Abstract
The anti-inflammatory activity of licorice (LE) and roated licorice (rLE) extracts determined in the murine phorbol ester-induced acute inflammation model and collagen-induced arthritis (CIA) model of human rheumatoid arthritis. rLE possessed greater activity than LE in inhibiting phorbol ester-induced ear edema. Oral administration of LE or rLE reduced clinical arthritis score, paw swelling, and histopathological changes in a murine CIA. LE and rLE decreased the levels of proinflammatory cytokines in serum and matrix metalloproteinase-3 expression in the joints. Cell proliferation and cytokine secretion in response to type II collagen or lipopolysaccharide stimulation were suppressed in spleen cells from LE or rLE-treated CIA mice. Furthermore, LE and rLE treatment prevented oxidative damages in liver and kidney tissues of CIA mice. Taken together, LE and rLE have benefits in protecting against both acute inflammation and chronic inflammatory conditions including rheumatoid arthritis. rLE may inhibit the acute inflammation more potently than LE.
1. Introduction
Human rheumatoid arthritis (RA) is an autoimmune disease associated with painful joints that affects approximately 1% of the worldwide population, for which there is no effective cure available [1, 2]. It arises from chronic joint inflammation, which causes structural damage to cartilage, bone, and ligaments [3]. Rheumatoid synovial tissue is central to the pathogenesis of RA, as it becomes hyperplastic as a result of the accumulation and proliferation of inflammatory cells, including B cells, T cells, macrophages, and synovial fibroblasts [3]. Infiltrating immune cells produce and release a variety of cytokines, predominantly tumor necrosis factor (TNF)-α and interleukin (IL)-1β, which attract and activate other inflammatory cells, propagating a vicious inflammatory cycle that leads to bone damage [4]. These cytokines also increase the production of matrix metalloproteinases (MMPs), enzymes that can degrade all components of the extracellular matrix, leading to destruction of cartilage [5].
RA is widely treated with nonsteroidal anti-inflammatory drugs, immunosuppressive disease-modifying anti-rheumatic drugs and corticosteroids, which normalize the immune system and reduce the expression of inflammatory mediators [6, 7]. However, these drugs are undesirable for long-term treatment due to low efficacy, delayed onset of action, long-term side effects and toxicity [8]. Therapeutic targeting of cytokines in RA using TNF-α and IL-1β antagonists has substantial efficacy, but this strategy is expensive and has many side effects, including hypersensitivity to medications and infections [8, 9]. Therefore, it is necessary to develop preventive and therapeutic agents that have minimal toxicity, and oriental natural medicinal plants with anti-inflammatory activities are a useful resource to obtain novel antiarthritic agents.
Licorice is an esteemed crude drug that originates from the dried roots of several Glycyrrhiza species [10]. Chinese traditional medicine practitioners have used licorice to treat various ailments ranging from tuberculosis to peptic ulcers [11], and licorice has been employed as a flavoring and sweetening agent as well as a demulcent and expectorant in Western countries [12]. The roasted form of licorice has been reported to possess anti-allergic [13], neuroprotective [14], antioxidative [15], and anti-inflammatory activities [16]. In the present study, we found that roasted licorice extract (rLE) was more potent than unroasted licorice extract (LE) in inhibiting ear edema in mice treated with phorbol ester, which induces acute inflammation due to the increased activity of TNF-α and IL-1β [17]. Furthermore, we investigated the antiarthritic effects of rLE in a murine collagen-induced arthritis (CIA) model that has been widely accepted as an appropriate animal model of human rheumatoid arthritis [18].
2. Materials and Methods
2.1. Reagents
LE and rLE were generously provided by Lim, a coauthor [16]. Bovine collagen type II (CII), complete Freund's adjuvant (CFA) and incomplete Freund's adjuvant (IFA) were obtained from Chondrex (Redmond, WA). 12-O-tetradecanoylphorbol-13-acetate (TPA), dimethyl sulfoxide (DMSO), lipopolysaccharide (LPS), 1,1,3,3-tetramethoxypropane, 5,5-dithiobis (2-nitrobenzoic acid) (DTNB), and 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (purpald) were purchased from Sigma-Aldrich Chemical. (St. Louis, MO).
2.2. Animals
Female ICR mice (5 weeks old) and male DBA/1J mice (7–9 weeks old) were obtained from Central Laboratory Animal (Seoul, Korea) and the Jackson Laboratory (Bar Harbor, ME), respectively. All mice were permitted free access to a normal standard chow diet and tap water, and were housed at 20–22°C, with a relative humidity of 55 ± 5% and a 12-h light-dark cycle. Animal studies were conducted in accordance with the guidelines for the use and care of animals of Yonsei University (Seoul, Korea).
2.3. Determination of Anti-Inflammatory Activity
The right ear of each ICR mouse (5 per group) was topically treated with LE or rLE (1.0 and 2.0 mg/ear) in 50 μl vehicle (DMSO-acetone: 15–85, v/v) 30 minutes prior to the application of 5 nmol TPA in 50 μl vehicle. The left ears were treated with vehicle alone, and control mice received only vehicle on both ears. 4 hours later, right and left ear punches 6 mm in diameter were taken from each mouse, and then weighed. Edema was represented as the increase in weight of the right ear punch over that of the left.
2.4. Determination of Antiarthritic Activity
2.4.1. Induction of CIA and Administration of Extracts
DBA/1J mice were intradermally injected with 100 μg CII in CFA (1 : 1, w/v) to the tail base, and boosted with an intradermal injection of 100 μg CII in IFA (1 : 1, w/v) on day 21. CIA mice were orally administered the vehicle (PBS containing 1% DMSO), LE or rLE at 10 mg/kg once daily from day 25, when the first clinical signs of arthritis were observed, to day 45. Control mice were neither immunized with CII nor treated with extracts. The development of arthritis was evaluated by macroscopic scoring as follows: 0, normal; 1, swelling and/or redness in only one joint; 2, swelling and/or redness in more than one joint; 3, swelling and/or redness in the entire paw; and 4, severe swelling of the entire paw with deformity and/or ankylosis. The macroscopic arthritis score of each mouse was presented as the sum of each score of the four limbs, with the maximum score being 16. Paw swelling was assessed by measuring the thickness of the two hind paws with a digital caliper every other day from day 25. On day 45, all mice were sacrificed after serum collection. Hind paw and knee joints, spleens, livers and kidneys were removed from the control and CIA mice.
2.4.2. Histopathological and Immunohistochemical Analysis
The joint tissue sections were prepared and stained with hematoxylin and eosin after routine fixation, decalcification, and paraffin embedding. For immunohistochemical analysis, the sections were sequentially incubated with a specific primary antibody against MMP-3 (Santa Cruz Biotechnology, Santa Cruz, CA), and the appropriate horseradish peroxidase-linked secondary antibody. All sections were counterstained with hematoxylin.
2.4.3. Preparation of Spleen Single-Cell Suspensions
Spleens were washed with cold PBS and dissociated into single cell suspensions using cell strainers. Cells were cultured with RPMI 1640 medium containing 50 μM 2-mercaptoethanol, 1% antibiotic-antimycotic mixture and 10% FBS.
2.4.4. Measurement of Cytokine Levels in Blood Serum and in the Conditioned Medium of Spleen Cells
Serum was isolated from all mice on day 45 as described previously [19]. Single-cell suspensions (2 × 106 cells/well) were stimulated with 50 μg/ml denatured-CII or 5 μg/ml LPS for 48 hours at 37°C in a 5% CO2-humidified incubator. The conditioned media was collected. TNF-α and IL-1β levels in the serum and in the conditioned medium were assayed using commercially available ELISA kits (R&D systems, Minneapolis, MN).
2.4.5. Spleen Cell Proliferation Assay
Spleen single-cell suspensions (2 × 105 cells/well) were stimulated with 50 μg/ml denatured-CII for 72 hours at 37°C in a 5% CO2 humidified incubator. Cell proliferation was measured using a BrdU cell proliferation ELISA kit (Roche Diagnostics, Mannheim, Germany).
2.4.6. Determination of the Protective Activity on CIA-Induced Oxidative Tissue Damages
Lipid peroxidation, reduced glutathione (GSH) level and catalase activity in the liver and kidney homogenates were measured as described previously [15]. Protein concentrations were measured using the BCA protein assay kit (Pierce, Rockford, IL). Malondialdehyde (MDA) levels were measured in form of thiobarbituric acid reactive substances at 532 nm. A standard curve was established using 1,1,3,3-tetramethoxypropane, and the MDA level was expressed as nmol/mg protein. GSH levels were estimated at 412 nm colorimetrically after its complex formation with DTNB, and expressed as nmol/mg protein. The determination of catalase activity was based on the reaction of the enzyme with methanol in an optimal concentration of hydrogen peroxide. The formaldehyde produced was measured with purpald at 540 nm, and catalase activity was expressed as units/mg protein. One unit (U) of catalase was defined as the amount of enzyme that results in the formation of 1 nmol of formaldehyde per minutes at 25°C.
2.5. Statistical Analysis
Data are expressed as mean ± standard error (SE), and analyzed via one-way ANOVA with multiple comparisons, followed by Tukey test. P values less than .05 were considered to be statistically significant.
3. Results and Discussion
The present study is the first report to determine and compare the antiarthritic activities and underlying mechanism of LE and rLE in the CIA mouse model of human RA. Licorice is used in alternative medicine to treat individuals with gastric ulcers, bronchitis, arthritis, adrenal insufficiency and allergies [10, 20]. It contains diverse chemical components including glycyrrhizin and its aglycone, as well as species-specific flavonoids such as licochalcone A, liquiritin, isoliquiritin and their corresponding aglycons [10, 21, 22]. These flavonoids possess antioxidative [23, 24] and anticarcinogenic activities [25]. Recent studies demonstrated that rLE showed more potent anti-allergic, anti-diabetic, and anti-inflammatory effects than LE [13, 20, 26], and had higher glycyrrhetinic acid and lower glycyrrhizin content than LE [26]. We have confirmed, using high-performance liquid chromatographic analysis, that rLE contained more licochalcone A and less glycyrrhizin and isoliquiritigenin. The amount of glycyrrhizin and isoliquiritigenin was reduced by half but the amount of licochalcone A was increased to 5-fold by roasting (data not shown). In this study, we first evaluated the anti-inflammatory activity of LE and rLE on TPA-induced ear edema to predict their antiarthritic potential. Mouse ear edema induced with topically applied TPA is an excellent acute inflammation animal model, closely related with the infiltration of neutrophil and macrophages, the induction of proinflammatory cytokines including TNF-α and IL-1, and the generation of ROS including superoxide anion, thereby can be very useful short-term test to detect the agents with anti-arthritic potential [27]. Pretreatment with LE or rLE dose-dependently suppressed mouse ear edema induced with topical application of TPA for 4 hours, and rLE showed more potent inhibition of inflammation than LE, as shown in Figure 1. Pretreatment with licochalcone A and glycyrrhizin, not isoliquiritigenin, significantly inhibited TPA-induced ear edema in mice (data not shown). The increased licochalcone A by roasting may contribute to better anti-inflammatory of rLE on TPA-induced acute inflammation as compared with LE.
Figure 1.
Effects of LE and rLE on TPA-induced mouse ear edema. The right ear of each ICR mouse was topically treated with LE and rLE (1.0 and 2.0 mg/ear) in 50 μl vehicle (DMSO-acetone: 15–85, v/v) 30 minutes prior to the application of 5 nmol TPA in 50 μl vehicle. The left ears were treated with vehicle alone. Four hours later, edema was measured as the increase in the weight of the right ear punch over that of the left. Data are expressed as means ± SE of 5 mice per group. *P < .005, **P < .0001 versus TPA alone-treated group.
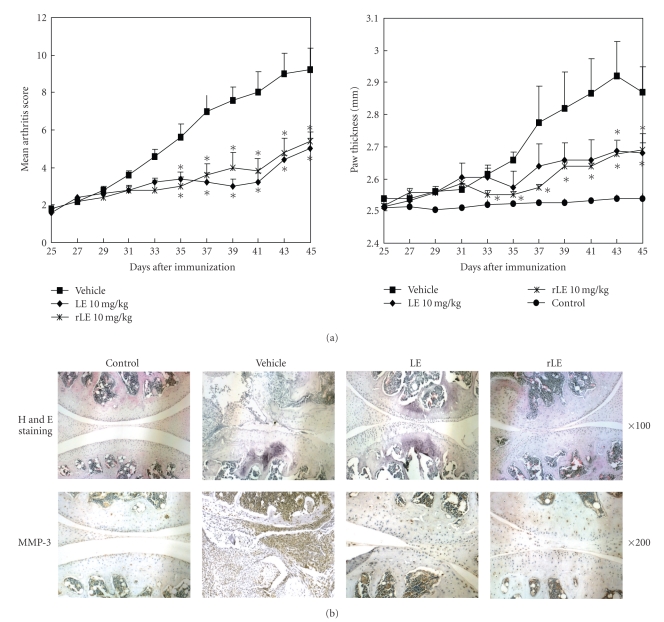
We further determined the effects of LE and rLE on chronic inflammatory diseases using a mouse CIA model. Although the murine CIA model does not completely recapitulate human RA, it is a useful tool for studying inflammation, autoimmune arthritis, cartilage destruction, and bone erosion [18]. Our data indicated that oral administration of LE and rLE to CII-immunized mice reduced the progression of arthritis by inhibiting the increase in arthritis score and paw swelling, as compared to the vehicle-treated immunized mice (Figure 2(a)). There was no significant difference between the ability of LE and rLE to inhibit CIA severity, but rLE seemed to suppress early paw swelling more effectively than LE. The joints of the vehicle-treated mice had noticeable pathological changes, including CIA-characteristic synovial hyperplasia, infiltration of inflammatory cells into the joint cavity, and extensive pannus formation. The destruction of articular cartilage and subchondral bone was also observed in the joint tissues of vehicle-treated CIA mice. The pathological events apparent in vehicle-treated CIA mice were substantially reduced in the joints of LE- and rLE-treated CIA mice (Figure 2(b)). Furthermore, immunohistochemical analysis indicated that the joint specimens from vehicle-treated CIA mice strongly stained for MMP-3, which is a key contributor to the destruction of cartilage, but treatment with LE and rLE reduced the expression of MMP-3 in inflammatory articular cartilage (Figure 2(b)). Therefore, rLE and LE are novel potential anti-arthritic agents.
Figure 2.
Effects of LE and rLE on the progression and severity of CIA in mice. LE and rLE at 10 mg/kg were orally administered to CII-immunized mice once daily from day 25 to day 45. (a) The severity of arthritis was evaluated by clinical arthritis score and hind paw thickness. Data are expressed as mean ± SE of 5 mice per group. *P < .05 versus vehicle-treated CIA mice. (b) Joint tissues from all mice were stained with H&E for histopathological examination (upper) and immunohistochemically stained for MMP-3 expression (lower).
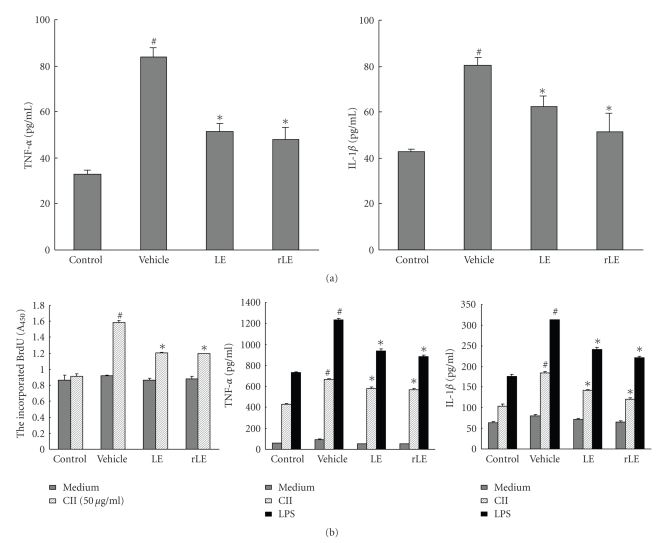
Similarly to human RA, the binding and accumulation of anti-CII antibodies in the articular region of CII-immunized mice may initiate inflammatory responses by activating the complement cascade. The activation of the complement cascade results in the recruitment of neutrophils and macrophages, which secrete chemotoxic materials and pro-inflammatory cytokines, such as IL-1β, TNF-α, IL-8, IL-6, nitric oxide, and prostaglandin E2 [18]. Of these, TNF-α drives early joint inflammation and inflammatory cell infiltration into the synovial tissue, while IL-1β plays a pivotal role in mediating cartilage degradation and bone erosion. In particular, TNF-α has been considered as a therapeutic target for RA [28]. Inflammatory cytokines such as TNF-α and IL-1β stimulate the production of MMPs. Therefore, controlling MMP activity as well as the production of pro-inflammatory cytokines is required for the effective treatment of arthritis. In this study, TNF-α and IL-1β levels were measured in the sera of control mice and CIA mice treated with vehicle, LE or rLE on day 45. While TNF-α and IL-1β levels were significantly increased in the sera of vehicle-treated CIA mice, orally administrated LE and rLE inhibited the CIA-induced increases in the serum levels of these cytokines (Figure 3(a)). These results suggest that LE and rLE may ameliorate CIA by blocking the increased levels of TNF-α and IL-1β in CIA mice.
Figure 3.
Effects of LE and rLE on the serum TNF-α and IL-1β levels, and pro-inflammatory immune response of spleen cell in CIA mice. CIA mice with the clinical signs of arthritis were orally administered the vehicle (PBS containing 1% DMSO), LE (10 mg/kg) or rLE (10 mg/kg) once daily from day 25 to day 45. Control mice were neither immunized with CII nor treated with extracts. (a) The serum levels of TNF-α and IL-1β were measured by ELISA assay. (b) Single-cell suspensions from spleens were obtained from control and all CIA mice. The proliferation of spleen cells stimulated with 50 μg/ml denatured CII for 72 hours was assessed using a BrdU cell proliferation ELISA kit (left), and TNF-α and IL-1β levels were measured in conditioned media of spleen cells stimulated with 50 μg/ml denatured CII or 5 μg/ml LPS for 48 hours, using each specific ELISA kit (middle and right). Data is expressed as mean ± SE of 5 mice per group. #P < .01 versus control mice, *P < .01 versus vehicle-treated CIA mice.
The prevalence of CII-specific T cells, the primary mediators of CIA induction, has been observed in the early phase of RA [29]. Although the pathways that drive cytokine release in RA synovium are still undefined, T cells can regulate cytokine secretion by directly releasing IL-17 into the extracellular space or via cellular contacts with synovial macrophages [28, 30]. To estimate the effects of our extracts on CII-specific T cell responses, cell proliferation was investigated with spleen cells isolated from control mice and CIA mice treated with vehicle, LE or rLE. The spleen is the major site of adaptive immune response to blood-borne antigens due to the high population of resident phagocytes and lymphocytes. Spleen cells are stimulated with either CII to activate T cells or LPS to potently activate macrophages [16]. The proliferation of spleen cells from vehicle-treated CIA mice was increased by CII stimulation for 72 hours, but spleen cells from LE- and rLE-treated CIA mice exhibited significantly less proliferation than those from vehicle-treated CIA mice (see Figure 3(b)). Next, CII- and LPS-induced cytokine levels were measured in spleen cells from CIA mice treated with vehicle, LE and rLE. CII- or LPS-stimulated secretion of TNF-α and IL-1β was also reduced in spleen cells isolated from CIA mice treated with LE or rLE, as compared to spleen cells from vehicle-treated CIA mice (Figure 3(b)). These results suggest that LE and rLE alleviate CIA by reducing the immune responses of spleen cells, which results in decreased cytokine production.
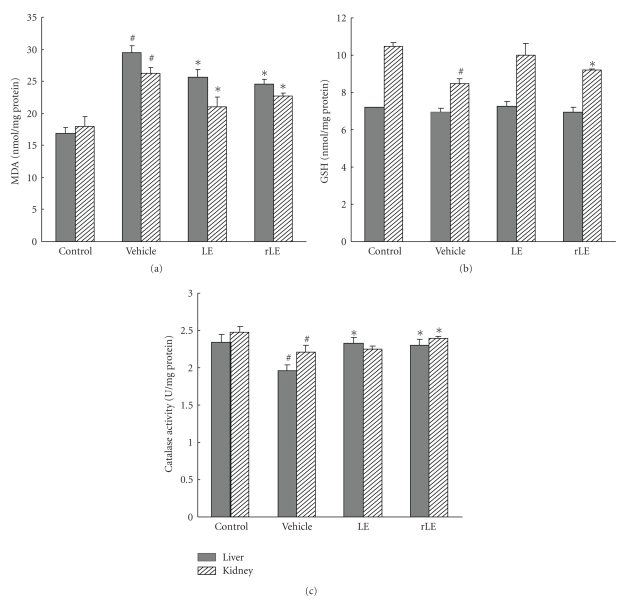
ROS released from infiltrating inflammatory cells is implicated in the breakdown of cartilage and bone in RA, and elevated levels of MDA have been observed in the serum and synovial fluid of RA patients [31]. Moreover, chronic inflammatory conditions reduce a body's antioxidant capacity by affecting a variety of endogenous ROS scavenging proteins, enzymes, and chemical compounds. This leads to oxidative stress, which damages other tissues and organs [32]. Therefore, control of ROS levels is also required to reduce CIA severity and tissue damage. To study the protective effects of LE and rLE on tissue oxidative damages in CIA mice, MDA level as a marker of lipid peroxidation, GSH content, and antioxidant enzyme activity were measured in liver and kidney tissue homogenates from control mice and CIA mice treated with vehicle, LE or rLE. MDA levels were elevated in liver and kidney tissues from vehicle-treated CIA mice, but the increase in MDA levels was inhibited in those from LE or rLE-treated CIA mice (Figure 4(a)). In addition, the decreased tissue GSH levels (Figure 4(b)) and catalase activities (Figure 4(c)) in vehicle-treated CIA mice were rescued by oral administration of LE or rLE. These results suggest that LE and rLE can prevent tissue damage by blocking oxidative stress in CIA mice.
Figure 4.
Effects of LE and rLE on oxidative tissue damages in CIA mice. MDA (a) and GSH (b) levels, and catalase activities (c) were determined in liver and kidney tissues from control and CIA mice treated with vehicle, LE (10 mg/kg) or rLE (10 mg/kg) on day 45. Data is expressed as mean ± SE of 5 mice per group. #P < .01 versus control mice, *P < .05 versus vehicle-treated mice.
4. Conclusion
Topical application of LE or rLE onto the mouse ear prior to TPA treatment inhibited TPA-induced acute inflammation, and oral administration of LE or rLE effectively suppressed the inflammatory response and tissue damage in the CIA mouse model. rLE exhibited a more potent inhibition on TPA-induced acute inflammation than LE, but the anti-arthritic effect of rLE was similar to that of LE in the CIA model. Overall, these data suggest that supplementation with LE and rLE may be beneficial in preventing and treating both acute and chronic inflammatory conditions.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (R13-2003-013-03002-0), and in part by the Yonsei University College of Dentistry Research Fund of 2008. Ki Rim Kim and Chan-Kwon Jeong contributed equally to this work.
References
- 1.Lee DM, Weinblatt ME. Rheumatoid arthritis. The Lancet. 2001;358(9285):903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann M. Pathogenesis of arthritis: recent research progress. Nature Immunology. 2001;2(9):771–773. doi: 10.1038/ni0901-771. [DOI] [PubMed] [Google Scholar]
- 3.Tak PP, Smeets TJM, Daha MR, et al. Analysis of the synovial cell infiltrate in early rheumatoid synovial tissue in relation to local disease activity. Arthritis and Rheumatism. 1997;40(2):217–225. doi: 10.1002/art.1780400206. [DOI] [PubMed] [Google Scholar]
- 4.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annual Review of Immunology. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 5.Muller-Ladner U, Pap T, Gay RE, Neidhart M, Gay S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nature Clinical Practice Rheumatology. 2005;1(2):102–110. doi: 10.1038/ncprheum0047. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nature Reviews Drug Discovery. 2003;2(6):473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 7.Henderson NK, Sambrook PN. Relationship between osteoporosis and arthritis and effect of corticosteroids and other drugs on bone. Current Opinion in Rheumatology. 1996;8(4):365–369. doi: 10.1097/00002281-199607000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. The New England Journal of Medicine. 2006;355(7):704–712. doi: 10.1056/NEJMct055183. [DOI] [PubMed] [Google Scholar]
- 9.Bernatsky S, Hudson M, Suissa S. Anti-rheumatic drug use and risk of serious infections in rheumatoid arthritis. Rheumatology. 2007;46(7):1157–1160. doi: 10.1093/rheumatology/kem076. [DOI] [PubMed] [Google Scholar]
- 10.Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regulatory Toxicology and Pharmacology. 2006;46(3):167–192. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Huang KH. Use of liquid extract of liquorice for pulmonary tuberculosis. Shandong Yi Kan. 1959;21:17–18. [PubMed] [Google Scholar]
- 12.Asl MN, Hosseinzadeh H. Review of pharmacological effects of glycyrrhiza sp. and its bioactive compounds. Phytotherapy Research. 2008;22(6):709–724. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majima T, Yamada T, Tega E, Sakurai H, Saiki I, Tani T. Pharmaceutical evaluation of liquorice before and after roasting in mice. Journal of Pharmacy and Pharmacology. 2004;56(5):589–595. doi: 10.1211/0022357023286. [DOI] [PubMed] [Google Scholar]
- 14.Hwang I-K, Lim S-S, Choi K-H, et al. Neuroprotective effects of roasted licorice, not raw form, on neuronal injury in gerbil hippocampus after transient forebrain ischemia. Acta Pharmacologica Sinica. 2006;27(8):959–965. doi: 10.1111/j.1745-7254.2006.00346.x. [DOI] [PubMed] [Google Scholar]
- 15.Choi Y-J, Lim SS, Jung J-Y, et al. Blockade of nitroxidative stress by roasted licorice extracts in high glucose-exposed endothelial cells. Journal of Cardiovascular Pharmacology. 2008;52(4):344–354. doi: 10.1097/FJC.0b013e3181888898. [DOI] [PubMed] [Google Scholar]
- 16.Kim J-K, Oh S-M, Kwon H-S, Oh Y-S, Lim SS, Shin H-K. Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochemical and Biophysical Research Communications. 2006;345(3):1215–1223. doi: 10.1016/j.bbrc.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 17.Lee DY, Choo BK, Yoon T, et al. Anti-inflammatory effects of Asparagus cochinchinensis extract in acute and chronic cutaneous inflammation. Journal of Ethnopharmacology. 2009;121(1):28–34. doi: 10.1016/j.jep.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Cho Y-G, Cho M-L, Min S-Y, Kim H-Y. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmunity Reviews. 2007;7(1):65–70. doi: 10.1016/j.autrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Lee CK, Park KK, Lim SS, Park JHY, Chung WY. Effects of the licorice extract against tumor growth and cisplatin-induced toxicity in a mouse xenograft model of colon cancer. Biological and Pharmaceutical Bulletin. 2007;30(11):2191–2195. doi: 10.1248/bpb.30.2191. [DOI] [PubMed] [Google Scholar]
- 20.Li YS, Tong PJ, Ma HZ. Toxicity attenuation and efficacy potentiation effect of liquorice on treatment of rheumatoid arthritis with Tripterygium wilfordii. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26(12):1117–1119. [PubMed] [Google Scholar]
- 21.Kiso Y, Tohkin M, Hikino H, Hattori M, Sakamoto T, Namba T. Mechanism of antihepatotoxic activity of glycyrrhizin, I: effect on free radical generation and lipid peroxidation. Planta Medica. 1984;50(4):298–302. doi: 10.1055/s-2007-969714. [DOI] [PubMed] [Google Scholar]
- 22.Vaya J, Belinky PA, Aviram M. Antioxidant constituents from licorice roots: isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radical Biology & Medicine. 1997;23(2):302–313. doi: 10.1016/s0891-5849(97)00089-0. [DOI] [PubMed] [Google Scholar]
- 23.Fuhrman B, Buch S, Vaya J, et al. Licorice extract and its major polyphenol glabridin protect low-density lipoprotein against lipid peroxidation: in vitro and ex vivo studies in humans and in atherosclerotic apolipoprotein E-deficient mice. American Journal of Clinical Nutrition. 1997;66(2):267–275. doi: 10.1093/ajcn/66.2.267. [DOI] [PubMed] [Google Scholar]
- 24.Haraguchi H, Ishikawa H, Mizutani K, Tamura Y, Kinoshita T. Antioxidative and superoxide scavenging activities of retrochalcones in Glycyrrhiza inflata. Bioorganic & Medicinal Chemistry. 1998;6(3):339–347. doi: 10.1016/s0968-0896(97)10034-7. [DOI] [PubMed] [Google Scholar]
- 25.Jung JI, Lim SS, Choi HJ, et al. Isoliquiritigenin induces apoptosis by depolarizing mitochondrial membranes in prostate cancer cells. Journal of Nutritional Biochemistry. 2006;17(10):689–696. doi: 10.1016/j.jnutbio.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Ko B-S, Jang JS, Hong SM, et al. Changes in components, glycyrrhizin and glycyrrhetinic acid, in raw Glycyrrhiza uralensis Fisch, modify insulin sensitizing and insulinotropic actions. Bioscience, Biotechnology and Biochemistry. 2007;71(6):1452–1461. doi: 10.1271/bbb.60533. [DOI] [PubMed] [Google Scholar]
- 27.Murakami A, Nakamura Y, Torikai K, et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion mice. Cancer Research. 2000;60(18):5059–5066. [PubMed] [Google Scholar]
- 28.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nature Reviews Immunology. 2007;7(6):429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 29.Kim H-Y, Kim W-U, Cho M-L, et al. Enhanced T cell proliferative response to type II collagen and synthetic peptide CII (255–274) in patients with rheumatoid arthritis. Arthritis and Rheumatism. 1999;42(10):2085–2093. doi: 10.1002/1529-0131(199910)42:10<2085::AID-ANR8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 30.Lubberts E, Koenders M, van den Berg WB. The role of T cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Research and Therapy. 2005;7(1):29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostrakhovitch EA, Afanas’ev IB. Oxidative stress in rheumatoid arthritis leukocytes: suppression by rutin and other antioxidants and chelators. Biochemical Pharmacology. 2001;62(6):743–746. doi: 10.1016/s0006-2952(01)00707-9. [DOI] [PubMed] [Google Scholar]
- 32.Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal ions in human disease: an overview. Methods in Enzymology. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]