Abstract
Formation of a heterotrimeric IPP complex composed of integrin-linked kinase (ILK), the LIM domain protein PINCH, and parvin is important for signaling through integrin adhesion receptors. Mammals possess two PINCH genes that are expressed simultaneously in many tissues. PINCH1 and PINCH2 have overlapping functions and can compensate for one another in many settings; however, isoform-specific functions have been reported and it is proposed that association with a PINCH1- or PINCH2-containing IPP complex may provide a bifurcation point in integrin signaling promoting different cellular responses. Here we report that the LIM1 domains of PINCH1 and PINCH2 directly compete for the same binding site on the ankyrin repeat domain (ARD) of ILK. We determined the 1.9 Å crystal structure of the PINCH2 LIM1 domain complexed with the ARD of ILK, and show that disruption of this interface by point mutagenesis reduces binding in vitro and alters localization of PINCH2 in cells. These studies provide further evidence for the role of the PINCH LIM1 domain in association with ILK and highlight direct competition as one mechanism for regulating which PINCH isoform predominates in IPP complexes. Differential regulation of PINCH1 and PINCH2 expression may therefore provide a means for altering cellular integrin signaling pathways.
Keywords: Integrin signaling, Ankyrin repeat domain, LIM domain, IPP complex
Introduction
The integrin-linked kinase (ILK) is an essential cytoplasmic protein important for signaling to and from integrin adhesion receptors (Legate et al., 2006; Hannigan et al., 2005; Wu, 2005; McDonald et al., 2008). ILK has critical roles in anchorage-dependent cell growth and survival, cell cycle progression, epithelial to mesenchymal transition, cell motility, contractility and early development (Yasunaga et al., 2005; Sakai et al., 2003; Hannigan et al., 2005). ILK is also required for cardiac, vascular, brain, kidney, muscle, skin, platelet, chondrocyte and T cell function and plays important roles in tumor angiogenesis (Legate et al., 2006; McDonald et al., 2008). ILK contains an N-terminal ankyrin-repeat domain (ARD), composed of 5 ankyrin repeats (Chiswell et al., 2008; Yang et al., 2009) followed by a predicted kinase domain (Fig 1A). Genetic analyses in flies, worms, fish and mice show the importance of ILK as a signaling and cytoskeletal scaffold but the kinase activity of ILK remains controversial (Zervas et al., 2001; Postel et al., 2008; Mackinnon et al., 2002; Legate et al., 2006; Sakai et al., 2003). Indeed recent data indicate that ILK kinase activity is dispensable for mouse development (Lange et al 2009). Numerous ILK binding partners have been identified, including PINCH, parvin, β integrins, paxillin, ILK-associated phosphatase and kindlins (Harburger and Calderwood, 2009; Legate et al., 2006; McDonald et al., 2008).
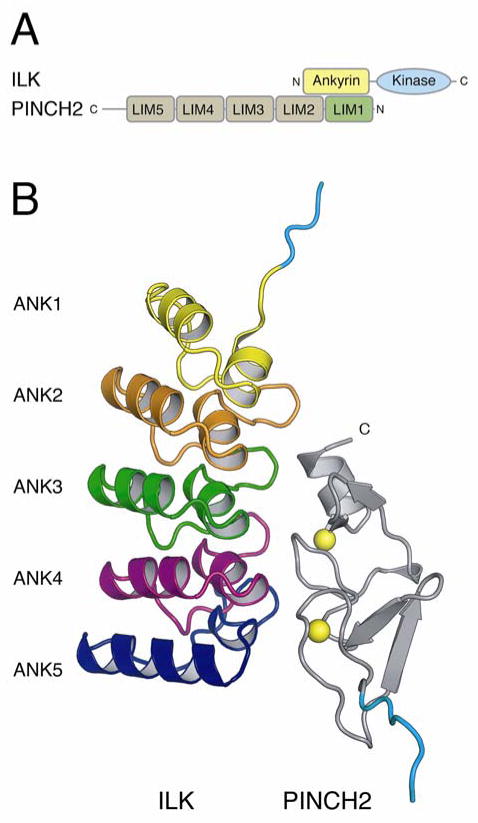
Figure 1. Structure of PINCH2 LIM1 in complex with ILK ARD.
A) Schematic representation of the domain interactions of ILK and PINCH2. B) Cartoon of the ILK ARD domain in complex with PINCH2 LIM1. ILK is colored according to ARD repeat (ANK1 yellow, ANK2 red, ANK3 green, ANK4 purple, ANK5 blue). PINCH2 is shown in grey with zinc atoms as yellow spheres. For both proteins, vector derived sequence at the N-terminus is in light blue.
ILK is normally found in complex with two other proteins: PINCH and parvin (Legate et al., 2006; Wu, 2004), and while ILK kinase activity is not essential for mouse development the formation of a complex with parvin is required (Lange et al 2009). The heterotrimeric complex between ILK, PINCH and parvin, termed the IPP complex, is an essential signaling platform that regulates cell adhesion, spreading and migration. In mammals, formation of the IPP complex stabilizes expression of the constituent proteins, at least in part by reducing their degradation by the proteosome (Fukuda et al., 2003; Stanchi et al., 2005), and is required for their correct targeting to adhesions (Zhang et al., 2002b; Stanchi et al., 2009). Mammals contain two PINCH proteins, PINCH1 and PINCH2, and three parvins, α-, β- and γ-parvin, thus several different IPP complexes may be formed depending on which combination of PINCH and parvin isoforms bind to ILK. While the physiological significance and specificity determinants of complex formation of these different complexes is poorly understood it is proposed that the signaling specificity of the IPP complex depends on which PINCH isoform it contains (Legate et al., 2006). The formation of different IPP complexes containing PINCH1 or PINCH2 may therefore be a bifurcation point in integrin signaling.
PINCH1 and PINCH2 bind ILK (Braun et al., 2003; Zhang et al., 2002a) and consist of five LIM (Lin11, Isl1 and Mec3) domains followed by a short C-terminal tail. PINCH1 and 2 share 85% sequence identity (Braun et al., 2003; Zhang et al., 2002a) although PINCH2 contains an 11 amino acid extension on the C-terminal tail. PINCH1 is widely expressed throughout development, and PINCH1−/− mice die at the peri-implantation stage with defects in cell-matrix adhesions, cell polarity and cell survival (Li et al., 2005; Liang et al., 2005). In contrast, PINCH2 is expressed later during development than PINCH1 (Braun et al., 2003; Liang et al., 2009; Fukuda et al., 2003) and, possibly due to compensation by up-regulated PINCH1, PINCH2−/− mice exhibit no overt phenotype (Stanchi et al., 2005). PINCH1 and 2 exhibit overlapping expression patterns and in some tissues PINCH2 may compensate for loss of PINCH1 and vice versa, suggesting that PINCH1 and PINCH2 have some functional redundancy (Liang et al., 2009; Liang et al., 2005; Stanchi et al., 2005). Consistent with this, ectopic PINCH2 expression can rescue some cellular phenotypes associated with loss of PINCH1 and protects ILK from degradation (Zhang et al., 2002a; Braun et al., 2003; Fukuda et al., 2003; Stanchi et al., 2005). However, in some cell types over-expressed PINCH2 inhibits spreading and migration, possibly by competing with PINCH1 (Zhang et al., 2002a; Shi et al., 2008). The C-terminal tail of PINCH is important for the differential effects (Xu et al., 2005) which are likely to be due to differential binding of accessory proteins to the two PINCH isoforms (Legate et al., 2006; Dougherty et al., 2005). Signaling may therefore depend on which PINCH is present in the IPP complex.
The ILK-binding site in PINCH is localized primarily in the first LIM domain, which interacts with the ARD of ILK (Stanchi et al., 2005; Legate et al., 2006; Tu et al., 1999; Zhang et al., 2002c; Chiswell et al., 2008; Yang et al., 2009) (Fig 1A). We previously used X-ray crystallography, point mutagenesis and protein-protein interactions studies to reveal the structural basis of ILK-PINCH1 interactions (Chiswell et al., 2008). Here we report the crystal structure of the ILK ARD - PINCH2 LIM1 complex, which shows a striking similarity to the equivalent ILK-PINCH1 complex. Consistent with this, we demonstrate that the LIM1 domains of PINCH1 and PINCH2 compete for binding to ILK. Furthermore, we identify mutations in ILK and PINCH2 that disrupt complex formation in vitro and prevent proper localization of ILK/PINCH to integrin-rich focal adhesions in cells. Overall we show that ILK can interact in an experimentally identical manner with a conserved surface on PINCH1 or PINCH2. Thus, the formation of PINCH1- or PINCH2-containing IPP complexes is likely to be largely determined by competition between available levels of PINCH1 or 2.
Materials and Methods
Protein expression and purification
An ILK ARD-PINCH2 LIM1 complex was produced and purified using the same strategy previously described for the ILK ARD-PINCH1 LIM complex (Chiswell et al., 2008). Briefly, recombinant GST-tagged human ILK 1-192 (Swiss-Prot Q13418) and His-tagged human PINCH2 6-68 (numbered according to Swiss-Prot Q7Z4I7-3) were produced separately in Escherichia coli BL21-Gold(DE3) (Stratagene) using pGEX-4T and a modified pET32 expression vectors respectively. Following induction cells were pelleted, resuspended, mixed together and co-lysed using a freeze-thaw protocol and sonication. Following clarification the complex was affinity purified using the PINCH2 N-terminal 6xHis-tag on His-bind resin (Novagen), eluted with 500 mM imidazole and then bound to glutathione-Separose 4 Fast Flow medium (Amersham Biosciences) using the N-terminal ILK GST-tag. The glutathione-Sepharose-bound complex was washed and the GST and 6xHis tags were removed simultaneously with thrombin (Enzyme Research Laboratories). Mass spectrometry and N-terminal sequencing revealed there to be an internal thrombin proteolysis of ILK1-192 that generates a fragment spanning ILK1-174 (Chiswell et al., 2008). The cleaved ILK 1-174 - PINCH2 6-68 complex was further purified with anion exchange using a MonoQ 5/50 GL column (GE Healthcare) and fractions containing the complex were pooled and concentrated.
Crystallization and structure determination
The ILK ARD complex with PINCH2 LIM1 crystallized from a 1:1 mixture of 20 mg/mL protein with a reservoir of 8% PEG 550 MME (Fluka), 0.1 M MES, pH 6.5, and 0.2 μL of 20% benzamidine hydrochloride hydrate (Hampton Research). Crystals appeared after 5 days and grew to a size of approximately 20 × 20 × 100 μm. 181° of data were collected using NSLS beamline X6A to 1.9 Å resolution and processed using the HKL2000 package (Otwinowski and Minor, 1997) in space group P212121 with unit cell dimensions of a = 41.4 Å, b = 72.0 Å, c = 83.9 Å. Molecular replacement used the ILK ARD complex with PINCH1 LIM1 as a search model (PDB ID: 3F6Q) yielded a translation function Z-score of 26.7 with Phaser (McCoy et al., 2005). Automated model building using ARP/wARP (Perrakis et al., 2001) built and docked 214 residues, and manual model building and refinement using Coot (Emsley and Cowtan, 2004) and REFMAC5 (Murshudov et al., 1997) yielded final R and R free values of 17.0% and 22.0% respectively for a total of 241 residues in the asymmetric unit (Table 1). Two zinc atoms were built in the structure, both with tetrahedral coordination. The final structure showed no residues in disallowed or generously allowed regions of the Ramachandran plot (Laskowski et al., 1993).
Table 1.
Data collection and refinement statistics for the ILK-PINCH2 complex.
|
Data collection | |
| Number of crystals | 1 |
| Space group | P212121 |
| Crystal size (μm) | 20 × 20 × 100 |
| Wavelength (Å) | 0.9763 |
| Cell dimensions, a, b, c (Å) | 41.4, 72.0, 83.9 |
| α, β, γ (°) | 90, 90, 90 |
| Resolution range (Å)a | 20.0–1.9 (1.97–1.90) |
| Unique reflectionsa | 20416 (2002) |
| Completeness (%)a | 100 (100) |
| Rsym (%)a,b | 13.6 (41.4) |
| Mn (I/σI)a | 9.7 (3.3) |
| Redundancya | 7.2 (7.3) |
|
Refinement | |
| Resolution range (Å)a | 20.00–1.90 (1.95–1.90) |
| Rfactor (%)a,c | 17.0 (19.7) |
| Free Rfactor (%)a,c | 22.0 (23.5) |
| Residue range built, ILK | 1-166 |
| Residue range built, PINCH | 6-68 |
| Free R reflections (%)a | 5.1 |
| Free R reflections, no.a | 1040 |
| No. non-hydrogen protein atoms | 2370 |
| No. water molecules | 369 |
| No. Zn atoms | 2 |
|
Model quality | |
| RMSD bond lengths (Å) | 0.008 |
| RMSD bond angles (°) | 1.071 |
| Mean B-factors | |
| Overall (Å2) | 16.2 |
| Protein atoms, ILK (Å2) | 12.2 |
| Protein atoms, PINCH2 (Å2) | 18.7 |
| Water (Å2) | 26.9 |
| Zinc B-values (Å2) | 10.6, 8.3 |
| Ramachandran plot (%) favored/allowed/outlier regions | 99.6/0.4/0.0 |
Parentheses indicate the highest-resolution shell.
Rsym = Σi|I − <I>|/Σi <I>, where Ii is the intensity of the ith term and <I> is the mean observed intensity.
Rfactor = Σ||Fobs|−|Fcalc||/Σ|Fobs|, where Fobs and Fcalc are observed and calculated structure factor amplitudes.
Pull-down competition assays
Binding of wild-type and mutant ILK ARD to wild-type and mutant His-tagged PINCH2 LIM1 was assessed in pull-down assays as previously described for ILK-PINCH1 interactions (Chiswell et al., 2008). His-tagged PINCH2 LIM1 was expressed in E. coli, cells were lysed and the PINCH was bound to His-bind Resin as described for the purification of the complex for crystallization. The PINCH-coated resin was washed with 50 mM sodium phosphate, 20 mM imidazole, 300 mM NaCl, pH 8.0 and stored at 4°C until use. Wild-type and mutant GST-ILK ARD was expressed, purified and cleaved from glutathione-Sepharose beads with thrombin as described previously (Chiswell et al., 2008). PINCH2-coated resin was incubated with soluble ILK ARD in 500 μl of lysis buffer for 1 h at 23°C with constant rocking. The resin was washed with 50 mM sodium phosphate, 5 mM imidazole, 300 mM NaCl, pH 8.0 and protein was eluted from the resin with SDS-PAGE sample buffer containing β-mercaptoethanol. Bound protein was analyzed by SDS-PAGE. Protein binding was quantified by densitometry and is reported as a percentage of wild-type binding in each experiment.
To investigate competition between PINCH1 LIM1 and PINCH2 LIM1 increasing amounts of soluble PINCH1 LIM1 or PINCH2 LIM1 were premixed with ILK ARD protein, and ILK ARD binding to His-PINCH2 LIM1-coated beads or His-PINCH1 LIM1-coated beads. These were assessed by pull-down assays and Coomassie staining using proteins purified as described above. Soluble PINCH LIM1 proteins were prepared by tobacco etch virus (TEV) protease treatment of His-PINCH proteins adsorbed to His-bind resin followed by a single wash to recover PINCH LIM1 protein. Increasing amounts (up to 80 μg) of soluble PINCH1 or PINCH2 LIM1 proteins were premixed with ILK ARD in 500 μl of wash buffer for 15 min at room temperature with constant rocking, followed by addition of His-PINCH LIM1-coated beads (His-bind resin, Novagen) and additional incubation for 40 min at room temperature with rocking. The binding reactions were washed twice with wash buffer, and bound proteins eluted from the beads with Laemmli sample buffer containing reducing agent. Bound proteins were resolved by SDS-PAGE and visualized by Coomassie staining. Protein binding was quantified by densitometry and is reported as a percentage of maximal binding (in the absence of inhibitor) in each experiment.
Localization assays
CHO cells transfected with expression vectors encoding GFP- ILK, ILK H99D, PINCH1, PINCH1 F42A, PINCH2 and PINCH2 F42A were plated on fibronectin-coated coverslips and stained with a mouse monoclonal anti-vinculin antibody (as a marker of focal adhesion, red). Chinese Hamster Ovary (CHO) cells were cultured in Dulbecco’s Modified Essential Medium (Invitrogen) containing 9% Fetal bovine serum (Atlanta Biological), sodium pyruvate (Gibco) and penicillin/streptomycin (Invitrogen) and incubated at 37°C in a humidified atmosphere containing 5% CO2. Cells were transfected using Lipofectamine 2000 (Invitrogen). 20 h after transfection, cells were detached and replated on glass coverslips coated with 5 μg/ml fibronectin, fixed after 24 hours in 4% paraformaldehyde in phosphate buffered saline (PBS) pH 7.4 for 15 minutes and permeabilized for 30 min with PBS containing 0.2% bovine serum albumin (BSA), 0.3% triton X-100 and 50 mM NH4Cl. Cells were then incubated with mouse monoclonal anti-vinculin antibody (Sigma) for 1 hour at room temperature, then, incubated with Alexa Fluor 568 goat anti-mouse secondary antibody (Molecular Probes) for 45 minutes and washed in PBS. Coverslips were mounted using the ProLongGold anti-fade mounting agent (Invitrogen). Images were acquired using Nikon TE2000 with a 100X objective using IPLab (version 3.5.2; Scanalytics, Fairfax VA) software and analyzed using ImageJ (U. S. National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/).
Results
Structure of PINCH2 LIM1 domain in complex with ILK ARD
The structure of ILK ARD in complex with the LIM1 domain of PINCH2 reveals a stoichiometric 1:1 complex (Fig 1B). In the current structure, the ARD of ILK contains 5 ankyrin repeats as previously described (Chiswell et al., 2008; Yang et al., 2009). The LIM1 domain of PINCH2 contains tandem zinc finger domains connected by a 2-residue flexible liker. These two type VII treble clef zinc fingers overlay onto one another with an RMSD of 1.48 Å over 27 residues (using secondary structure matching (CCP4, 1994)). Zinc is tetrahedrally coordinated by residues Cys10, Cys13, His32 and Cys35 of the N-terminal zinc finger and Cys38, Cys41, Cys59, Asp62 of the C-terminal zinc finger, maintaining in PINCH2 LIM1 the CCHC and CCCD modules previously seen in the structure of ILK ARD in complex with PINCH1 LIM1 (Chiswell et al., 2008). The structure shows that interaction of ILK ARD with the LIM1 domain of PINCH2 is broadly mediated by the C-terminal zinc finger of PINCH2 LIM1 nestling into the concave ankyrin grove of ILK formed by ankyrin repeats 2 to 5. The interaction buries a total of 919 Å2 in ILK and 1008 Å2 in PINCH2 to give an average lost accessible surface area of 964 Å2 (calculated using PDBsum (Laskowski, 2001)) (Fig 2A). A total of 20 residues from ILK and 19 from PINCH2 LIM1 directly interact, forming 14 intermolecular hydrogen bonds and 146 intermolecular non-bonded contacts (PDBsum) to make an interface with both hydrophobic and polar interactions.
Figure 2. Comparison of PINCH2 and PINCH1 interactions with ILK ARD.
A) Open-book format showing residues that interact between PINCH2 LIM1 and ILK ARD. Surfaces are colored yellow for residues that interact and grey for residues that do not interact between ILK and PINCH2. B) Superposition of the crystal structures of ILK ARD in complex with PINCH2 LIM1 (yellow) and PINCH1 LIM1 (PDB:3F6Q) (salmon). C) Close-up superposition of the ILK-PINCH interface. Residues mutated in this study F42 and D62 (PINCH1 and PINCH2) and H99 (ILK) are indicated. Q43 in PINCH1 and R43 in PINCH2 are indicated. ILK-PINCH1 colored in yellow and ILK-PINCH2 colored in salmon. D) Sequence alignment of PINCH2 LIM domains and PINCH1 LIM1. Crystallographically determined residues that mediate a direct interaction with ILK are highlighted yellow. Sequence conservation in LIM domains 2–5 suggests that these domains do not interact with ILK ARD. Figure made using pymol (www.pymol.org).
The complex of ILK ARD with PINCH2 LIM1 is very similar to that seen for ILK ARD with PINCH1 LIM1. Superposition using secondary structure matching (CCP4, 1994) of the entire complex structure reveals an RMSD of 0.60 Å over 226 residues, 0.55 Å over 164 residues for the ILK ankyrin repeat domains and 0.65 Å over 62 residues for the PINCH1 and PINCH2 LIM1 domains (Fig 2B,C). The similarities of the two complex structures is, however, most striking when the C-terminal zinc fingers of the PINCH LIM1 domains are compared; over 33 residues the RMSD value of superposition is 0.2 Å. Along the interface, there are only 2 residue substitutions in PINCH2 compared to PINCH1: Ala15 and Arg43 in PINCH2 are Gly and Gln in PINCH1 (Fig 2D). These substitutions are marginal and do not alter the interaction interface. We conclude from our comparisons of the two crystal structures that the LIM1 domains of both PINCH1 and PINCH2 interact with ILK ARD via an experimentally identical interface, suggesting that PINCH1 and PINCH2 will directly compete for binding to ILK.
As a further comparison of the ILK-PINCH1 and ILK-PINCH2 interactions we assessed the effect of point mutations in ILK ARD and PINCH2 LIM1 on their interactions. As previously observed for PINCH1 interactions, ILK H99D mutants were dramatically impaired in binding to PINCH2 LIM1, while ILK ARD W110A retained the ability to bind PINCH2 LIM1 (Fig 3A). Likewise, as was the case for mutations in PINCH1 LIM1, F42A and D62A mutations in PINCH2 LIM1 inhibited binding to ILK ARD while mutation of had R12A little effect (Fig 3A). Thus point mutants confirm the significance of our crystallographic data and point to a highly similar mode of interaction of ILK with PINCH1 or PINCH2.
Figure 3. PINCH1 and PINCH2 LIM1 domains compete for binding to the ILK ARD.

A) Binding of wild-type and mutant ILK ARD to wild-type and mutant His-tagged PINCH2 LIM1 assessed in pull-down assays. Bound protein was analyzed by SDS-PAGE. Protein binding was quantified by densitometry and is reported as a percentage of wild-type binding in each experiment. B,C) Increasing amounts of soluble PINCH1 LIM1 or PINCH2 LIM1 were premixed with ILK ARD protein, and ILK ARD binding to His-PINCH2 LIM1-coated beads (B) or His-PINCH1 LIM1-coated beads (C) was assessed by pull-down assays and Coomassie staining. Protein binding was quantified by densitometry and is reported as a percentage of maximal binding (in the absence of inhibitor) in each experiment. Error bars indicate the standard error of the mean for three independent experiments. Curve fitting and IC50 determination was performed with Sigma Plot using the ligand binding one-site competition module with maximum and minimum values set to 100 and 0, respectively.
The LIM1 domains of PINCH1 and PINCH2 compete for binding with ILK ARD
The crystal structures of ILK-PINCH1 and ILK-PINCH2 reveal that the LIM1 binding site on the surface of ILK ARD is identical for the LIM1 domains of these proteins. Therefore, it is expected that PINCH1 and PINCH2 will compete for this binding site. To address this experimentally, we asked whether soluble PINCH1 LIM1 can inhibit the binding of soluble ILK ARD to His-tagged PINCH2 LIM1 coated beads. Figure 2b shows that the binding of ILK ARD to PINCH2 is inhibited in a dose-dependant manner in the presence of increasing concentrations of PINCH1 LIM1. The calculated IC50 for inhibition by PINCH1 is 2.9 μM (log IC50 = 3.47 ± 0.06 (S.E.)), compared to 2.3 μM for inhibition by soluble PINCH2 LIM1 of ILK ARD binding to immobilized PINCH2 LIM1 (log IC50 = 3.37 ± 0.06). These data establish that the LIM1 domains of PINCH1 and PINCH2 compete for binding to the ILK ARD, as expected given their high degree of sequence conservation and the structural similarity in the LIM1-ARD interface. In addition these binding studies suggest that the affinities of PINCH1 and PINCH2 LIM1 for ILK ARD binding are similar (Fig 3B). To confirm the specificity of the assay we tested the ability of the non-binding PINCH2 LIM1 F42A mutant to inhibit ILK ARD binding to PINCH2 LIM1 coated beads and saw no significant inhibition even at the highest dose tested (Fig 3B). We also conducted binding assays testing soluble PINCH2 LIM1 inhibition of ILK ARD binding to PINCH1 LIM1-coated beads (Fig 3C). PINCH2 LIM1 inhibited with an IC50 of 4.4 μM (log IC50 = 3.65 ± 0.05). Again, the control PINCH2 F42A mutant failed to inhibit (Fig 3C). Taken together, these data demonstrate that PINCH1 and PINCH2 compete for the same binding site on ILK with similar affinities.
Disruption of PINCH2 interaction with ILK ARD alters sub-cellular localization
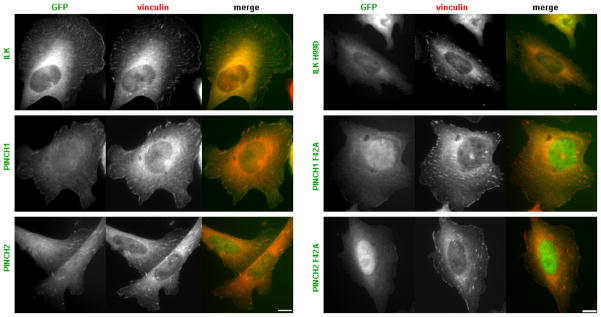
To assess the effect of mutations that perturb ILK-PINCH interactions in full-length proteins we examined focal adhesion localization of wild-type and mutant ILK, PINCH1 and PINCH2. Localization studies were performed using full-length ILK, PINCH1 or PINCH2 constructs that contain GFP at the N-terminus. Consistent with previous reports (Yang et al., 2009; Zhang et al., 2002a; Zhang et al., 2002c; Zhang et al., 2002b; Li et al., 1999; Braun et al., 2003), when expressed in Chinese hamster ovary (CHO) cells, transiently expressed wild-type ILK, PINCH1 or PINCH2 each localized to focal adhesions, identified by co-staining with vinculin (Fig 4). However, point mutations that inhibit ILK ARD - PINCH LIM1 interactions (H99D in ILK and F42A in PINCH1 and PINCH2) impaired focal adhesion localization (Fig 4). Western blotting of cell lysates confirmed that mutants were expressed as full-length proteins (not shown). Vinculin staining showed that focal adhesions were still present in cells expressing the mutant ILK or PINCH proteins, indicating that in the context of cells expressing endogenous IPP components, the over-expressed mutant proteins did not dramatically alter the number or size of focal adhesions formed. Thus, mutations generated on the basis of structural data and shown to inhibit direct ILK-PINCH interactions in vitro, also prevent normal focal adhesion localization of ILK and PINCH in cells, confirming the importance of ILK-PINCH interactions for correct subcellular localization.
Figure 4. Disruption of PINCH - ILK interactions alters sub-cellular localization.
CHO cells transfected with expression vectors encoding GFP- ILK, ILK H99D, PINCH1, PINCH1 F42A, PINCH2 and PINCH2 F42A were plated on fibronectin-coated coverslips and stained with a mouse monoclonal anti-vinculin antibody (as a marker of focal adhesion, red). Scale bar: 10μm. Point mutations that inhibit ILK ARD - PINCH LIM1 interactions (H99D in ILK and F42A in PINCH1 and PINCH2) impair focal adhesion localization.
Discussion
Unlike the invertebrate species examined to date, mammals possess two separate but well conserved PINCH genes that are both expressed in many tissues (Liang et al., 2009). The evolutionary reasons for mammalian expression of alternate genomic isoforms of PINCH is not fully clear, but it may permit differential regulation of signaling downstream of integrins. PINCH1 and PINCH2 have similar functions and can compensate for one another in many settings (Liang et al., 2009; Stanchi et al., 2005) however isoform-specific interactions and functions have been reported (Dougherty et al., 2005; Fukuda et al., 2003; Shi et al., 2008), suggesting that association with a PINCH1 or PINCH2 containing IPP complex may provide a bifurcation point in integrin signaling. Here we have shown the molecular basis for PINCH2 association with ILK and demonstrated that localization of PINCH2 to focal adhesions is ablated by a mutation that severely impairs PINCH2 binding to ILK. Most significantly, comparison of PINCH2 and PINCH1 binding to ILK reveals that both proteins bind ILK in an experimentally identical manner and compete directly with one another for binding to ILK. Our data therefore suggest that in vivo the relative expression levels of PINCH1 or PINCH2 will determine which IPP complex predominates. Differential regulation of PINCH1 and PINCH2 expression, for example by epigenetic silencing (Kim et al., 2006) or transforming growth factor β1 stimulation (Shi et al., 2008), may therefore provide a means for altering integrin signaling pathways.
Acknowledgments
We thank Ewa Folta-Stogniew, Vivian Stojanoff and the staff of NSLS beamline X6A. Modified pET32 was designed by F. Poy. This work was supported by a Swebelius Cancer Research Award from the Yale Cancer Center and by R01 GM088240 from the NIH. B.P.C. was supported by Neuropharmacology (T32NS007136) and Vascular Research (T32HL007950) and A.L.S. by Cancer (T32CA09085) postdoctoral training grants from the NIH.
Footnotes
Accession numbers
The crystal structure of PINCH2 LIM1 domain in complex with ILK ARD has been deposited in the Protein Data Bank with an accession number 3IXE.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Braun A, Bordoy R, Stanchi F, Moser M, Kostka G, Ehler E, Brandau O, Fassler R. PINCH2 is a new five LIM domain protein, homologous to PINCH and localized to focal adhesions. Exp Cell Res. 2003;284:239–250. doi: 10.1016/s0014-4827(02)00039-3. [DOI] [PubMed] [Google Scholar]
- 2.CCP4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 3.Chiswell BP, Zhang R, Murphy JW, Boggon TJ, Calderwood DA. The structural basis of integrin-linked kinase-PINCH interactions. Proc Natl Acad Sci U S A. 2008;105:20677–20682. doi: 10.1073/pnas.0811415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dougherty GW, Chopp T, Qi SM, Cutler ML. The Ras suppressor Rsu-1 binds to the LIM 5 domain of the adaptor protein PINCH1 and participates in adhesion-related functions. Exp Cell Res. 2005;306:168–179. doi: 10.1016/j.yexcr.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda T, Chen K, Shi X, Wu C. PINCH-1 is an obligate partner of integrin-linked kinase (ILK) functioning in cell shape modulation, motility, and survival. J Biol Chem. 2003;278:51324–51333. doi: 10.1074/jbc.M309122200. [DOI] [PubMed] [Google Scholar]
- 7.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 8.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SK, Jang HR, Kim JH, Noh SM, Song KS, Kim MR, Kim SY, Yeom YI, Kim NS, Yoo HS, Kim YS. The epigenetic silencing of LIMS2 in gastric cancer and its inhibitory effect on cell migration. Biochem Biophys Res Commun. 2006;349:1032–1040. doi: 10.1016/j.bbrc.2006.08.128. [DOI] [PubMed] [Google Scholar]
- 10.Lange A, Wickstrom SA, Jakobson M, Zent R, Sainio K, Fassler R. Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature. 2009;461:1002–1006. doi: 10.1038/nature08468. [DOI] [PubMed] [Google Scholar]
- 11.Laskowski RA. PDBsum: summaries and analyses of PDB structures. Nucleic Acids Research. 2001;29:221–222. doi: 10.1093/nar/29.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst. 1993;26:283–291. [Google Scholar]
- 13.Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Zhang Y, Wu C. Integrin-linked kinase is localized to cell-matrix focal adhesions but not cell-cell adhesion sites and the focal adhesion localization of integrin-linked kinase is regulated by the PINCH-binding ANK repeats. J Cell Sci. 1999;112:4589–4599. doi: 10.1242/jcs.112.24.4589. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Bordoy R, Stanchi F, Moser M, Braun A, Kudlacek O, Wewer UM, Yurchenco PD, Fassler R. PINCH1 regulates cell-matrix and cell-cell adhesions, cell polarity and cell survival during the peri-implantation stage. J Cell Sci. 2005;118:2913–2921. doi: 10.1242/jcs.02422. [DOI] [PubMed] [Google Scholar]
- 16.Liang X, Sun Y, Ye M, Scimia MC, Cheng H, Martin J, Wang G, Rearden A, Wu C, Peterson KL, Powell HC, Evans SM, Chen J. Targeted ablation of PINCH1 and PINCH2 from murine myocardium results in dilated cardiomyopathy and early postnatal lethality. Circulation. 2009;120:568–576. doi: 10.1161/CIRCULATIONAHA.109.864686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang X, Zhou Q, Li X, Sun Y, Lu M, Dalton N, Ross J, Jr, Chen J. PINCH1 plays an essential role in early murine embryonic development but is dispensable in ventricular cardiomyocytes. Mol Cell Biol. 2005;25:3056–3062. doi: 10.1128/MCB.25.8.3056-3062.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol. 2002;12:787–797. doi: 10.1016/s0960-9822(02)00810-2. [DOI] [PubMed] [Google Scholar]
- 19.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Likelihood-enhanced fast translation functions. Acta Crystallographica Section D-Biological Crystallography. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 20.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase--essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 21.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 22.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Macromolecular Crystallography, Pt A. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 23.Perrakis A, Harkiolaki M, Wilson KS, Lamzin VS. ARP/wARP and molecular replacement. Acta Crystallographica Section D-Biological Crystallography. 2001;57:1445–1450. doi: 10.1107/s0907444901014007. [DOI] [PubMed] [Google Scholar]
- 24.Postel R, Vakeel P, Topczewski J, Knoll R, Bakkers J. Zebrafish integrin-linked kinase is required in skeletal muscles for strengthening the integrin-ECM adhesion complex. Dev Biol. 2008;318:92–101. doi: 10.1016/j.ydbio.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fassler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi X, Qu H, Kretzler M, Wu C. Roles of PINCH-2 in regulation of glomerular cell shape change and fibronectin matrix deposition. Am J Physiol Renal Physiol. 2008;295:F253–F263. doi: 10.1152/ajprenal.00070.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanchi F, Bordoy R, Kudlacek O, Braun A, Pfeifer A, Moser M, Fassler R. Consequences of loss of PINCH2 expression in mice. J Cell Sci. 2005;118:5899–5910. doi: 10.1242/jcs.02686. [DOI] [PubMed] [Google Scholar]
- 28.Stanchi F, Grashoff C, Nguemeni Yonga CF, Grall D, Fassler R, Obberghen-Schilling E. Molecular dissection of the ILK-PINCH-parvin triad reveals a fundamental role for the ILK kinase domain in the late stages of focal-adhesion maturation. J Cell Sci. 2009;122:1800–1811. doi: 10.1242/jcs.044602. [DOI] [PubMed] [Google Scholar]
- 29.Tu Y, Li F, Goicoechea S, Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol. 1999;19:2425–2434. doi: 10.1128/mcb.19.3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu C. The PINCH-ILK-parvin complexes: assembly, functions and regulation. Biochim Biophys Acta. 2004;1692:55–62. doi: 10.1016/j.bbamcr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Wu C. PINCH, N(i)ck and the ILK: network wiring at cell-matrix adhesions. Trends Cell Biol. 2005;15:460–466. doi: 10.1016/j.tcb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Xu Z, Fukuda T, Li Y, Zha X, Qin J, Wu C. Molecular dissection of PINCH-1 reveals a mechanism of coupling and uncoupling of cell shape modulation and survival. J Biol Chem. 2005;280:27631–27637. doi: 10.1074/jbc.M504189200. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Wang X, Hawkins CA, Chen K, Vaynberg J, Mao X, Tu Y, Zuo X, Wang J, Wang YX, Wu C, Tjandra N, Qin J. Structural basis of focal adhesion localization of LIM-only adaptor PINCH by integrin-linked kinase. J Biol Chem. 2009;284:5836–5844. doi: 10.1074/jbc.M805319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yasunaga T, Kusakabe M, Yamanaka H, Hanafusa H, Masuyama N, Nishida E. Xenopus ILK (integrin-linked kinase) is required for morphogenetic movements during gastrulation. Genes Cells. 2005;10:369–379. doi: 10.1111/j.1365-2443.2005.00841.x. [DOI] [PubMed] [Google Scholar]
- 35.Zervas CG, Gregory SL, Brown NH. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol. 2001;152:1007–1018. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Chen K, Guo L, Wu C. Characterization of PINCH-2, a new focal adhesion protein that regulates the PINCH-1-ILK interaction, cell spreading, and migration. J Biol Chem. 2002a;277:38328–38338. doi: 10.1074/jbc.M205576200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Chen K, Tu Y, Velyvis A, Yang Y, Qin J, Wu C. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J Cell Sci. 2002b;115:4777–4786. doi: 10.1242/jcs.00166. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Guo L, Chen K, Wu C. A critical role of the PINCH-integrin-linked kinase interaction in the regulation of cell shape change and migration. J Biol Chem. 2002c;277:318–326. doi: 10.1074/jbc.M108257200. [DOI] [PubMed] [Google Scholar]