doi: 10.1007/978-3-540-74805-2_3. [DOI] [PubMed] [Google Scholar]
- 68.Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel alpha2delta subunit. J Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kochegarov AA. Pharmacological modulators of voltage-gated calcium channels and their therapeutical application. Cell Calcium. 2003;33:145–162. doi: 10.1016/s0143-4160(02)00239-7. [DOI] [PubMed] [Google Scholar]
- 70.Lacerda AE, Kim HS, Ruth P, Perez-Reyes E, Flockerzi V, Hofmann F, Birnbaumer L, Brown AM. Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature. 1991;352:527–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- 71.Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T, Catterall WA. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;399:155–159. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- 72.Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated Cl- channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 73.Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, Luo ZD. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- 76.Luebke JI, Dunlap K. Sensory neuron N-type calcium currents are inhibited by both voltage-dependent and -independent mechanisms. Pflugers Arch. 1994;428:499–507. doi: 10.1007/BF00374571. [DOI] [PubMed] [Google Scholar]
- 77.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maguire J, Santoro T, Jensen P, Siebenlist U, Yewdell J, Kelly K. Gem: an induced, immediate early protein belonging to the Ras family. Science. 1994;265:241–244. doi: 10.1126/science.7912851. [DOI] [PubMed] [Google Scholar]
- 79.Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- 80.Mintz IM, Venema VJ, Swiderek KM, Lee TD, Bean BP, Adams ME. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature. 1992;355:827–829. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- 81.Molkentin JD. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J Clin Invest. 2006;116:623–626. doi: 10.1172/JCI27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mori MX, Vander Kooi CW, Leahy DJ, Yue DT. Crystal structure of the CaV2 IQ domain in complex with Ca2+/calmodulin: high-resolution mechanistic implications for channel regulation by Ca2+ Structure. 2008;16:607–620. doi: 10.1016/j.str.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murata M, Cingolani E, McDonald AD, Donahue JK, Marban E. Creation of a genetic calcium channel blocker by targeted gem gene transfer in the heart. Circ Res. 2004;95:398–405. doi: 10.1161/01.RES.0000138449.85324.c5. [DOI] [PubMed] [Google Scholar]
- 84.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 85.Newcomb R, Szoke B, Palma A, Wang G, Chen X, Hopkins W, Cong R, Miller J, Urge L, Tarczy-Hornoch K, Loo JA, Dooley DJ, Nadasdi L, Tsien RW, Lemos J, Miljanich G. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- 86.Olcese R, Qin N, Schneider T, Neely A, Wei X, Stefani E, Birnbaumer L. The amino terminus of a calcium channel beta subunit sets rates of channel inactivation independently of the subunit's effect on activation. Neuron. 1994;13:1433–1438. doi: 10.1016/0896-6273(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 87.Olivera BM, Miljanich GP, Ramachandran J, Adams ME. Calcium channel diversity and neurotransmitter release: the omega-conotoxins and omega-agatoxins. Annu Rev Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- 88.Opatowsky Y, Chen CC, Campbell KP, Hirsch JA. Structural analysis of the voltage-dependent calcium channel beta subunit functional core and its complex with the alpha 1 interaction domain. Neuron. 2004;42:387–399. doi: 10.1016/s0896-6273(04)00250-8. [DOI] [PubMed] [Google Scholar]
- 89.Opatowsky Y, Chomsky-Hecht O, Kang MG, Campbell KP, Hirsch JA. The voltage-dependent calcium channel beta subunit contains two stable interacting domains. J Biol Chem. 2003;278:52323–52332. doi: 10.1074/jbc.M303564200. [DOI] [PubMed] [Google Scholar]
- 90.Paradis S, Harrar DB, Lin Y, Koon AC, Hauser JL, Griffith EC, Zhu L, Brass LF, Chen C, Greenberg ME. An RNAi-based approach identifies molecules required for glutamatergic and GABAergic synapse development. Neuron. 2007;53:217–232. doi: 10.1016/j.neuron.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 92.Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain beta subunit of the L-type calcium channel. J Biol Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- 93.Perez-Reyes E, Lory P. Molecular biology of T-type calcium channels. CNS Neurol Disord Drug Targets. 2006;5:605–609. doi: 10.2174/187152706779025508. [DOI] [PubMed] [Google Scholar]
- 94.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 95.Peterson BZ, Tanada TN, Catterall WA. Molecular determinants of high affinity dihydropyridine binding in L-type calcium channels. J Biol Chem. 1996;271:5293–5296. doi: 10.1074/jbc.271.10.5293. [DOI] [PubMed] [Google Scholar]
- 96.Plummer MR, Logothetis DE, Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989;2:1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- 97.Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel beta-subunit binds to a conserved motif in the I-II cytoplasmic linker of the alpha 1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 98.Pragnell M, Sakamoto J, Jay SD, Campbell KP. Cloning and tissue-specific expression of the brain calcium channel beta-subunit. FEBS Lett. 1991;291:253–258. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- 99.Qin N, Yagel S, Momplaisir ML, Codd EE, D'Andrea MR. Molecular cloning and characterization of the human voltage-gated calcium channel alpha(2)delta-4 subunit. Mol Pharmacol. 2002;62:485–496. doi: 10.1124/mol.62.3.485. [DOI] [PubMed] [Google Scholar]
- 100.Reynet C, Kahn CR. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science. 1993;262:1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- 101.Ruth P, Rohrkasten A, Biel M, Bosse E, Regulla S, Meyer HE, Flockerzi V, Hofmann F. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989;245:1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- 102.Schuster A, Lacinova L, Klugbauer N, Ito H, Birnbaumer L, Hofmann F. The IVS6 segment of the L-type calcium channel is critical for the action of dihydropyridines and phenylalkylamines. EMBO J. 1996;15:2365–2370. [PMC free article] [PubMed] [Google Scholar]
- 103.Scriven DR, Dan P, Moore ED. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys J. 2000;79:2682–2691. doi: 10.1016/S0006-3495(00)76506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shistik E, Ivanina T, Puri T, Hosey M, Dascal N. Ca2+ current enhancement by alpha 2/delta and beta subunits in Xenopus oocytes: contribution of changes in channel gating and alpha 1 protein level. J Physiol. 1995;489:55–62. doi: 10.1113/jphysiol.1995.sp021029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- 106.Snutch TP, Sutton KG, Zamponi GW. Voltage-dependent calcium channels--beyond dihydropyridine antagonists. Curr Opin Pharmacol. 2001;1:11–16. doi: 10.1016/s1471-4892(01)00012-1. [DOI] [PubMed] [Google Scholar]
- 107.Stern MD. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Striessnig J, Grabner M, Mitterdorfer J, Hering S, Sinnegger MJ, Glossmann H. Structural basis of drug binding to L Ca2+ channels. Trends Pharmacol Sci. 1998;19:108–115. doi: 10.1016/s0165-6147(98)01171-7. [DOI] [PubMed] [Google Scholar]
- 109.Sun XH, Protasi F, Takahashi M, Takeshima H, Ferguson DG, Franzini-Armstrong C. Molecular architecture of membranes involved in excitation-contraction coupling of cardiac muscle. J Cell Biol. 1995;129:659–671. doi: 10.1083/jcb.129.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Takahashi SX, Mittman S, Colecraft HM. Distinctive modulatory effects of five human auxiliary beta 2 subunit splice variants on L-type calcium channel gating. Biophys J. 2003;84:3007–3021. doi: 10.1016/S0006-3495(03)70027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Talavera K, Nilius B. Biophysics and structure-function relationship of T-type Ca2+ channels. Cell Calcium. 2006;40:97–114. doi: 10.1016/j.ceca.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 112.Tombler E, Cabanilla NJ, Carman P, Permaul N, Hall JJ, Richman RW, Lee J, Rodriguez J, Felsenfeld DP, Hennigan RF, Diverse-Pierluissi MA. G protein-induced trafficking of voltage-dependent calcium channels. J Biol Chem. 2006;281:1827–1839. doi: 10.1074/jbc.M508829200. [DOI] [PubMed] [Google Scholar]
- 113.Tottene A, Volsen S, Pietrobon D. alpha(1E) subunits form the pore of three cerebellar R-type calcium channels with different pharmacological and permeation properties. J Neurosci. 2000;20:171–178. doi: 10.1523/JNEUROSCI.20-01-00171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Triggle DJ. Calcium channel antagonists: clinical uses--past, present and future. Biochem Pharmacol. 2007;74:1–9. doi: 10.1016/j.bcp.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 115.Uchitel OD. Toxins affecting calcium channels in neurons. Toxicon. 1997;35:1161–1191. doi: 10.1016/s0041-0101(96)00210-3. [DOI] [PubMed] [Google Scholar]
- 116.Van Petegem F, Chatelain FC, Minor DL., Jr. Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain-Ca2+/calmodulin complex. Nat Struct Mol Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van Petegem F, Clark KA, Chatelain FC, Minor DL., Jr. Structure of a complex between a voltage-gated calcium channel beta-subunit and an alpha-subunit domain. Nature. 2004;429:671–675. doi: 10.1038/nature02588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vitko I, Shcheglovitov A, Baumgart JP, Arias O, II, Murbartian J, Arias JM, Perez-Reyes E. Orientation of the calcium channel beta relative to the alpha(1)2.2 subunit is critical for its regulation of channel activity. PLoS ONE. 2008;3:e3560. doi: 10.1371/journal.pone.0003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang HG, George MS, Kim J, Wang C, Pitt GS. Ca2+/calmodulin regulates trafficking of CaV1.2 Ca2+ channels in cultured hippocampal neurons. J Neurosci. 2007;27:9086–9093. doi: 10.1523/JNEUROSCI.1720-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 121.Wier WG, Balke CW. Ca2+ release mechanisms, Ca2+ sparks, and local control of excitation-contraction coupling in normal heart muscle. Circ Res. 1999;85:770–776. doi: 10.1161/01.res.85.9.770. [DOI] [PubMed] [Google Scholar]
- 122.Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24:8818–8822. doi: 10.1523/JNEUROSCI.2915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu W, Lipscombe D. Neuronal CaV1.3 alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang T, Suhail Y, Dalton S, Kernan T, Colecraft HM. Genetically encoded molecules for inducibly inactivating CaV channels. Nat Chem Biol. 2007;3:795–804. doi: 10.1038/nchembio.2007.42. [DOI] [PubMed] [Google Scholar]
- 125.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 126.Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]