Abstract
We explored the nature of the tumor-initiating cell in osteosarcoma, a bone malignancy that predominately occurs in children. Previously we observed expression of Oct-4, an embryonal transcriptional regulator, in osteosarcoma cell cultures and tissues. To examine the relationship between Oct-4 and tumorigenesis, cells from an osteosarcoma biopsy (OS521) were stably transfected with a plasmid containing the human Oct-4 promoter driving a GFP reporter, to generate the transgenic line OS521Oct-4p. In culture, only ∼24% of the OS521Oct-4p cells were capable of activating the transgenic Oct-4 promoter; yet, xenograft tumors generated in NOD/SCID mice contained approximately 67% GFP+ cells, which selectively expressed the MSC-associated surface antigens CD105 and ICAM-1. Comparison of the tumor-forming capacity of GFP-enriched and GFP-depleted cell fractions revealed that the GFP-enriched fractions were at least 100-fold more tumorigenic, capable of forming tumors at doses of less than 300 cells, and formed metastases in the lung. Clonal populations derived from a single Oct-4/GFP+ cell were capable of forming tumors heterogeneous for Oct-4/GFP expression. These data are consistent with the cancer stem cell model of tumorigenesis in osteosarcoma and implicate a functional link between the capacity to activate an exogenous Oct-4 promoter and tumor formation. This osteosarcoma tumor-initiating cell appears highly prolific and constitutes a majority of the cell population in a primary xenograft tumor, which may provide a biological basis for the particular virulence of this type of cancer.
Keywords: Oct-4, Osteosarcoma, Cancer Stem Cell, Embryonic Stem Cell, Tumorigenicity
Introduction
Osteosarcoma is a malignant mesenchymal tumor in which the cancerous cells produce osteoid, the organic extracellular matrix of bone. It is the most common primary, non-hematologic malignancy in children, occurring most frequently in patients between the ages of 10 and 25 (1). Prior to multi-agent chemotherapy, amputation provided a long-term survival rate of only about 20%. Currently, the use of chemotherapeutics in combination with aggressive surgery has improved the long-term survival in these patients to approximately 60% (2, 3). Despite intensive efforts to improve both surgical and medical management, this survival rate has not improved over the last 30 years, and fully 40% of osteosarcoma patients die of their disease (4).
Despite the supposed clonal origin of cancer (5), the constituent cells of a tumor can demonstrate significant heterogeneity with respect to surface antigens, proliferation kinetics, colony forming activity, as well as tumorigenic and metastatic potential (6, 7). The biological basis for this heterogeneity remains unclear and has potential therapeutic implications, as many anti-neoplastic agents were developed under the assumption that cancer cells are functionally homogeneous.
Two models have been proposed to explain intra-tumoral heterogeneity (6, 8). The stochastic model, predicts that all cells in a tumor are homogeneous for tumorigenic and metastatic potential, and that heterogeneity arises from intrinsic and extrinsic factors that impact cell behavior in a random fashion. In contrast, cancer stem cell model proposes that heterogeneity occurs as a result of a hierarchal organization reminiscent of normal stem cell driven organogenesis. In this model, tumor-initiating cells constitute a distinct sub-population and share important properties with normal tissue stem cells, including self-renewal and differentiation (9).
In considering the types of cancer likely to arise from stem/progenitor cells, osteosarcoma seems a favorable candidate. Bone is a rich reservoir of growth factors and adult stem and progenitor cells. It is one of the few human organs with the capacity for regeneration, and children, more so than adults, are capable of regenerating large segments of bone lost to trauma or surgery. Osteosarcoma occurs most commonly near active growth plates in long bones during adolescence. During this phase of post-natal bone development, stem and progenitor cells are highly active in expansion and differentiation (10, 11). If stem/progenitor cells are indeed vulnerable to oncogenic disruption, this time and site of vigorous organogenesis would seem opportune for the development of a malignant stem-like cell.
Previously, we found that cultures derived from osteosarcoma biopsies contained a subpopulation of cells capable of self-renewal as spherical clones (“sarcospheres”) under anchorage-independent, serum-starved culture conditions (1). We also detected expression of the embryonic stem cell (ES cell)-specific transcription factors Oct-4 and Nanog in monolayer culture, which was markedly enhanced in sarcospheres. These cells also expressed genes associated with multiple lineages and could be differentiated toward several mesenchymal phenotypes. Our results suggested that subpopulations of cells in bone sarcomas possessed stem-like properties.
Here, we worked to explore the nature of the tumor-initiating cell in osteosarcoma, hypothesizing that tumorigenesis is driven by a defined subset of cells that utilize regulatory networks of ES cells. To test this, we stably transfected a tumorigenic osteosarcoma cell line (OS521) with a plasmid in which the human Oct-4 promoter drives expression of green fluorescent protein (GFP). Fractionation of cells from tumors based on the activity of the fluorescent reporter identified a functionally distinct, stem-like tumor-initiating cell population.
Materials and Methods
Cell Culture
Osteosarcoma cultures were established from patient biopsies as described (12). Samples were obtained with consent, using protocols approved by the IRB of University of Florida, College of Medicine. Human mesenchymal stem cells (hMSC) were obtained from the Tulane University Center for Gene Therapy. Human fetal osteoblasts (hFOB) obtained from ATCC (CRL-11372 ATCC; Manassas, VA). Cultures were maintained in complete culture medium (DMEM/F12, 1% penicillin/streptomycin, 10% FBS (Gibco BRL; Grand Island, NY) with the exception of hFOB and OS521Oct4p (complete culture medium with 0.3 mg/ml G418 (Mediatech; Hernondon, VA).
Generation of Oct-4/GFP transgenic osteosarcoma cell lines
OS521Oct-4p cell line was generated by transfection of OS521 with phOct-4/GFP (a gift from Dr. Wei Cui; Rosyln Institute, UK (13)) using lipofectamine 2000 (Invitrogen Corporation; Carlsbad, CA) according to the manufacturer's protocol. Selection was performed using standard culture media with 1.0 - 0.5 mg/ml G418 [Mediatech; Herndon, VA]).
Xenotransplantation/Tumorigenicity Assays
Animal experiments were approved by the University of Florida Institutional Animal Use and Care Committee. Tumors were grown in 6 week-old female NOD/SCID mice (Jackson Labs; Bar Harbor, Maine) by subcutaneous inoculation with 3 × 102 to 3 × 106 cells suspended in OptiMEM. Tumor onset was set at 0.5 cm diameter, and tumors were harvested at a diameter of 0.8-1.0 cm. Recovered cells were cultured overnight in complete medium. For FACS, cultures were trypsinized and resuspended in PBS/2.0%BSA. Fractionated cell populations were resuspended in OptiMEM for subsequent injection into NOD/SCID mice.
Isolation/Expansion of OS521Oct-4p, GFP+ Clones
Tumors from transplant of unsorted OS521Oct-4p cells were harvested and dissociated as above. Cultures were fractionated into GFP-enriched and GFP-depleted populations, resuspended at 1 × 102 cells/ml. 0.01 ml of the cell suspension was then seeded to each well of a flat-bottom 96-well plate. Wells verified to contain a single cell were monitored for growth of colonies. Selected clones were expanded and characterized for GFP, surface antigens and tumorigenicity.
Immunohistochemistry
Tumor specimens were fixed in 4°C in 4% paraformaldehyde, incubated overnight in 20% sucrose/PBS at 4°C and embedded in O.C.T. (Sakura Finetek; Torrence, CA). 5μm sections were immunostained using rabbit anti-GFP (ab290, Abcam; Cambridge, MA). Slides were heat retrieved in 10mM Citra Buffer, pH 6.0, blocked with normal serum then stained overnight at 4°C. Signal was detected with anti-rabbit Alexafluor 488 (Molecular Probes; Eugene, OR).
Microscopy
Expression of Oct-4/GFP and CMV/GFP was visualized using a Leica DMIL inverted fluorescence microscope (Leica Microsystems; Wetzlar, Germany). Images were captured using the Retiga 1300R camera (Q Imaging; Pleasanton, CA) and analyzed using the manufacturers' software. Histologic images were captured using 3.3 MPX Camera (Imaging Planet; Goleta, CA) mounted on a Zeiss Axioskop 40 microscope (Carl Zeiss Microimaging Inc; Thornwood, NY) and analyzed using Image Planet Capture software (Imaging Planet; Goleta, CA).
Flow Cytometry
Monolayer
Cells were trypsinized, resuspended in PBS/0.05%BSA at 5 × 106 cells/ml and blocked with human IgG (1μg/105 cells) prior to incubation with the specified antibodies and isotype controls (1:10 dilution). Analyses were performed using a LSRII Flow Cytometer (BD Biosciences; Franklin Lakes, NJ).
Tumors
Tumors were removed at 0.8-1.0cm and dissociated as indicated for FACS enrichment and resuspended in PBS/0.05%BSA (5 × 106 cells/ml). Blocking and antibody/isotype hybridization were performed as above.
Antibodies
Anti-CD44 (550989), CD106 (555647), and HLA-A,B,C, (MHC-I, 555553) were purchased from BD Pharmigen (Franklin Lakes, NJ); anti-CD133/2 (130-090-053) from Miltenyi Biotech (Auburn, CA); anti-CD56 (FAB240P), CD90 (FAB10971P), CD105 (FAB2067P), CXCR-4 (FAB170P), SSEA-4 (FAB1435P), and EpCAM (FAB9601A) from R&D Systems (Minneapolis, MN): anti-CD29 from eBioscience (12-0299-71, San Diego, CA), and anti-ICAM1 from Abcam (ab27298-1, Cambridge, MA).
Results
Development of an Assay for Tumorigenesis
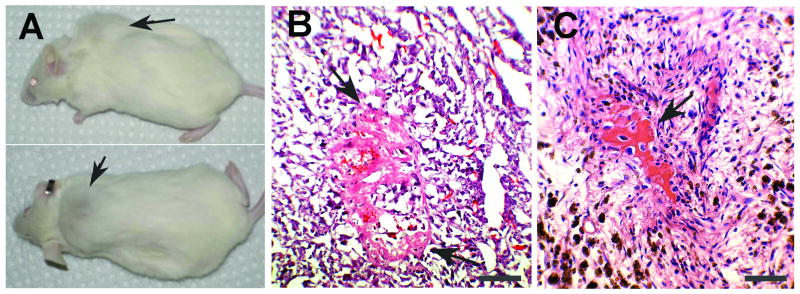
Cell lines established from human osteosarcoma biopsies were evaluated for the capacity to generate tumors following subcutaneous transplantation into NOD/SCID mice. One cell line, OS521, which originated from a high-grade distal femoral osteosarcoma, was capable of reliable, robust tumor formation. Resulting tumors showed evidence of osteoid arising from malignant spindle cells, recapitulating the histologic phenotype of the patient's osteosarcoma. (Fig. 1).
Figure 1. OS521 cells are tumorigenic and regenerate the histologic phenotype of the primary tumor in NOD/SCID xenografts.
(A) NOD/SCID mouse with a tumor (arrows) from subcutaneous injection of 3 × 106 OS521 cells. (B) Xenograft OS521 tumor stained with H&E. (C) H&E stained section of the primary OS521 osteosarcoma. For B and C, arrows designate osteoid. Images are at 200× magnification. Bars represent 100μm.
In later experiments we found that delivery of 3 × 106 and 3 × 105 OS521 cells produced tumors of >1.0 cm diameter with 100% efficiency. At a dose of 3 × 104 cells, the efficiency of tumor formation was reduced to approximately 75%. We selected this as the starting cell dose for subsequent tumorigenicity assays since it represented the highest dose at which differences in tumorigenic potential could be detected.
Phenotypic Characterization of OS521 Cells In Vitro
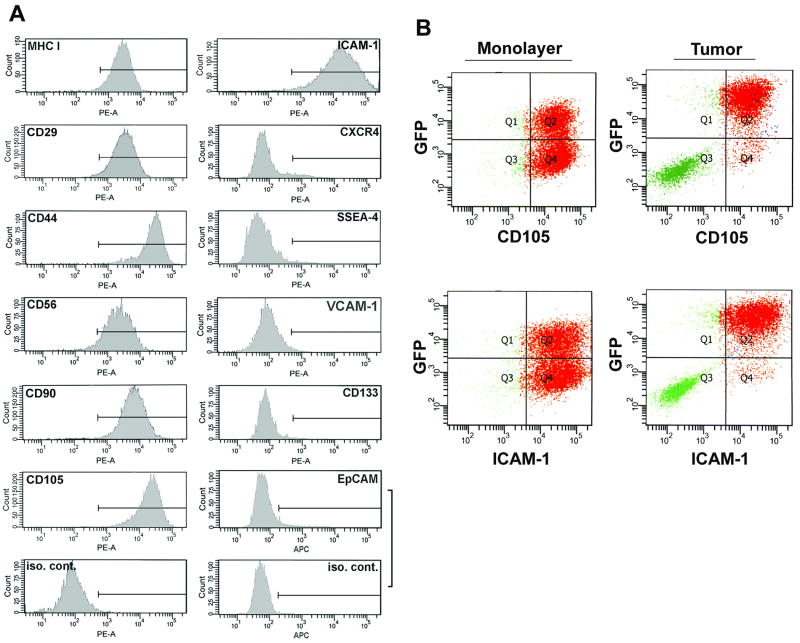
To attempt to identify phenotypically distinct subpopulations within the OS521 line for comparison in tumorigenicity assays, we characterized these cells for expression of a variety of surface antigens associated with normal and malignant stem cells in other tissues (14, 15). Monolayer cultures of OS521 were comprised of a largely homogenous population (Fig 2A); they uniformly expressed MHC Class I and the mesenchymal stem cell (MSC)-associated antigens CD29, CD90, CD105 and CD44, implicated as a marker for breast and colon cancer stem cells (14, 16-18). They also expressed adhesion molecules, ICAM-1 and CD56. OS521 cells were negative for the ES cell-specific surface antigen SSEA-4 and the chemokine receptor CXCR4. They also were negative for the tumor-associated adhesion molecule VCAM-1 and the colon cancer stem cell marker EpCAM, as well as the neural and brain tumor stem-cell associated marker CD133 (16, 19-21). This apparent lack of heterogeneity prohibited fractionation of OS521 cells based on antigen phenotype.
Figure 2. Analysis of OS521 cells for expression of stem-cell associated surface antigens.
(A) Cultures of OS521 incubated with specific antibodies or isotype controls (iso. cont.) conjugated with either phycoerythrin (PE-A) or allophycocyanin (APC), were analyzed for surface antigen expression by flow cytometry. OS521 was uniformly positive for expression of MHC I, CD29, CD44, CD56, CD90, CD105 and ICAM-1 but negative for others tested. Vertical axes represent cell number; horizontal axes the relative levels of fluorescence. Gating indicating fluorescence exceeding 95% of isotype controls designated by a horizontal bar. (B) OS521Oct-4p cells analyzed by flow cytometry for co-expression of GFP with CD105 or ICAM-1, in monolayer or xenograft tumor. Oct-4/GFP activation in tumors was closely associated with cells expressing CD105 and ICAM-1. For each plot the vertical axis represents GFP fluorescence, and the horizontal axis CD105 or ICAM-1.
A Subset of OS521 Cells Activates the Exogenous Oct-4 Promoter
We devised an alternative approach to identify distinct subpopulations based on the capacity to activate an exogenous human Oct-4 promoter element. We obtained the plasmid phOct-4/GFP, containing the entire 4 kb human Oct-4 promoter sequence positioned upstream of a GFP reporter (13). This plasmid also contains an independent SV40 promoter driven, neomycin resistance cassette, which allows for positive selection of cells that acquire the plasmid, irrespective of the capacity to activate the Oct-4 promoter.
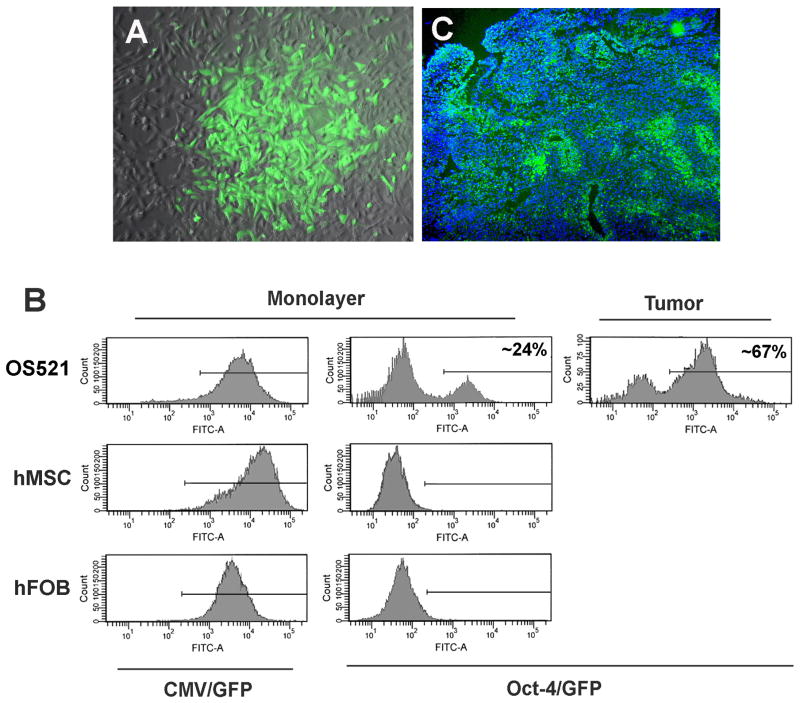
phOct-4/GFP was transfected into three cell cultures: OS521, human mesenchymal stem cells (hMSC), and a human fetal osteoblast line (hFOB). As positive controls, parallel cultures were similarly transfected with a plasmid, pEGFP-N1 containing a CMV promoter driven GFP reporter. Despite the homogeneity of the cells in culture with regard to surface proteins, following stable transfection of the OS521 line (hereafter termed OS521Oct-4p), only 24% of the G418-resistant population expressed Oct-4/GFP (Fig. 3A and B). Despite expression of the CMV/GFP reporter in >95% of the cells in all 3 cultures, Oct-4/GFP expression was observed only in OS521 (Fig. 3B).
Figure 3. OS521 is heterogeneous for expression of the Oct-4/GFP reporter.
Cultures of OS521 cells, human mesenchymal stem cells (hMSC) and fetal osteoblast cells (hFOB) were stably transfected with plasmids containing a GFP reporter, driven by the CMV-promoter/enhancer or the human Oct-4 promoter (CMV/GFP and Oct-4/GFP, respectively) (A) Fluorescence microscopy of OS521 cells stably transfected with phOct-4/GFP (OS521Oct-4p cells) shows variable GFP expression. (B) Fluorescence of the respective transfected cultures (Monolayer) using flow cytometry, and cells isolated from xenograft tumors in NOD/SCID mice generated by subcutaneous injection of 3 × 104 unfractionated OS521Oct-4p cells (Tumor). In monolayer, ∼24% of the OS521Oct-4p cells expressed the GFP reporter; while in tumors, ∼67% were GFP+. (C) Immunohistochemical detection of GFP expression in frozen sections of a xenograft tumor generated from unfractionated OS521Oct-4p cells. Discrete foci of cells expressing the Oct-4 promoter reporter (GFP+) are seen throughout the tumor.
Oct-4/GFP+ Cells from Tumors Selectively Express CD105 and ICAM-1
To explore the role of the Oct-4/GFP+ cells in tumor initiation, we injected 3 × 104 OS521Oct-4p cells (both GFP+ and GFP- cells) as well as non-transfected OS521 cells into separate groups of six NOD/SCID mice. At ∼five weeks, tumors >0.5 cm diameter had formed in 5/6 and 4/6 animals of the respective groups, indicating that integration of the phOct-4/GFP plasmid did not influence the tumor-initiating potential of the OS521 cells. Histologic examination showed the tumors were heterogeneous for Oct-4/GFP expression, exhibiting discrete regions of GFP+ and GFP- cells (Fig. 3C). Flow cytometry showed the proportion of GFP+ cells to be ∼67%, increasing nearly three-fold over the 24% observed in monolayer culture (Fig. 3B), suggesting a selective amplification of cells that are able to activate the Oct-4 promoter during tumorigenesis.
Interestingly, analysis of surface antigen expression of the cells recovered from tumors, produced results similar to those from monolayer cells shown in Figure 2A, with the notable exceptions of CD105 and ICAM-1. Expression of these antigens corresponded closely with that of Oct-4/GFP, indicating that in cells isolated directly from tumors, the capacity to transcriptionally activate the exogenous Oct-4 promoter is linked with a distinct cellular phenotype.
Oct-4/GFP+ Cell Fractions Exhibit Heightened Tumorigenicity
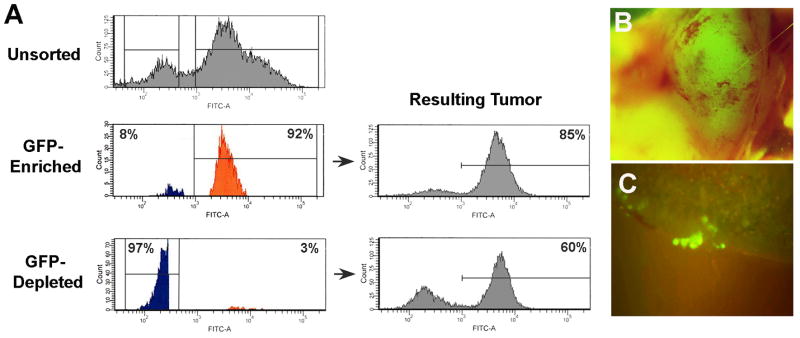
To test if the GFP+, OS521Oct-4p cells showed enhanced tumorigenesis, cells recovered from OS521Oct-4p xenograft tumors were pooled and fractionated by FACS into GFP-enriched and GFP-depleted populations. Secondary analyses showed that the enriched fraction was composed of ∼92% GFP+ cells; while in the GFP-depleted fraction, GFP+ cells numbered about 3% (Fig. 4A). Starting from a dose of 3 × 104 cells, we injected serial ten-fold dilutions of the respective fractions, as well as equivalent numbers of unfractionated OS521Oct-4p cells, into groups of eight NOD/SCID mice and examined the rate of tumor formation over a period of 90 days.
Figure 4. GFP+, OS521Oct-4p cells show heightened tumorigenicity and metastases.
(A) Fluorescence of OS521Oct-4p cells prior (Unsorted) and following fractionation. Gates used to deline GFP+ and GFP- cells are designated by brackets. The GFP-enriched fraction contained ∼92% GFP+ cells (orange); he depleted fraction was comprised of ∼97% GFP- cells (blue) and ∼3% GFP+ cells. (B) Regardless of dose, resulting tumors from both fractions were highly fluorescent. Shown is a fluorescent image of a tumor formed from 3 × 104 cells from the GFP-depleted fraction. Resulting tumors following delivery of 3 × 104 GFP-enriched or GFP-depleted cells were composed of 85% and 60% GFP+ cells, respectively. (C) Clusters of fluorescent metastatic cells in the lungs of mice following serial passage of GFP-enriched cells.
Cells in the GFP-enriched fraction proved to be >100-fold more tumorigenic than those in the GFP-depleted fraction (Table 1). At doses as low as 3 × 102 cells, the GFP-enriched fraction formed tumors in all of the mice inoculated. For the GFP-depleted group, at 3 × 103 cells, only one of eight mice developed a tumor, and at 3 × 102 cells, none of the mice developed tumors. At the lowest dose the GFP-enriched fraction was also significantly more tumorigenic than the unsorted population as only three of eight animals formed tumors. Curiously, all tumors formed from the GFP-enriched and GFP-depleted fractions were highly GFP+ (Fig. 4A and 4B). In both cases, similar to that shown in Figure 2B, expression of CD105 and ICAM-1 was restricted to the GFP+ cells. Altogether, these results indicated that tumorigenesis in OS521 is functionally linked with the capacity to activate the exogenous Oct-4 promoter.
Table 1. Tumor incidence and time to onset following xenotransplantion of fractionated OS521Oct-4p cells or an OS521Oct-4p, GFP+ clone.
The Fisher-Irwin exact test was used to determine if the probability of tumor formation for the GFP-enriched fraction was greater than the GFP-depleted or unsorted (OS521Oct-4p only) fractions. Tumor incidence = number of mice with tumors/mice injected. * indicates p-values for enriched versus unsorted populations; ˆ indicates p-values for enriched versus depleted populations.
| OS521Oct-4p | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell Dose | GFP-enriched | Unsorted | p-value* | GFP-depleted | p-valueˆ | ||||
| Incidence | Onset | Incidence | Onset | Incidence | Onset | ||||
| 3 × 104 | 8/8 | 22 days | 6/8 | 26 days | 0.233 | 5/8 | 47 days | 0.10 | |
| 3 × 103 | 8/8 | 34 days | 6/8 | 44 days | 0.233 | 1/8 | 51 days | <0.001 | |
| 3 × 102 | 8/8 | 45 days | 3/8 | 60 days | 0.012 | 0/8 | 90 days | <0.001 | |
| OS521Oct-4 p, GFP+ clone | |||||||||
| Cell Dose | GFP-enriched | GFP-depleted | p-valueˆ | ||||||
| Incidence | Onset | Incidence | Onset | ||||||
| 3 × 104 | 4/4 | 23 days | 2/4 | 47 days | 0.2143 | ||||
| 3 × 103 | 4/4 | 36 days | 1/4 | 50 days | 0.0714 | ||||
| 3 × 102 | 4/4 | 44 days | 0/4 | 90 days | 0.0142 | ||||
We passaged the OS521Oct-4p, GFP+ cells three additional times in mice, whereby the cells were injected, harvested from tumors, fractionated and re-injected at 3 × 102 cells. We found that the tumors appeared to increase in virulence with passage, producing tumors with shorter time to onset and more rapid growth rate. By the third passage we noted the formation of multiple tumor nodules following a single injection. Analysis of the lungs of these mice showed clear evidence of metastases, with clusters of GFP+ cells readily identified throughout (Fig. 4C). The formation of multiple tumor nodules or lung metastases had not been observed in any of the prior experiments suggesting that these changes were the result of selection by serial passage in vivo.
OS521Oct-4p, GFP+ Clones Generate Heterogeneous Tumors
To determine if the heterogeneity in Oct-4/GFP expression in tumors reflected differences in growth rates of pre-existing GFP+ and GFP- cell populations in the inocula, or signaled asymmetric division by GFP+, OS521Oct-4p tumor-initiating cells. Cells from OS521Oct-4p xenograft tumors were sorted by FACS and seeded at single-cell density into individual wells of 96 well plates. Oct-4/GFP+ clones arose with approximately 90% efficiency, and three were selected for expansion and characterization. We were unable to isolate GFP- cells capable of growth at low density, suggesting these cells were non-clonogenic in vitro (22-25). The disparate in vitro clonogenic potential of the GFP+ and GFP- populations was consistent with our in vivo results regarding tumorigenicity.
Each of the three Oct-4/GFP+ clonal populations was highly uniform for GFP expression in vitro (Fig. 5A), which was maintained with passage. Following delivery of 3 × 104 cells of the respective clones into NOD/SCID mice, tumors readily formed within two to three weeks. Analysis by flow cytometry showed that the tumors had re-established heterogeneity with respect to Oct-4/GFP expression as well as CD105 and ICAM-1. Upon first passage, GFP+ clones generated tumors composed of cells whose fluorescence intensity ranged over 3 logs, showing that GFP- cell populations could arise from GFP+ cells in vivo. Interestingly, by the third serial passage of unfractionated cells, the total cell population had resolved into discrete GFP+ and GFP- populations of approximately equal proportions.
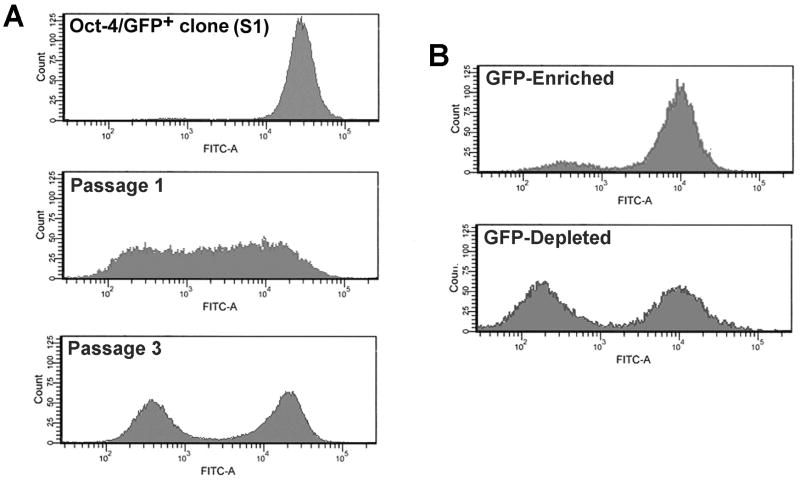
Figure 5. OS521Oct-4p GFP+ clones are capable of self-renewal and generate heterogeneous tumors following xenotransplantation.
Cells from OS521Oct-4p GFP-enriched fractions were seeded at single cell density into multi-well plates. (A) Analysis by flow cytometry of a representative clone expanded in culture (S1) shows that, in monolayer, cells uniformly expressed the exogenous Oct-4/GFP reporter. Xenotransplantation of S1 cells into NOD/SCID mice generated tumors comprised of cells that heterogeneously express the Oct-4/GFP reporter (Passage 1). Following the third passage in vivo tumors are composed of distinct populations of GFP+ and GFP- cells which were fractionated into GFP-enriched and -depleted populations and tested for relative tumor forming capacity. The GFP-enriched fraction was significantly more tumorigenic (see Table 1) (B) Tumors formed from both GFP-enriched and GFP-depleted fractions contained a significant proportion of GFP+ cells (∼ 90% and 45%, respectively).
To assay for differences in tumorigenic capacity of the GFP+ and GFP- cells we harvested third passage tumors from one of the clones (S1), fractionated the cells based on fluorescence and transplanted them at decreasing doses, similar to that described earlier. Consistent with our previous results, the GFP-enriched cell fraction was significantly more tumorigenic than the GFP-depleted fraction (Table 1). Analysis of the resulting tumor cell populations for Oct-4/GFP expression showed that the GFP-enriched fractions formed tumors composed of ∼90% GFP+ cells (Fig. 5B). Tumors derived from transplantation of GFP-depleted fractions contained ∼45% GFP+ cells. Altogether, these findings support the idea that OS521Oct-4p, GFP+ cells are capable of tumor initiation and self-renewal, and can give rise to functionally disparate cell populations in xenografts.
Discussion
Characteristics of Osteosarcoma Tumor-Initiating Cells
Among cells from a primary tumor biopsy, the sub-population capable of activating an Oct-4 promoter/GFP reporter construct showed significantly enhanced tumorigenic activity. Following ectopic transplantation, these cells reliably formed tumors at doses of 300 cells or less, representing greater than 100-fold enrichment of tumorigenic capacity. Phenotypic characterization showed that the tumor-initiating cells selectively expressed surface antigens CD105 and ICAM-1.
This tumorigenic osteosarcoma cell appears both highly prolific and plastic, such that it not only comprises a majority of the cells in a tumor, but gives rise to phenotypically divergent progeny (CD105 and ICAM-1 negative) that are incapable of activating the Oct-4 promoter or efficiently forming tumors in the xenograft model. Despite the finding that this cell activates an ES cell-specific promoter element, we did not detect expression of ES cell surface markers SSEA-4 and CXCR4. Instead, tumor-initiating cells remained intrinsically mesenchymal, expressing surface antigens commonly associated with MSCs (CD29, CD44, CD56, CD90 and CD105). Moreover, the tumors formed displayed pleomorphic, malignant, spindle cells that secrete osteoid, the characteristic osteosarcoma phenotype (26). We found no link between tumorigenicity and expression of CD133, EpCAM, or CD44, markers associated with tumor-initiating cells in brain, breast and colon cancer (14, 17, 18, 21).
Generation of Oct-4/GFP+ Tumors from GFP-depleted Cell Populations
We routinely observed tumors that were highly GFP+ arising from transplantation of GFP-depleted cell fractions. This is likely attributable to one of two scenarios. The first is that GFP- cells within the Oct-4/GFP-depleted fractions acquired the capacity to re-activate the Oct-4 promoter/reporter, resulting in a high percentage of GFP+ cells in the tumor. We feel a more plausible explanation is that the ∼3% GFP+ cells contaminating the GFP-depleted fractions were sufficient to initiate tumor formation, and thereby generated tumors that were largely GFP+. Since as few as 300 cells from the GFP-enriched fractions readily formed tumors in mice, it seems reasonable then that the ∼900 contaminating GFP+ cells in the 3 × 104 cell dose (3% of 3 × 104) of the GFP-depleted fraction would likewise be capable of tumorigenesis (see Table 1). If the contaminating GFP+ cells are indeed responsible for generating the tumors in the GFP-depleted fractions, then our approximation of 100-fold increased tumorigenic activity in these cells is a vast underestimate.
Oct-4/GFP Expression and Tumorigenesis in Osteosarcoma
The expression of the Oct-4/GFP reporter appears directly linked to tumorigenesis in our xenograft model. While we have not yet elucidated the exact nature of this relationship, it suggests the molecular machinery of ES cells is active in osteosarcoma and is critical to the phenotype of the tumor-initiating cell.
The Oct-4/GFP reporter construct used in these studies contains the elements critical for Oct-4 tissue-specific gene expression and includes both the distal and proximal enhancers. Gerrard et al. (13) showed that in human ES cells stably transfected with this construct, GFP expression faithfully represented expression of endogenous Oct-4 protein in undifferentiated ES cells and its subsequent loss during neural differentiation. Similarly, in our studies the loss of Oct-4/GFP expression was associated with an apparent differentiation event, evidenced by a reduction in tumorigenic potential and a change in cell surface phenotype. Furthermore, the promoter/reporter was only active in our tumor-initiating cells and not in the normal MSCs or the more differentiated osteoblast cell line.
We have attempted to characterize Oct-4 expression in OS521Oct-4p cells using several methods, including RT-PCR, Western blot and immune staining of cells and tumor sections; however, discrepancies were noted in the results among these assays. Indeed, definitive detection of Oct-4 expression remains an area of active controversy in the cancer literature (27, 28). The identification of numerous Oct-4 pseudogenes and splice variants, as well as reports questioning the specificity of the commercially available antibodies used to detect this protein cloud the issue surrounding Oct-4 expression (27, 28).
Regardless of our inability to conclusively correlate endogenous Oct-4 protein with the activation of our reporter, other groups using similar Oct-4 promoter driven constructs have shown that activation of the exogenous promoter is restricted to ES and primordial germ cells in vitro (29, 30). Moreover, in transgenic mice, expression of the exogenous Oct-4 promoter is specifically limited to germ line cells in embryos and adult mice (30, 31). Additionally, Oct-4/GFP expression is used routinely as an indicator of cellular reprogramming following transplant of somatic nuclei into ooplasms and during fusion of somatic and ES cells (32-37). Therefore, whether or not Oct-4 specifically contributes to oncogenesis in osteosarcoma, activation of the Oct-4 promoter/reporter in the osteosarcoma initiating cells suggests that these cells have likewise undergone cellular reprogramming and possess a transcriptional profile related to that of embryonic cells (38, 39). This implies that the regulatory networks controlling stem cell function are active in osteosarcoma and are functionally linked to tumorigenesis.
Although the data shown here are derived from a single osteosarcoma, we have since established three additional primary osteosarcoma cultures capable of forming tumors in the NOD/SCID mouse. These tumor lines have been stably transfected with the Oct-4/GFP construct, and ongoing studies demonstrate heterogeneous GFP expression, suggesting that the activation of exogenous Oct-4 promoter may be a generalized phenomenon in this type of cancer. We are currently evaluating the relative tumorigenicity of the respective GFP-enriched and depleted cell fractions for these lines.
An Osteosarcoma Stem Cell?
Tumorigenesis in osteosarcoma appears most consistent with the cancer stem cell model, as defined by the prospective selection of a discrete sub-population of cells within a tumor with enhanced tumorigenic capacity (8). In addition to activating the Oct-4 promoter and bearing surface antigens frequently associated with MSCs, the tumor-initiating cells we identified possess several stem-like properties. Clonal populations generate antigenically distinct progeny and give rise to heterogeneous tumors comprised of tumorigenic and non-tumorigenic cell populations. These tumor-initiating cells also self-renew, as demonstrated through serial transplantation in NOD/SCID mice, and spontaneously metastasize.
In contrast to other malignancies in which cancer stem cells are described as rare and slowly dividing (16), osteosarcoma tumor-initiating cells appear highly proliferative and comprise much of the tumor cell population. This may be a manifestation of their enhanced capacity for self-renewal and a more plastic cellular phenotype, enabling the initiating cells to adapt to the stringent environment of the xenograft. This is supported by the observation that in vitro the percentage of cells expressing Oct-4/GFP remains stable; yet, significant changes in the proportion of GFP+ and GFP- cells are observed following a single passage in vivo. Further, following serial transplantation we observed an increase in virulence and the acquisition of metastatic capability, suggesting a selective adaptation analogous to the process of tumor progression (5, 40, 41).
The preponderance of GFP+ cells in our xenografts may therefore represent selection and early expansion of the tumor-initiating population by symmetric division. Once a critical mass has been achieved and a suitable microenvironment formed, the cells then begin to differentiate, producing the GFP- cells. This is supported by the image shown in Figure 3, displaying dense zones of Oct-4/GFP+ cells distributed throughout a xenograft tumor. Using this interpretation, it is possible that the xenograft tumors exhibit a disproportionately high percentage of tumor-initiating cells because of their small size at the time of harvest, which may not have permitted sufficient time for both expansion and generation of a mature cellular hierarchy.
Alternatively, it may mean that osteosarcomas in general are comprised of a large percentage of highly-proliferative, tumor-initiating cells. This could explain the extreme virulence of this form of cancer, and of this cell line in particular. The aggressiveness of a specific sarcoma, is directly related to its' histologic grade, determined predominantly by the degree of differentiation. Grade has been shown to be the single most predictive variable related to survival in patients not already having metastases (42). Interestingly, OS521 was derived from a poorly differentiated osteosarcoma that exhibited scant osteoid production. The clinical course of this patient was one of rapid progression to metastases and death despite chemotherapy, reflecting the aggressive appearance of the histology.
Acknowledgments
Grant Support: National Cancer Institute R21CA123421 (C. Parker Gibbs) National Institute of Arthritis and Musculoskeletal and Skin Diseases R01AR050249 (Steven C. Ghivizzani)
Special thanks to the Jay Shields Osteosarcoma Foundation and the McKnight Brain Institute Cancer Stem Cell Initiative
Footnotes
Potential Conflicts of Interest: There are no conflicts of interest.
References
- 1.Gibbs CP, Kukekov VG, Reith JD, et al. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7(11):967–76. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffe N, Carrasco H, Raymond K, Ayala A, Eftekhari F. Can cure in patients with osteosarcoma be achieved exclusively with chemotherapy and abrogation of surgery? Cancer. 2002;95(10):2202–10. doi: 10.1002/cncr.10944. [DOI] [PubMed] [Google Scholar]
- 3.Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005;23(9):2004–11. doi: 10.1200/JCO.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Bruland OS, Pihl A. On the current management of osteosarcoma. A critical evaluation and a proposal for a modified treatment strategy. Eur J Cancer. 1997;33(11):1725–31. doi: 10.1016/s0959-8049(97)00252-9. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 6.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112(13):4793–807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 7.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 8.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 9.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3(12):895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 10.Klein MJ, Siegal GP. Osteosarcoma: anatomic and histologic variants. Am J Clin Pathol. 2006;125(4):555–81. doi: 10.1309/UC6K-QHLD-9LV2-KENN. [DOI] [PubMed] [Google Scholar]
- 11.Unni KK. Osteosarcoma of bone. J Orthop Sci. 1998;3(5):287–94. doi: 10.1007/s007760050055. [DOI] [PubMed] [Google Scholar]
- 12.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 13.Gerrard L, Zhao D, Clark AJ, Cui W. Stably transfected human embryonic stem cell clones express OCT4-specific green fluorescent protein and maintain self-renewal and pluripotency. Stem Cells. 2005;23(1):124–33. doi: 10.1634/stemcells.2004-0102. [DOI] [PubMed] [Google Scholar]
- 14.Cho RW, Clarke MF. Recent advances in cancer stem cells. Curr Opin Genet Dev. 2008;18(1):48–53. doi: 10.1016/j.gde.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 16.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 17.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104(24):10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidemann J, Maaser C, Lugering A, et al. Expression of vascular cell adhesion molecule-1 (CD 106) in normal and neoplastic human esophageal squamous epithelium. Int J Oncol. 2006;28(1):77–85. [PubMed] [Google Scholar]
- 20.Osta WA, Chen Y, Mikhitarian K, et al. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64(16):5818–24. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 22.Hamburger A, Salmon SE. Primary bioassay of human myeloma stem cells. J Clin Invest. 1977;60(4):846–54. doi: 10.1172/JCI108839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197(4302):461–3. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 24.Hamburger AW, Salmon SE, Kim MB, et al. Direct cloning of human ovarian carcinoma cells in agar. Cancer Res. 1978;38(10):3438–44. [PubMed] [Google Scholar]
- 25.Page RH, Tilchen EJ, Davis HL. Effects of tumor cell viability and inoculum density on growth parameters in the human tumor, soft-agar clonogenic assay. Cancer Res. 1988;48(14):3944–8. [PubMed] [Google Scholar]
- 26.Gibbs CP, Jr, Weber K, Scarborough MT. Malignant bone tumors. Instr Course Lect. 2002;51:413–28. [PubMed] [Google Scholar]
- 27.Liedtke S, Stephan M, Kogler G. Oct4 expression revisited: potential pitfalls for data misinterpretation in stem cell research. Biol Chem. 2008;389(7):845–50. doi: 10.1515/BC.2008.098. [DOI] [PubMed] [Google Scholar]
- 28.Zuk PA. The intracellular distribution of the ES cell totipotent markers OCT4 and Sox2 in adult stem cells differs dramatically according to commercial antibody used. J Cell Biochem. 2009;106(5):867–77. doi: 10.1002/jcb.22054. [DOI] [PubMed] [Google Scholar]
- 29.Veraitch FS, Scott R, Wong JW, Lye GJ, Mason C. The impact of manual processing on the expansion and directed differentiation of embryonic stem cells. Biotechnol Bioeng. 2008;99(5):1216–29. doi: 10.1002/bit.21673. [DOI] [PubMed] [Google Scholar]
- 30.Yeom YI, Fuhrmann G, Ovitt CE, et al. Germline regulatory element of Oct-4 specific for the totipotent cycle of embryonal cells. Development. 1996;122(3):881–94. doi: 10.1242/dev.122.3.881. [DOI] [PubMed] [Google Scholar]
- 31.Ohmura M, Yoshida S, Ide Y, Nagamatsu G, Suda T, Ohbo K. Spatial analysis of germ stem cell development in Oct-4/EGFP transgenic mice. Arch Histol Cytol. 2004;67(4):285–96. doi: 10.1679/aohc.67.285. [DOI] [PubMed] [Google Scholar]
- 32.Boiani M, Eckardt S, Scholer HR, McLaughlin KJ. Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev. 2002;16(10):1209–19. doi: 10.1101/gad.966002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Do JT, Scholer HR. Nuclei of embryonic stem cells reprogram somatic cells. Stem Cells. 2004;22(6):941–9. doi: 10.1634/stemcells.22-6-941. [DOI] [PubMed] [Google Scholar]
- 34.Han DW, Do JT, Gentile L, Stehling M, Lee HT, Scholer HR. Pluripotential reprogramming of the somatic genome in hybrid cells occurs with the first cell cycle. Stem Cells. 2008;26(2):445–54. doi: 10.1634/stemcells.2007-0553. [DOI] [PubMed] [Google Scholar]
- 35.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269–75. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 36.Tada M, Tada T, Lefebvre L, Barton SC, Surani MA. Embryonic germ cells induce epigenetic reprogramming of somatic nucleus in hybrid cells. Embo J. 1997;16(21):6510–20. doi: 10.1093/emboj/16.21.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11(19):1553–8. doi: 10.1016/s0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 38.Miremadi A, Oestergaard MZ, Pharoah PD, Caldas C. Cancer genetics of epigenetic genes. Hum Mol Genet. 2007;16(Spec No 1):R28–49. doi: 10.1093/hmg/ddm021. [DOI] [PubMed] [Google Scholar]
- 39.Shukla V, Vaissiere T, Herceg Z. Histone acetylation and chromatin signature in stem cell identity and cancer. Mutat Res. 2008;637(12):1–15. doi: 10.1016/j.mrfmmm.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Dalerba P, Clarke MF. Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell. 2007;1(3):241–2. doi: 10.1016/j.stem.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–8. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 42.Peabody TD, Gibbs CP, Jr, Simon MA. Evaluation and staging of musculoskeletal neoplasms. J Bone Joint Surg Am. 1998;80(8):1204–18. doi: 10.2106/00004623-199808000-00016. [DOI] [PubMed] [Google Scholar]