Abstract
Pro-inflammatory cytokines IL-1β and TNFα play important roles in the manifestation of arthritis by disrupting the anabolic and catabolic activities of the chondrocytes. We observed a novel mechanism of cartilage regulation by which muscle cells diminish the response of chondrocytes to IL-1β and TNFα. We found that chondrocytes cocultured with muscle cells or cultured in muscle cell-conditioned medium significantly enhanced the expression of cartilage matrix proteins (collagen II and collagen IX) and resisted IL-1β and TNFα-induced cartilage damage. Our data suggest that this effect is achieved by inhibiting the expression of key components of the signaling pathways of pro-inflammatory cytokines (including NFκB, ESE-1, Cox-2 and GADD45β), leading to attenuated expression of cartilage degrading enzymes (MMPs and ADAMTS4). Therefore, our work unveils a potential role of muscle in regulating cartilage homeostasis and response to pro-inflammatory stimuli, and provides insights on designing treatment strategies for joint degenerative diseases such as arthritis.
Keywords: muscle, pro-inflammatory cytokines, cartilage, matrix
INTRODUCTION
Inflammation of the joint leads to chronic pain and swelling in arthritis [1]. Pro-inflammatory cytokines in the inflamed joint, most notably IL-1β and TNFα, disrupt the catabolic and anabolic balance of the cartilage cells (i.e. chondrocytes). This leads to the destruction of cartilage extracellular matrix (ECM), whose major protein components are collagens and proteoglycans [2]. Collagen type II is the major collagen in cartilage that makes up over 90% of collagen mass [1]. Other collagens, including collagen IX and XI, play important roles in stabilizing the structure of collagen type II [3]. Pro-inflammatory cytokine-induced destruction of collagen and other ECM is mediated by the signaling pathways of MAP kinases and NFκB [2]. These events subsequently lead to two effects on cartilage gene expression. One effect is the induction of metalloproteinases MMPs and ADAMTS, which leads to the degradation of collagen and aggrecan at the protein level. This effect is mediated by transcription factors such as AP-1 [2]. The other effect is the inhibition of transcription of cartilage matrix proteins such as collagen II, which reduces the cartilage matrix production at the mRNA level [2]. This effect is mediated by transcription factors EGR-1, ESE-1 and GADD45β, as well as by cyclooxygenase-2 (Cox-2) [4–6].
Under normal conditions, cartilage homeostasis is regulated by factors expressed within the cartilage tissue as well as tissues surrounding the cartilage [2]. Muscle is a tissue that lies immediately next to the developing cartilage in the embryo [7]. While cartilage does not directly contact muscle tissue in the adult joint (except at the temporomandibular joint), it remains in close proximity to cartilage throughout life [8]. Multiple pieces of evidence suggest that muscle may regulate skeletal development. For example, when muscle in the chick embryo was paralyzed by botulinum toxin, which abolished muscle contraction and caused muscle atrophy, the embryo showed abnormal joint formation and shortened bones [9]. Mouse mutants that lack muscle-specific proteins such as dystrophin/utrophin or myogenin also exhibited skeletal abnormalities including a curved spine or a reduced skeletal size [10, 11]. Similarly, mouse knockouts of Pax7, a marker for muscle progenitor cells, exhibited reduced body sizes as well [12]. Consistent with the phenotype of these mouse mutants, short stature and scoliosis are common features of children with Duchenne Muscular Dystrophy [13, 14]. More recently, we found that chondrocytes cocultured with muscle cells exhibited increased expression of cartilage matrix proteins [15]. Since these muscle cell cultures do not contract, we believe that muscle cells provide biochemical signals to regulate cartilage gene expression [15].
In addition to regulating skeletal development, muscle has also been proposed to be an immunogenic organ [16]. Muscle produces a variety of cytokines (such as IL-6 and IL-15), matrix metalloproteinases (MMPs) and MMP inhibitors (TIMPs) [16–18]. Some of these factors have been shown to mediate exercise-associated anti-inflammatory responses [16]. Furthermore, patients with congenital myopathy showed deformation and fibrosis of the temporomandibular joint, and it has been suggested that reduced muscle strength may be a risk factor for knee arthritis [19, 20].
Despite the implication of muscle in regulating inflammation, the role of muscle cells on the response of chondrocytes to pro-inflammatory cytokines has never been reported. Here we show that chondrocytes cocultured with muscle cells are more resistant to cartilage destruction induced by pro-inflammatory cytokines, suggesting a novel role of muscle cells in regulating catabolic and anabolic processes in cartilage tissue.
MATERIALS AND METHODS
Cell culture
Murine myoblasts (C2C12) and NIH3T3 cells were purchased from American Type Culture Collection. RCS chondrocytes were a generous gift from Dr. Andrew Lassar (Harvard Medical School). The cells were seeded at a density of 104/well of 24 well plates. All cocultures were seeded at a ratio of 2:1 (RCS:C2C12). C2C12 muscle cell-conditioned medium was prepared as described [15]. Cells were cultured in DMEM with 10% FBS (Hyclone) and 1% pen/strep for 3 days before 3 days of IL-1β or TNFα (Peprotech) treatment.
Immunocytochemistry
Cultures were fixed with 4% paraformaldehyde and incubated with primary antibodies overnight. The primary antibodies were: mouse anti-Collagen II (generous gift from Dr. Tom Linsenmayer, Tufts University); mouse anti-Collagen IX (D1–9, Dev. Stud. Hybr. Bank); rabbit anti-Desmin (Abcam Cat#12500). The secondary antibodies were: goat anti-mouse/rabbit conjugated with Alexa 488 (green) or 594 (red) (Invitrogen). Images were taken using the Olympus IX71 inverted microscope and Olympus DP70 digital camera. Relative protein levels were quantified by analyzing pixel intensities of fluorescent signals using the program Image J [21]. Values of pixel intensity were normalized to the total chondrocyte numbers, which were determined by their round cell morphology and collagen II protein expression. Three repeats were carried out for each experiment, and for each experiment 3–10 views were photographed for quantification. For statistical analysis, the mean and standard deviation were calculated. Statistically significant differences (i.e. P<0.05) were determined by one-way analysis of variance (ANOVA).
Western Blot analysis
For Western Blot analysis, total protein lysates were obtained from confluent 6cm tissue culture plates that contained roughly 3×106 cells, following a standard protocol. The proteins were separated by SDS-PAGE and blotted using the BioRad apparatus. The membranes were hybridized with the following antibodies overnight: rabbit anti-Collagen II (Abcam, Ab34712), rabbit anti-Desmin (Abcam, Ab12500), mouse anti-Collagen IX (Chemicon/Millipore, MAB3304) and mouse anti-GAPDH (Abcam, Ab8245). After repeated washing, the membranes were hybridized with secondary antibodies of goat anti-mouse or anti-rabbit HRP-conjugated antibodies (Calbiochem). The signals were developed using ECL substrate (Pierce, cat# 32106), and films exposed to chemiluminescent signals were developed in Kodak M35A-X-OMAT processor.
RT-PCR analysis
Total RNA was isolated using the RNeasy mini kit (Qiagen) [15]. All PCR analyses were normalized based on GAPDH expression using the iQ5 Real Time PCR Detection System (BioRad). Rat primer sequences are (5’ to 3’): GAPDH (NCBI#NM_017008), 1131-GTTGCTGAGGAGTCCCCA-1147 (Forw) and 1258-CCTATTCGAGAGAAGGGA-1241 (Rev); Col IIa1 (NCBI#NM_012929.2), 1972-AAGCAAGGTGACCAGGGTATTCCT-1995 (Forw) and 2255-TTCTCGCCAACATCACCTCTGTCT-2232 (Rev); Col IX (NCBI#NM_001108675), 1961-TCGTGGATGTGGTGCTGAAGATGA-1984 (Forw) and 2100-ATTGGGTCCCTGTTTGCCTGGATA-2083 (Rev); aggrecan (NCBI#NM_0221190.1), 1363-AAGGACTGTCTATCTGCACGCCAA-1386 (Forw) and 1487-TCACCACCCACTCCGAAGAAGTTT-1465 (Rev). MMP13 (NCBI#NM_133530.1), 1705-GCTGAAGAGTGATCATAA-1722 (Forw) and 1904-GCTATCTGTTAATGTGTGT-1886 (Rev). MMP9 (NCBI#NM-031055.1), 2869-AGCCCGTTTAAAGTGCATGTGTGC-2892 (Forw) and 2959-GAGTGTCCGAGGAAGATACTTGGT-2936 (Rev). ADAMTS4 (NCBI#XM_341154.1), 1818-AGCAGATGGTTCTTACGCCCTCAA-1841 (Forw) and 2012-AGACGTACATTCTGTGGGTTGCCA-1989 (Rev). NF-κB (NCBI#XM_342346), 3419-AAGGCCATCATATCGTTCCG-3438 (Forw) and 3619-CATACCCCAAGCCACACCGA-3600 (Rev). ESE-1 (NCBI#NM_001024768), 221-CCTGAACAACCAACACATGTCCC-243 (Forw) and 302-CTCAGTTCTGTCCCTTTGGGATCT-279 (Rev). COX-2 (NCBI#NM_017232.3) 1596-ACCTAGCACCTTCGGAGGA-1614 (Forw) and 1694-GAGGCAAAGGGACACCCTT-1676 (Rev). GADD45β (NCBI#NM_001008321), 2-AACCCGGGATCCGTGTCA-19 (Forw) and 187-GCAAAGCACTCGTCCAGATCC-167 (Rev).
RESULTS
Chondrocytes cocultured with muscle cells maintained collagen protein expression upon pro-inflammatory cytokine challenge
We tested the effect of muscle cells on the response to pro-inflammatory cytokines in chondrocytes by coculturing these two cell types. We have previously used the same coculture system to evaluate the effect of muscle cells on cartilage matrix production [15]. In this system, we selected RCS cell line (rat chondrosarcoma cells), which is a commonly used chondrocyte cell line for studying cartilage gene expression [22]. For muscle cells, we selected C2C12 mouse muscle cell line, which is a widely used cell line for studying muscle differentiation [23]. RCS cells generally have a compact morphology and express cartilage marker collagen II, while C2C12 muscle cells have a fibroblast-like morphology and express muscle marker desmin (Fig.1A). In addition, C2C12 muscle cells do not express cartilage markers even when cocultured with chondrocytes (Fig.1A)[15]. This distinction between the two cell types allows us to evaluate protein expression in chondrocytes through immunocytochemistry.
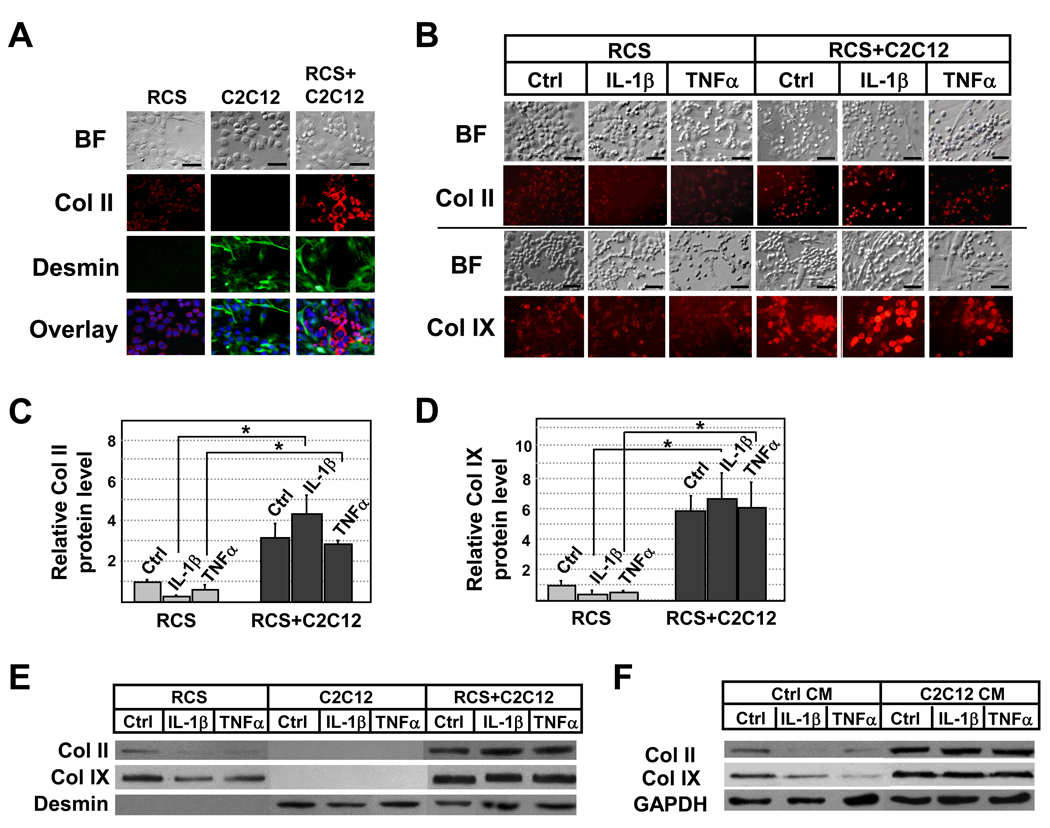
Figure 1. Cartilage-specific collagen expression in muscle and cartilage cell cocultures upon IL-1β (1ng/ml) and TNFα (2ng/ml) treatment.
A. Immunocytochemistry analysis confirming that in cocultures, RCS chondrocytes and C2C12 muscle cells continue to express markers of cartilage (Col II) and muscle (Desmin) respectively. Overlay images of Collagen II (red), Desmin (green) and Dapi (blue) indicate no overlapping expression of Collagen II and Desmin. B. Immunocytochemistry analysis of collagen II and collagen IX proteins upon IL-1β and TNFα treatment. BF, bright field. C and D, Quantification of relative collagen II and collagen IX protein levels based on immunofluorescent intensity (pixels) using the program “Image J”. Values were normalized to total chondrocyte number. Grey bars, RCS alone. Black bars, RCS and C2C12 cocultures. E. Western Blot analysis of collagen II and collagen IX expression on rat chondrocyte lysates. As we were unable to obtain a rat-specific internal control for loading, we normalized protein loading to the number of chondrocytes. Desmin, but not collagen II or collagen IX, in only present in lysates of C2C12 cells. F. Western Blot analysis of collagen II and collagen IX expression in RCS chondrocytes cultured in C2C12 muscle cell-conditioned medium (C2C12 CM). Note: scale bars, 40µm; samples with the same cytokine treatments were compared in statistical analysis, * denotes: P<0.05.
We first assayed cartilage marker Collagen protein expression in chondrocytes treated with pro-inflammatory cytokines IL-1β and TNFα. We found that chondrocytes cultured alone showed reduced Collagen II and Collagen IX expression after the administration of 1ng/ml of IL-1β or 2ng/ml of TNFα (Fig.1B), which is consistent with prior reports [24]. Strikingly, Collagen II and Collagen IX protein expression remained high upon IL-1β or TNFα treatment in chondrocytes that were cocultured with muscle cells (Fig.1B). This result was further quantified using Image J, a program that measures the fluorescent intensity of immunostained images [21] (Fig.1C and 1D). We performed Western Blot analysis and further confirmed that indeed chondrocytes cocultured with muscle cells expressed higher levels of collagen II and collagen IX proteins, which were maintained in the presence of IL-1β or TNFα (Fig. 1E). Since C2C12 cells do not express cartilage markers (Fig.1A and 1F) [15], our analysis suggests that muscle cells enhance the resistance of chondrocytes to pro-inflammatory cytokine-induced collagen reduction. We confirmed the specificity of this effect by coculturing chondrocytes with non-muscle cells NIH3T3 and found that coculturing with NIH3T3 cells did not lead to enhanced collagen II expression in chondrocytes upon IL1β treatment (S-Fig.1). Furthermore, we found that chondrocytes cultured in cell-free muscle cell-conditioned medium exhibited significantly enhanced resistance to pro-inflammatory cytokine-induced collagen destruction, suggesting that this effect is mediated by factors secreted by muscle cells (Fig.1F).
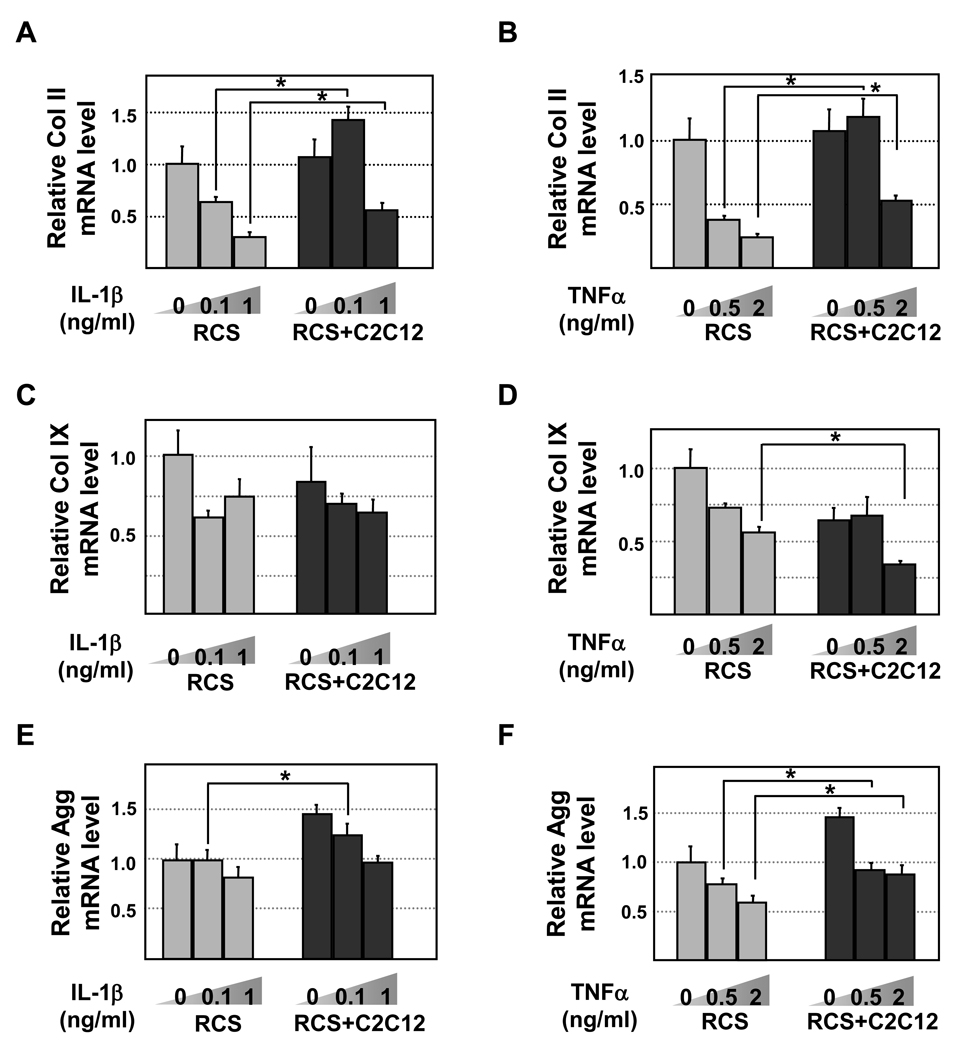
Increased resistance to IL-1β and TNFα-induced reduction of collagen II and collagen IX can be achieved by maintained mRNA production or by reduced protein degradation. To evaluate whether coculturing with muscle cells leads to maintained mRNA expression in chondrocytes treated with IL-1β or TNFα, we performed qRT-PCR analysis on collagen and aggrecan genes. We purposely chose cells from two different species so that the presence of mouse C2C12 cells would not interfere with our RT-PCR analysis of rat chondrocytes when we use rat-specific primers with GAPDH as a loading control. From this analysis, we found that coculturing with muscle cells did not alter the mRNA level of collagen II in the absence of IL-1β and TNFα (Fig.2A and 2B). However, in the presence of increasing amounts of IL-1β (0.1-1ng/ml) or TNFα (0.5–2ng/ml), chondrocytes cocultured with C2C12 muscle cells exhibited significantly higher levels of collagen II mRNA, while control chondrocytes expressed a progressively reduced amount of collagen II mRNA (Fig.2A and 2B). In contrast to the results of collagen II, coculturing with muscle cells did not prevent IL-1β or TNFα-induced collagen IX mRNA downregulation (Fig.2C–2D). Since our earlier results indicated that collagen IX protein expression was maintained in co-cultured chondrocytes upon IL-1β or TNFα treatment (Fig.1D–1F), it suggests that muscle cells may regulate collagen IX expression primarily through a post-transcriptional mechanism. While we were unable to assay aggrecan protein expression due to the lack of a suitable antibody, we found that aggrecan mRNA was maintained at a higher level in cocultured chondrocytes than in chondrocytes cultured alone when treated with low levels of IL-1β or TNFα, although both cytokines did lead to significant reduction of aggrecan expression in cocultured chondrocytes (Fig.2E and 2F).
Figure 2. Quantitative RT-PCR analysis of Col II, Col IX and Aggrecan mRNA levels in chondrocytes.
RCS and RCS-C2C12 co-cultures were subject to two different levels of IL-1β (0.1 and 1ng/ml) or TNFα (0.5 and 2ng/ml). GAPDH was used to for normalization. Solid bars, RCS culture alone. Grey bars, RCS alone. Black bars, RCS and C2C12 cocultures. Samples with the same cytokine treatments were compared in statistical analysis, * denotes: P<0.05. A and B, Collagen II levels upon IL-1β and TNFα treatment, respectively. C and D, Collagen IX levels upon IL-1β and TNFα treatment, respectively. E and F, Aggrecan levels upon IL-1β and TNFα treatment, respectively.
Taken together, we found that chondrocytes cocultured with muscle cells exhibited resistance to IL-1β and TNFα-induced collagen reduction. In addition, muscle cells may regulate cartilage gene expression at both the transcriptional level and the post-transcriptional level.
Coculturing with muscle cells leads to reduced expression of cartilage degrading enzymes
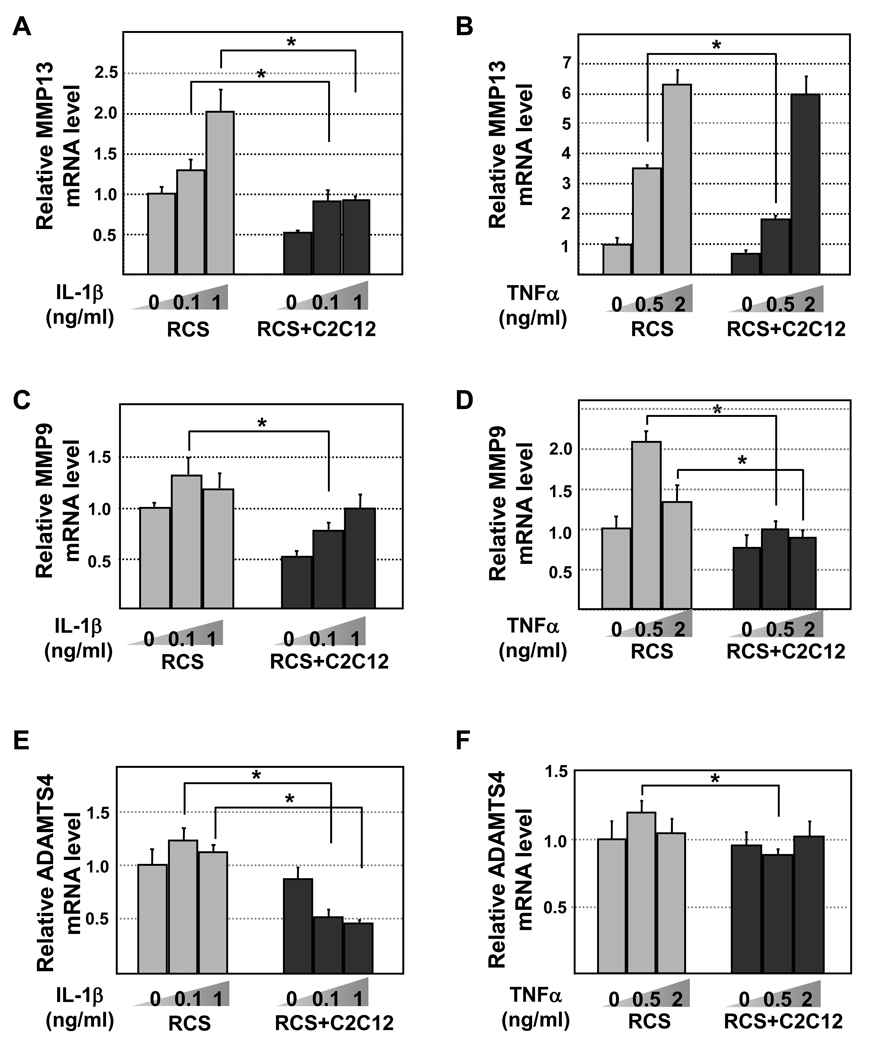
Cartilage degrading enzymes play important roles in regulating cartilage matrix expression at the post-transcriptional level [25]. Thus we evaluated the expression of three such enzymes MMP13, MMP9 and ADAMTS4 [2]. We found that increasing amounts of IL-1β (from 0.1ng/ml to 1ng/ml) or TNFα (from 0.5 to 2ng/ml) led to a progressive increase in MMP13 expression in RCS chondrocytes cultured alone (Fig. 3A and 3B). However, IL-1β or TNFα (especially at low concentrations) did not induce a significant increase in MMP13 expression in chondrocytes cocultured with muscle cells (Fig.3A and 3B). Similarly, the expression level of other metalloproteinases MMP9 and ADAMTS-4 were also generally lower in chondrocytes co-cultured with muscle cells (Fig.3C–3F).
Figure 3. Quantitative RT-PCR analysis of MMP13, MMP9 and ADAMTS4 mRNA levels in chondrocytes.
RCS and RCS-C2C12 co-cultures were subject to two different levels of IL-1β (0.1 and 1ng/ml) or TNFα (0.5 and 2ng/ml). GAPDH was used to for normalization. Grey bars, RCS alone. Black bars, RCS and C2C12 cocultures. Samples with the same cytokine treatments were compared in statistical analysis, * denotes: P<0.05. A and B, MMP13 levels upon IL-1β and TNFα treatment, respectively. C and D, MMP9 levels upon IL-1β and TNFα treatment, respectively. E and F, ADAMTS4 levels upon IL-1β and TNFα treatment, respectively.
Coculturing with muscle cells reduces pro-inflammatory cytokine signaling in chondrocytes
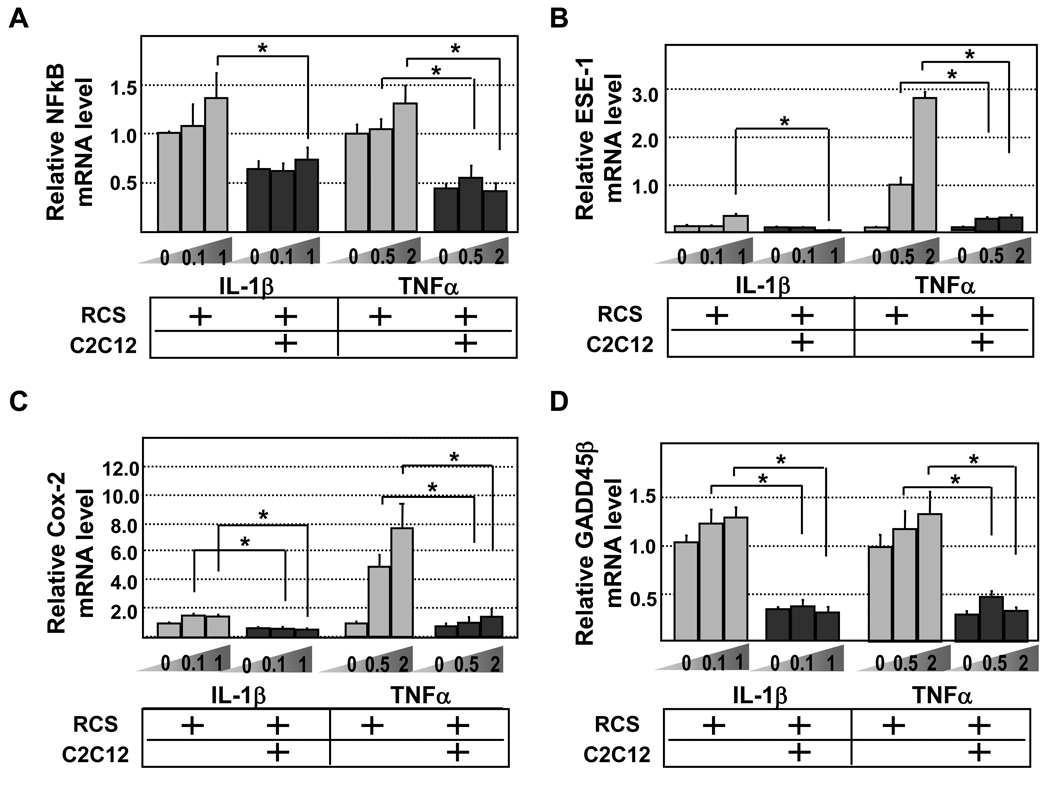
We next evaluated whether reduced expression of cartilage degrading enzymes in chondrocytes cocultured with muscle cells correlates with reduced expression of downstream mediators of pro-inflammatory cytokines. As NFκB plays a key role in the signal transduction of pro-inflammatory cytokines [2], we evaluated the mRNA level of p105, which encodes a crucial NFκB subunit. We found that chondrocytes cocultured with muscle cells generally expressed lower levels of NFκB either in the absence or presence of IL-1β or TNFα (Fig.4A). To evaluate the activation of NFκB signaling, we assayed the expression of NFκB-induced factors. ESE1 (epithelial-specific ETS factor1) is a direct target of NFκB, and can inhibit collagen II expressionby binding to its promoter [2]. Indeed, we found that ESE-1 expression in control chondrocytes was induced by both IL-1β and TNFα (Fig.4B). Interestingly, ESE-1 expression was significantly lower in cocultured chondrocytes treated with IL-1β or TNFα (Fig. 4B). Furthermore, Cox-2, a factor induced by ESE-1 and mediates the IL-1β-induced inflammatory response, was also expressed at a lower level upon pro-inflammatory cytokine challenge in chondrocytes cocultured with muscle cells (Fig.4C). Finally, we assayed the expression of another NFκB-induced factor, GADD45β, which also inhibits collagen II mRNA expression [2, 6]. Similar to ESE-1 and Cox-2, GADD45β was expressed at a much lower level in chondrocytes cocultured with muscle cells upon IL-1β or TNFα challenge (Fig.4D). Therefore, we concluded that chondrocytes cocultured with muscle cells elicited an attenuated response to pro-inflammatory cytokines, leading to the reduced expression of cartilage-degrading enzymes, and the enhanced resistance to cytokine-induced reduction of cartilage matrix at both the mRNA and protein level.
Figure 4. Quantitative RT-PCR analysis of NFκB, ESE-1, Cox-2 and GADD45β mRNA levels in chondrocytes.
RCS and RCS-C2C12 co-cultures were subject to two different levels of IL-1β (0.1 and 1ng/ml) or TNFα (0.5 and 2ng/ml). GAPDH was used to for normalization. Grey bars, RCS alone. Black bars, RCS and C2C12 cocultures. Samples with the same cytokine treatments were compared in statistical analysis, * denotes: P<0.05. A. NFκB levels upon IL-1β and TNFα treatment, respectively. B. ESE-1 levels upon IL-1β and TNFα treatment, respectively. C. Cox-2 levels upon IL-1β and TNFα treatment, respectively. D. GADD45β levels upon IL-1β and TNFα treatment, respectively.
DISCUSSION
We observed a novel mechanism of muscle-mediated cartilage regulation. We found that in an inflammatory environment, coculturing with muscle cells significantly enhanced the expression of collagen II and collagen IX proteins in chondrocytes, while reducing the expression of cartilage-degrading enzymes. We suggest that this effect is achieved by inhibiting the expression of key components of the signaling pathways (such as NFκB) that mediate the activity of pro-inflammatory cytokines in chondrocytes. Since culturing in muscle cell-conditioned medium led to significantly enhanced resistance to pro-inflammatory cytokine-induced collagen destruction in chondrocytes, we suggest that the enhanced resistance to pro-inflammatory cytokine damage is conferred by factors secreted from the muscle cells alone, and is not due to the method of coculturing itself. Therefore, our work indicates a potential role of muscle in regulating cartilage homeostasis in an inflammatory environment [20].
Several factors have shown antagonistic effects to pro-inflammatory cytokine-induced cartilage degradation. One such factor is TGFβ, which inhibited IL-1β-induced collagen II reduction and MMP13 induction, but did not inhibit the expression of aggrecanase ADAMTS-4 [26]. In contrast, in this study, the effect of chondrogenic factor BMP2 on IL-1β-induced cartilage destruction was minimal [26]. While IGF-I has been reported to inhibit pro-inflammatory cytokine-induced collagen degradation, often a large amount of IGF-I is required for this inhibitory activity [27–29]. In addition, components of cartilage ECM such as glucosamine and chondroitin sulfate were shown to be anti-inflammatory, as they inhibit NFκB signaling [30, 31]. Thus it will be of interest to examine the effect of muscle cells on the expression of glucosamine and chondroitin sulfate in chondrocytes.
While it is not yet clear which specific factors in muscle cells contribute to the anti-inflammatory effect we observed in our cocultures, proteomic analyses of muscle cells and muscle cell-conditioned medium have uncovered an array of muscle-derived proteins including IGFs and Wnts [32–34], which may be involved in the anti-inflammatory response. In this investigation, we evaluated our cultures two days after cytokine treatment, a timeline that did not allow us to analyze the expression of immediate early mediators of IL-1β (such as Egr-1 and c-Jun), whose induction occurs quickly (within one hour of IL-1β treatment) but subsequently tapers off over the course of one day [35]. Evaluation of early response genes that are involved in cartilage degradation upon treatment with pro-inflammatory cytokines in our system will further our understanding on the mechanisms of muscle cell-mediated cartilage regulation [2] [36].
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr. David Kaplan (Tufts) for helpful discussions. We thank Drs. Andrew Lassar (Harvard) and Tom Linsenmayer (Tufts) for providing reagents and antibodies. We thank Derrick Hwu and Anthony Bartley for their excellent technical help. This work has been supported by grants to LZ from the Arthritis National Research Foundation and from the NIH (AR054611).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring MB, Otero M, Tsuchimochi K, et al. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis. 2008;67 Suppl 3:iii75–iii82. doi: 10.1136/ard.2008.098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaschke UK, Eikenberry EF, Hulmes DJ, et al. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J Biol Chem. 2000;275:10370–10378. doi: 10.1074/jbc.275.14.10370. [DOI] [PubMed] [Google Scholar]
- 4.Mastbergen SC, Jansen NW, Bijlsma JW, et al. Differential direct effects of cyclooxygenase-1/2 inhibition on proteoglycan turnover of human osteoarthritic cartilage: an in vitro study. Arthritis Res Ther. 2006;8:R2. doi: 10.1186/ar1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng H, Tan L, Osaki M, et al. ESE-1 is a potent repressor of type II collagen gene (COL2A1) transcription in human chondrocytes. J Cell Physiol. 2008;215:562–573. doi: 10.1002/jcp.21338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ijiri K, Zerbini LF, Peng H, et al. A novel role for GADD45beta as a mediator of MMP-13 gene expression during chondrocyte terminal differentiation. J Biol Chem. 2005;280:38544–38555. doi: 10.1074/jbc.M504202200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckingham M, Bajard L, Chang T, et al. The formation of skeletal muscle: from somite to limb. J Anat. 2003;202:59–68. doi: 10.1046/j.1469-7580.2003.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubota E, Kubota T, Matsumoto J, et al. Synovial fluid cytokines and proteinases as markers of temporomandibular joint disease. J Oral Maxillofac Surg. 1998;56:192–198. doi: 10.1016/s0278-2391(98)90868-0. [DOI] [PubMed] [Google Scholar]
- 9.Drachman D, Sokoloff l. The role of movement in embryonic joint development. Dev Biol. 1966;14:401. [Google Scholar]
- 10.Deconinck AE, Rafael JA, Skinner JA, et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 11.Knapp JR, Davie JK, Myer A, et al. Loss of myogenin in postnatal life leads to normal skeletal muscle but reduced body size. Development. 2006;133:601–610. doi: 10.1242/dev.02249. [DOI] [PubMed] [Google Scholar]
- 12.Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. Embo J. 2004;23:3430–3439. doi: 10.1038/sj.emboj.7600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapaport D, Colletto GM, Vainzof M, M C, et al. Short stature in Duchenne muscular dystrophy. Growth Regul. 1991;1:11–15. [PubMed] [Google Scholar]
- 14.Eiholzer U, Boltshauser E, Frey D, et al. Short stature: a common feature in Duchenne muscular dystrophy. Eur J Pediatr. 1988;147:602–605. doi: 10.1007/BF00442472. [DOI] [PubMed] [Google Scholar]
- 15.Cairns D, Lee P, Uchimura T, et al. The role of muscle cells in regulating cartilage matrix production. J Orthop Res. 2009 doi: 10.1002/jor.21014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nielsen S, Pedersen BK. Skeletal muscle as an immunogenic organ. Curr Opin Pharmacol. 2008;8:346–351. doi: 10.1016/j.coph.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Goetsch SC, Hawke TJ, Gallardo TD, et al. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics. 2003;14:261–271. doi: 10.1152/physiolgenomics.00056.2003. [DOI] [PubMed] [Google Scholar]
- 18.Sun GL, Zhao S, Li P, Jiang HK. Expression of tissue inhibitor of metalloproteinase-1 in progression muscular dystrophy. Neurosci Bull. 2006;22:85–90. [PubMed] [Google Scholar]
- 19.Zanoteli E, Guimaraes AS, Martins RJ, et al. Temporomandibular joint involvement in a patient with centronuclear myopathy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:118–121. doi: 10.1067/moe.2000.107051. [DOI] [PubMed] [Google Scholar]
- 20.Slemenda C, Heilman DK, Brandt KD, et al. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Abramoff M, Magelhaes P, Ram S. Image processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 22.Krejci P, Masri B, Fontaine V, et al. Interaction of fibroblast growth factor and C-natriuretic peptide signaling in regulation of chondrocyte proliferation and extracellular matrix homeostasis. J Cell Sci. 2005;118:5089–5100. doi: 10.1242/jcs.02618. [DOI] [PubMed] [Google Scholar]
- 23.Andres V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132:657–666. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 25.Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res. 2004:S37–S46. doi: 10.1097/01.blo.0000144484.69656.e4. [DOI] [PubMed] [Google Scholar]
- 26.Sandell LJ, Xing X, Franz C, et al. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthritis Cartilage. 2008;16:1560–1571. doi: 10.1016/j.joca.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui W, Cawston T, Rowan AD. Transforming growth factor beta 1 and insulin-like growth factor 1 block collagen degradation induced by oncostatin M in combination with tumour necrosis factor alpha from bovine cartilage. Ann Rheum Dis. 2003;62:172–174. doi: 10.1136/ard.62.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui W, Rowan AD, Cawston T. Modulation of the expression of matrix metalloproteinase and tissue inhibitors of metalloproteinases by TGF-beta1 and IGF-1 in primary human articular and bovine nasal chondrocytes stimulated with TNF-alpha. Cytokine. 2001;16:31–35. doi: 10.1006/cyto.2001.0950. [DOI] [PubMed] [Google Scholar]
- 29.Im HJ, Pacione C, Chubinskaya S, et al. Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1beta-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem. 2003;278:25386–25394. doi: 10.1074/jbc.M302048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gouze JN, Gouze E, Popp MP, et al. Exogenous glucosamine globally protects chondrocytes from the arthritogenic effects of IL-1beta. Arthritis Res Ther. 2006;8:R173. doi: 10.1186/ar2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jomphe C, Gabriac M, Hale TM, Heroux L, Trudeau LE, Deblois D, Montell E, Verges J, du Souich P. Chondroitin sulfate inhibits the nuclear translocation of nuclear factor-kappaB in interleukin-1beta-stimulated chondrocytes. Basic Clin Pharmacol Toxicol. 2008;102:59–65. doi: 10.1111/j.1742-7843.2007.00158.x. [DOI] [PubMed] [Google Scholar]
- 32.Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 33.Kislinger T, Gramolini AO, Pan Y, et al. Proteome dynamics during C2C12 myoblast differentiation. Mol Cell Proteomics. 2005;4:887–901. doi: 10.1074/mcp.M400182-MCP200. [DOI] [PubMed] [Google Scholar]
- 34.Chan XC, McDermott JC, Siu KW. Identification of secreted proteins during skeletal muscle development. J Proteome Res. 2007;6:698–710. doi: 10.1021/pr060448k. [DOI] [PubMed] [Google Scholar]
- 35.Goldring MB, Birkhead JR, Suen LF, et al. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J Clin Invest. 1994;94:2307–2316. doi: 10.1172/JCI117595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandell LJ. Anabolic factors in degenerative joint disease. Curr Drug Targets. 2007;8:359–365. doi: 10.2174/138945007779940142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.