Abstract
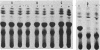
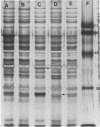
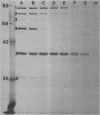
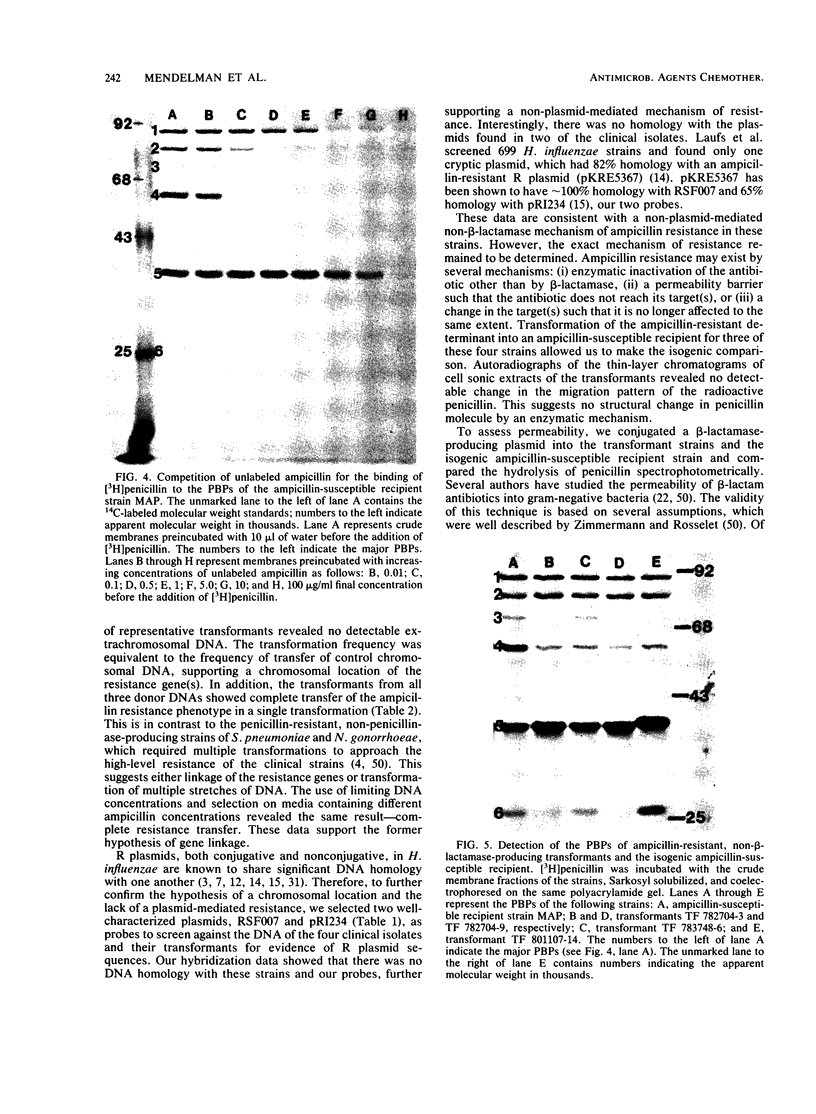
Ampicillin resistance in Haemophilus influenzae is most often due to the plasmid-mediated production of TEM beta-lactamase. We studied four strains with high-level ampicillin resistance (MIC of 32 micrograms/ml with an inoculum of 10(5) CFU on solid media) which did not produce detectable beta-lactamase activity with two different detection methods. Two of the four strains contained extrachromosomal DNA by agarose gel electrophoresis. Conjugation failed to transfer ampicillin resistance; in contrast, transformation yielded ampicillin-resistant transformants in three of the four strains. These transformants did not contain detectable extrachromosomal DNA. In addition, mobilization of the resistance determinant by transformation to, or conjugation with, recombination-deficient strains was unsuccessful. DNA-DNA hybridization experiments revealed no homology of the DNA of these strains with two R plasmids (one coding for ampicillin resistance, the other for chloramphenicol and tetracycline resistance). We conclude that the genetic basis of the non-beta-lactamase ampicillin resistance in these strains appears to be chromosomally mediated. We investigated the mechanism of resistance in these strains. Enzymatic modification of penicillin was not detected by autoradiography of a thin-layer chromatogram of cell sonic extracts of three ampicillin-resistant transformant strains incubated with [14C]penicillin. To assess changes in permeability of the cell envelope, a plasmid coding for beta-lactamase was conjugated into these strains, and the hydrolysis of penicillin by intact cells and cell sonic extracts was compared. Only one of three transformant strains had significantly diminished permeability. Outer membrane proteins of these strains analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed apparent differences in comparison with the isogenic ampicillin-susceptible recipient strain. Autofluorography of a sodium dodecyl sulfate-polyacrylamide gel electrophoresis of Sarkosyl-solubilized crude membrane (the putative inner membranes) from these ampicillin-resistant transformant strains incubated with [3H]penicillin compared with the isogenic ampicillin-susceptible recipient strain revealed reduced binding to PBP 3 and 6, 3 and 4, or 4. In addition, affinity binding studies revealed decreased affinity of PBP 4 for ampicillin of all four transformants tested. We conclude that the major mechanism of resistance in these strains is altered penicillin-binding proteins; however, other mechanisms, including permeability, may also play a role.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell S. M., Plowman D. Mechanisms of ampicillin resistance in Haemophilus influenzae from respiratory tract. Lancet. 1980 Feb 9;1(8163):279–280. doi: 10.1016/s0140-6736(80)90778-3. [DOI] [PubMed] [Google Scholar]
- Catlin B. W., Bendler J. W., 3rd, Goodgal S. H. The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol. 1972 May;70(3):411–422. doi: 10.1099/00221287-70-3-411. [DOI] [PubMed] [Google Scholar]
- De Graaff J., Elwell L. P., Falkow S. Molecular nature of two beta-lactamase-specifying plasmids isolated from Haemophilus influenzae type b. J Bacteriol. 1976 Apr;126(1):439–446. doi: 10.1128/jb.126.1.439-446.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty T. J., Koller A. E., Tomasz A. Penicillin-binding proteins of penicillin-susceptible and intrinsically resistant Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1980 Nov;18(5):730–737. doi: 10.1128/aac.18.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Saunders J. R., Richmond M. H., Falkow S. Relationships among some R plasmids found in Haemophilus influenzae. J Bacteriol. 1977 Jul;131(1):356–362. doi: 10.1128/jb.131.1.356-362.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Laufs R., Kaulfers P. M., Kolenda H. Molecular nature of two Haemophilus influenzae R factors containing resistances and the multiple integration of drug resistance transposons. J Bacteriol. 1979 May;138(2):584–597. doi: 10.1128/jb.138.2.584-597.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laufs R., Kaulfers P. M. Molecular characterization of a plasmid specifying ampicillin resistance and its relationship to other R factors from Haemophilus influenzae. J Gen Microbiol. 1977 Dec;103(2):277–286. doi: 10.1099/00221287-103-2-277. [DOI] [PubMed] [Google Scholar]
- Makover S. D., Wright R., Telep E. Penicillin-binding proteins in Haemophilus influenzae. Antimicrob Agents Chemother. 1981 Apr;19(4):584–588. doi: 10.1128/aac.19.4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S. M. Isolation of an ampicillin-resistant, non-beta-lactamase-producing strain of Haemophilus influenzae. Antimicrob Agents Chemother. 1980 Jan;17(1):80–83. doi: 10.1128/aac.17.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros A. A., Kent R. L., O'Brien T. F. Characterization and prevalence of the different mechanisms of resistance to beta-lactam antibiotics in clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1974 Dec;6(6):791–801. doi: 10.1128/aac.6.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Yoshida T. Penetration of cephalosporins and corresponding 1-oxacephalosporins through the outer layer of Gram-negative bacteria and its contribution to antibacterial activity. Antimicrob Agents Chemother. 1982 Feb;21(2):254–258. doi: 10.1128/aac.21.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Joshi V. R., Allison D. P. Molecular basis for the transformation defects in mutants of Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):1171–1180. doi: 10.1128/jb.110.3.1171-1180.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Campos J. M., Plotkin S. A. Ampicillin-resistant, beta-lactamase-negative haemophilus influenzae type b. Pediatrics. 1982 Feb;69(2):230–232. [PubMed] [Google Scholar]
- Peterson G. L. Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall. Anal Biochem. 1979 Dec;100(2):201–220. doi: 10.1016/0003-2697(79)90222-7. [DOI] [PubMed] [Google Scholar]
- Philpott-Howard J., Williams J. D. Increase in antibiotic resistance in Haemophilus influenzae in the United Kingdom since 1977: report of study group. Br Med J (Clin Res Ed) 1982 May 29;284(6329):1597–1599. doi: 10.1136/bmj.284.6329.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., Smith A. L. Molecular characterization of "plasmid-free" antibiotic-resistant Haemophilus influenzae. J Bacteriol. 1980 Oct;144(1):476–479. doi: 10.1128/jb.144.1.476-479.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Swenson C. D., Owens L. M., Smith A. L. Characterization of chloramphenicol-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 1980 Oct;18(4):610–615. doi: 10.1128/aac.18.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Stull T. L., Smith A. L. Comparative virulence of Haemophilus influenzae with a type b or type d capsule. Infect Immun. 1981 May;32(2):518–524. doi: 10.1128/iai.32.2.518-524.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C. Novel resistance selected by the new expanded-spectrum cephalosporins: a concern. J Infect Dis. 1983 Mar;147(3):585–589. doi: 10.1093/infdis/147.3.585. [DOI] [PubMed] [Google Scholar]
- Scheifele D. W. Ampicillin-resistant Hemophilus influenzae in Canada: nationwide survey of hospital laboratories. Can Med Assoc J. 1979 Jul 21;121(2):198–202. [PMC free article] [PubMed] [Google Scholar]
- Scheifele D. W., Syriopoulou V. P., Harding A. L., Emerson B. B., Smith A. L. Evaluation of a rapid beta-lactamase test for detecting ampicillin-resistant strains of Hemophilus influenzae type b. Pediatrics. 1976 Sep;58(3):382–387. [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. Chromosomally integrated conjugative plasmids are common in antibiotic-resistant Haemophilus influenzae. J Bacteriol. 1980 Jun;142(3):925–930. doi: 10.1128/jb.142.3.925-930.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syriopoulou V. P., Scheifele D. W., Sack C. M., Smith A. L. Effect of inoculum size on the susceptibility of Haemophilus influenzae b to beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Oct;16(4):510–513. doi: 10.1128/aac.16.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syriopoulou V., Scheifele D., Smith A. L., Perry P. M., Howie V. Increasing incidence of ampicillin resistance in Hemophilus influenzae. J Pediatr. 1978 Jun;92(6):889–892. doi: 10.1016/s0022-3476(78)80354-0. [DOI] [PubMed] [Google Scholar]
- Thomas W. J., McReynolds J. W., Mock C. R., Bailey D. W. Letter: Ampicillin-resistant Haemophilus influenzae meningitis. Lancet. 1974 Feb 23;1(7852):313–313. doi: 10.1016/s0140-6736(74)92617-8. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., Baker C. N., Kirven L. A., Swenson J. M. Susceptibility of ampicillin-resistant Haemophilus influenzae to seven penicillins. Antimicrob Agents Chemother. 1976 Jan;9(1):70–73. doi: 10.1128/aac.9.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Kirven L. A. Ampicillin resistance in Haemophilus influenzae as determined by a rapid test for beta-lactamase production. Antimicrob Agents Chemother. 1974 Nov;6(5):653–654. doi: 10.1128/aac.6.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. I., Tsai T. F., Filice G. A., Fraser D. W. Prevalence of ampicillin- and chloramphenicol-resistant strains of Haemophilus influenzae causing meningitis and bacteremia: national survey of hospital laboratories. J Infect Dis. 1978 Sep;138(3):421–424. doi: 10.1093/infdis/138.3.421. [DOI] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Klingeren B., van Embden J. D., Dessens-Kroon M. Plasmid-mediated chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1977 Mar;11(3):383–387. doi: 10.1128/aac.11.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]