Abstract
Background
Irritability is prevalent and impairing in pediatric bipolar disorder (BD) but has been minimally studied using neuroimaging techniques. We used magnetoencephalography (MEG) to study theta band oscillations in the anterior cingulate cortex (ACC) during frustration in BD youth. ACC theta power is associated with attention to emotional stimuli, and the ACC may mediate responses to frustrating stimuli.
Methods
We used the affective Posner task, an attention paradigm that uses rigged feedback to induce frustration, to compare 20 medicated BD youth (14.9±2.0 years; 45% male) and 20 healthy controls (14.7±1.7 years; 45% male). MEG measured neuronal activity following negative and positive feedback; we also compared groups on reaction time, response accuracy, and self-reported affect. Patients met strict DSM-IV BD criteria and were euthymic. Controls had no psychiatric history.
Results
BD youth reported more negative affective responses than controls. Following negative feedback, BD subjects, relative to controls, displayed greater theta power in the right ACC and bilateral parietal lobe. Following positive feedback, BD subjects displayed lower theta power in the left ACC than did controls. Correlations between MEG, behavior, and affect were nonsignificant.
Conclusion
In this first MEG study of BD youth, BD youth displayed patterns of theta oscillations in the ACC and parietal lobe in response to frustration-inducing negative feedback that differed from healthy controls. These data suggest that BD youth may display heightened processing of negative feedback and exaggerated self-monitoring following frustrating emotional stimuli. Future studies are needed with unmedicated bipolar youth, and comparison ADHD and anxiety groups.
Keywords: irritability, attention, neuroimaging, anterior cingulate cortex, parietal lobe, theta
Introduction
Of the many impairing symptoms associated with pediatric bipolar disorder (BD), irritability is one of the most debilitating (1-3). Despite the importance of irritability in pediatric BD, little work examines its pathophysiology.
In prior research, we studied irritability in pediatric BD by adding an emotional component to a standard attention task, the affective Posner (4), to induce frustration (5;6). During an initial non-emotional condition, BD and control subjects displayed comparable affect, behavior, and psychophysiology (electroencephalography, EEG, event-related potentials, ERPs). However, when rigged negative feedback was introduced, BD youth, relative to controls, reported more negative affect, exhibited slower reaction time, and displayed decreased P300 amplitude, an index of attention allocation. These results suggest that BD youth had attention deficits when frustrated, perhaps because of their heightened negative emotional state.
Here we extend this work using magnetoencephalography (MEG) in an independent sample of euthymic youth with BD to clarify the neuronal activity of frustration. MEG measures instantaneous changes in neuronal activity (7), and thus has excellent temporal resolution, i.e. to the millisecond (8;9). While MEG and EEG have comparable temporal resolution, MEG data analytic techniques such as Synthetic Aperture Magnetometry (SAM), allow superior spatial resolution. SAM can localize neuronal oscillations to specific brain regions, within different frequency bands, and in response to particular stimuli (10-13), e.g. those which elicit frustration.
Our MEG analyses focused on theta band (4-8 Hz) power generated by the anterior cingulate cortex (ACC). Theta power generated by frontal-midline structures such as the ACC is thought to facilitate attention to, and processing of, emotional stimuli (14-23), including evaluative feedback (24), and is also associated with self-monitoring (25;26); i.e. the conditions involved in the affective Posner task. In addition, the ACC has been implicated in the pathophysiology of frustration (27;28), and ACC structural (29;30) and functional (31;32) aberrations have been documented in BD youth.
Using MEG and the affective Posner task, we sought to identify how theta oscillations in the ACC differed in euthymic youth with BD vs. controls following the presentation of negative or positive feedback, in the context of frustration. We predicted that, when frustrated, BD youth would display greater theta power in the ACC than would controls, indicating heightened attention to negatively-valenced events.
Materials and Methods
Participants
Twenty BD youth and 20 healthy controls enrolled in an IRB-approved study at the National Institute of Mental Health (NIMH). Subjects and a guardian provided written informed assent/consent.
All subjects and a parent completed the Kiddie-Schedule for Affective Disorders-Present and Lifetime Version (K-SADS-PL) (33), a diagnostic interview administered to parents and children separately by graduate level clinicians with established reliability (i.e. kappa ≥ 0.9). All BD subjects met strict DSM-IV (34) criteria for BD, including a history of at least one hypomanic or manic episode meeting full duration criteria —i.e. lasting ≥ 4 days-- characterized by elevated mood (35). Comorbid diagnoses were diagnosed on the basis of symptoms present during euthymia (Table 1). Medicated patients were included because the high rates of medication use in bipolar youth made enrolling only unmedicated patients extremely challenging. Also, it was not ethical for us to require medication cessation solely for research purposes
Table 1.
Demographic Data
| BD | Control | p value | |
|---|---|---|---|
| Age, years | 14.9±2.0 | 14.7±1.7 | .73 |
| Sex (male): % (N) | 45 (9) | 45 (9) | 1 |
| IQ | 108.9±17.7 | 111.5±9.2 | .58 |
| CGAS | 51.3 ± 11.1 | — | — |
| Euthymic Mood State: % (N) | 100 (20) | — | — |
| Diagnoses: % (N) | |||
| BP I | 80 (16) | — | — |
| BP II | 20 (4) | — | — |
| ADHD | 60 (12) | — | — |
| GAD | 20 (4) | — | — |
| Separation anxiety | 25 (5) | — | — |
| Simple phobia | 10 (2) | — | — |
| Any anxiety | 40 (8) | — | — |
| ODD | 20 (4) | — | — |
| Average Number of Diagnoses | 2.7±1.6 | ||
| Medicated: % (N) | |||
| mood stabilizers | 55 (11) | — | — |
| antipsychotics | 60 (12) | — | — |
| lithium | 40 (8) | — | — |
| stimulants | 25 (5) | — | — |
| antidepressants | 40 (8) | — | — |
| Average Number of Medications | 2.4±1.4 | — | — |
Note. BD = Bipolar Disorder; CGAS = Children's Global Assessment Scale; ADHD = Attention Deficit Hyperactivity Disorder; GAD = Generalized Anxiety Disorder; ODD = Oppositional Defiant Disorder; All diagnoses are current.
Control subjects had no psychiatric history in themselves or first-degree relatives, as determined using the KSADS and a review of family psychiatric history.
All participants had normal physical and neurological history. Exclusion criteria included I.Q. < 70 [as measured with the Wechsler Abbreviated Scale of Intelligence (WASI) (36)], pervasive developmental disorder, unstable medical illness, or substance abuse within two months.
BD patients' mood was evaluated with the Children's Depression Rating Scale (CDRS) (37) and the Young Mania Rating Scale (YMRS) (38). The Children's Global Assessment Scale (CGAS) (39) measured general level of function.
Procedure
Affective Posner Task
All subjects completed the affective Posner task (4-6) (Figure 1), a modified version of the standard Posner paradigm (40). The task consisted of three conditions of 100 trials each. Conditions involved the same stimuli and instructions, but differed in feedback. A fixation cross appeared in the center of the screen (750 msec), followed by two boxes arranged horizontally (300 msec). The cue consisted of one box illuminating blue (200 msec). Following this, a target square appeared inside one of the boxes (maximum 1260 msec, depending on response). Subjects were instructed to press the button (i.e. left, right) matching the target location. Feedback was presented (2000 msec) following the response.
Figure 1. Affective Posner task.
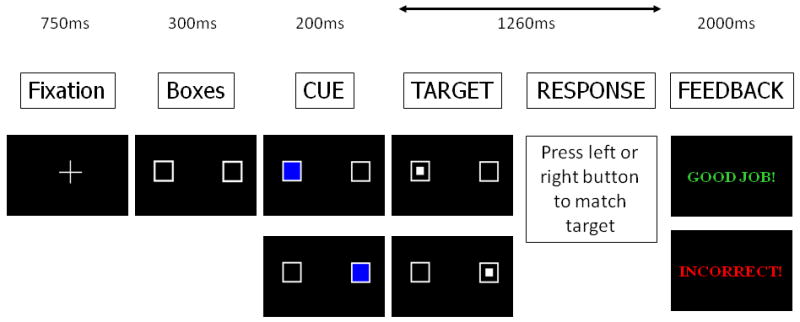
Figure details the affective Posner task, which examines attention during different emotional contexts, including frustration in response to rigged negative feedback.
Condition 1 was the non-emotional baseline; feedback told subjects of their response accuracy (“Good job!” or “Incorrect!”). Condition 2 introduced contingencies: subjects won or lost twenty-five cents on each trial, based on performance (“Great Job! Win 25 Cents” or “Wrong! Lose 25 Cents”). Condition 3 had the same contingencies as condition 2, but rigged negative feedback was added to induce frustration. After 44% of correct responses, subjects received accurate feedback and reward (“You're Quick! Win 25 Cents”). However, after 56% of correct responses, rigged feedback informed the subject that he/she was too slow and lost money (“Too Slow! Lose 25 Cents”), even though there was no timing component to the task. Incorrect responses always resulted in punishment feedback (“Wrong! Lose 25 Cents”). The frustration elicited by the task did not exceed minimal risk standards of pediatric research i.e., it was no greater than that encountered in typical daily experience. Further, no participant displayed affect or behavior necessitating the cessation of the task, nor did any subject request that the task be terminated.
Data collected included reaction time (RT) and response accuracy (i.e. percentage of responses matching target location). Self-reported mood was collected after each condition, with subjects rating two aspects of their affective response [valence: happy/sad; arousal: calm/excited] using the Self-Assessment Manikin (41) line-drawings.
MEG Recording
Neuromagnetic data were collected with a whole-head 275-sensor MEG system (CTF Systems Inc., Vancouver, Canada), located in a magnetically shielded room (Vacuumschmelze, Germany). The MEG signal was recorded with a sampling rate of 600 Hz (bandwidth: 0–150 Hz). Each sensor was configured as a first-order axial gradiometer with 18 mm coils and 50 mm baseline, with an average spacing of 22 mm. Head positioning was measured continuously to monitor the participant's movement. Subjects included in the analyses did not have movement exceeding 6 mm.
Anatomical MRI Recording and Coregistration
Each participant received a high resolution T-1 weighted structural magnetic resonance image (sMRI) with a 3-Tesla or 1.5-Tesla scanner (GE Signa, Milwaukee, WI.) We used a standardized magnetization prepared gradient echo sequence (180 1.2 mm sagittal slices; FOV = 24; NEX = 1; TR = 11.4 ms; TE = 4.4 ms, matrix = 256×256×256; TI= 300 ms; bandwidth = 130 Hz/pixel, 33 kHz/256 pixels). Anatomical scans were transformed into Talairach space using AFNI (42).
To facilitate localization and spatial coregistration of the MRI and MEG data, MRIs were converted into AFNI format and co-registered with the MEG data by aligning fiducial points. MEG source localizations were calculated using multi-sphere head models derived from individual participants' MRIs. Group 3D-maps of event-related activity were calculated with Talairach-aligned volumes using AFNI (42) software. We employed standard 6 mm voxel spacing.
Data Analysis
Behavioral and Affective Data
Analyses focused on the rigged-feedback condition 3 because: 1) our a priori hypotheses concerned performance in a frustrating context, and; 2) we were unable to examine performance following negative feedback in conditions 1 and 2 because of high performance accuracy (i.e. ≥ 98% in both groups). Such high levels of performance generate an insufficient number of post-negative feedback trials to meaningfully analyze behavioral or neural responses. Thus, we used a series of 2 (group: BD vs. CON) × 2 (positive vs. negative feedback) ANOVAs to compare self-reported mood and behavioral performance during the frustration-inducing condition 3.
MEG Data
MEG raw data was filtered in 3rd gradient mode for noise reduction using reference coils with fixed weights along with DC offset removal, minimal highpass filter (0.61 Hz), and powerline (60 Hz) filtering.
To determine the optimal time-frequency windows for analysis, we conducted Stockwell Transformations (ST) (43). ST produces a time-frequency (TF) representation of a real signal with absolute phase information. The ST was performed on raw MEG signals for channels overlying areas of interest (e.g. frontal channels for ACC) for each subject. The complex ST was then squared, yielding power for each channel. The resulting TF arrays of power were then averaged across channels and trials. We performed this operation in the active (post feedback) and control (fixation) time windows beginning 200 msec before the onset of feedback and extending to 900 msec after feedback. Visual inspection of this window indicated it was of sufficient length to capture theta oscillations that might be evoked by feedback (25;44-46). We used a non-parametric Wilcoxon test (42) to identify TF regions showing a significant difference between the active and control arrays both within and between groups. Results of the ST supported the use of a 700 msec window beginning immediately following feedback presentation as the basis for analysis. As an example, Figure 2 depicts the comparison between BD and CON samples of the TF representations of channels overlying the right medial frontal sensors (i.e. sensors likely to record ACC activity).
Figure 2. Between-group (BD vs. CON) comparison of Stockwell transformations of raw theta power at right medial frontal sensors.
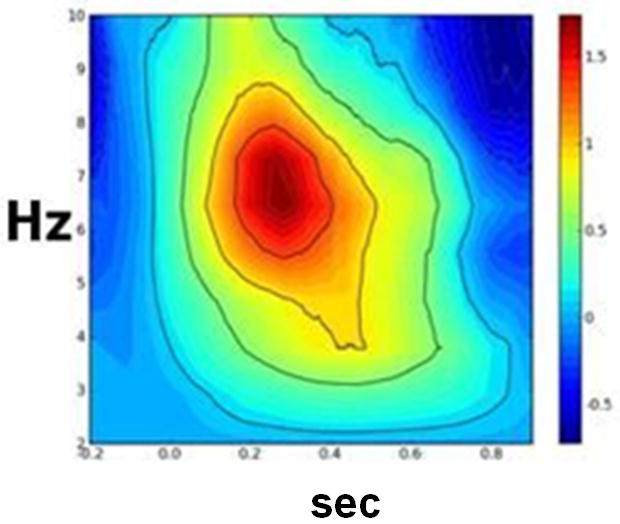
Note: BD = bipolar disorder; CON = control. Figure displays the comparison between BD and CON samples of the time-frequency representations of the averaged raw theta power in response to the presentation of negative feedback as compared to theta power in response to the presentation of the fixation cross. 0.0 on the X-axis reflects the onset of feedback or fixation cross.
To investigate condition-related cortical activation, the Synthetic Aperture Magnetometry (SAM) beamformer technique was used to calculate the electromagnetic source power distribution for individual voxels (10-12). SAM produces a 3D representation of brain activity by using the recorded magnetic field across the entire array of 275 sensors and computing a set of beamformer weights to estimate activation. At each voxel an optimal spatial filter (beamformer) is determined through a minimum variance procedure that reduces extraneous power sources but preserves signal from the voxel to limit potential artifacts. The orientation of the source dipole is estimated by the vector based approach of Sekihara et al. (47).
For each subject the SAM procedure calculated pseudo F-values (10;48;49) to provide a ratio of power between the active state (700 msec immediately following feedback presentation) and control state (700 msec window in which the fixation cross appeared). At the individual-subject level, SAM volumes were normalized to Z-scores by subtracting the mean and dividing by the standard deviation of the entire volume. They were then averaged and transformed into common Talairach coordinate space.
Then, at the between-subjects level, the normalized pseudo F-values were compared between the BD and control samples. Specifically, we examined the neural response to both negative and positive feedback in the frustration-inducing condition 3 to determine if our results reflected activation to feedback generally or, alternatively, if there was a differential response to feedback type in BD and control subjects. We conducted a 2 × 2 ANOVA on theta band power with group (BD vs. control) and feedback type (negative vs. positive) as our independent variables. Increased theta power (i.e. synchronization) indicated neuronal activation (50-53).
We conducted whole brain analyses and interpreted results that survived false discovery rate (FDR) and cluster detection analysis (CDA) procedures. These are methods standard to neuroimaging research to account for the number of analyses and brain voxels and thereby reduce the possibility of Type I and II error (48;54-59). FDR analyses correct for multiple comparisons by estimating the percentage of false positives across the entire brain volume for a given statistical threshold. This is based on the observed distribution of p values; we choose an FDR of .1 (or 10%) based on previous recommendations (60;61). CDA analyses identified connected voxels of activation that survived the threshold determined by FDR analyses. This best identifies and controls for voxels more likely to be false positives given the extent to which they are contiguous with other voxels that survived the FDA threshold procedure. We defined a cluster connection radius (rmm) of 11 mm, based on our 6 mm voxel size, and a minimum cluster volume (vmul) of 648 micro-liters (3 voxels). This procedure is consistent with, or more conservative than, recent MEG studies using cognitive/behavioral tasks (7;49;62-65). We report those regions that survived this procedure for the 2-way ANOVA interaction (group × feedback type). All results are reported in Left, Posterior, Inferior (LPI) coordinates and reflect the peak activation voxel of that region. The presence of outliers was defined using the SPSS (66) convention of data points greater than three standard deviations.
Secondary analyses used independent-sample t-tests to compare theta power in BD subjects with and without comorbid ADHD or anxiety (separately) as well as those taking vs. not taking various classes of psychotropic medications.
Results
Participant Demographics and Clinical Data
BD (14.9±2.0 years; 45% male; IQ=108.9±17.7) and control (CON) (14.7±1.7 years; 45% male; IQ=111.5±9.2) subjects did not differ in age (t38=.35, p=.73)], gender (X240=.00, p=1.00), or IQ (t38=.-56, p=.58).
Within the BD sample, 80% (N=16) had BDI, while the other 20% had BDII. Patients had an average of 2.7 ± 1.6 diagnoses and 90% (N=18) of patients were medicated (Table 1). CDRS (mean= 24.4 ± 5.7) and YMRS (mean= 5.8 ± 3.6) scores indicated that 100% (N=20) of our BD subjects were euthymic (i.e. CDRS ≤ 40 and YMRS ≤ 12). CGAS scores (51.3 ± 11.1) indicated that the BD sample was moderately to severely impaired.
Affective Data
We conducted two 2×2 ANOVAs (group; feedback type) examining self-report mood after condition 3: one for valence (i.e. happy/sad), the other for arousal (i.e. calm/excited). For valence, the group × feedback valence interaction was significant [F(1,38)=5,48, p=.02] as was the group main effect [F(1,38)=8.60, p=.006]. BD subjects were significantly more upset during condition 3 than controls, and more specifically, BD subjects were significantly more upset than controls following negative feedback (p=.01) but not following positive feedback (p=.20). We should note that there was one outlier in the BD sample for valence after negative feedback. Removal of this subject did not change our results.
The ANOVA for arousal during condition 3 found a nonsignificant interaction [F(1,38)=1.52, p=.23] and a nonsignificant main effect for group [F(1,38)=1.13, p=.29].
Behavioral Data
The between-group ANOVA comparing RT following negative vs. positive feedback on condition 3 found a nonsignificant group × feedback valence interaction [F(1,38)=.81, p=.37]. The group main effect was significant [F(1,38)=5.85, p=.02], indicating that BD subjects were slower to respond than controls regardless of feedback valence.
The ANOVA comparing response accuracy found a nonsignificant group × feedback valence interaction [F(1,38)=.04, p=.83], and a nonsignificant group main effect [F(1,38)=.69, p=.41]. There were no outliers in the behavioral data.
MEG Data
Whole-brain analyses revealed a significant group (BD vs. control) × feedback (negative vs. positive) interaction in the theta band in two regions: the bilateral ACC and parietal lobe.
Specifically, the two clusters in the ACC displaying a significant group × feedback interaction were: 1) left ACC [Brodmann Area (BA) 10/9; 1,47,14], [F(1,76)=11.13, p=.001]; and 2) right ACC [BA 32; 17,31,28], [F(1,76)=11.91, p=.001] (Table 2; Figures 3 and 4).
Table 2.
Regions in which theta power differed between youth with bipolar disorder and healthy controls following presentation of negative and positive feedback
| Region | BA | X,Y,Z | Between-group comparison of neural activation | t value | p value |
|---|---|---|---|---|---|
| Anterior Cingulate Cortex (ACC): L | 10/9 | 1,47,14 | Pos. fdbk.: CON > BD | -3.42 | .0009 |
| Anterior Cingulate Cortex (ACC): R | 32 | 17,31,28 | Neg. fdbk.: BD > CON | 2.99 | .004 |
| Inferior Parietal Lobule (IPL): L | 40 | -55,-43,35 | Neg. fdbk.: BD > CON | 2.83 | .006 |
| Superior Parietal Lobule (SPL): R | 7 | 20,-67,45 | Neg. fdbk.: BD > CON | 3.16 | .002 |
Note. Regions identified based on significant group (BD vs. CON) × feedback type (negative vs. positive) ANOVA interaction. Greater theta band power = greater neural activation; BA=Brodmann Area; X,Y,Z = Talairach space coordinates reflecting the peak voxel of that region; L = left; R = right; Pos. fdbk. = after positive feedback; Neg. fdbk. = after negative feedback; BD = bipolar disorder; CON = control.
Figures 3 & 4. Theta band power differences between youth with bipolar disorder and healthy controls in the left and right anterior cingulate cortex (ACC).
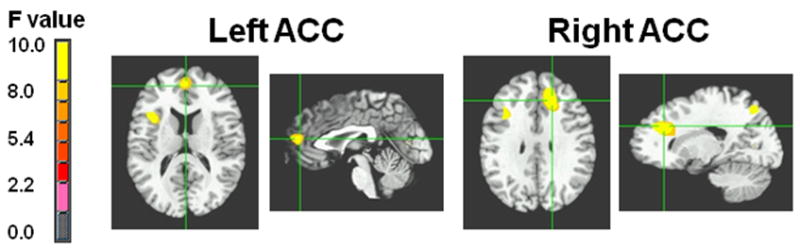
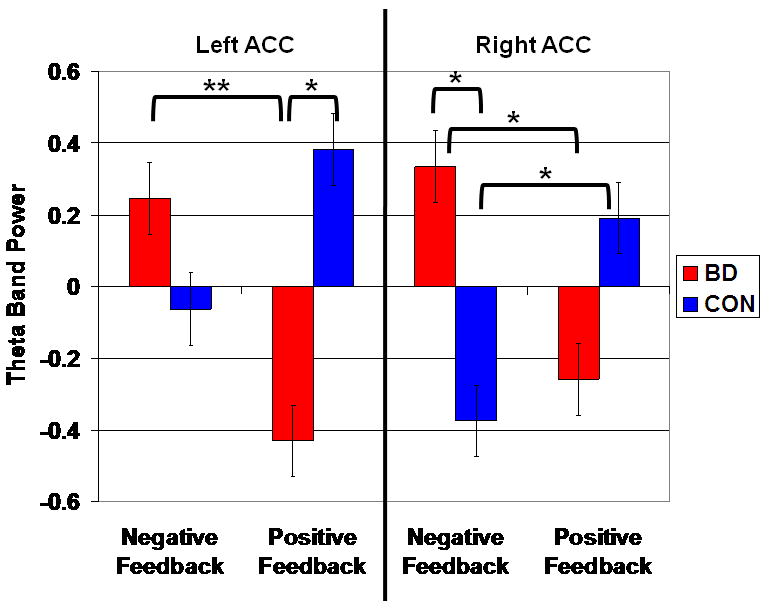
Note: BD = bipolar disorder; CON = control. Figure displays the locations in the left ACC (BA 10/9; 1,47,14) where BD subjects (N=20) had lower theta power than controls (N=20) following positive feedback, and the right ACC (BA 32; 17,31,28) where BD subjects had greater theta power than controls following negative feedback.
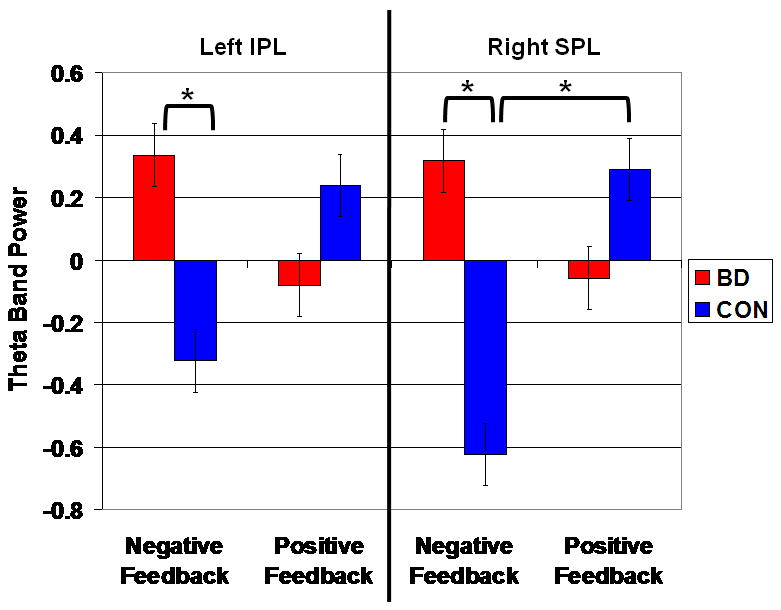
Post hoc analyses of the left ACC found that, following positive feedback, BD subjects had significantly lower theta power (i.e. lower activation) than controls (t38=-3.42, p=.0009). The between-group comparison following negative feedback was nonsignificant (t38=1.30, p=.20).
Post hoc analyses of the right ACC data found that, following negative feedback, BD subjects had significantly greater theta power than controls (t38=2.99, p=.004). Following positive feedback, the between-group comparison was non-significant, with a trend toward greater theta power in controls (t38=-1.88, p=.06).
There was a significant group × feedback interaction at two parietal clusters: 1) left Inferior Parietal Lobule (IPL) [BA 40; -55,-43, 35], [F(1,76)=8.80, p=.004], and; 2) right Superior Parietal Lobule (SPL) [BA 7; 20,-67,45], [F(1,76)=9.34, p=.003] (Figures 5 and 6). Post hoc analyses of the left IPL found that, following negative feedback, BD subjects had significantly greater theta power than controls (t38=2.83, p=.006). Post hoc analyses of the right SPL found that, following negative feedback, BD subjects had significantly greater theta power than controls (t38=3.16, p=.002). All other between-group comparisons for parietal theta were nonsignificant.
Figures 5 & 6. Theta band power differences between youth with bipolar disorder and healthy controls in the left inferior parietal lobule (IPL) and right superior parietal lobule (SPL).
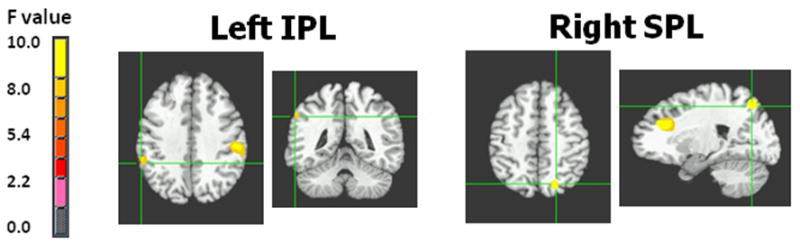
Note: BD = bipolar disorder; CON = control. Figure displays the locations in the left IPL (BA 40; -55,-43,35) and right SPL (BA 7; 20,-67,45) where BD subjects (N=20) had greater theta power than controls (N=20) following negative feedback.
Analyses found that there were no outliers for left ACC power after negative feedback, right ACC power after negative and positive feedback, and SPL power after positive feedback. For left ACC power after positive feedback, IPL power after positive and negative feedback, and SPL power after negative feedback, there was a different single outlier from the BD sample for each condition. When these subjects were removed from analyses, our results were unchanged.
Secondary Analyses: Theta Associations Comorbid Diagnoses and Medication
All secondary analyses focused on the four above-noted regions where BD and control samples differed in theta power. Pearson bivariate correlations within each group failed to identify significant associations between theta power and accuracy, RT, or self-reported affect.
An ANOVA comparing BD subjects with comorbid ADHD (N=12), BD subjects without ADHD (N=8), and controls found that BD subjects both with and without ADHD differed from controls as described previously for the BD sample as a whole in terms of ACC and parietal theta power. Similarly, an ANOVA comparing BD subjects with comorbid anxiety (N=8), BD subjects without anxiety (N=12), and controls yielded identical results as with the whole BD sample except that left IPL theta power in BD subjects without anxiety did not differ from controls (p=.16).
We did not have enough unmedicated BD subjects to compare those on vs. off medications. Correlations found nonsignificant relationships between theta power in the ACC or parietal lobe and the number of medications taken by BD subjects. ANOVA comparisons of theta power in BD subjects taking vs. not taking specific classes of medications (lithium, anti-depressants, or atypical antipsychotics) revealed no between group differences.
Discussion
Irritability is often considered the most impairing symptom of pediatric BD (1-3), yet there is minimal research on its pathophysiology. Prior work demonstrated affective, behavioral, and psychophysiological deficits in BD youth in a frustration-inducing context (5;6). As in that work, here we used the affective Posner task, modified by rigging feedback to induce frustration, and we now examined the neural mechanisms of frustration in BD youth using magnetoencephalography (MEG). We predicted greater ACC theta power in BD youth following negative feedback because increased ACC theta power (i.e. synchronization), which reflects neuronal activation (50-53), is seen during the processing of emotional stimuli (16;67).
Replicating our previous finding (5;6), BD youth reported a more adverse affective response to negative feedback in the frustrating context than controls. While not a measure specifically of irritability, this result suggests that BD youth were more upset by the frustration-inducing condition than were controls. BD youth were also slower to respond than controls in the frustration condition, though this deficit was seen in response to both positive and negative feedback. Our MEG results indicated that, in response to frustration-inducing negative feedback, BD youth displayed greater theta power relative to controls in the right ACC and bilateral parietal lobe (i.e. left IPL and right SPL). In contrast, compared to BD youth, controls displayed greater left ACC theta power following positive feedback, with a trend in the same direction in the right ACC.
ACC-generated theta power is associated with self-monitoring and emotion processing, often in response to evaluative feedback (25;26;68-73). Thus, our results suggest that, compared to controls, BD youth display heightened processing of negative feedback and exaggerated self-monitoring following this aversive stimulus. Information-processing theories (74-77) would suggest that disproportionate cognitive engagement by negative stimuli, such as that seen in BD youth, might sustain and/or exacerbate negative mood (i.e. irritability and frustration). In contrast to results in BD youth, controls engaged the ACC more robustly following positive feedback. Thus, in BD youth positive information which might otherwise diminish negative mood may be filtered out.
Our finding that ACC theta synchronization patterns in BD patients differed from controls adds to structural (29;30;78-80) and functional (31;32;81;82) MRI literature implicating the ACC in adult and pediatric BD. In pediatric BD specifically, data suggest ACC volumetric deficits and ACC hyperactivation during non-emotional tasks (i.e. behavioral inhibition (32) and working memory (31)), as well as during emotional tasks involving processing both positive (31) and, in our study, negative stimuli. Continued work is necessary to elucidate the extent to which these patterns of ACC theta oscillations are specific to bipolar youth or frustration in general, and how variables such as age and gender impact MEG data. Developmental studies have not been conducted using MEG, and the data using other imaging techniques are mixed. For example, while some fMRI (83) and EEG studies (84-87) find greater ACC activation in adults compared to youth, a number of studies report the opposite finding (83;88;89).
In addition to the ACC result, BD youth displayed greater theta power than controls in the inferior and superior parietal lobe (IPL and SPL) in response to negative feedback. The parietal patterns identified here are consistent with our previous EEG-based findings of a parietal P300 deficit in a different sample of BD youth using the affective Posner task (5;6). These results, along with prior studies finding volumetric deficits (90) and hyperactivity during attention (91), implicate parietal perturbations in the pathophysiology of pediatric BD. In addition, our finding in BD youth of increased theta power in both the ACC and parietal lobe is consistent with prior work identifying concomitant activation of these regions. Specifically, coordinated activation of the ACC and parietal lobe is thought to reflect attention (92) and performance monitoring, in particular in response to unexpected conflicts (93;94) and feedback (95). In sum, prior ACC-parietal results further support the suggestion that, in response to negative feedback, the neural mechanisms mediating self-monitoring and attention to emotional stimuli differ between euthymic BD youth and controls.
A strength of this study is that all BD participants were euthymic when tested. This may in part be attributable to the medicated status of most of our patients, which is our primary study limitation. Because it is unethical to discontinue medication solely for research purposes, it was not feasible for us to limit the sample to unmedicated patients. A recent MEG study of adults with schizophrenia found comparable theta activity between unmedicated patients and those receiving neuroleptic medication (96). Previous fMRI studies with BD subjects suggest that differences between unmedicated BD subjects and controls are greater than those between medicated patients and controls (32;97-99), suggesting that the inclusion of medicated subjects may inflate the possibility of Type II, rather than Type I, error. In addition, a previous EEG study found that psychotropic medications may reduce the power of neuronal oscillations (100). Despite this, we documented increased theta synchronization in BD youth compared to controls, and this was specific to negative, and not positive, feedback. However, there is documentation of altered theta power resulting from lithium, (101-103), mood stabilizers (104-106), and serotonin-specific reuptake inhibitors (SSRIs) (107;108). Clearly, additional research is needed to differentiate neural perturbations associated with BD from those associated with medication.
An additional limitation is that correlations between theta power and behavior and affect were nonsignificant. Considerable previous work demonstrates discordance among between-group differences in clinical characteristics, task-related performance, and brain function in studies of psychiatrically-impaired children, adolescents, and adults. Indeed, the current result is comparable to prior data that found inconsistent behavioral and neural results, i.e. neural differences in the absence of behavioral differences (109-114), or incongruent behavioral and neural results (109;112;115). The field continues to debate the advantages and disadvantages of performing research with tasks that do or do not generate between-group differences in behavior, in the context of brain imaging. On the one hand, some argue that the absence of group differences in task performance is preferred, because group differences in neural responses can not reflect potential subject performance deficits (116;117). Other researchers, however, view differences in task performance as aiding interpretation of differences in activation (118). This is because such differences provide on-line evidence that a perturbed behavioral response style is specifically engaged in the context of the imaging experiment. We should also note that the inconsistency in our study may also reflect methodological issues. Namely, behavior and imaging data reflect processes occurring at related but clearly differentiable points in time: behavior only was measured at one point in time, in response to targets on trials following positive and negative feedback; this was potentially 2800 ms after the continuously monitored patterns of theta responding showed between-group differences. Moreover, emotional response was measured at the completion of the 8-minute condition 3, rather than on a trial-by-trial, let alone millisecond-by-millisecond, basis. It is important for future work to explore the relative contributions of affective, behavioral, and neural data to understanding cognitive-emotional functioning in psychiatric populations.
Consistent with other neuroimaging studies of bipolar youth (30;90;91;119-124), our patient sample presented with high rates of comorbidity, most notably ADHD and anxiety disorders. Post hoc analyses found that ADHD did not change the nature of our results, though BD subjects without anxiety failed to display the left IPL theta power deficits seen in the BD sample as a whole. However, these analyses are severely limited by the small sample size, and therefore our results should be considered preliminary. Understanding our data is also limited by the lack of strong normative population data. Future work should explore the impact of comorbid disorders and compare youth with different diagnoses (e.g. BD, ADHD, anxiety) to elucidate the specificity of neural perturbations.
Conclusion
To our knowledge, this is the first MEG study in youth with BD. Using a frustration-induction paradigm, we found euthymic BD youth have increased ACC and parietal theta power in response to negative feedback. These neural perturbations suggest heightened attention to negative emotional stimuli and self-monitoring in a frustrating context. These cognitive deficits might contribute to the irritability and affective-dysregulation that is both prevalent and impairing in BD youth.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIMH. This funding organization was not involved in research aspects such as study design and conduct; data collection, analysis, or interpretation; nor manuscript preparation or approval. We gratefully acknowledge the subjects and families of patients and controls without whose participation this research would not have been possible. We also thank the staff of the Section on Bipolar Spectrum Disorders at the NIMH.
Reference List
- 1.Geller B, Williams M, Zimerman B, Frazier J, Beringer L, Warner KL. Prepubertal and early adolescent bipolarity differentiate from ADHD by manic symptoms, grandiose delusions, ultra-rapid or ultradian cycling. J Affect Disord. 1998 November;51(2):81–91. doi: 10.1016/s0165-0327(98)00175-x. [DOI] [PubMed] [Google Scholar]
- 2.Carlson GA, Jensen PS, Findling RL, Meyer RE, Calabrese J, Delbello MP, Emslie G, Flynn L, Goodwin F, Hellander M, Kowatch R, Kusumakar V, Laughren T, Leibenluft E, McCracken J, Nottelmann E, Pine D, Sachs G, Shaffer D, Simar R, Strober M, Weller EB, Wozniak J, Youngstrom EA. Methodological issues and controversies in clinical trials with child and adolescent patients with bipolar disorder: report of a consensus conference. J Child Adolesc Psychopharmacol. 2003;13(1):13–27. doi: 10.1089/104454603321666162. [DOI] [PubMed] [Google Scholar]
- 3.Biederman J, Faraone SV, Wozniak J, Monuteaux MC. Parsing the association between bipolar, conduct, and substance use disorders: a familial risk analysis. Biol Psychiatry. 2000 December 1;48(11):1037–44. doi: 10.1016/s0006-3223(00)00906-9. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Edgar K, Fox N. A behavioral and electrophysiological study of children's selective attention under neutral and affective conditions. Journal of Cognition and Development. 2005;6:89–116. [Google Scholar]
- 5.Rich BA, Schmajuk M, Perez-Edgar KE, Pine DS, Fox NA, Leibenluft E. The impact of reward, punishment, and frustration on attention in pediatric bipolar disorder. Biol Psychiatry. 2005 October 1;58(7):532–9. doi: 10.1016/j.biopsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am J Psychiatry. 2007 February;164(2):309–17. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- 7.Hirata M, Koreeda S, Sakihara K, Kato A, Yoshimine T, Yorifuji S. Effects of the emotional connotations in words on the frontal areas--a spatially filtered MEG study. Neuroimage. 2007 March;35(1):420–9. doi: 10.1016/j.neuroimage.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Reite M, Teale P, Rojas DC. Magnetoencephalography: applications in psychiatry. Biol Psychiatry. 1999 June 15;45(12):1553–63. doi: 10.1016/s0006-3223(99)00062-1. [DOI] [PubMed] [Google Scholar]
- 9.Ioannides AA. Magnetoencephalography as a research tool in neuroscience: state of the art. Neuroscientist. 2006 December;12(6):524–44. doi: 10.1177/1073858406293696. [DOI] [PubMed] [Google Scholar]
- 10.Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001 October;25(2):249–71. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- 11.Ishii R, Shinosaki K, Ukai S, Inouye T, Ishihara T, Yoshimine T, Hirabuki N, Asada H, Kihara T, Robinson SE, Takeda M. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport. 1999 March 17;10(4):675–9. doi: 10.1097/00001756-199903170-00003. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi M, Kato A, Fujita N, Hirata M, Tanaka H, Kihara T, Ninomiya H, Hirabuki N, Nakamura H, Robinson SE, Cheyne D, Yoshimine T. Movement-related desynchronization of the cerebral cortex studied with spatially filtered magnetoencephalography. Neuroimage. 2000 September;12(3):298–306. doi: 10.1006/nimg.2000.0611. [DOI] [PubMed] [Google Scholar]
- 13.Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005 April;6(4):285–96. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- 14.Aftanas LI, Varlamov AA, Pavlov SV, Makhnev VP, Reva NV. Affective picture processing: event-related synchronization within individually defined human theta band is modulated by valence dimension. Neurosci Lett. 2001 May 4;303(2):115–8. doi: 10.1016/s0304-3940(01)01703-7. [DOI] [PubMed] [Google Scholar]
- 15.Adinoff B, Devous MD, Sr, Best SM, George MS, Alexander D, Payne K. Limbic responsiveness to procaine in cocaine-addicted subjects. Am J Psychiatry. 2001 March;158(3):390–8. doi: 10.1176/appi.ajp.158.3.390. [DOI] [PubMed] [Google Scholar]
- 16.Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci Lett. 2001 September 7;310(1):57–60. doi: 10.1016/s0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- 17.Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci Biobehav Rev. 2007;31(3):377–95. doi: 10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Aftanas LI, Reva NV, Varlamov AA, Pavlov SV, Makhnev VP. Analysis of evoked EEG synchronization and desynchronization in conditions of emotional activation in humans: temporal and topographic characteristics. Neurosci Behav Physiol. 2004 October;34(8):859–67. doi: 10.1023/b:neab.0000038139.39812.eb. [DOI] [PubMed] [Google Scholar]
- 19.Aftanas LI, Varlamov AA, Reva NV, Pavlov SV. Disruption of early event-related theta synchronization of human EEG in alexithymics viewing affective pictures. Neurosci Lett. 2003 April 3;340(1):57–60. doi: 10.1016/s0304-3940(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 20.Salminen M, Ravaja N. Increased oscillatory theta activation evoked by violent digital game events. Neurosci Lett. 2008 April 11;435(1):69–72. doi: 10.1016/j.neulet.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Nishitani N. Dynamics of cognitive processing in the human hippocampus by neuromagnetic and neurochemical assessments. Neuroimage. 2003 September;20(1):561–71. doi: 10.1016/s1053-8119(03)00280-5. [DOI] [PubMed] [Google Scholar]
- 22.Sauseng P, Hoppe J, Klimesch W, Gerloff C, Hummel FC. Dissociation of sustained attention from central executive functions: local activity and interregional connectivity in the theta range. Eur J Neurosci. 2007 January;25(2):587–93. doi: 10.1111/j.1460-9568.2006.05286.x. [DOI] [PubMed] [Google Scholar]
- 23.Doppelmayr M, Finkenzeller T, Sauseng P. Frontal midline theta in the pre-shot phase of rifle shooting: differences between experts and novices. Neuropsychologia. 2008 April;46(5):1463–7. doi: 10.1016/j.neuropsychologia.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003 January;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- 25.Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004 August;115(8):1821–35. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 26.Sammer G, Blecker C, Gebhardt H, Bischoff M, Stark R, Morgen K, Vaitl D. Relationship between regional hemodynamic activity and simultaneously recorded EEG-theta associated with mental arithmetic-induced workload. Hum Brain Mapp. 2007 August;28(8):793–803. doi: 10.1002/hbm.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegrist J, Menrath I, Stocker T, Klein M, Kellermann T, Shah NJ, Zilles K, Schneider F. Differential brain activation according to chronic social reward frustration. Neuroreport. 2005 November 28;16(17):1899–903. doi: 10.1097/01.wnr.0000186601.50996.f7. [DOI] [PubMed] [Google Scholar]
- 28.Abler B, Walter H, Erk S. Neural correlates of frustration. Neuroreport. 2005 May 12;16(7):669–72. [Google Scholar]
- 29.Wilke M, Kowatch RA, Delbello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004 May 30;131(1):57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Kaur S, Sassi RB, Axelson D, Nicoletti M, Brambilla P, Monkul ES, Hatch JP, Keshavan MS, Ryan N, Birmaher B, Soares JC. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. 2005 September;162(9):1637–43. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- 31.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004 August;61(8):781–92. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 32.Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007 January;164(1):52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 33.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997 July;36(7):980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 35.Geller B, Zimerman B, Williams M, Delbello MP, Bolhofner K, Craney JL, Frazier J, Beringer L, Nickelsburg MJ. DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol. 2002;12(1):11–25. doi: 10.1089/10445460252943533. [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- 37.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the Children's Depression Rating Scale. J Am Acad Child Psychiatry. 1984 March;23(2):191–7. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978 November;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 39.Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S. A Children's Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983 November;40(11):1228–31. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 40.Posner MI. Chronometric Explorations of the Mind. Hillsdale NJ: 1978. [Google Scholar]
- 41.McManis MH, Bradley MM, Berg WK, Cuthbert BN, Lang PJ. Emotional reactions in children: verbal, physiological, and behavioral responses to affective pictures. Psychophysiology. 2001 March;38(2):222–31. [PubMed] [Google Scholar]
- 42.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996 June;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 43.Drabycz S, Stockwell RG, Mitchell JR. Image Texture Characterization Using the Discrete Orthonormal S-Transform. J Digit Imaging. 2008 August 2; doi: 10.1007/s10278-008-9138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aftanas LI, Varlamov AA, Reva NV, Pavlov SV. Disruption of early event-related theta synchronization of human EEG in alexithymics viewing affective pictures. Neurosci Lett. 2003 April 3;340(1):57–60. doi: 10.1016/s0304-3940(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 45.Salminen M, Ravaja N. Increased oscillatory theta activation evoked by violent digital game events. Neurosci Lett. 2008 April 11;435(1):69–72. doi: 10.1016/j.neulet.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Hanslmayr S, Pastotter B, Bauml KH, Gruber S, Wimber M, Klimesch W. The electrophysiological dynamics of interference during the Stroop task. J Cogn Neurosci. 2008 February;20(2):215–25. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- 47.Sekihara K, Nagarajan SS, Poeppel D, Marantz A, Miyashita Y. Reconstructing spatio-temporal activities of neural sources using an MEG vector beamformer technique. IEEE Trans Biomed Eng. 2001 July;48(7):760–71. doi: 10.1109/10.930901. [DOI] [PubMed] [Google Scholar]
- 48.Cornwell BR, Baas JM, Johnson L, Holroyd T, Carver FW, Lissek S, Grillon C. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. Neuroimage. 2007 August 1;37(1):282–9. doi: 10.1016/j.neuroimage.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo Q, Holroyd T, Jones M, Hendler T, Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007 January 15;34(2):839–47. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aftanas LI, Varlamov AA, Pavlov SV, Makhnev VP, Reva NV. Time-dependent cortical asymmetries induced by emotional arousal: EEG analysis of event-related synchronization and desynchronization in individually defined frequency bands. Int J Psychophysiol. 2002 April;44(1):67–82. doi: 10.1016/s0167-8760(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 51.Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999 November;110(11):1842–57. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- 52.Basar E, Schurmann M, Sakowitz O. The selectively distributed theta system: functions. Int J Psychophysiol. 2001 January;39(2-3):197–212. doi: 10.1016/s0167-8760(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 53.Pizzagalli DA, Oakes TR, Davidson RJ. Coupling of theta activity and glucose metabolism in the human rostral anterior cingulate cortex: an EEG/PET study of normal and depressed subjects. Psychophysiology. 2003 November;40(6):939–49. doi: 10.1111/1469-8986.00112. [DOI] [PubMed] [Google Scholar]
- 54.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, Manji HK. Increased Anterior Cingulate Cortical Activity in Response to Fearful Faces: A Neurophysiological Biomarker that Predicts Rapid Antidepressant Response to Ketamine. Biol Psychiatry. 2008 September 24; doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cornwell BR, Carver FW, Coppola R, Johnson L, Alvarez R, Grillon C. Evoked amygdala responses to negative faces revealed by adaptive MEG beamformers. Brain Res. 2008 December 9;1244:103–12. doi: 10.1016/j.brainres.2008.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J Neurosci. 2008 June 4;28(23):5983–90. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogawa H, Wakita M, Hasegawa K, Kobayakawa T, Sakai N, Hirai T, Yamashita Y, Saito S. Functional MRI detection of activation in the primary gustatory cortices in humans. Chem Senses. 2005 September;30(7):583–92. doi: 10.1093/chemse/bji052. [DOI] [PubMed] [Google Scholar]
- 58.Lapalme E, Lina JM, Mattout J. Data-driven parceling and entropic inference in MEG. Neuroimage. 2006 March;30(1):160–71. doi: 10.1016/j.neuroimage.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 59.Ossadtchi A, Baillet S, Mosher JC, Thyerlei D, Sutherling W, Leahy RM. Automated interictal spike detection and source localization in magnetoencephalography using independent components analysis and spatio-temporal clustering. Clin Neurophysiol. 2004 March;115(3):508–22. doi: 10.1016/j.clinph.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 60.Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003 October;12(5):419–46. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- 61.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002 April;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 62.Kahkonen S, Yamashita H, Rytsala H, Suominen K, Ahveninen J, Isometsa E. Dysfunction in early auditory processing in major depressive disorder revealed by combined MEG and EEG. J Psychiatry Neurosci. 2007 September;32(5):316–22. [PMC free article] [PubMed] [Google Scholar]
- 63.Henson RN, Mattout J, Singh KD, Barnes GR, Hillebrand A, Friston K. Population-level inferences for distributed MEG source localization under multiple constraints: Application to face-evoked fields. Neuroimage. 2007 November 15;38(3):422–38. doi: 10.1016/j.neuroimage.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 64.Brunetti M, Della PS, Ferretti A, Del Gratta C, Cianflone F, Belardinelli P, Caulo M, Pizzella V, Olivetti BM, Romani G. A Frontoparietal Network for Spatial Attention Reorienting in the Auditory Domain: A Human fMRI/MEG Study of Functional and Temporal Dynamics. Cereb Cortex. 2007 August 23; doi: 10.1093/cercor/bhm145. [DOI] [PubMed] [Google Scholar]
- 65.Onoda K, Okamoto Y, Shishida K, Hashizume A, Ueda K, Yamashita H, Yamawaki S. Anticipation of affective images and event-related desynchronization (ERD) of alpha activity: an MEG study. Brain Res. 2007 June 2;1151:134–41. doi: 10.1016/j.brainres.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 66.Statistical Package for Social Sciences for Windows, Version 16 [computer program] Chicago: SPSS; 2007. [Google Scholar]
- 67.Adinoff B, Devous MD, Sr, Best SM, George MS, Alexander D, Payne K. Limbic responsiveness to procaine in cocaine-addicted subjects. Am J Psychiatry. 2001 March;158(3):390–8. doi: 10.1176/appi.ajp.158.3.390. [DOI] [PubMed] [Google Scholar]
- 68.Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003 January;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- 69.Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, Himle JA, Gehring WJ. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006 April 12;26(15):4063–70. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MG, Cohen JD. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat Neurosci. 2004 May;7(5):497–8. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- 71.Luu P, Pederson SM. The anterior cingulate cortex: Regulating actions in context. In: Posner MI, editor. Cognitive neuroscience of attention. New York: Guilford Publication, Inc.; 2004. [Google Scholar]
- 72.Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001 September;38(5):752–60. [PubMed] [Google Scholar]
- 73.Tzur G, Berger A. When things look wrong: theta activity in rule violation. Neuropsychologia. 2007 October 1;45(13):3122–6. doi: 10.1016/j.neuropsychologia.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 75.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 76.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 77.Crick NR, Dodge KA. Social information-processing mechanisms in reactive and proactive aggression. Child Dev. 1996 June;67(3):993–1002. [PubMed] [Google Scholar]
- 78.Wang F, Jackowski M, Kalmar JH, Chepenik LG, Tie K, Qiu M, Gong G, Pittman BP, Jones MM, Shah MP, Spencer L, Papademetris X, Constable RT, Blumberg HP. Abnormal anterior cingulum integrity in bipolar disorder determined through diffusion tensor imaging. Br J Psychiatry. 2008 August;193(2):126–9. doi: 10.1192/bjp.bp.107.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiu S, Widjaja F, Bates ME, Voelbel GT, Pandina G, Marble J, Blank JA, Day J, Brule N, Hendren RL. Anterior cingulate volume in pediatric bipolar disorder and autism. J Affect Disord. 2008 January;105(1-3):93–9. doi: 10.1016/j.jad.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 80.Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004 October 1;56(7):467–75. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Blumberg HP, Stern E, Martinez D, Ricketts S, de Asis J, White T, Epstein J, McBride PA, Eidelberg D, Kocsis JH, Silbersweig DA. Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry. 2000 December 1;48(11):1045–52. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- 82.Gruber SA. Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. Journal of Affective Disorders. 2004;82(2):191–201. doi: 10.1016/j.jad.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 83.Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 2007 May 30; doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jonkman LM, Lansbergen M, Stauder JE. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003 September;40(5):752–61. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- 85.Johnstone SJ, Pleffer CB, Barry RJ, Clarke AR, Smith JL. Development of inhibitory processing during the go/nogo task. Journal of Psychophysiology. 2005;19(1):11–23. [Google Scholar]
- 86.Dimoska A, Johnstone SJ, Chiswick D, Barry RJ, Clarke AR. A developmental investigation of stop-signal inhibition: Dissociating low- and higher-frequency activity in the event-related potential. Journal of Psychophysiology. 2007;21(2):109–26. [Google Scholar]
- 87.Bekker EM, Kenemans JL, Verbaten MN. Source analysis of the N2 in a cued Go/NoGo task. Brain Res Cogn Brain Res. 2005 February;22(2):221–31. doi: 10.1016/j.cogbrainres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 88.Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000 January;24(1):13–9. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- 89.Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Neural development of selective attention and response inhibition. Neuroimage. 2003 October;20(2):737–51. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- 90.Frazier JA, Breeze JL, Makris N, Giuliano AS, Herbert MR, Seidman L, Biederman J, Hodge SM, Dieterich ME, Gerstein ED, Kennedy DN, Rauch SL, Cohen BM, Caviness VS. Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disord. 2005 December;7(6):555–69. doi: 10.1111/j.1399-5618.2005.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adler CM, Delbello MP, Mills NP, Schmithorst V, Holland S, Strakowski SM. Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disord. 2005 December;7(6):577–88. doi: 10.1111/j.1399-5618.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 92.Posner MI, Sheese BE, Odludas Y, Tang Y. Analyzing and shaping human attentional networks. Neural Netw. 2006 November;19(9):1422–9. doi: 10.1016/j.neunet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 93.Satpute AB, Lieberman MD. Integrating automatic and controlled processes into neurocognitive models of social cognition. Brain Res. 2006 March 24;1079(1):86–97. doi: 10.1016/j.brainres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Lieberman MD, Jarcho JM, Satpute AB. Evidence-based and intuition-based self-knowledge: an FMRI study. J Pers Soc Psychol. 2004 October;87(4):421–35. doi: 10.1037/0022-3514.87.4.421. [DOI] [PubMed] [Google Scholar]
- 95.Labudda K, Woermann FG, Mertens M, Pohlmann-Eden B, Markowitsch HJ, Brand M. Neural correlates of decision making with explicit information about probabilities and incentives in elderly healthy subjects. Exp Brain Res. 2008 June;187(4):641–50. doi: 10.1007/s00221-008-1332-x. [DOI] [PubMed] [Google Scholar]
- 96.Fehr T, Kissler J, Wienbruch C, Moratti S, Elbert T, Watzl H, Rockstroh B. Source distribution of neuromagnetic slow-wave activity in schizophrenic patients--effects of activation. Schizophr Res. 2003 September 1;63(1-2):63–71. doi: 10.1016/s0920-9964(02)00213-x. [DOI] [PubMed] [Google Scholar]
- 97.Caligiuri MP, Brown GG, Meloy MJ, Eberson SC, Kindermann SS, Frank LR, Zorrilla LE, Lohr JB. An fMRI study of affective state and medication on cortical and subcortical brain regions during motor performance in bipolar disorder. Psychiatry Res. 2003 July 30;123(3):171–82. doi: 10.1016/s0925-4927(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 98.Nelson EE, Vinton DT, Berghorst L, Towbin KE, Hommer RE, Dickstein DP, Rich BA, Brotman MA, Pine DS, Leibenluft E. Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disord. 2007 December;9(8):810–9. doi: 10.1111/j.1399-5618.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 99.Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, Gueorguieva R, Fulbright RK, McGlashan TH, Gore JC, Krystal JH. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005 December;183(3):308–13. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 100.Smith ME, Gevins A, McEvoy LK, Meador KJ, Ray PG, Gilliam F. Distinct cognitive neurophysiologic profiles for lamotrigine and topiramate. Epilepsia. 2006 April;47(4):695–703. doi: 10.1111/j.1528-1167.2006.00508.x. [DOI] [PubMed] [Google Scholar]
- 101.Rohayem J, Bayle JF, Richa S. Predictors of prophylactic response to lithium. Encephale. 2008 September;34(4):394–9. doi: 10.1016/j.encep.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 102.Suslov NI, Fedorov AA, Pershina OV, Borodin YI, Rachkovskaya LN. Effect of Noolit, a novel lithium preparation, on electrophysiological activity of rat cerebral cortex. Bull Exp Biol Med. 2004 February;137(2):155–8. doi: 10.1023/b:bebm.0000028128.18241.51. [DOI] [PubMed] [Google Scholar]
- 103.Schulz C, Mavrogiorgou P, Schroter A, Hegerl U, Juckel G. Lithium-induced EEG changes in patients with affective disorders. Neuropsychobiology. 2000;42 1:33–7. doi: 10.1159/000054850. [DOI] [PubMed] [Google Scholar]
- 104.Gholmieh GI, Courellis SH, Chen LS. Screening for the effects of antiepileptic drugs on short term plasticity using a time efficient bioassay. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:2247–52. doi: 10.1109/IEMBS.2007.4352772. [DOI] [PubMed] [Google Scholar]
- 105.Clemens B, Menes A, Piros P, Bessenyei M, Altmann A, Jerney J, Kollar K, Rosdy B, Rozsavolgyi M, Steinecker K, Hollody K. Quantitative EEG effects of carbamazepine, oxcarbazepine, valproate, lamotrigine, and possible clinical relevance of the findings. Epilepsy Res. 2006 August;70(2-3):190–9. doi: 10.1016/j.eplepsyres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 106.Clemens B. Valproate decreases EEG synchronization in a use-dependent manner in idiopathic generalized epilepsy. Seizure. 2008 April;17(3):224–33. doi: 10.1016/j.seizure.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 107.Iosifescu DV, Greenwald S, Devlin P, Mischoulon D, Denninger JW, Alpert JE, Fava M. Frontal EEG predictors of treatment outcome in major depressive disorder. Eur Neuropsychopharmacol. 2009 June 30; doi: 10.1016/j.euroneuro.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 108.Dumont GJ, de Visser SJ, Cohen AF, van Gerven JM. Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. Br J Clin Pharmacol. 2005 May;59(5):495–510. doi: 10.1111/j.1365-2125.2005.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry. 2008 February;65(2):179–88. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tucker DM, Luu P, Frishkoff G, Quiring J, Poulsen C. Frontolimbic response to negative feedback in clinical depression. J Abnorm Psychol. 2003 November;112(4):667–78. doi: 10.1037/0021-843X.112.4.667. [DOI] [PubMed] [Google Scholar]
- 111.Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. Am J Psychiatry. 2007 April;164(4):608–16. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- 112.Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia. 2008 October;46(12):2904–13. doi: 10.1016/j.neuropsychologia.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Santesso DL, Steele KT, Bogdan R, Holmes AJ, Deveney CM, Meites TM, Pizzagalli DA. Enhanced negative feedback responses in remitted depression. Neuroreport. 2008 July 2;19(10):1045–8. doi: 10.1097/WNR.0b013e3283036e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Santesso DL, Dillon DG, Birk JL, Holmes AJ, Goetz E, Bogdan R, Pizzagalli DA. Individual differences in reinforcement learning: behavioral, electrophysiological, and neuroimaging correlates. Neuroimage. 2008 August 15;42(2):807–16. doi: 10.1016/j.neuroimage.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chiu PH, Holmes AJ, Pizzagalli DA. Dissociable recruitment of rostral anterior cingulate and inferior frontal cortex in emotional response inhibition. Neuroimage. 2008 August 15;42(2):988–97. doi: 10.1016/j.neuroimage.2008.04.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Callicott JH, Weinberger DR. Neuropsychiatric dynamics: The study of mental illness using functional magnetic resonance imaging. European Journal of Radiology. 2000;30:95–104. doi: 10.1016/s0720-048x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 117.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003 December;160(12):2209–15. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 118.Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nat Rev Neurosci. 2004 January;5(1):67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- 119.Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, Pittman B, Martin A, Peterson BS, Fulbright RK, Krystal JH. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord. 2005 December;7(6):570–6. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005 June;44(6):565–73. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 121.Sanches M, Sassi RB, Axelson D, Nicoletti M, Brambilla P, Hatch JP, Keshavan MS, Ryan ND, Birmaher B, Soares JC. Subgenual prefrontal cortex of child and adolescent bipolar patients: a morphometric magnetic resonance imaging study. Psychiatry Res. 2005 January 30;138(1):43–9. doi: 10.1016/j.pscychresns.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 122.Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M, Brambilla P, Keshavan MS, Ryan ND, Birmaher B, Soares JC. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry. 2004 September 15;56(6):399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 123.Delbello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004 February;6(1):43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 124.Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005 July;162(7):1256–65. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]


