Abstract
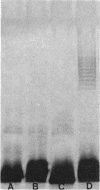
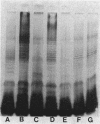
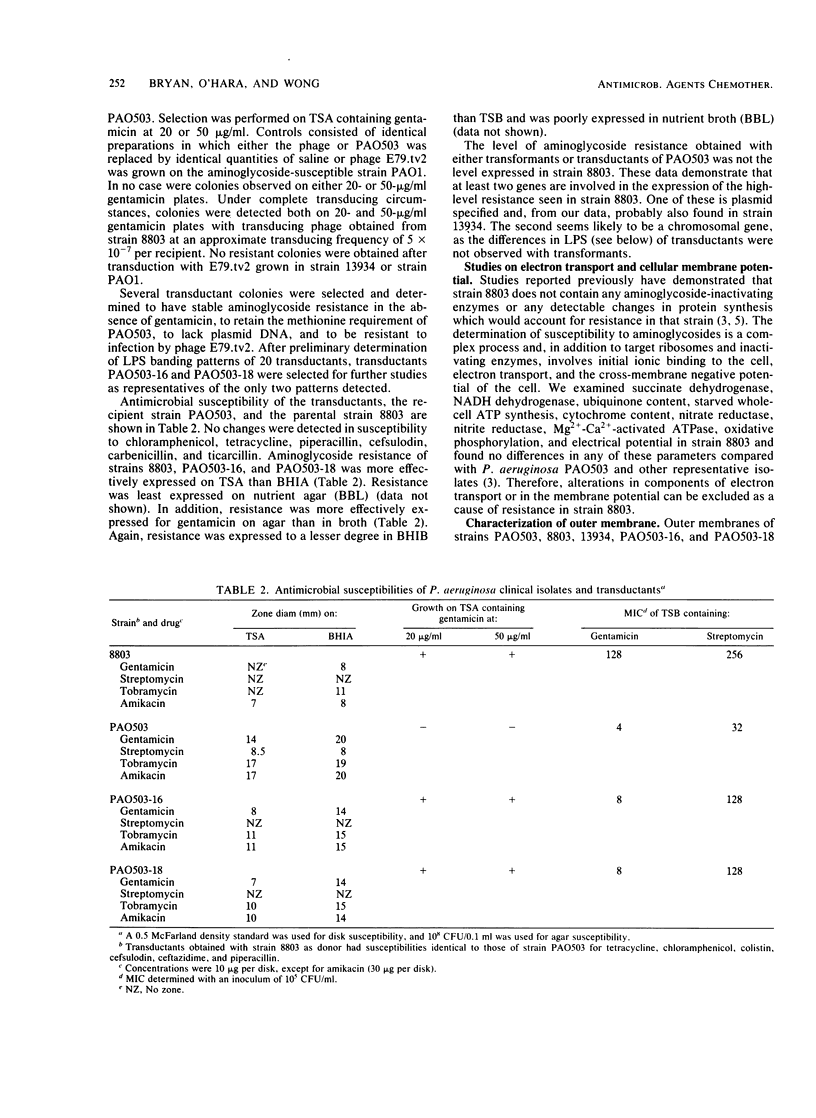
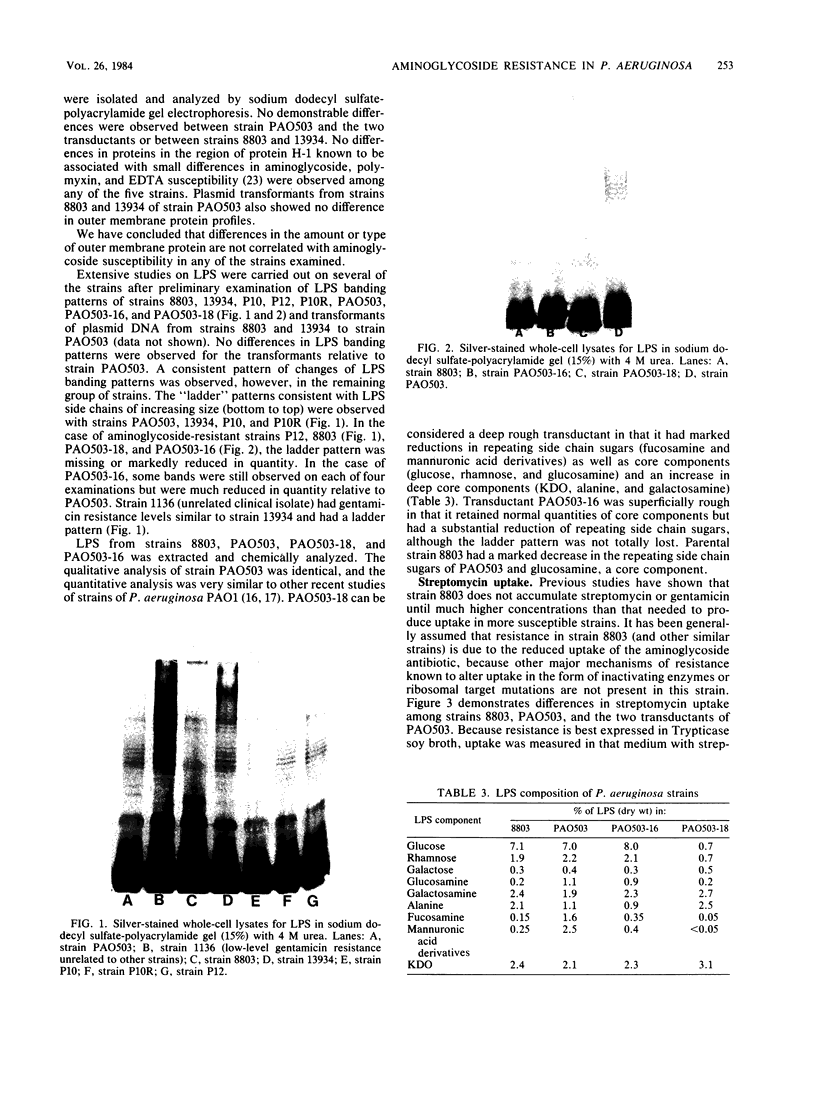
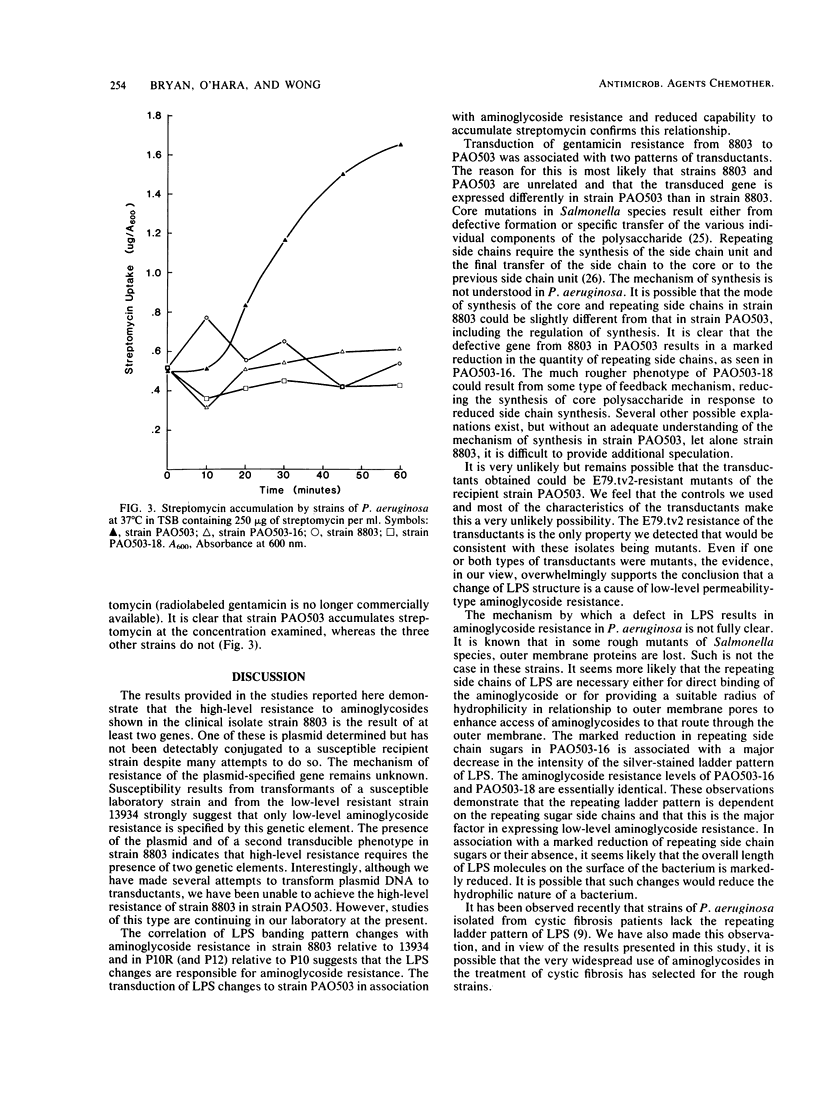
Clinical isolates of Pseudomonas aeruginosa were examined for the basis of impermeability-type aminoglycoside resistance. Two apparently related burn isolate strains with high-level (strain 8803) and low-level (strain 13934) gentamicin resistance each had a plasmid. Transformation of the plasmid from either strain to P. aeruginosa PAO503 resulted in low-level gentamicin resistance. No mechanism for this resistance could be determined. Low-level gentamicin and streptomycin resistance from strain 8803 (but not 13934) was transduced with phage E79.tv2 to PAO503 without transfer of plasmid DNA. Transductants like strain 8803 showed absence or reduction of the lipopolysaccharide (LPS) "ladder" pattern of PAO503, had a change in chemical composition of LPS, and, like strain 8803, had a reduced capability to accumulate streptomycin. Comparison of the resistant clinical isolates 8803 and P10 with the apparently related but less-resistant strains 13934 and P10R, respectively, showed the latter strains had LPS ladder patterns and the former strains did not. Strain 8803 had normal outer membrane protein profiles, electron transport components, and transmembrane electrical potential relative to PAO503 and has been previously shown to have no detectable gentamicin-modifying enzymes and normal protein synthesis. We conclude that low-level impermeability-type aminoglycoside resistance in P. aeruginosa results from conversion of smooth LPS to superficial or deeper rough LPS phenotypes. High-level resistance apparently results from a plasmid-specified, but as yet unknown, mechanism combined with the preceding change in LPS structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan L. E., Haraphongse R., Van den Elzen H. M. Gentamicin resistance in clinical-isolates of Pseudomonas aeruginosa associated with diminished gentamicin accumulation and no detectable enzymatic modification. J Antibiot (Tokyo) 1976 Jul;29(7):743–753. doi: 10.7164/antibiotics.29.743. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Kwan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother. 1983 Jun;23(6):835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Nicas T., Holloway B. W., Crowther C. Aminoglycoside-resistant mutation of Pseudomonas aeruginosa defective in cytochrome c552 and nitrate reductase. Antimicrob Agents Chemother. 1980 Jan;17(1):71–79. doi: 10.1128/aac.17.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Shahrabadi M. S., van den Elzen H. M. Gentamicin resistance in Pseudomonas aeruginosa: R-factor-mediated resistance. Antimicrob Agents Chemother. 1974 Aug;6(2):191–199. doi: 10.1128/aac.6.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan L. E., Van Den Elzen H. M. Gentamicin accumulation by sensitive strains of Escherichia coli and Pseudomonas aeruginosa. J Antibiot (Tokyo) 1975 Sep;28(9):696–703. doi: 10.7164/antibiotics.28.696. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Van den Elzen H. M. Streptomycin accumulation in susceptible and resistant strains of Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1976 Jun;9(6):928–938. doi: 10.1128/aac.9.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R., Gillies R. R. Further studies in the pyocine typing of Pseudomonas pyocyanea. J Med Microbiol. 1969 Feb;2(1):17–25. doi: 10.1099/00222615-2-1-17. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Mutharia L. M., Chan L., Darveau R. P., Speert D. P., Pier G. B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983 Oct;42(1):170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy D. J., Legeai R. J., O'Callaghan R. J. Klebsiella neonatal injections: mechanism of broadening aminoglycoside resistance. Antimicrob Agents Chemother. 1980 Oct;18(4):542–548. doi: 10.1128/aac.18.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Genetics of Pseudomonas. Bacteriol Rev. 1969 Sep;33(3):419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Knirel YuA, Vinogradov E. V., Shashkov A. A., Dmitriev B. A., Kochetkov N. K., Stanislavsky E. S., Mashilova G. M. Somatic antigens of Pseudomonas aeruginosa. The structure of O-specific polysaccharide chains of P. aeruginosa O:3(a),c and O:3a,d,e lipopolysaccharides. Eur J Biochem. 1983 Aug 1;134(2):289–297. doi: 10.1111/j.1432-1033.1983.tb07564.x. [DOI] [PubMed] [Google Scholar]
- Kuzio J., Kropinski A. M. O-antigen conversion in Pseudomonas aeruginosa PAO1 by bacteriophage D3. J Bacteriol. 1983 Jul;155(1):203–212. doi: 10.1128/jb.155.1.203-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Mercer A. A., Loutit J. S. Transformation and transfection of Pseudomonas aeruginosa: effects of metal ions. J Bacteriol. 1979 Oct;140(1):37–42. doi: 10.1128/jb.140.1.37-42.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills B. J., Holloway B. W. Mutants of Pseudomonas aeruginosa that show specific hypersensitivity to aminoglycosides. Antimicrob Agents Chemother. 1976 Sep;10(3):411–416. doi: 10.1128/aac.10.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. F. Isolation and characterization of Pseudomonas aeruginosa R' plasmids constructed by interspecific mating. J Bacteriol. 1982 Feb;149(2):654–661. doi: 10.1128/jb.149.2.654-661.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Moellering R. C., Jr In-vivo acquisition of two different types of aminoglycoside resistance by a single strain of Klebsiella pneumoniae causing severe infection. Ann Intern Med. 1982 Feb;96(2):176–180. doi: 10.7326/0003-4819-96-2-176. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini F., Peters B. P. Fluorometric analysis of amino sugars and derivatized neutral sugars. Anal Biochem. 1982 Jul 1;123(2):357–363. doi: 10.1016/0003-2697(82)90458-4. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Bray D., Dankert B. M., Wright A. Direction of chain growth in polysaccharide synthesis. Science. 1967 Dec 22;158(3808):1536–1542. doi: 10.1126/science.158.3808.1536. [DOI] [PubMed] [Google Scholar]
- Shannon K., Phillips I. Mechanisms of resistance to aminoglycosides in clinical isolates. J Antimicrob Chemother. 1982 Feb;9(2):91–102. doi: 10.1093/jac/9.2.91. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Weinstein R. A., Nathan C., Gruensfelder R., Kabins S. A. Endemic aminoglycoside resistance in gram-negative bacilli: epidemiology and mechanisms. J Infect Dis. 1980 Mar;141(3):338–345. doi: 10.1093/infdis/141.3.338. [DOI] [PubMed] [Google Scholar]