Abstract
The DPM (Dose Planning Method) Monte Carlo electron and photon transport program, designed for fast computation of radiation absorbed dose in external beam radiotherapy, has been adapted to the calculation of absorbed dose in patient-specific internal emitter therapy. Because both its photon and electron transport mechanics algorithms have been optimized for fast computation in 3D voxelized geometries (in particular, those derived from CT scans), DPM is perfectly suited for performing patient-specific absorbed dose calculations in internal emitter therapy. In the updated version of DPM developed for the current work, the necessary inputs are a patient CT image, a registered SPECT image, and any number of registered masks defining regions of interest. DPM has been benchmarked for internal emitter therapy applications by comparing computed absorption fractions for a variety of organs using a Zubal phantom with reference results from the Medical Internal Radionuclide Dose (MIRD) Committee standards. In addition, the β decay source algorithm and the photon tracking algorithm of DPM have been further benchmarked by comparison to experimental data. This paper presents a description of the program, the results of the benchmark studies, and some sample computations using patient data from radioimmunotherapy studies using 131I.
Index Terms: Biomedical applications of nuclear radiation, biomedical nuclear imaging, dosimetry, Monte Carlo methods
I. Introduction
Optimization of treatment plans for internal emitter therapy requires accurate determination of absorbed dose in both targeted tumors (for estimation of the efficacy of the treatment) and critical organs (to preclude toxicity). Conventional methods for determining absorbed dose, such as those based on Medical Internal Radionuclide Dose (MIRD) S values [1], ignore details of patient-specific anatomy and so are inherently approximate. Such methods typically employ a single, standard human form and so assume uniform activity distributions in standardized source organs, yielding uniform absorbed dose distributions in standardized target organs. Such results can be in error when patient anatomy significantly deviates from the standard. In contrast, the accuracy of Monte Carlo-based dosimetry methods is limited only by errors in the specification of patient anatomy (typically obtained from CT) and in the determination of source activity distributions (typically derived from PET or SPECT studies). A number of investigators have reported efforts to develop dosimetry platforms for internal emitter therapy applications based on Monte Carlo computation of absorbed dose [2]–[5].
While Monte Carlo simulations offer the prospect of greatly improved accuracy, they are computationally expensive and can involve approximations of their own. For example, Monte Carlo dosimetry platforms which rely on CT data to describe patient anatomy usually treat the body as an essentially homogeneous medium (composed of water) but with spatially varying density. This approximation can lead to errors in absorbed dose computations in regions where high Z materials (such as bone) are present. In the current work, we report of the adaptation of the Monte Carlo program DPM (Dose Planning Method) [6] (originally developed specifically for fast absorbed dose computations in external beam radiotherapy applications), to internal emitter therapy applications.
DPM is well-suited as a Monte Carlo engine for internal emitter dosimetry problems. Because it was never meant to be a general-purpose code, during development DPM was optimized specifically for efficient computation of absorbed dose distributions in CT-derived, voxelized geometries, and so is faster than conventional Monte Carlo programs for this application. DPM achieves some of its speed from numerous small optimization practices that were followed during its construction. For example, since the form of the geometry, source, and required output is known and fixed (i.e., voxel-based maps), only the most rudimentary user options are available for specifying physics models, and no “hooks” exist for interrogating the program for problem tallies. Thus, DPM avoids evaluating scores of conditional tests inside the transport loops, and it instead calls tallying routines only at those instances when energy is deposited. Similarly, DPM mandates that the energy thresholds for secondary particle production and transport cut-off energies be equivalent, also avoiding conditional tests inside its transport loops.
These and other minor, localized conventions, provide a small part of the speedup realized by DPM. Two global features of the program account for the bulk of its efficiency. First, DPM employs the well-known δ-scattering method of Woodcock [7] in its treatment of photon transport. Woodcock tracking allows for the transport of photons across geometric boundaries and is most efficient with regular geometries and where interaction cross sections are fairly uniform over the entire volume. Both of these conditions are fulfilled in internal emitter therapy simulations. In addition, DPM achieves significant speed-up in electron transport problems through its use of a novel “transport mechanics” model which permits DPM to take much longer (and so far fewer) electron transport steps relative to conventional Monte Carlo programs. Because of the way it has been formulated and implemented, the transport mechanics model in DPM also permits electron transport across several region boundaries in a single step, even when those regions are composed of differing materials. In conventional Monte Carlo algorithms, step-sizes (and hence computational efficiency) for energetic electrons can be limited by region size. The details of the DPM transport mechanics model, labeled the “modified random-hinge,” and related implementation issues in the program have been described in detail by Sempau et al. [6].
Taken together, δ-scattering for photons, the random hinge mechanics with cross-boundary transport for electrons, and its many other small optimizations provide DPM with significant speed advantages over other Monte Carlo programs in voxelized geometries, particularly when the voxel dimensions are small in relation to the mean free paths of source and secondary photons and the total range of source and secondary electrons. For the simulation of electron beams in the 10–20 MeV range in typical external beam radiotherapy problems, DPM is roughly 5–10 times faster than the widely used EGS4 program [8] with 1 mm voxels, primarily because the random-hinge transport mechanics permits the accurate modeling of very long electron transport steps [6]. When voxel sizes increase (or electron and photon energies decrease), however, the speed benefits of both δ-scattering and the random hinge mechanics diminish. In particular, because of the relatively low energies of the β's emitted by isotopes typically used in internal emitter therapy applications, the speedup advantages of DPM over EGS4 are expected to be less then factor of 5–10 seen in external beam simulations. The average and maximum β particle energies following a 131I disintegration are .192 and .807 MeV, respectively, and the corresponding maximum electron ranges in water are roughly .4 mm at the average energy and 3 mm at the highest energy. Thus, electron transport plays little role in absorbed dose simulations when this isotope is used. Even for 90Y, in which there has been significant recent interest as a radioimmunotherapy isotope [9]–[11], the maximum electron ranges corresponding to the β endpoint energy (2.28 MeV) and average energy (.94 MeV) are still only 1 cm and 4 mm, respectively, meaning that some of the speedup DPM achieves through its electron transport model will not be realized except for small voxel sizes. Further, in many cases, the resolution of SPECT images used for activity quantification may be of the same order as electron range, thus mandating large voxels and so virtually negating all of DPM's potential electron transport speedup. Results of a study of the relative efficiency of DPM vs. EGS4 for internal emitter therapy absorbed dose computations are presented in the Results section of this work.
In order to reduce memory requirements and at the same time preclude post-processing, DPM does not employ batch tallies, but instead computes absorbed dose and variance on a particle-by-particle basis. Performing Monte Carlo calculations with batches of histories is convenient in that it permits computation of variance in arbitrary regions of interest after the final batches are complete, but because a minimum of 30 batches must be run to provide accurate estimates of variance, batch-based programs require either memory allocation of at least 35 times the total of number voxels or post-processing of the entire absorbed dose distribution computed for each individual batch. The memory requirements of DPM, in contrast, are roughly six times the number of voxels, (density, deposited energy, variance, material index and two temporary variables that score history-by-history data), and so problems of dimension of 256 × 256 × 256 can be run on machines with 2 GB of RAM. The disadvantage of not using batch tallies is that special scoring must be done to provide estimates of variance (though, not, of course, estimates of absorbed dose) for regions of interest.
DPM is written in FORTRAN and has been installed and run under Linux, Digital Unix, HP Unix, and VMS, among other operating systems. The standard source code and all associated data files and auxiliary files, including the pre-processing programs based on the physics and data models in PENELOPE, can be downloaded from http://www-personal.engin.umich.edu/bielajew/DPM.
II. Methods
The primary modifications necessary to adapt DPM for internal emitter therapy applications involve the source and scoring algorithms. In the current work, DPM has been modified to treat β decay radiation from generic isotopes, as defined by user through the input of branching ratio and energy level data. The implementation is generic, though thus far appropriate input data has been compiled only for 131I sources. The basic unit of radiation corresponding to a DPM Monte Carlo “history” is a single β decay—all radiation emitted subsequent to the initial decay is treated as part of a single history. Decay schemes and branching ratios have been taken from the Lawrence Berkeley/Lund University Isotopes Project [12], and are input in terms of the isotope energy levels and branching ratios for each level. Beta-particle energies are sampled from the normalized Fermi distribution using a rejection scheme. The normalized Fermi distribution is given as
| (1) |
where E0 is the maximum total energy of the line, E and p are the energy and momentum, respectively, of the emitted electron, and η is given by Ze2/hv, with v the ejected electron velocity and h Planck's constant.
The spatial distribution of the radiation source is input by the user as a PET or SPECT image registered to a CT image, which is used to define the patient anatomy. DPM reads and stores relative source intensities in each voxel. During execution, voxel ID numbers are rapidly chosen using the alias sampling technique of Walker [13], and the actual positions of the disintegrations are assumed to be uniformly distributed inside each voxel.
DPM permits the user to specify up to 100 regions of interest in the CT image (usually tumors and organs) via maps which overlay the CT geometry. All voxels not specifically assigned to be part of a user-defined ROI are taken as an aggregate “rest of body” organ. Absorbed dose due to all daughter particles originating from the initial β emission and from the subsequent gamma emission following each decay is tallied distinctly for all combinations of source and target organs defined in the user input ROIs.
The geometry data required is a map of the actual voxel densities, and in the default case, DPM assigns each voxel to be either air or water. However, if segmentation has been done a priori on the reference geometry, exact materials can be specified for chosen voxels when the tallying regions of interest are input.
SPECT images supplied to DPM are typically single time-frame snapshots of source distributions which actually have non-uniform time-dependencies, and so DPM provides a facility for organ-by-organ corrections to the source intensity distribution based on measured or assumed residence times for the defined regions of interest. This method does, of course, assume that the time-dependence of the activity curve is uniform for each voxel within each specific organ.
A facility also is provided for studying the effects of misregistration. Offsets in (x, y, z) can be input by the user and the SPECT image will be shifted relative to the CT image by the specified amounts. A study of the magnitude of misregistration effects using DPM and phantoms has been reported recently [15].
DPM computes and prints absorbed dose rate maps, absorbed dose rate uncertainty maps, and summary tables for the various ROIs. A sample input activity map is shown in Fig. 1, a sample input CT in Fig. 2, and the resultant computed absorbed dose rate map in Fig. 3. A sample output summary table is shown in Fig. 4. Note that in this example, the SPECT activity was not corrected for the variable residence times in the ROIs. Computations were performed using a 256 × 256 × 38 CT image with voxel dimensions of 0.2 × 0.2 × 1 cm. This particular data set included one extremely large and elongated tumor, which evinces higher than usual self-dose due to gammas.
Fig. 1.
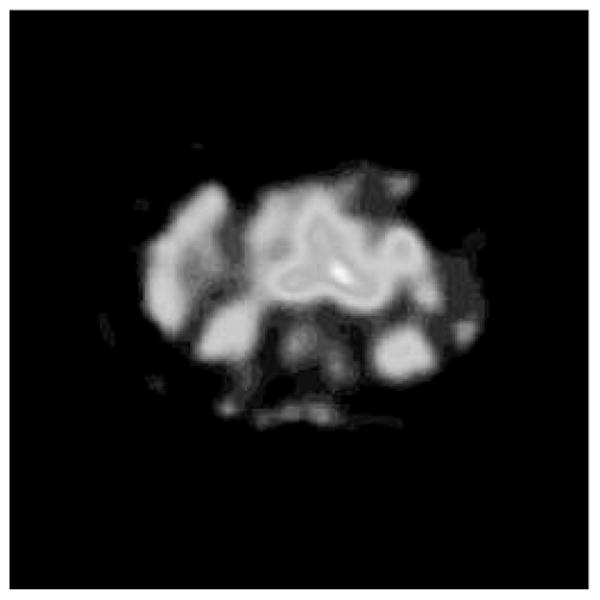
Patient Activity Map.
Fig. 2.
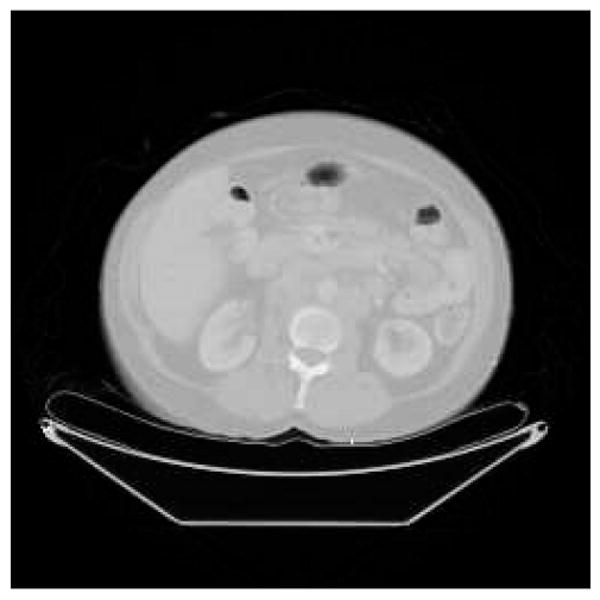
Patient CT Map.
Fig. 3.
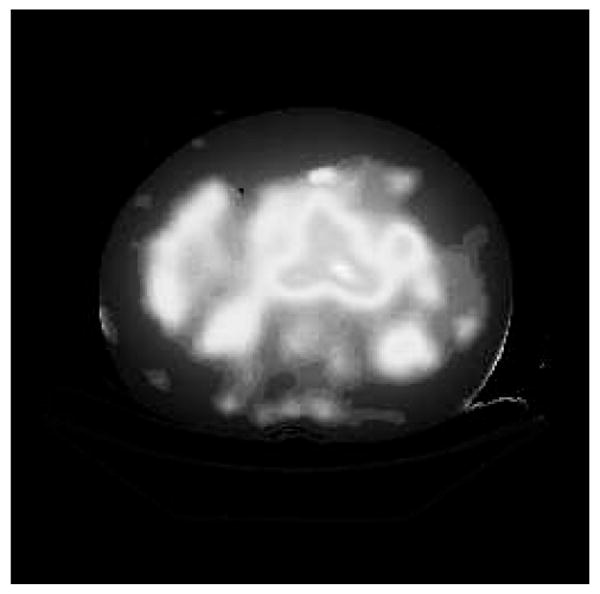
Patient Absorbed Dose Rate Map.
Fig. 4.
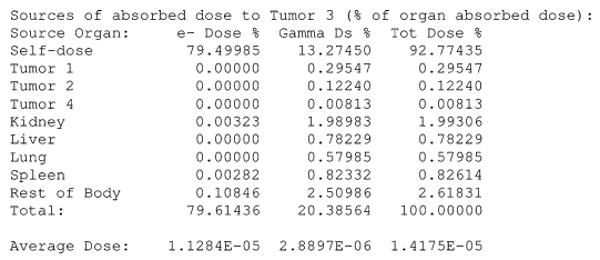
DPM output of target absorbed dose from various sources.
III. Results and Benchmarking
The initial, external source, version of DPM was originally validated by comparison against the EGS4 [8] and PENELOPE [14] Monte Carlo programs [6]. DPM has since been bench-marked against measurements for external beam therapy applications (with both electron and photon beams), in homogeneous and heterogeneous media [16]–[18]. DPM is currently being used extensively in research investigations addressing clinical problems (see for example, [19]–[21]). Thus, the accuracy of the basic physics and transport and models of DPM is well established for external sources. We present here results of exercises designed to validate the features added to DPM for modeling internal emitter therapies.
As reported previously [22], DPM has been validated for internal emitter studies by comparing conventional MIRD S factors with those calculated by DPM using a Zubal phantom [23] as the reference geometry. Some of those earlier results are reproduced in Table I. As noted in the prior work, because some of the organs in the voxelized Zubal phantom differ considerably in mass and geometry from the organs in the Standard Man mathematical phantom employed in the MIRD methodology, organ self-dose S factors from DPM must be corrected to account for this. When this is done using a linear mass-weighting factor, DPM and MIRD agree to within a few percent. Since organ cross-doses are dependent on photon transport, however, no simple corrections for the geometric differences in the two phantoms can be applied, and DPM and MIRD S factors results disagree by up to 30%, as seen in the table.
TABLE I.
Comparison of DPM and Conventional (MIRD) S Factors (in mGy/MBq-s). The Liver-Liver, Kidney-Kidney, and Spleen-Spleen Factors Have Been Adjusted for Differences in the Sizes of the Zubal and Standard Man Organs by Mass-Weighting
| Organ | S factor | ||
|---|---|---|---|
| Source | Target | DPM | MIRD |
| Liver | Liver | 2.15E-05 | 2.12E-05 |
| Kidney | Kidney | 1.19E-04 | 1.17E-04 |
| Spleen | Spleen | 2.00E-04 | 1.93E-04 |
| Liver | Kidney | 1.18E-06 | 8.13E-07 |
| Liver | Spleen | 2.71E-07 | 2.14E-07 |
| Kidney | Liver | 1.17E-06 | 8.13E-07 |
| Kidney | Spleen | 1.83E-06 | 1.85E-06 |
| Spleen | Liver | 2.72E-07 | 2.14E-07 |
| Spleen | Kidney | 1.83E-06 | 1.85E-06 |
The photon portion of the β decay routines in DPM were further benchmarked against experiment. A sphere of volume 16.4 cm3 was filled with 15 cm3 of a solution containing 936.55μCi of 131I and placed in an ellipsoidal phantom made of tissue-equivalent plastic and filled with water. The phantom had a height of 18.5 cm, a major axis of 31.0 cm (taken in the y direction) and minor axis of 23.0 cm, and the center of the source sphere was at (0, 2.86, 13.15). Lithium fluoride TLD detectors (dimensions of .32 × .32 × .09 cm) were arrayed in the shape of a cross centered directly above the source sphere and attached to the outside of the phantom at positions given in Table II. Total exposure time for the experiment was 67 hours and 45 minutes.
TABLE II.
TLD Locations on the Phantom
| TLD ID | x (cm) | y(cm) | z (cm) |
|---|---|---|---|
| 1 | -6.0 | 2.86 | 18.5 |
| 2 | -4.0 | 2.86 | 18.5 |
| 3 | -2.0 | 2.86 | 18.5 |
| 4 | 0.0 | 2.86 | 18.5 |
| 5 | 2.0 | 2.86 | 18.5 |
| 6 | 4.0 | 2.86 | 18.5 |
| 7 | 6.0 | 2.86 | 18.5 |
| 8 | 0.0 | 6.86 | 18.5 |
| 9 | 0.0 | 8.86 | 18.5 |
| 10 | 0.0 | 10.86 | 18.5 |
| 11 | 0.0 | -5.14 | 18.5 |
| 12 | 0.0 | -7.14 | 18.5 |
| 13 | 0.0 | -9.14 | 18.5 |
Because of the difficulty in making precise measurements of the positions of the dosimeters and the source, the TLDs were placed in symmetric locations about the source to provide redundancy in the experimental data and to help illuminate positional measurement errors. Given the small size of the TLDs, even with the modest absorbed dose gradients expected for the photon-driven experiment, errors in the assumed positions could result in large discrepancies between measurements and computations. This problem was encountered by Gardin, et al [24] in comparing TLD measurements with absorbed dose computations (not Monte Carlo) for internal emitters.
For consistency with the way DPM is used in modeling patients, the phantom was “voxelized” into a simulated CT-map of an 256 × 256 × 64 array of cells, and the source sphere was similarly converted into a simulated registered SPECT activity map of the same size.
To simulate the experiment, several modifications were made to DPM, primarily because of the implications of using Woodcock tracking. Because of the way Woodcock methods are typically implemented, when a particle escapes a surface, unlike in conventional Monte Carlo tracking methodologies, its final position is not exactly on that surface, but rather at some random distance along its direction vector outside the phantom. Thus DPM was first modified to retrace the paths of escaping photons and put them back on the surface of phantom. Further, since the TLD dimensions did not conform to the sizes of the voxelized version of the phantom, they had to be treated as “add-ons,” and not integral parts of the simulation. Thus DPM was modified to search though the list of TLDs arrayed on the surface of the phantom whenever a photon escaped and to determine whether the photon would pass through any TLD. This includes intersections with the side faces of the TLDs, as, even though the devices were less than a millimeter thick, for those TLDs at large distances from the source sphere, a substantial fraction of the absorptions come from photons impacting the side faces. While complete particle transport was not simulated in the dosimeters, initial photon interaction probabilities and positions were computed and some portion of the resultant Compton or photo-electron energy, as determined by the proximity of the collision to the edges of the TLD, was tallied.
Measured and computed absorbed doses for the experimental setup are given in Table III. Included in the table in the column labeled “ΔR” is the lateral (x and y only) displacement of each TLD relative to the center of the source.
TABLE III.
Comparison of Measured and Calculated Absolute Absorbed Doses. The Column Labeled “ΔR” Represents the Lateral Displacement of the TLD Relative to the Center of the Source
| TLD | ΔR (cm) |
Expt Dose (cGy) |
DPM Dose (cGy) |
|---|---|---|---|
| 4 | 0.0 | 2.43 | 3.23 |
| 3 | 2.0 | 2.26 | 2.76 |
| 5 | 2.0 | 2.14 | 2.75 |
| 2 | 4.0 | 1.65 | 1.92 |
| 6 | 4.0 | 1.54 | 1.91 |
| 8 | 4.0 | 1.54 | 1.92 |
| 1 | 6.0 | 1.08 | 1.23 |
| 7 | 6.0 | 1.06 | 1.23 |
| 9 | 6.0 | 1.06 | 1.24 |
| 10 | 8.0 | 0.72 | 0.79 |
| 11 | 8.0 | 0.74 | 0.80 |
| 12 | 10.0 | 0.48 | 0.52 |
| 13 | 12.0 | 0.32 | 0.35 |
As seen in the table, DPM overestimates the absorbed dose in all cases, by as much as 30%, with the largest discrepancies seen for TLDs nearest the source. It was noted earlier that possible sources of errors lie in inaccuracies in the measured positions of the TLDs and the source. For this experiment, the source position was difficult to characterize precisely, as the sphere containing the activity had to be situated and measured, then removed and filled with the active solution, and later replaced, with the assumption that it was replaced in the exact location at which the position measurements were taken. In addition, the sphere wall thickness was not precisely known, and had to be inferred from measurement of the diameter and then known volume of injected liquid, under the assumption that the sphere was completely filled, and that no air was present.
Evidence of possible errors in precisely locating the source can be seen in the asymmetry in the measurement data. Despite having been nominally sited at the same distance from the center of the source sphere, TLDs 3 and 5 yielded measured absorbed doses which differed by 5%. Likewise, the absorbed dose measured in TLD 2 was 7% different from the absorbed doses reported for TLDs 6 and 8, even though all three dosimeters were thought to be at equivalent distances from the assumed center of the sphere. A consistent pattern can be found in the differences in the measured absorbed doses for the various sets of TLDs placed at equivalent distances, from which it can be inferred that the center of the source may have been misaligned slightly in both the negative x and negative y directions from its nominal location. In Table IV we present results from DPM when it is assumed that the source center was shifted 2 mm in both the negative x and negative y directions, and that the actual effective center of the sphere was 6 mm below the measured center. With this assumption, absolute values of absorbed dose computed by DPM agree to within 4% of experimental measurements.
TABLE IV.
Comparison of Measured and Calculated Absolute Absorbed Doses Assuming that the Center of the Source Sphere was Mis-Measured by 2 mm in the Negative x and y Dimensions, and 6 mm in the Negative z Dimension
| TLD | ΔR (cm) |
Expt Dose (cGy) |
DPM Dose (cGy) |
|---|---|---|---|
| 4 | 0.0 | 2.43 | 2.52 |
| 3 | 2.0 | 2.26 | 2.27 |
| 5 | 2.0 | 2.14 | 2.16 |
| 2 | 4.0 | 1.65 | 1.68 |
| 6 | 4.0 | 1.54 | 1.56 |
| 8 | 4.0 | 1.54 | 1.56 |
| 1 | 6.0 | 1.08 | 1.13 |
| 7 | 6.0 | 1.06 | 1.05 |
| 9 | 6.0 | 1.06 | 1.04 |
| 10 | 8.0 | 0.72 | 0.69 |
| 11 | 8.0 | 0.74 | 0.75 |
| 12 | 10.0 | 0.48 | 0.50 |
| 13 | 12.0 | 0.32 | 0.34 |
To demonstrate the speed-up advantages of DPM, comparisons of CPU time used by DPM and by a version of EGS4 (based on the XYZDOS(PRESTA) program distributed with EGS4) adapted for identical internal emitter dosimetry applications have been performed. Table V shows the relative speed of DPM vs. EGS4 as a function of voxel size. The voxel sizes are expressed in terms of fractions of the range of the maximum energy β particle in water (.33 cm for 131I). Even though this meant that the simulations used voxels which are quite small in an absolute sense, the benchmark was set up in this manner because such results can be applied directly to higher energy isotopes for which electron transport across voxel boundaries could be important to model.
TABLE V.
Relative Speed of DPM vs. EGS4 as a Function of Voxel Size (Relative to the Range of Maximum Energy β Particles) in Absorbed Dose Computations
| Voxel size (X/R(E0)) |
Cutoffs (MeV) | Speedup | |
|---|---|---|---|
| e− | γ | ||
| .25 | .100 | .004 | 6.09 |
| .50 | .100 | .004 | 5.16 |
| 1.00 | .100 | .004 | 3.93 |
IV. Conclusion
The fast Monte Carlo electron and photon transport DPM has been modified to model radiation absorption in internal emitter therapy problems, and to track electron and photon contributions to absorbed dose for user defined source and target organs. It has been benchmarked against results using the MIRD formalism and against experiment. DPM is now being used to perform accurate absorbed dose computations for a retrospective analysis attempting to correlate calculated absorbed dose with tumor response in patients who have undergone 131I radioimmunotherapy for non-Hodgkin's lymphoma.
Acknowledgments
This work was supported in part under Grant R01 EB001994 awarded by the National Institutes of Health.
Contributor Information
S. J. Wilderman, Department of Nuclear Engineering and Radiologic Sciences, University of Michigan, Ann Arbor, MI 48109 USA (sjwnc@umich.edu)
Y. K. Dewaraja, Department of Radiology, University of Michigan, Ann Arbor, MI 48109 USA
References
- 1.Snyder WS, Ford MR, Wagner GG, Watson SB. Transl.: MIRD Pamphlet No. 11. Society for Nuclear Medicine; New York: 1975. “S.” Absorbed Dose Per Unit Cumulated Activity for Selected Radionuclides and Organs. [Google Scholar]
- 2.Furhang EE, Chui CS, Kolbert KS, Larson SM, Sgouros G. Implementation of a Monte Carlo dosimetry method for patient-specific internal emitter therapy. Med Phys. 1997;24:1163–1172. doi: 10.1118/1.598018. [DOI] [PubMed] [Google Scholar]
- 3.Yoriyaz H, Santos A, Stabin M, Cabezas R. Absorbed fractions in a voxel-based phantom calculated with the MCNP-4B code. Med Phys. 2000;27:1555–1562. doi: 10.1118/1.599021. [DOI] [PubMed] [Google Scholar]
- 4.Ljungberg M, Green KS, Liu X, Frey E, Dewaraja Y, Strand SE. A 3-dimensional Absorbed dose calculation method based on quantitative SPECT for radionuclide therapy: Evaluation for 131I using Monte Carlo simulation. J Nucl Med. 2002;43:1101–1109. [PMC free article] [PubMed] [Google Scholar]
- 5.Descalle MA, Hartman Siantar CL, Dauffy L, Nigg DW, Wemple CA, Yuan A, DeNardo GL. Application of MINERVA Monte Carlo simulations to targeted radionuclide therapy. Cancer Biotherapy Radiopharm. 2003;18:71–79. doi: 10.1089/108497803321269340. [DOI] [PubMed] [Google Scholar]
- 6.Sempau J, Wilderman SJ, Bielajew AF. DPM—A fast, accurate Monte Carlo code optimized for photon and electron radiotherapy treatment planning dose computations. Phys Med Biol. 2000;45:2262–2291. doi: 10.1088/0031-9155/45/8/315. [DOI] [PubMed] [Google Scholar]
- 7.Woodcock E, Murphy T, Hemmings P, Longworth S. Techniques used in the GEM code for Monte Carlo neutronics calculations in reactors and other systems of complex geometry. Proc. Conf. Applications of Computing Methods to Reactor Problems; 1965. p. 557. [Google Scholar]
- 8.Nelson WR, Hirayama H, Rogers DWO. Report SLAC–265. Stanford, CA: Stanford Linear Accelerator Center; 1985. The EGS4 Code System. [Google Scholar]
- 9.Wiseman GA. Phase I/II 90Y-Zevalin (yttrium-90 ibritumomab tiuxetan, IDEC-Y2B8) radioimmunotherapy dosimetry results in relapsed or refractory non-Hodgkin's lymphoma. Eur J Nucl Med. 2000;27:766–777. doi: 10.1007/s002590000276. [DOI] [PubMed] [Google Scholar]
- 10.Sharkey RM. Radioimmunotherapy of non-Hodgkin's lymphoma with 90Y-DOTA humanized anti-CD22 IgG (90Y-epratuzumab): Do tumor targeting and dosimetry predict therapeutic response? J Nucl Med. 2003;44:766–2018. [PubMed] [Google Scholar]
- 11.Pauwels S, Barone R, Walrand S, Borson Chazot F, Valkema R, Kvols LK, Krenning EP, Jamar F. Practical dosimetry of peptide receptor radionuclide therapy with 90Y-labeled somatosatin analogs. J Nucl Med. 2005;46:92S–98S. [PubMed] [Google Scholar]
- 12.Ekström LP, Firestone RB. WWW Table of Radioactive Isotopes. [Online]. Available: http://ie.lbl.gov/toi/index.htm, database version 2/28/99 from URL.
- 13.Walker AJ. An efficient method for generating discrete random variables with general distributions. ACM Trans Math Softw. 1977;3:253–256. [Google Scholar]
- 14.Salvat F, Fernández-Varea JM, Baró J, Sempau J. Informes Tecnicos CIEMAT n. 799. Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas; Madrid: 1996. PENELOPE, an Algorithm and Computer Code for the Monte Carlo Simulation of Electron-Photon Showers. [Google Scholar]
- 15.Dewaraja Y, Wilderman SJ. Patient specific dosimetry in radionuclide therapy: Effect of CT/SPECT misregistration and limited SPECT field-of-view. J Nucl Med. 2005;46:89P. [PMC free article] [PubMed] [Google Scholar]
- 16.Chetty IJ, Moran JM, McShan DL, Frass BA, Wilderman SJ, Bielajew AF. Benchmarking of the Dose Planning Method (DPM) Monte Carlo code using electron beams from a racetrack microtron. Med Phys. 2002;29:1035–1041. doi: 10.1118/1.1481512. [DOI] [PubMed] [Google Scholar]
- 17.Chetty IJ, Moran JM, Nurushev TS, McShan DL, Frass BA, Wilderman SJ, Bielajew AF. Experimental validation of the DPM Monte Carlo code using minimally scattered electron beams in heterogeneous media. Phys Med Biol. 2002;47:1837–1851. doi: 10.1088/0031-9155/47/11/301. [DOI] [PubMed] [Google Scholar]
- 18.Chetty IJ, Charland PM, Tyagi N, McShan DL, Frass BA, Bielajew AF. Photon beam relative dose validation of the DPM Monte Carlo code in lung-equivalent media. Med Phys. 2003;30:563–573. doi: 10.1118/1.1555671. [DOI] [PubMed] [Google Scholar]
- 19.Chetty IJ, Rosu M, McShan DL, Frass BA, Balter JM, Ten Haken RK. Accounting for center-of-mass target motion using convolution methods in Monte Carlo-based dose calculations of the lung. Med Phys. 2004;31:925–932. doi: 10.1118/1.1669083. [DOI] [PubMed] [Google Scholar]
- 20.Chetty IJ, Rosu M, McShan DL, Frass BA, Ten Haken RK. The influence of beam model differences in the comparison of dose calculation algorithms for lung cancer treatment planning. Phys Med. 2005;50:801–815. doi: 10.1088/0031-9155/50/5/006. [DOI] [PubMed] [Google Scholar]
- 21.Rosu M, Chetty IJ, Balter JM, Kessler ML, McShan DL, Ten Haken RK. Dose reconstruction in deforming lung anatomy: Dose grid size effects and clinical implications. Med Phys. 2005;32:2487–2495. doi: 10.1118/1.1949749. [DOI] [PubMed] [Google Scholar]
- 22.Dewaraja Y, Wilderman SJ, Ljungberg M, Koral KF, Zasadny KR, Kaminiski M. Accurate Dosimetry in 131I radionuclide therapy using patient-specific, 3-dimensional methods for SPECT reconstruction and absorbed dose calculation. J Nucl Med. 2005;46:840–849. [PMC free article] [PubMed] [Google Scholar]
- 23.Zubal G, Harrell C, Smith E, Ratner Z, Gindi G, Hoffer P. Computerized three-dimensional segmented human anatomy. Math Phys. 1994;21:299–302. doi: 10.1118/1.597290. [DOI] [PubMed] [Google Scholar]
- 24.Gardin I, Bouchet LG, Assie K, Caron J, Lisbona A, Ferrer L, Bloch WE, Vera P. Voxeldose: A computer program for 3-D dose calculation in therapeutic nuclear medicine. Cancer Biotherapy Radiopharm. 2003;18:109–115. doi: 10.1089/108497803321269386. [DOI] [PubMed] [Google Scholar]


