Abstract
Objective
An attenuation of the nighttime decline in blood pressure (BP) predicts cardiovascular disease and cardiovascular-related mortality, over and above daytime BP levels. We investigated whether positive and negative psychological attributes were associated with sleep-wake BP ratios and examined sleep parameters as potential mediators of these relationships.
Design
Two hundred twenty-four participants (50% men; 43% Black; mean age = 60 years) underwent ambulatory BP monitoring for two days and nights. Self-reports of positive and negative psychological attributes were collected. In-home polysomnography was conducted for two nights, and a wrist actigraph was worn for nine nights.
Main Outcome Measures
Sleep-wake mean arterial pressure (MAP) ratios.
Results
After adjustment for demographics, body mass index, and hypertensive status, low life purpose and high hostility were associated with high sleep-wake MAP ratios. Depression, anxiety, and optimism were not related to MAP ratios. Sleep latency, fragmentation, architecture, and the apnea-hypopnea index were examined as potential mediators between psychological attributes and MAP ratios; only long sleep latency mediated the relationship between hostility and MAP ratios.
Conclusion
Low life purpose and high hostility are associated with high sleep-wake BP ratios in Black and White adults, and these relationships are largely independent of sleep.
Keywords: life purpose, hostility, ambulatory blood pressure, nondipping, sleep
The majority of individuals exhibit a diurnal pattern of blood pressure (BP) variation, in which nighttime BP decreases relative to daytime BP. Individuals who show less of a nighttime decline in BP are at increased risk for cardiovascular events (Ohkubo et al., 2002; Staessen et al., 1999; Verdecchia, Angeli, Borgioni, Gattobigio, & Reboldi, 2007), declining renal function (Davidson, Hix, Vidt, & Brotman, 2006), and mortality (Fagard et al., 2008). The prognostic value of night-to-day BP ratios for health outcomes is independent of, and at times stronger than, daytime BP levels (Davidson et al., 2006; Fagard et al., 2008; Ohkubo et al., 2002; Staessen et al., 1999). Thus, identification of factors that disrupt the normal diurnal pattern of BP may help to inform strategies for the prevention and treatment of cardiovascular disease and related disorders.
Negative emotions may be among the factors that have a detrimental effect on night-day BP ratios, as they may occur in conjunction with cognitive rumination and impaired recovery of the sympathetic nervous system (Pieper & Brosschot, 2005). On the other hand, positive traits and emotions may facilitate a healthier rhythm due to an increased ability to cope with life stressors or remain optimistic in the face of challenges. Indeed, available data offer support for associations between individual differences in psychological attributes and diurnal BP rhythms. Negative emotions, including depression, anxiety, and hostility and anger, have been related to an attenuated decline in nighttime BP (Kario, Schwartz, Davidson, & Pickering, 2001; Steffen, McNeilly, Anderson, & Sherwood, 2003; Thomas, Nelesen, & Dimsdale, 2004), although not all studies have found significant relationships (Ituarte, Kamarck, Thompson, & Bacanu, 1999; Linden, Klassen, & Phillips, 2008). Preliminary evidence for an association between positive psychological characteristics and a healthy diurnal BP rhythm comes from the finding that daily mood ratings of pleasantness and happiness were related to lower absolute levels of diastolic BP during the night in healthy young adults (Shapiro, Jamner, & Goldstein, 1997). Studies have also shown that positive emotions and traits are associated with lower resting BP and faster BP recovery following stressful tasks (Ostir, Berges, Markides, & Ottenbacher, 2006; Steptoe, Gibson, Hamer, & Wardle, 2007). However, the relationship between positive attributes and night-day BP ratios has not been evaluated directly. As positive and negative emotions and attitudes are not simply opposite ends of a single continuum, it is reasonable to examine both of these aspects separately in relation to BP rhythms.
One potential pathway linking psychological factors to night-day BP ratios is disturbed sleep. Ryff, Singer, and Love (2004) observed an inverse association between purpose in life and movement throughout the night in older women, and a recent study reported that both positive affect and eudaimonic well-being were related to better self-reported sleep quality (Steptoe, O'Donnell, Marmot, & Wardle, 2008). On the contrary, elevated negative affect is related to longer sleep latency, increased fragmentation throughout the night, and a decreased percentage of time spent in slow-wave sleep (Grano, Vahtera, Virtanen, Keltikangas-Jarvinen, Kivimaki, 2008; Paudel et al., 2008; Spira, Stone, Beaudreau, Ancoli-Israel, & Yaffe, 2009; Thase, Fasiczka, Berman, Simons, & Reynolds, 1998). The sleep of individuals with higher nighttime BP is characterized by similar disturbances in sleep latency, fragmentation, and architecture (Loredo, Nelesen, Ancoli-Israel, & Dimsdale, 2004; Mansoor, 2002; Matthews et al., 2008). Moreover, sleep apnea is associated with a blunted decline in nocturnal BP in both cross-sectional and prospective studies (Hla, Young, Finn, Peppard, Szklo-Coxe, & Stubbs, 2008; Suzuki, Guilleminault, Ostuka, Shiomi, 1996). Given these associations, it is possible that sleep may partially account for the reported links between psychological attributes and diurnal BP rhythms. However, most previous studies either have not assessed sleep or have assessed sleep with self-report measures of sleep quality. Other dimensions – such as sleep continuity, architecture, or disordered breathing – can be measured with techniques such as actigraphy or polysomnography, and could plausibly affect BP rhythms as well. Whether or not these sleep parameters act as mediators linking psychological attributes with BP rhythms is unknown.
We have previously reported in the present sample that higher sleep-wake BP ratios are associated with poorer sleep continuity and increased Stage 1 sleep (Matthews et al., 2008). Here, we investigate the hypotheses that negative (depression, anxiety, and hostility) and positive (optimism and life purpose) psychological attributes are associated with sleep-wake BP ratios and evaluate whether such associations are independent of sleep continuity, architecture, and sleep-disordered breathing.
Method
Research Participants
Participants were recruited from the Heart Strategies Concentrating On Risk Evaluation (Heart SCORE) study, which is a single center, prospective, community-based participatory research cohort study investigating mechanisms for racial disparities in cardiovascular risk and attempting to decrease these disparities via a community-based intervention. Eligibility criteria included age 45 to 75 years, residence in the greater Pittsburgh metropolitan area, ability to undergo baseline and annual follow-up visits, and absence of known co-morbidities expected to limit life expectancy to less than five years. The Institutional Review Board at the University of Pittsburgh approved the study protocol and all study participants provided written informed consent. Data collection included demographics, medical history, anthropometrics, lipids/lipoproteins, physical activity, and psychological status as previously described (Aiyer, Kip, Marroquin, Mulukutla, Edmundowicz, & Reis, 2007).
The sleep study, entitled Sleep SCORE, enrolled approximately equal ratios of male to female and Black to White participants from the Heart SCORE study sample. Exclusionary criteria for Sleep SCORE included pregnancy; use of continuous positive airway pressure treatment for sleep-disordered breathing; medication for sleep problems on a regular basis; nighttime work schedule; medication for diabetes; and prior diagnosis of stroke, myocardial infarction, or interventional cardiology procedures.
Measures and Procedure
Overview
Participants were recruited during Heart SCORE assessment visits. Study personnel approached potential participants and if interested and eligible, provided them with detailed information while obtaining written informed consent approved by the University of Pittsburgh Institutional Review Board. The study protocol lasted 10 days. Actigraphs, which are watch-like activity monitors worn on the wrist to track rest and activity patterns via physical movement, were worn on nights 1–9 to provide behavioral data regarding sleep duration and nighttime mobility. Two nights of in-home polysomnography (PSG) were performed on nights 1 and 2 of the study to provide data on a variety of sleep parameters, including sleep architecture and sleep-disordered breathing. Twenty-four hour ambulatory blood pressure monitoring was conducted on days/nights 3 and 4 of the study. Participants also completed written questionnaires assessing positive and negative psychological attributes (see below).
Ambulatory Blood Pressure Assessment
A Spacelabs Monitor #90217 was used to measure ambulatory blood pressure for two days and nights. This oscillometric monitor has been certified according to the British Hypertension Society criteria as being a reliable and valid measure of ambulatory BP. A BP reading was taken every 30 minutes from 8:00 a.m. to 9:00 p.m. and hourly thereafter. Measurements were averaged across the sleep period (starting when participants reported going to sleep and stopping when they reported awakening, confirmed by sleep diary and actigraphy) and across the remaining wake period. To be included, individuals had to have a minimum of 16 awake and six night BP readings across each measurement period. The sleep-wake ratio was a continuous variable defined as the average sleep mean arterial pressure (MAP) divided by the average wake MAP (e.g., Stolarz, Staessen, & O'Brien, 2002), averaged across the two recording periods. The MAP ratios across the two nights and days were correlated at r = .51, p < .0001. Although different methods can be used to characterize diurnal BP patterns, we chose a sleep-wake ratio for several reasons. This method results in a continuous variable, which maximizes statistical power, and incorporates all three days of BP data (participants had different start and end times, given their personal schedules). Moreover, epidemiological studies frequently use a similar ratio when reporting relationships between BP rhythms and disease risk or cardiovascular co-morbidities (e.g., de la Sierra et al., 2009; Fagard, Thijs, Staessen, Clement, De Buyzere, & De Bacquer, 2009). We present results using the MAP ratio only, as opposed to systolic or diastolic BP, for the sake of parsimony. However, the findings regarding psychological attributes and BP ratios reported below were identical whether we used MAP, systolic, or diastolic BP as an outcome.
Psychological Attributes
We examined three negative psychological attributes (depression, anxiety, and hostility) and two positive psychological attributes (sense of life purpose and optimism) in relation to sleep-wake MAP ratios. Depressive symptoms were measured by the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977), and the sleep item was excluded from the total score. Although the CES-D inquires about symptoms over the past week, we have observed the test-retest reliability of this measure to remain fairly stable over a period of one year in other data sets (average r = .50 across eight annual assessments). Trait anxiety was assessed using the Spielberger Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, & Lushene, 1970), and trait hostility was assessed using the total of the Cynicism, Hostile Affect, and Aggressive Responding sub-scales of the Cook-Medley Hostility Scale (CM; Barefoot, Dodge, Peterson, Dahlstrom, & Williams, 1989; Cook & Medley, 1954). Both of these measures have good stability over time (test-retest correlations > .8; Barefoot, Dahlstrom, & Williams, 1983; Barnes, Harp, & Jung, 2002), suggesting that they are more reflective of trait-like attributes rather than states. A sense of life purpose was measured by the Life Engagement Test (LET; Scheier et al., 2006). This scale measures the extent to which a person reports engaging in activities that are personally valued, and it has been found to have adequate internal consistency and validity. Optimism was assessed using the Revised Life Orientation Test (LOT; Scheier, Carver, & Bridges, 1994). Both the LET and the LOT have acceptable stability over time (test-retest correlations range from .61 – .76 for the LET and from .56 – .79 for the LOT; Scheier et al., 1994; Scheier et al., 2006).
Sleep Parameters
Participants wore an Actiwatch-64 (Phillips Respironics, Inc.) on the non-dominant wrist continuously for 9 nights and 10 days to monitor sleep and wake patterns. Data were stored in 1-minute epochs and validated MiniMitter software (Phillips Respironics, Inc.) algorithms were used in conjunction with sleep diaries to estimate sleep parameters. Sleep diaries were completed by participants each night and morning to record sleep and wake times, which were then used to identify relevant actigraphy epochs for examination of movement. Actigraphy variables used in analyses included sleep latency (time required for onset of sleep after first attempting to fall asleep) and the fragmentation index, a measure of nocturnal movement that was calculated as follows: (the sum of % mobile bouts and % immobile bouts less than 1 minute duration to the number of immobile bouts). Higher levels of fragmentation indicate more movement. Mean latency and fragmentation values were calculated by averaging the values from nights 1–9 of the study.
On study nights 1 and 2, PSG recording was conducted in participants' homes using a Compumedics Siesta monitor. The PSG montage included bilateral central and occipital electro-encephalogram (EEG) channels, bilateral electro-oculograms (EOG), bipolar submentalis electromyograms (EMG), and one channel of electrocardiogram (EKG) recording. On the first night of PSG, participants were monitored for sleep-disordered breathing using nasal pressure, inductance plethysmography, and fingertip oximetry. High frequency filter settings were 100 Hz for EEG and EOG and 70 Hz for EMG. Low frequency filter settings were .3 Hz for EEG and 10 Hz for EMG. Trained PSG technologists scored sleep records using standard sleep stage scoring criteria for each 20-second epoch (Rechtschaffen & Kales, 1986). American Academy of Sleep Medicine Task Force (1999) definitions were used to identify apneas and hypopneas; oximetry readings were used to quantify average and minimum oxygen saturation levels. The PSG variables used in analyses included percentage of total sleep time spent in Stage 1 sleep (averaged across two nights of recording) and the apnea-hypopnea index (AHI; number of breathing pauses or shallow breathing episodes per hour of sleep) recorded on the first night of the study.
Covariates
Covariates for analyses included age, sex, race, body mass index (BMI), and hypertensive status, due to the potential associations between these variables and BP or sleep parameters. Age, sex, and race were determined by self-report. BMI was assessed in the Heart SCORE study protocol. Participants coded as hypertensive were those who 1) reported a history of hypertension and 2) either had resting BP measurements consistent with a hypertensive diagnosis (systolic BP ≥ 140 or diastolic BP ≥ 90) or used anti-hypertensive medications (ACE inhibitors, angiotensin II blockers, beta blockers, calcium channel blockers, alpha I blockers, alpha II agonists, and diuretics). Reports of anti-hypertensive medication use were collected during in-home PSG studies.
Statistical Analysis
Multivariate linear regression was used to test the hypotheses that negative and positive psychological attributes were associated with sleep-wake MAP ratios. All models were adjusted for age, sex, race, BMI, and hypertensive status, before examining each psychological attribute as a predictor of sleep-wake MAP ratios. We tested for curvilinear relationships between attributes and sleep-wake ratios by squaring each psychological attribute and entering it into the second step of the model. Due to previous reports of race differences in diurnal BP patterns (Profant & Dimsdale, 1999), we investigated whether the relationships between psychological attributes and sleep-wake MAP ratios were similar in Blacks and Whites by testing race × attribute interactions. There is both theoretical and statistical overlap among the psychological constructs examined in this study. Because we were interested in observing the individual associations between each psychological attribute and MAP ratios, and inter-correlations among these variables suggested they were relatively independent of each other, we first entered each psychological measure in separate statistical models. Psychological attributes that were associated with MAP ratios at the p ≤ .05 statistical significance level were retained and combined in a final model. This allowed us to examine the unique association of each psychological attribute with MAP ratios, independent of the others.
In order to determine whether potential associations between psychological attributes and sleep-wake BP ratios were due to differences in daytime, rather than nocturnal, BP, we performed additional analyses using nighttime BP as the criterion variable after adjusting for daytime BP. None of the psychological attributes were associated with daytime BP; however, associations between psychological attributes and nocturnal BP were identical to those obtained when using the sleep-wake BP ratio as the criterion variable. Therefore, we report only the results from analyses using an outcome of sleep-wake BP ratios, as described above.
Mediation analyses tested whether sleep parameters accounted for the main effects of any of the psychological attributes that were found to be associated with MAP ratios. Candidate mediators included sleep latency, sleep fragmentation, percentage of time spent in Stage 1 sleep, and the AHI index, due to their associations with nocturnal BP in previous literature (Hla et al., 2008; Loredo, Nelesen, Ancoli-Israel, & Dimsdale, 2004; Mansoor, 2002), as well as in an earlier analysis of this sample (Matthews et al., 2008). The indirect effects of psychological attributes on MAP ratios via sleep parameters were examined in a series of multiple linear regression analyses in which the relevant psychological construct and covariates were entered in the first step, and the potential mediating variable was entered in the second step. The statistical significance of the indirect effects of psychological attributes on MAP ratios via an individual mediator was evaluated using the Sobel method (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002). All analytic procedures used two-tailed tests of significance and were conducted with SPSS, v. 15 (SPSS Inc., Chicago, IL).
Results
Participant Characteristics, Psychological Attributes, and Sleep Parameters
The study sample included 224 participants, half of whom were male, with a mean age around 60 years. Ninety-seven participants reported their race as Black, 123 as non-Hispanic White, and four as Asian. Asian and White participants were combined for analytical purposes; excluding the four Asian participants resulted in identical findings. Participant characteristics are shown in Table 1.
Table 1.
Participant Characteristics
| Measure | No. (%) | M (SD) | Range |
|---|---|---|---|
| Men | 113 (50.4) | ||
| Black | 97 (43.3) | ||
| White or Asian | 127 (56.7) | ||
| Hypertensivea | 133 (59.4) | ||
| Age (years) | 60.0 (7.2) | 46 – 78 | |
| Body Mass Index | 29.5 (5.0) | 17.9 – 45.3 | |
| Mean Arterial Pressure | |||
| Sleep | 84.2 (9.9) | 64.3 – 125.7 | |
| Awake | 96.0 (9.3) | 71.2 – 126.7 | |
| Ratio | .88 (.07) | .63 – 1.16 | |
| Depression (CES-D) | 5.3 (6.6) | 0 – 32 | |
| Anxiety (STAI) | 6.0 (5.0) | 0 – 21 | |
| Hostility (CM) | 8.5 (4.3) | 1 – 20 | |
| Optimism (LOT) | 17.2 (4.1) | 2 – 24 | |
| Life Purpose (LET) | 25.1 (3.5) | 14 – 30 | |
| Actigraphy Sleep Latency | 18.3 (14.5) | 1.1 – 64.6 | |
| Actigraphy Fragmentation Index | 32.9 (12.5) | 12.4 – 89.5 | |
| PSG % Stage 1 Sleep | 9.5 (5.9) | 1.3 – 33.6 | |
| PSG Apnea-Hypopnea Index | 13.2 (14.9) | 0 – 92.9 |
Note. CES-D = Center for Epidemiological Studies Depression Scale, minus the sleep item; STAI = Spielberger Trait Anxiety Inventory; CM = Cook-Medley Hostility Scale; LOT = Revised Life Orientation Test; LET = Life Engagement Test; PSG = polysomnography
Participants coded as hypertensive were those who 1) reported a history of hypertension and 2) had resting BP measurements consistent with a hypertensive diagnosis or were using anti-hypertensive medications.
The bivariate correlations between each of the psychological attributes and sleep parameters are shown in Table 2. Higher hostility and higher depression were associated with longer sleep latency, and higher life purpose was associated with less Stage 1 sleep. Prior analyses in a sub-set of this sample showed that Blacks had poorer sleep continuity than Whites (Mezick et al., 2008). Similar results were found in the current sample, as Blacks had longer latency (β = −.29, p < .001) and more fragmented sleep (β = −.24, p = .001) as measured by actigraphy than Whites. There were no race differences in Stage 1 sleep (β = −.03, p = .71) or the apnea-hypopnea index (β = −.01, p = .88). Blacks reported higher life purpose than Whites (β = −.23, p = .002); the other psychological attributes were similar between races (ps > .20).
Table 2.
Correlations between Psychological Attributes and Sleep Variables
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Depression (CES-D) | -- | .66** | .28** | −.39** | −.40** | .22** | .05 | .002 | −.05 |
| 2. Anxiety (STAI) | -- | .32** | −.59** | −.57** | .04 | .003 | .03 | −.08 | |
| 3. Hostility (CM) | -- | −.33** | −.19** | .18** | .04 | .08 | .07 | ||
| 4. Optimism (LOT) | -- | .48** | −.05 | .06 | −.06 | −.01 | |||
| 5. Life Purpose (LET) | -- | .04 | .01 | −.14*. | .04 | ||||
| 6. Sleep Latency | -- | .35** | .04 | .09 | |||||
| 7. Sleep Fragmentation | -- | .27** | .20** | ||||||
| 8. % Stage 1 Sleep | -- | .17*. | |||||||
| 9. Apnea-Hypopnea Index | -- |
Note. CES-D = Center for Epidemiological Studies Depression Scale, minus the sleep item; STAI = Spielberger Trait Anxiety Inventory; CM = Cook-Medley Hostility Scale; LOT = Revised Life Orientation Test; LET = Life Engagement Test
Sleep-Wake MAP Ratios
The mean sleep-wake MAP ratio across the two nights was .88. Using the standard criterion of <10% decrease in sleep BP relative to daytime BP, approximately one-third of the sample (35.7%) would be classified as MAP nondippers. Results from linear regression models that only included covariates as predictors of sleep-wake MAP ratios are shown in Table 3. Participants with high resting BP/taking anti-hypertensive medications had higher sleep-wake MAP ratios. There were no differences in MAP ratios by race, sex, age, or BMI.
Table 3.
Results of Multiple Linear Regression Testing Psychological Attributes' Associations with Sleep-Wake Mean Arterial Pressure (MAP) Ratios
| Variable | Base Model |
Negative |
Positive |
|||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| Age | .05 | .44 | ||||
| Sex (1=male; 0=female) | .12 | .07 | ||||
| Race (1=White; 0=Black) | −.06 | .40 | ||||
| Body Mass Index | .03 | .68 | ||||
| Hypertension Status (1=yes; 0nNo) | .19 | .007 | ||||
| Negative Psychological Attributes a | ||||||
| CES-D Depression | .09 | .16 | ||||
| STAI Anxiety | .13 | .06 | ||||
| CM Hostility | .15 | .03 | ||||
| Positive Psychological Attributes b | ||||||
| LOT Optimism | −.08 | .22 | ||||
| LET Purpose | −.18 | .009 | ||||
Regression coefficients are presented for depression, anxiety, and hostility scores when they were entered into separate linear regression models. When the three scores were entered simultaneously into one model, hostility remained associated with sleep-wake MAP ratios (β = .15, p = .04).
Regression coefficients are presented for optimism and life purpose scores when they were entered into two separate linear regression models. When they were included in the same model, life purpose remained associated with sleep-wake MAP ratios (β = −.17, p = .03).
Note. CES-D = Center for Epidemiological Studies Depression Scale, minus the sleep item; STAI = Spielberger Trait Anxiety Inventory; CM = Cook-Medley Hostility Scale; LOT = Revised Life Orientation Test; LET = Life Engagement Test
Negative Psychological Attributes
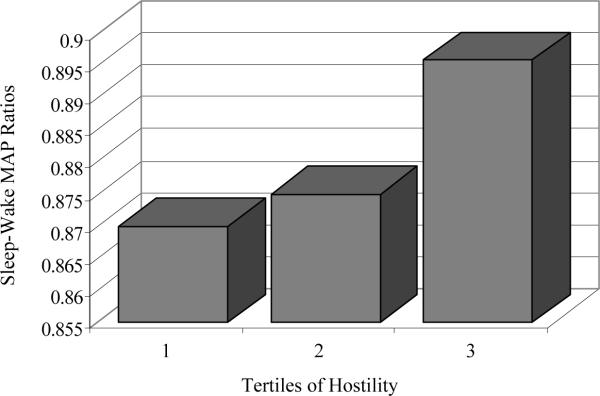
The results of linear regression models testing the associations between negative psychological attributes and sleep-wake MAP ratios are displayed in Table 3. After adjustment for covariates, higher hostility was associated with higher sleep-wake MAP ratios (Figure 1). The association between anxiety and sleep-wake MAP ratios approached statistical significance. Depression was not associated with sleep-wake MAP ratios. No curvilinear relationships between negative psychological attributes and MAP ratios were observed. The associations between negative attributes and MAP ratios were similar in Blacks and Whites (interactions: ps > .59).
Figure 1.
Associations between Cook-Medley hostility and sleep-wake mean arterial pressure (MAP) ratios. Adjusted means of sleep-wake MAP ratios are shown at each tertile of hostility. Tertile grouping is for depictive purposes only; hostility was analyzed as a continuous variable in regression models.
Positive Psychological Attributes
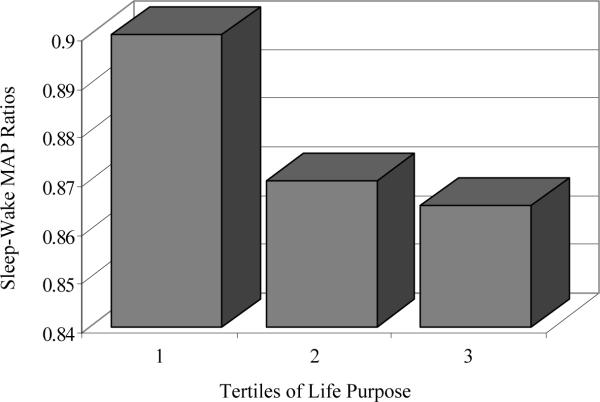
The results for linear regression models testing the associations between positive psychological attributes and sleep-wake MAP ratios are displayed in Table 3. After adjustment for covariates, a higher sense of life purpose was associated with lower sleep-wake MAP ratios (Figure 2). Optimism was not associated with sleep-wake MAP ratios. No curvilinear relationships between positive psychological attributes and MAP ratios were observed. The associations between positive attributes and MAP ratios were similar in Blacks and Whites (interactions: ps > .38).
Figure 2.
Associations between Life Engagement Test purpose scores and sleep-wake mean arterial pressure (MAP) ratios. Adjusted means of sleep-wake MAP ratios are shown at each tertile of life purpose. Tertile grouping is for depictive purposes only; life purpose was analyzed as a continuous variable in regression models.
In order to assess whether the associations of hostility and life purpose with sleep-wake ratios were independent of or confounded by the other, we entered both of these measures simultaneously in one model. Both hostility (β = .13, p = .05) and life purpose (β = −.15, p = .03) were uniquely associated with sleep-wake MAP ratios. These two factors accounted for approximately 4% of the variance in sleep-wake ratios.
Sleep Parameters as Potential Mediators
Actigraphy
In linear regression models adjusted for covariates, longer sleep latency (β = .17, p = .02) and greater fragmentation (β = .25, p < .001) were associated with higher sleep-wake MAP ratios. Longer sleep latency was a statistical mediator of the association between higher hostility and higher sleep-wake MAP ratios using the Sobel method (z = 2.02, p = .04); however, the size of the indirect effect was small (approximately 16%). Sleep fragmentation did not mediate the relationship between hostility and sleep-wake ratios (z = .43, p = .66). Neither latency nor fragmentation reduced the strength of the association between life purpose and sleep-wake ratios (zs < 1.96, ps > .05).
PSG
In linear regression models adjusted for covariates, a greater percentage of time spent in Stage 1 sleep (β = .19, p = .01) and a higher AHI index (β = .15, p = .03) were associated with higher sleep-wake MAP ratios. However, neither time spent in Stage 1 sleep nor the AHI index reduced the strength of the associations between hostility or life purpose and sleep-wake MAP ratios.
Discussion
The aim of this study was to examine psychological attributes as they relate to sleep-wake BP ratios and to evaluate the potential mediating roles of sleep continuity, architecture, and sleep-disordered breathing in these relationships. We found that higher levels of hostility and lower levels of life purpose were associated with an attenuated nocturnal decline in BP measured across two nights. These associations were independent of sleep fragmentation, percentage of time spent in Stage 1 sleep, and sleep apnea. However, sleep latency was a statistical mediator of the association between high hostility and high sleep-wake MAP ratios.
Existing data on the links between hostility and/or anger (a separate, but overlapping, construct) and diurnal BP rhythms are mixed. In a study of Black and White adults, hostility and anger expression explained ethnic differences in nocturnal decreases in BP after taking social desirability into account (Thomas et al., 2004), and another study found that anger inhibition was related to a blunted decline in diastolic BP (Steffen et al., 2003). However, at least two other reports failed to find an association between hostility and night-day BP ratios (Ituarte et al., 1999; Linden et al., 2008). Methodological differences in the measurement of hostility and BP patterns may partially explain disparate findings. For example, we defined sleep and wake periods based upon participants' reports and actigraphy recordings as opposed to using standard night and day cut-off times, as is often done in other studies. We also calculated the ratio of nighttime BP relative to daytime BP instead of using a nighttime “difference” value, and averaged across two nights of recording to increase the reliability of sleep-wake estimates.
The fact that individuals higher in hostility took longer to fall asleep and had higher sleep-wake MAP ratios suggests one potential mechanism that may link hostility and/or anger to BP rhythms. A recent Finnish study reported a positive relationship between hostility and sleep disturbances (Grano et al., 2008), and trait anger was related to a variety of self-reported sleep problems, including longer sleep latency, in a large study of Korean adults (Shin, Kim, Yi, Lee, Lee, & Shin, 2005). Individuals high in hostility may have delayed onset of sleep due to rumination or elevated arousal following their daily interactions (Hall, Buysse, Dew, Prigerson, Kupfer, & Reynolds, 1997). If the sleep window is considered to be an extended restorative period, negative experiences during the day may hinder physiological recovery at night, resulting in difficulties initiating sleep and a blunted decline in BP (e.g., Hall et al., 2004).
We also observed an inverse association between life purpose scores and sleep-wake MAP ratios. Although individuals high in life purpose also spent less time in Stage 1 sleep, this did not influence the strength of the association between life purpose scores and nocturnal dips in BP. Moreover, life purpose remained a significant correlate of BP ratios after adjustment for levels of hostility. While this is the first study to relate an aspect of well-being with sleep-wake BP ratios, positive emotions and traits have been shown to augment cardiovascular recovery after exposure to stressful stimuli (Fredrickson, Mancuso, Branigan, & Tugade, 2000; Steptoe et al., 2007), and feelings of daytime happiness have been linked to lower BP at night (Shapiro et al., 1997). The life purpose scale used in this study assesses the degree to which one finds personal value in daily activities rather than the experience of positive emotions, but its association with nocturnal decreases in BP highlights the potential importance of assessing positive psychological factors when examining diurnal BP rhythms. The lower sleep-wake ratios among those with a high sense of life purpose may be due to a stress-buffering effect, in which fewer overall stressors are encountered, and/or potential stressors are perceived as less threatening due to increased coping resources (Pressman & Cohen, 2005). It is also possible that feeling life is devoid of meaning or without purpose may hinder cardiovascular recovery at night. However, depressive symptoms, which were correlated with low purpose, were unrelated to the sleep-wake ratios, suggesting that high purpose may be more likely to facilitate recovery.
In addition to using a recovery framework to understand the above results, several other explanations are possible. The chronic experience of interpreting events in a hostile manner may disrupt autonomic balance (Sloan, Shapiro, Bigger, Bagiella, Steinman, & Gorman, 1994), whereas perceptions of life purpose and engagement may protect against such disruptions (Ryff, Singer, & Love, 2004). If so, differences in sympathetic activity may be captured more accurately with nocturnal BP measurements, as there is less variability during the night in posture, activity, social interactions, diet, and other factors that influence BP than during the day. Psychological factors also may be associated with changes in neurohormonal systems that are related to BP rhythms, such as activation of the hypothalamic-pituitary-adrenal axis (Holt-Lunstad & Steffen, 2007). As our data were cross-sectional, conclusions regarding causality cannot be drawn. For example, heightened activity of the sympathetic nervous system may result in high levels of hostility or low purpose in life, perhaps via poor sleep. It is also feasible that individuals who endorse high negative or low positive psychological factors are more likely to be disturbed by study equipment, and subsequently experienced sleep disturbances and higher nocturnal BP. However, this is unlikely as statistical adjustment for sleep fragmentation did not alter the results.
Neither depression nor anxiety was related to sleep-wake BP ratios in the current sample. This is surprising given that these attributes might also be expected to co-vary with the cognitive (e.g., rumination, worry; Pieper & Brosschot, 2005) and physiological processes that have been implicated in impaired sympathetic recovery. It is possible that depression and anxiety need to occur above a certain threshold before associations with nocturnal BP can be observed, as the mean levels reported in our sample were relatively low. A previous study reported that both depression and anxiety were correlated with higher systolic night-day ratios in men, but not in women, suggesting that gender may act as a potential moderator of this relationship (Kario et al., 2001). In addition, the relationship between anxiety and sleep-wake ratios in the current sample was in the hypothesized direction but failed to reach significance, suggesting that we may have lacked sufficient statistical power to detect such an effect.
We used MAP ratios as the outcome in the current paper for ease of reporting results. However, associations between psychological attributes and sleep-wake ratios were consistent whether we examined systolic or diastolic BP. As both elevated night-day ratios and high nocturnal BP appear to be indicators of disease risk (Fagard et al., 2008; Fagard et al., 2009), we also tested whether individual attributes were associated with nocturnal BP after adjusting for daytime levels. Both life purpose and hostility were related to nocturnal BP (data not shown), supporting the theory that nighttime sympathetic activity may be driving the associations between psychological factors and diurnal BP rhythms.
In addition to the cross-sectional nature of the data, several other limitations of the study should be noted. Although one aim was to examine sleep parameters as potential pathways to elevated nocturnal BP, associations between psychological attributes and the selected sleep variables were somewhat limited, underscoring the need for replication in other samples. A related limitation is that our sample was drawn from a large study of volunteers who were free from diagnosed sleep disorders, and, therefore, these findings may not generalize to populations that include adults with diagnosed sleep disorders. Third, the stability of measurements of nocturnal variation in BP has been questioned (Parati & Staessen, 2007). We attempted to address this issue by collecting data over two nights, using a continuous BP ratio rather than a categorical breakdown, and using individual sleep and wake times to determine BP ratios (Stolarz et al., 2002). Finally, although participants were free from diabetes and prevalent heart disease, subclinical or other unmeasured disease may have affected the observed relationships.
In sum, the attributes of life purpose and hostility are associated with sleep-wake BP ratios in a sample of Black and White adults, supporting a potential pathway between psychological factors and disease. For the most part, these relationships appear to be independent of sleep disturbances; however, longer sleep latency may be one mediator linking hostility and high sleep-wake BP. Longitudinal data would be beneficial in determining the causal pathways between psychological factors, sleep, and diurnal BP rhythms.
Acknowledgements
This research was supported by grants RR024153, CTSA-RR024153, HL076379, HL065111, HL065112 (KAM), and HL07560 (EJM) from the National Institutes of Health, Bethesda, MD, and under a grant with the Pennsylvania Department of Health (Contract ME-02-384) (SER). The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/hea
Conflicts of Interest: Dr. Daniel Buysse serves as a consultant for: Actelion, Arena, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Respironics, sanofi-aventis, Sepracor, Servier, Stress Eraser, and Takeda Pharmaceuticals. No other author reports a conflict of interest.
References
- Aiyer AN, Kip KE, Marroquin OC, Mulukutla SR, Edmundowicz D, Reis SE. Racial differences in coronary artery calcification are not attributed to differences in lipoprotein particle sizes: the Heart Strategies Concentrating on Risk Evaluation (Heart SCORE) Study. American Heart Journal. 2007;153:328–334. doi: 10.1016/j.ahj.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Dahlstrom WG, Williams RB. Hostility, CHD incidence, and mortality: a 25-year follow-up of 225 physicians. Psychosomatic Medicine. 1983;45:59–63. doi: 10.1097/00006842-198303000-00008. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB., Jr. The Cook-Medley hostility scale: item content and ability to predict survival. Psychosomatic Medicine. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Barnes LLB, Harp D, Jung WS. Reliability generalization of scores on the Spielberger State-Trait Anxiety Inventory. Education and Psychological Measurement. 2002;62:603–618. [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. Journal of Applied Psychology. 1954;38:414–418. [Google Scholar]
- Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Archives of Internal Medicine. 2006;126:846–852. doi: 10.1001/archinte.166.8.846. [DOI] [PubMed] [Google Scholar]
- de la Sierra A, Redon J, Banegas JR, Segura J, Parati G, Gorostidi M, et al. Prevalance and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53:466–472. doi: 10.1161/HYPERTENSIONAHA.108.124008. [DOI] [PubMed] [Google Scholar]
- Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61. doi: 10.1161/HYPERTENSIONAHA.107.100727. [DOI] [PubMed] [Google Scholar]
- Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. Journal of Human Hypertension. 2009 doi: 10.1038/jhh.2009.9. in press. Epub ahead of print retrieved July 8, 2009, from http://www.nature.com/jhh/journal/vaop/ncurrent/abs/jhh20099a.html. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Mancuso RA, Branigan C, Tugade MM. The undoing effect of positive emotions. Motivation and Emotion. 2000;24:237–258. doi: 10.1023/a:1010796329158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grano N, Vahtera J, Virtanen M, Keltikangas-Jarvinen L, Kivimaki M. Association of hostility with sleep duration and sleep disturbances in an employee population. International Journal of Behavioral Medicine. 2008;15:73–80. doi: 10.1080/10705500801929510. [DOI] [PubMed] [Google Scholar]
- Hall M, Buysse DJ, Dew MA, Prigerson HG, Kupfer DJ, Reynolds CF. Intrusive thoughts and avoidance behaviors are associated with sleep disturbances in bereavement-related depression. Depression and Anxiety. 1997;6:106–112. [PubMed] [Google Scholar]
- Hall M, Vasko R, Buysse DJ, Ombao H, Chen Q, Cashmere JD, et al. Acute stress affects heart rate variability during sleep. Psychosomatic Medicine. 2004;66:56–62. doi: 10.1097/01.psy.0000106884.58744.09. [DOI] [PubMed] [Google Scholar]
- Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31:795–800. doi: 10.1093/sleep/31.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt-Lunstad J, Steffen PR. Diurnal cortisol variation is associated with nocturnal blood pressure dipping. Psychosomatic Medicine. 2007;69:339–343. doi: 10.1097/PSY.0b013e318050d6cc. [DOI] [PubMed] [Google Scholar]
- Ituarte PH, Kamarck TW, Thompson HS, Bacanu S. Psychosocial mediators of racial differences in nighttime blood pressure dipping among normotensive adults. Health Psychology. 1999;18:393–402. doi: 10.1037//0278-6133.18.4.393. [DOI] [PubMed] [Google Scholar]
- Kario K, Schwartz JE, Davidson KW, Pickering TG. Gender differences in associations of diurnal blood pressure variation, awake physical activity, and sleep quality with negative affect: the work site blood pressure study. Hypertension. 2001;38:997–1002. doi: 10.1161/hy1101.095009. [DOI] [PubMed] [Google Scholar]
- Linden W, Klassen K, Phillips M. Can psychosocial factors account for a lack of blood pressure dipping? Annals of Behavioral Medicine. 2008;36:253–258. doi: 10.1007/s12160-008-9069-0. [DOI] [PubMed] [Google Scholar]
- Loredo JS, Nelesen R, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in normal adults. Sleep. 2004;27:1097–1103. doi: 10.1093/sleep/27.6.1097. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor GA. Sleep actigraphy in hypertensive patients with the `non-dipper' blood pressure profile. Journal of Human Hypertension. 2002;16:237–242. doi: 10.1038/sj.jhh.1001383. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Kamarck TW, Hall MH, Strollo PJ, Owens JF, Buysse DJ, Lee L, Reis SE. Blood pressure dipping and sleep disturbance in African-American and Caucasian men and women. American Journal of Hypertension. 2008;21:826–831. doi: 10.1038/ajh.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. Journal of Hypertension. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- Ostir GV, Berges IM, Markides KS, Ottenbacher KJ. Hypertension in older adults and the role of positive emotions. Psychosomatic Medicine. 2006;68:727–733. doi: 10.1097/01.psy.0000234028.93346.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati G, Staessen JA. Day-night blood pressure variations: mechanisms, reproducibility and clinical relevance. Journal of Hypertension. 2007;25:2377–2380. doi: 10.1097/HJH.0b013e3282f2d116. [DOI] [PubMed] [Google Scholar]
- Paudel ML, Taylor BC, Diem SJ, Stone KL, Ancoli-Israel S, Redline S, et al. Association between depressive symptoms and sleep disturbances in community-dwelling older men. Journal of the American Geriatrics Society. 2008;56:1228–1235. doi: 10.1111/j.1532-5415.2008.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper S, Brosschot JF. Prolonged stress-related cardiovascular activation: is there any? Annals of Behavioral Medicine. 2005;30:91–103. doi: 10.1207/s15324796abm3002_1. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. Hypertension. 1999;33:1099–1104. doi: 10.1161/01.hyp.33.5.1099. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rechtschaffen A, Kales A. US Government Printing Office, Department of Health Education and Welfare; Washington, D.C.: A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects NIH Publication 204. 1986
- Ryff CD, Singer BH, Love GD. Positive health: connecting well-being with biology. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2004;359:1383–1394. doi: 10.1098/rstb.2004.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A re-evaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Wrosch C, Baum A, Cohen S, Martire LM, Matthews KA, et al. The Life Engagement Test: assessing purpose in life. Journal of Behavioral Medicine. 2006;29:291–298. doi: 10.1007/s10865-005-9044-1. [DOI] [PubMed] [Google Scholar]
- Shapiro D, Jamner LD, Goldstein IB. Daily mood states and ambulatory blood pressure. Psychophysiology. 1997;34:399–405. doi: 10.1111/j.1469-8986.1997.tb02383.x. [DOI] [PubMed] [Google Scholar]
- Shin C, Kim J, Yi H, Lee H, Lee J, Shin K. Relationship of trait-anger and sleep disturbances in middle-aged men and women. Journal of Psychosomatic Research. 2005;58:183–189. doi: 10.1016/j.jpsychores.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Sloan RP, Shapiro PA, Bigger JT, Bagiella E, Steinman RC, Gorman JM. Cardiac autonomic control and hostility in healthy subjects. American Journal of Cardiology. 1994;74:298–300. doi: 10.1016/0002-9149(94)90382-4. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto: 1970. [Google Scholar]
- Spira AP, Stone K, Beaudreau SA, Ancoli-Israel S, Yaffe K. Anxiety symptoms and objectively measured sleep quality in older women. The American Journal of Geriatric Psychiatry. 2009;17:136–143. doi: 10.1097/JGP.0b013e3181871345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staessen JA, Thijs L, Fagard R, O'Brien ET, Clement D, de Leeuw PW, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic Hypertension in Europe Trial Investigators. Journal of the American Medical Association. 1999;282:539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- Steffen PR, McNeilly M, Anderson N, Sherwood A. Effects of perceived racism and anger inhibition on ambulatory blood pressure in African Americans. Psychosomatic Medicine. 2003;65:746–750. doi: 10.1097/01.psy.0000079380.95903.78. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Gibson EL, Hamer M, Wardle J. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology. 2007;32:56–64. doi: 10.1016/j.psyneuen.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Steptoe A, O'Donnell K, Marmot M, Wardle J. Positive affect, psychological well-being, and good sleep. Journal of Psychosomatic Research. 2008;64:409–415. doi: 10.1016/j.jpsychores.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Stolarz K, Staessen JA, O'Brien ET. Night-time blood pressure: dipping into the future? Journal of Hypertension. 2002;20:2131–2133. doi: 10.1097/00004872-200211000-00006. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Guilleminault C, Otsuka K, Shiomi T. Blood pressure “dipping” and “non-dipping” in obstructive sleep apnea syndrome patients. Sleep. 1996;19:382–387. doi: 10.1093/sleep/19.5.382. [DOI] [PubMed] [Google Scholar]
- Thase ME, Fasiczka AL, Berman SR, Simons AD, Reynolds CF., III Electroencephalographic sleep profiles before and after cognitive behavior therapy of depression. Archives of General Psychiatry. 1998;55:138–144. doi: 10.1001/archpsyc.55.2.138. [DOI] [PubMed] [Google Scholar]
- Thomas KS, Nelesen RA, Dimsdale JE. Relationships between hostility, anger expression, and blood pressure dipping in an ethnically diverse sample. Psychosomatic Medicine. 2004;66:298–304. doi: 10.1097/01.psy.0000126196.82317.9d. [DOI] [PubMed] [Google Scholar]
- The Report of an American Academy of Sleep Medicine Task Force Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- Verdecchia P, Angeli F, Borgioni C, Gattobigio R, Reboldi G. Ambulatory blood pressure and cardiovascular outcome in relation to perceived sleep deprivation. Hypertension. 2007;49:777–783. doi: 10.1161/01.HYP.0000258215.26755.20. [DOI] [PubMed] [Google Scholar]